Abstract
Background
This study was performed to characterize the metabolic, functional, and structural cardiac changes in a canine model of radiation‐induced heart disease by serial in vivo imaging and ex vivo analyses.
Methods and Results
Thirty‐six dogs were randomly assigned to control or irradiated groups at 3 time points (months 3, 6, and 12 after radiation; each group comprised 6 dogs). The left anterior myocardium of dogs in irradiated groups was irradiated locally with a single dose of 20‐Gy X‐ray. The irradiated myocardial regions showed increased myocardial uptake of 18F‐FDG (18F‐fludeoxyglucose) in the irradiated beagles, but the increased uptake area decreased at months 6 and 12 compared with month 3 after radiation. Abnormality of myocardial perfusion and cardiac function were detected at month 6 after radiation. Compared with the control groups, the protein expression of GLUT4 (glucose transporter 4) was upregulated in the irradiated groups, correlating with significantly decreased CPT1 (carnitine acyltransferase 1) expression. Mitochondria degeneration, swelling, and count reduction in the irradiated groups were observed. The difference in CD68 of macrophage markers and the inflammatory cytokines (IL‐6 [interleukin 6], TNF‐α [tumor necrosis factor α]) between the irradiation and control groups was not significant. Furthermore, the progressive aggravation of apoptosis and fibrosis was displayed.
Conclusions
Elevated 18F‐FDG uptake occurred after irradiation and subsequently led to ventricular perfusion defects and dysfunction. The process was associated with myocardial metabolic substrate remodeling, cardiac muscle cell apoptosis, and myocardial fibrosis rather than inflammation.
Keywords: 18F‐FDG PET/CT, apoptosis, inflammation, metabolic substrate remodeling, myocardial fibrosis, radiation‐induced heart disease
Subject Categories: Nuclear Cardiology and PET
Nonstandard Abbreviations and Acronyms
- CPT1
carnitine acyltransferase 1
- 18F‐FDG
18F‐fludeoxyglucose
- GLUT4
glucose transporter 4
- RIHD
radiation‐induced heart disease
Clinical Perspective
What Is New?
Elevated 18F‐FDG (18F‐fludeoxyglucose) uptakes occurred after irradiation and subsequently led to ventricular perfusion defects and dysfunction.
The elevated 18F‐FDG uptakes were associated with changes in myocardial substrate metabolism rather than inflammation.
The ventricular perfusion defects and dysfunction were associated with cardiac muscle cell apoptosis and myocardial fibrosis, and adverse metabolic remodeling resulted in the cardiac remodeling of radiation‐induced heart disease.
What Are the Clinical Implications?
18F‐FDG positron emission tomography/computed tomography might be a sensitive method for detecting early radiation‐induced myocardial damage.
Substrate metabolism alternation precedes cardiac remodeling after radiation, which may provide new targets for the treatment of radiation‐induced heart disease.
Malignant tumors are an important cause of morbidity and mortality worldwide. 1 Radiotherapy was established as monotherapy or adjuvant therapy for different types of malignancies. Improved advances in radiation therapy could increase cancer survival. 2 Nevertheless, radiation‐induced heart disease (RIHD) related to micro‐ and macrovascular damage leads to cardiac manifestations including pericarditis, acute myocardial infarction, or cardiomyopathy, which were widely reported in patients with breast cancer 3 and Hodgkin lymphoma. 4 RIHD was also associated with an increased risk of long‐term mortality. 5 Consequently, it is of vital importance to assess potential cardiac complications induced by radiotherapy. Radiation‐induced myocardial damage was likely adverse myocardial remodeling in relation to decreased cardiac function without clinical symptoms and ultimately caused heart failure. 6
In the normal adult heart, fatty acid β‐oxidation provides almost 70% of cardiac ATP. However, the failing heart relies more on glucose oxidation than on fatty acid oxidation as the preferred metabolic substrate. 7 The glucose is transported to cardiomyocytes by binding with glucose transporter protein. GLUT4 (glucose transporter 4) is the most abundant glucose transporter in the heart in relation to environmental changes. 7 Proteome study of cardiac tissue response to irradiation found that the most obvious downregulation of proteins was related to fatty acid metabolism. 8 , 9
Sensitive and precise quantification for monitoring or predicting early heart adverse effects in the myocardium is demanding. 10 Positron emission tomography (PET) is the gold standard approach for quantitative evaluation of myocardial metabolism and perfusion by glucose analog 18F‐FDG (18F‐fludeoxyglucose) and 13N‐ammonia due to its superior sensitivity and accuracy in diagnosis. 11 There have been limited clinical studies applying cardiac PET to detect pathologic changes in association with RIHD occurrence.
In this study, we serially investigated myocardial 18F‐FDG uptake of myocardial glucose metabolism after radiation treatment in correlation with quantitative myocardial perfusion. In addition, biochemical and histologic analyses were performed to determine the tissular mechanism underlying imaging findings.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Animals and Experimental Procedures
Thirty‐six healthy, 1‐year‐old, male beagles were provided by Nanjing chaimen Biotechnology Co., Ltd. All animals were placed in stainless steel cages at the Experimental Animal Center of the China Institute for Radiation Protection, in an air‐conditioned facilities (18°C–25°C; relative humidity, 40%–60%; ≥12 air changes per hour), fed with standard certified commercial dog chow twice a day, with water available ad libitum. Thirty‐six beagles were assigned by random number table method to control groups or irradiated groups at each time point (months 3, 6, and 12 after radiation; each group comprised 6 dogs). The left anterior myocardium of dogs in irradiated groups was irradiated with an X‐ray dose of 20 Gy. The control groups underwent sham radiation. Cardiac 18F‐FDG PET/computed tomography (CT), 13N‐ammonia myocardial perfusion imaging, and biochemical and histologic analysis were performed serially. Experiments were performed in accordance with Chinese national legislation and local guidelines, and animal experimentation was conducted as approved by Shanxi Medical University.
Radiation Procedure
The left anterior myocardium of dogs (irradiated groups) was irradiated with an X‐ray dose of 20 Gy. The anterior wall of the left ventricle was outlined as a radiation target in irradiated animals. The targeted volume accounted for a quarter to one‐third of left ventricular volume (Figure 1A). Radiation was delivered in the supine position on a linear accelerator unit (Clinac IX; Varian Medical Systems) using a single 20‐Gy dose with 6MVX‐ray (Figure 1B). Treatment planning was performed by control‐enhanced CT (Discovery VCT64 PET/CT; General Electric), and the dose distribution was determined by a Varian Eclipse TPS system. The target area was verified by cone‐beam CT scanning with a flat‐panel detector (Figure 1C). The dogs in control groups were delivered on a linear accelerator unit without radiation over the same fraction duration.
Figure 1. Images of radiation procedure.
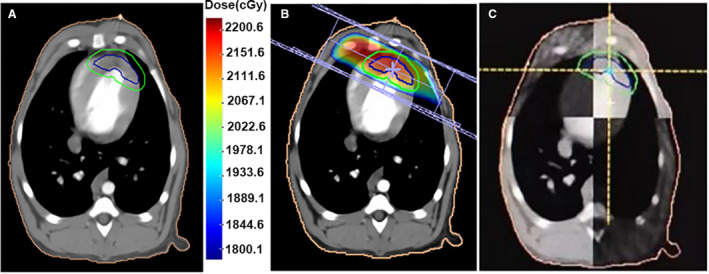
A, Irradiation target area. B, Radiation‐planning images. C, Images of position validation by cone‐beam computed tomography.
18F‐FDG and 13N‐Ammonia PET/CT
After overnight fasting, each dog was subjected to cardiac PET/CT (Discovery VCT; General Electric) after receiving the administration of 13N‐ammonia (148‐185 MBq) by intravenous bolus injection. The dogs then received 2 days of high‐fat diet and >12 hours of fasting. Subsequently, each dog was subjected to 18F‐FDG administration (3.7–5.5 MBq/kg), and 40 to 60 minutes later, a cardiac PET/CT scan was performed. The prescribed high‐fat diet was defined as a diet containing a high‐fat content (fat:protein ratio of 9:1) with almost no carbohydrates.
Image Acquisition and Reconstruction
The cardiac PET/CT scan was performed as described previously. 12 The CT scanning conditions were as follows: tube voltage of 120 kV, tube current of 30 mA, and slice thickness of 5 mm. Afterward, PET data (8 phases per cardiac cycle) were acquired using 3D electrocardiography gated mode and the following specifications: 10 minutes of acquisition time, 128 × 128 matrix, iterative reconstruction, 20 data subsets, 2 iterations, and 6‐mm full width at half maximum.
Image Analysis
In the semiquantitative analysis of 18F‐FDG imaging, the same sizes of the irradiated and nonirradiated fields were drawn manually as regions of interest. The maximal standardized uptake value of the irradiated field and the nonirradiated field were automatically measured by the Volumetrics software. Then the standardized uptake value ratio of the irradiation field to the nonirradiation field was calculated.
For myocardial perfusion imaging, the radioactivity count of the irradiated and the nonirradiated areas were also automatically measured by the Volumetrics software (Discovery VCT; General Electric). The irradiation:nonirradiation activity ratio was then used to calculate.
13N‐ammonia PET/CT images were analyzed using Myovation software to calculate the parameters, including left ventricular (LV) end‐diastolic volume, LV end‐systolic volume, and LV ejection fraction.
Real‐Time Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA from the heart tissue of the dogs was extracted according to the instructions for Trizol reagent (Takara Bio). The cDNA was synthesized using an iScript cDNA synthesis kit (Takara Bio). The cDNA was amplified by a multiple kit (SYBR Premix Ex Taq; Takara Bio) on a StepOnePlus Real‐Time PCR System (Thermo Fisher Scientific). The primers are indicated in Table 1. GAPDH served as an internal control.The 2-∆∆ C t method was used to analyze the relative changes in gene expression.
Table 1.
Primer Sequences for Real‐Time Polymerase Chain Reaction
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| CD68 | ACCCTGCTGCCCTCCTTCAC | GCCTGGTGAGTGGTGGTGTTG |
| IL6 | CGAGCCCACCAGGAACGAAAG | GAAAGCAGTAGCCATCACCAGGAG |
| TNF | GGCGTGGAGCTGACAGACAAC | CGAAGCGGCTGATGGTGTGG |
| GAPDH | AGGAGATGGAACGAGAGGCAGA | CGTGAAGCTGTCGGCATAAGTCC |
Western Blot Analysis
Total protein was isolated from the LV anterior myocardium of the dogs and the frozen sample using RIPA (Boster Bio). Protein concentration was quantified using the BCA Protein Assay Kit (Boster Bio). Equal amounts of proteins (30 μg) were separated by SDS‐PAGE and subsequently transferred electrophoretically to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% skimmed milk at radiotherapy for 4 hours, the membranes were incubated with anti‐CPT1 (1:5000; Abcam), anti‐SLC2A4 (1:1000; Sigma‐Aldrich), anti‐CD68 (1:1000; Abcam), and anti‐β‐actin (1:2000; Abcam) at 4°C overnight, followed by horseradish peroxidase–conjugated secondary antibodies at radiotherapy for 1 hour. Immunoblots were developed with an enhanced chemiluminescence system and quantified by ImageJ software.
Histology
Each dog’s heart was subjected to pathologic testing by Masson staining, tunnel staining, and electron microscopy. In detail, tissue samples from the LV anterior myocardium were obtained, fixed in 10% formalin, and embedded in a paraffin block. From each block, 5‐mm‐thick sections were prepared and stained with Masson trichrome stain and TUNEL (terminal deoxynucleotidal transferase–mediated biotin–deoxyuridine triphosphate nick‐end labeling) assay. Tissue samples (1 mm3) were obtained for electron microscopy, and the specimens were prepared as described previously. 12 Morphologic changes were observed by light microscopy (JEM‐2100; JEOL) and transmission electron microscope (JEM‐2100; JEOL).
Myocardial fibrosis was analyzed using a Leica‐Q 500MC image analysis system (Leica Microsystems) to identify zones of myocardial fibrosis, thereby semiquantitatively determining the collagen volume fraction. Apoptosis detection was performed by TUNEL assay. An apoptotic index expressed as percentage of TUNEL‐positive nuclei to total nuclei was calculated.
Statistical Analysis
Continuous variables are expressed as the mean with 95% confidence. Group data were analyzed using 1‐way and 2‐way ANOVA with Tukey post hoc corrections for multiple comparisons. Statistical analyses were performed using GraphPad Prism 7.0 software. P<0.05 was considered statistically significant.
Results
Animals
All dogs underwent the complete experimental process. Radiation was performed precisely, with the error of cone‐beam CT analysis consistently <5 mm. No heart failure or death occurred in any dogs.
Radiation Induced Increased Myocardial 18F‐FDG Uptake
Neglectable myocardial 18F‐FDG uptake was detected in the control groups. However, focal increased 18F‐FDG uptake in the anterior of the LV myocardium corresponding to the irradiated field was observed in the irradiated dogs (Figure 2A). Nevertheless, the area of the increased uptake gradually decreased over time (Figure 2B). The standardized uptake value ratio of irradiation related to nonirradiation in the irradiated dogs was significantly higher than in the control groups over time (P<0.01) (Figure 2C; Table S1).
Figure 2. 18F‐FDG and 13N‐ammonia PET/CT infused images of each group and parameters of PET/CT.
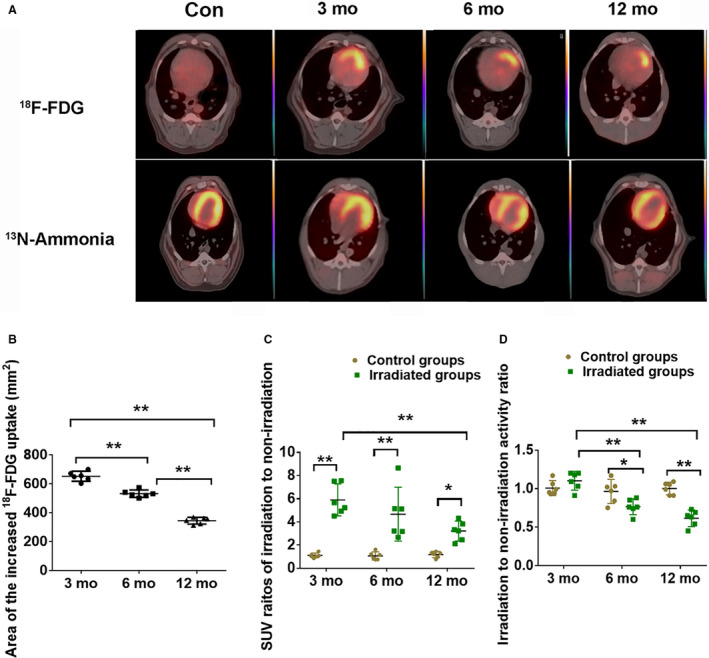
A, Focal increased 18F‐FDG uptake occurred in the irradiated field (upper panel), 13N‐ammonia uptake in the irradiated field decreased at month 6 after radiation and disappeared at month 12 after radiation (bottom panel). B, Area of increased 18F‐FDG uptake. C. Standardized uptake value ratios of irradiation to nonirradiation of 18F‐FDG PET/CT. D, Irradiation:nonirradiation activity ratio of 13N‐ammonia PET/CT. Data are mean with 95% confidence, *P<0.05, **P<0.01. Con indicates control; 18F‐FDG, 18F‐fludeoxyglucose; PET/CT indicates positron emission tomography/computed tomography; and SUV, standardized uptake value.
Myocardial 13N‐Ammonia Uptake Decreased After Irradiation
No abnormal myocardial perfusion was noted in the irradiated myocardial field in the control groups; however, perfusion reduction was observed 6 months after radiation, and perfusion defects were observed 12 months after radiation (Figure 2A). Regional irradiated wall motion abnormalities were observed in 4 dogs at 6 months after radiation and gradually worsened in 6 dogs by 12 months after radiation. In addition, there was a continuously decreased irradiation:nonirradiation activity ratio at 6 and 12 months after radiation compared with the control groups (Figure 2D; Table S1). The irradiated dogs displayed increased myocardial 18F‐FDG uptake and myocardial perfusion defects in the anterior wall of the left ventricle.
Radiation Affected Cardiac Dysfunction
Compared with the control groups, irradiated dogs showed that adverse cardiac remodeling in terms of enlarged end‐diastolic volume and end‐systolic volume and a decreased LV ejection fraction were observed at 6 months and gradually deteriorated by 12 months after radiation compared with the control groups (Figure 3; Table S1).
Figure 3. Comparison of functional parameters of the left ventricle in the control and irradiated groups.
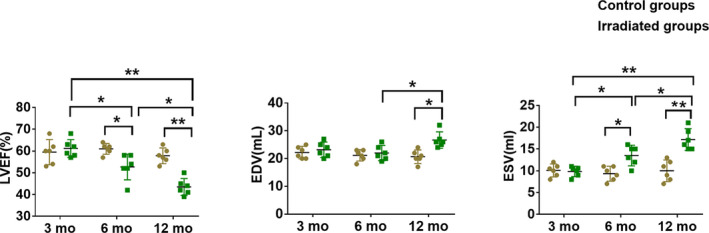
The left ventricular ejection fraction (LVEF) was progressively reduced after radiation (left). The ventricular end‐diastolic volume (EDV) was increased at month 12 after radiation (middle). The end‐systolic volume (ESV) was significantly increased after radiation (right). Data are mean with 95% confidence, *P<0.05, **P<0.01.
Expression of GLUT4 and CPT1 Proteins in Irradiated Myocardial Region
Compared with the control groups in dogs, the protein expression of GLUT4 increased in irradiated groups. In the meantime, we found that the level of GLUT4 was highest at 3 months after radiation and gradually decreased by 6 and 12 months after radiation. However, protein expression of CPT1 decreased when dogs were exposed to radiation (Figure 4A and 4B; Table S1).
Figure 4. Myocardial metabolic substrate remodeling induced increase of 18F‐FDG uptake.
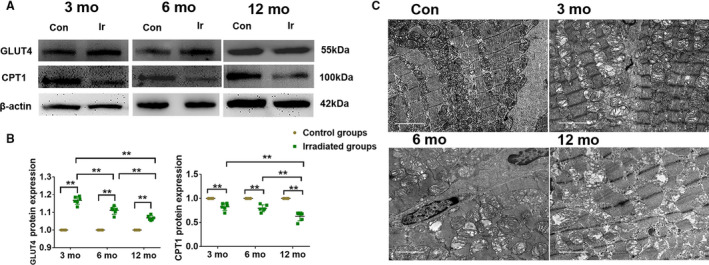
A, Expression of GLUT4 and CPT1 was determined by western blot. β‐actin was a load control. B, Data summary of GLUT4 (left) and CPT1 (right) were expressed as broken‐line graphs of means with 95% confidence of the independent experiments. *P<0.05, **P<0.01. C, Electron microscopy images of the irradiated myocardium of each group, scale bar = 2 μm. Con indicates control; CPT1, carnitine acyltransferase 1; 18F‐FDG, 18F‐fludeoxyglucose; GLUT4, glucose transporter 4; and Ir, irradiated.
Electron Microscopy After Radiation
The disordered arrangement of myofibrils and some mitochondria swelling were observed in the irradiated group at month 3 compared with the control groups. Myocardial injury was aggravated in the irradiated group at month 6, as shown by apparently disorganized myocardial fibers, interstitial fibrosis, and various mitochondrial damage (eg, swelling, degeneration, necrosis, and decreased count). In the irradiated group at month 12, the myocardial damage became more severe, and the interstitial and perivascular fibrosis was even more evident; the mitochondrial swelling and the number decreased were even more obvious (Figure 4C).
Effect of Radiation on CD68 and Inflammation Cytokines
To determine the role of inflammation in the process of increased 18F‐FDG uptake in irradiated myocardium, we also assessed CD68 of macrophage markers and the inflammatory cytokines (IL‐6 [interleukin 6], TNF‐α [tumor necrosis factor α]) between the irradiation and control groups. As shown in Figure 5 and Table S1, no significant differences in mRNA and protein expression of CD68, IL‐6, and TNF‐α were observed in irradiated groups compared with the control groups.
Figure 5. Potential mechanisms of the myocardial inflammation of increased 18F‐FDG uptake.
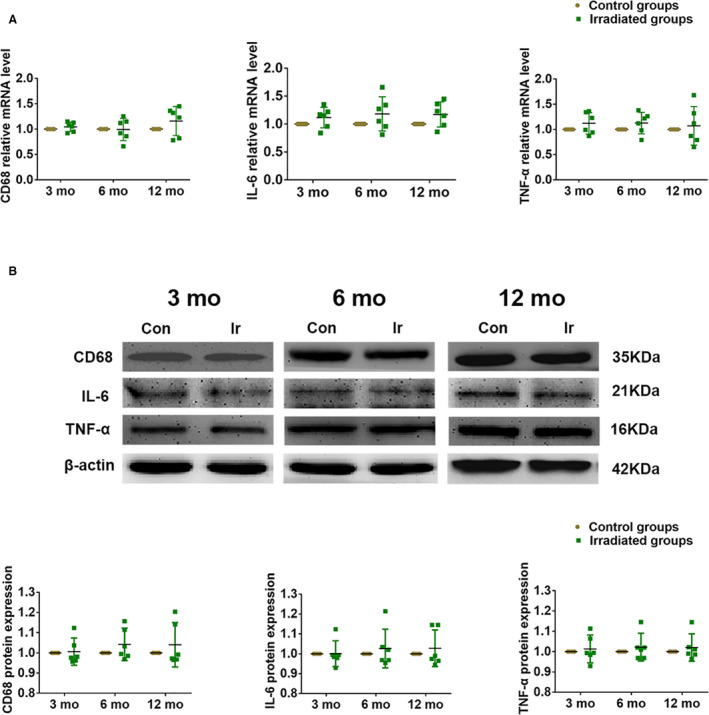
A, The mRNA levels of CD68, IL‐6, and TNF‐α were measured by quantitative reverse transcription polymerase chain reaction. B, The protein expression of CD68, IL‐6, and TNF‐α were determined by western blot. β‐actin was a load control. Quantitative data are presented as mean with 95% confidence. 18F‐FDG indicates 18F‐fludeoxyglucose; IL‐6, interleukin 6; and TNF‐α, tumor necrosis factor α.
Myocardial Fibrosis Aggravated After Irradiation
Our results showed that a clear myocardial fiber texture and almost no collagen staining were seen in the control groups. Pericardial and interstitial fibrosis appeared 3 months after irradiation, and fibrotic tissue gradually increased from 6 to 12 months after irradiation, as determined by Masson staining. Analysis of the images showed that in comparison to the collagen volume fraction of the control groups, those of the irradiated groups were gradually increased (P<0.01) (Figure 6; Table S1).
Figure 6. The Masson staining images and CVF of the irradiated myocardium of each group.
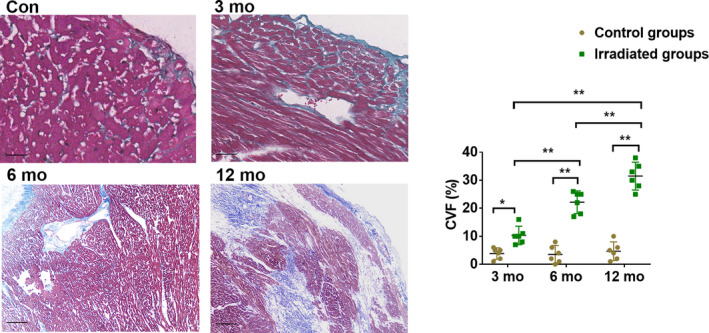
Blue staining of collagen fibers were gradually intensified over time. Scale bar = 100 μm, data are mean with 95% confidence, *P<0.05, **P<0.01. Con indicates control; and CVF, collagen volume fraction.
Radiation Exacerbated Cardiomyocyte Apoptosis
To further characterize radiation on cardiomyocyte apoptosis, we detected apoptotic cardiomyocytes using TUNEL reaction. TUNEL‐positive cells in the control groups were negligible. The number of TUNEL‐positive cells was significantly increased in the irradiated group at month 3. A most marked appearance of TUNEL‐positive cells was observed in the irradiation group at month 12 compared with other irradiated groups (Figure 7; Table S1).
Figure 7. TUNEL assay images and percentage of TUNEL‐positive cells of the irradiated myocardium of each group.
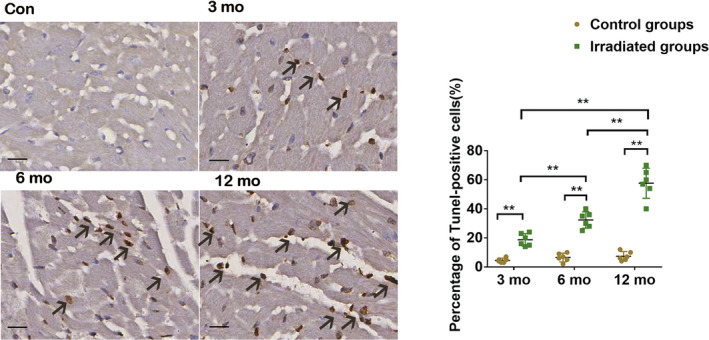
The myocardial apoptosis (arrows point to TUNEL‐positive nuclei) became aggravated in the irradiated groups at months 3, 6, and 12 compared with the control groups. Scale bar = 50 μm, data are mean with 95% confidence, **P<0.01. Con indicates control; and TUNEL, terminal deoxynucleotidal transferase–mediated biotin–deoxyuridine triphosphate nick‐end labeling.
Discussion
To our knowledge, this study is the first to investigate the metabolic and functional perspectives of coordinated imaging to determine cardiac damage at cellular and molecular levels. In this study, 18F‐FDG uptake serially increased in the irradiated myocardial area after radiation exposure over 12 months in association with the adverse alternation of cardiomyocytes. Meanwhile, adverse cardiac outcomes were exacerbated by gradually impaired myocardial blood flow reserve. Our observations indicated that the regional 18F‐FDG uptake in the RIHD might be a sensitive method for detecting early myocardial damage in association with myocardial metabolic substrate remodeling processes.
The cardiac radiation effects were well documented in patients with thoracic malignancy. Jingu et al found that 13 of 64 (20.3%) patients with esophageal cancer showed a focal increase in 18F‐FDG uptake in the basal myocardium corresponding to the irradiated fields after radiotherapy. 13 Similarly, increasing 18F‐FDG foci were detected in irradiated myocardial segments after thoracic radiotherapy. 14 , 15 Evans et al reported a 47% rate of nonmalignant increased 18F‐FDG uptake at irradiated myocardium after stereotactic radiotherapy (20‐Gy isodose line >5 cm3 of the heart). 16 In this study, we simulated clinical high‐dose radiation in large animals. Nevertheless, in correlation with the clinical setting, the beagle model of regional heart irradiation was simulated without clinical confounding factors, such as concurrent chemotherapy, and risk factors for cardiovascular disease. Fasting conditions before 18F‐FDG PET/CT, in theory, could harm the physiologic metabolism of 18F‐FDG in the myocardium. Nevertheless, inconsistent myocardial 18F‐FDG uptake was demonstrated under fasting conditions, 17 , 18 which could compromise the diagnosis of pathologic changes. We introduced a prescan scheme that included 2 days of high‐fat diet and subsequent fasting preparation, showing effective suppression of physiologic myocardial 18F‐FDG uptake in the control animals (Figure 2B) and removing negative interference with the diagnosis of pathologic changes. Moreover, we serially observed a moderately decreased myocardial flow and contractility at 6 months and then progressive worsening at 12 months after irradiation, suggesting that the increase in 18F‐FDG myocardial uptake corresponding to the irradiated field has predictive value regarding radiation‐induced myocardial damage.
RIHD began with inflammatory processes that occurred in response to the radiostimulus and accelerated and was sustained longitudinally. This proinflammatory pathogenesis induced by radiation eventually led to endothelial cell proliferation, myocardial collagen deposition, and late regional dysfunctions of the pericardium. The pioneering pathologic study of RIHD in animal models found that there was an inflammatory reaction in the early stage after myocardial radiation but that it was a self‐limiting process from the acute to chronic phases, and then it was a process of constant progressive reconstruction. 19 , 20 , 21 In our findings, no difference in proinflammation phenotype macrophage marker CD68 and the inflammatory cytokines was assessed in the irradiated groups compared with the control groups. This demonstrated that focal uptake in irradiated myocardium specifically came from cardiac apoptotic tissue during the chronic phase after cardiac injury.
Regarding the myocardial metabolic substrate, the increased 18F‐FDG uptake in irradiated myocardium suggested that the myocardium switched from fatty acid to glucose metabolism as the preferred metabolic substrate. GLUT4 and CPT1 were the major moderators of glucose and fatty acid metabolism, respectively. A radiation‐induced overexpression of GLUT4 and suppressed expression of CPT1 in myocardium were observed, resulting in the metabolic substrate alterations of the myocardium. And the reduction in fatty acid oxidation likely occurred because of reduction of overall mitochondrial function and oxidative capacity, as shown by electron microscopy. Under ischemic and hypoxic conditions, GLUT4 is rapidly transported from inside cardiomyocytes to the cell surface. 22 Correspondingly, Umezawa et al found a decrease in fatty acid metabolism in the irradiated myocardium after fatty acid imaging in patients undergoing thoracic radiotherapy for esophageal cancer. 23 Consequently, increased 18F‐FDG uptake at the irradiated myocardial area was the result, with myocardial metabolic substrate remodeling. We found that the myocardial area of elevated 18F‐FDG uptake was gradually decreased, which had not been reported previously. In concordance, GLUT4 expression steadily decreased over time, resulting in downregulation of glucose metabolism. This pattern of myocardial metabolic alternation was associated with aggravation of local myocardial apoptosis and fibrosis. Because apoptotic myocardium could not absorb 18F‐FDG, the area with increased 18F‐FDG within the irradiated myocardial area diminished.
Regarding long‐term cardiac outcome after radiation, myocardial perfusion defects are related to the time interval following radiation. Visual interpretation could detect perfusion abnormalities in the irradiated areas 6 to 12 months after radiation, which agrees with previous studies. 24 , 25 In addition, progressive cardiomyocyte apoptosis and myocardial interstitial fibrosis in the irradiated area were detected. Consequently, the ventricular perfusion defects and dysfunction might also be associated with cardiomyocyte apoptosis or myocardial interstitial fibrosis. Mitochondrial dysfunction led to progressive myocardial energy failure, along with decreased myocardial blood flow perfusion. This suggested that adverse metabolic remodeling and myocardial ischemia, which might directly or indirectly promote cardiac fibrosis formation and systolic myocardial dysfunction, could result in cardiac remodeling or heart failure.
Beyond monitoring the cardiac toxicity induced by antimalignant radiotherapy, cardiac 18F‐FDG PET might also be effective in the clinic after cardiac stereotactic body radiotherapy for terminating refractory ventricular tachycardias, which could result in adverse cardiac outcomes (eg, heart failure, pericarditis pericardial effusions). 26
Because symptoms are often nonspecific, patients with radiation‐induced cardiac diseases typically present long‐term delayed cardiac damage sustained for years after radiation exposure. Our study provides a valuable coordinated imaging method to monitor disease severity or therapeutic response.
Limitations
This study has the following limitations. First, no myocardial fatty acid imaging was performed at the same time. However, we observed that decreased the level of CPT1 in the irradiated groups and was sustained up to 12 months after radiation; the data initially reflected myocardial metabolic remodeling by radiation. Furthermore, tumors may occasionally undergo cardiac metastasis that results in an increase in 18F‐FDG uptake in the metastatic myocardial tissue 27 ; therefore, it is clinically necessary to determine the cause of increased 18F‐FDG uptake.
Conclusions
Elevated 18F‐FDG uptake occurred after irradiation and subsequently led to ventricular perfusion defects and dysfunction. Furthermore, the process was associated with myocardial metabolic changes rather than inflammation, which lead to the remodeling of cardiac muscle cell apoptosis and myocardial fibrosis. This study may provide new targets for the diagnosis and treatment of RIHD.
Sources of Funding
This study was supported by grants from the Natural Science Foundation of China (81671724), the Natural Science Foundation for Young Scientists of Shanxi Province, China (201701D221251), the Natural Science Foundation of Shanxi Province, China (201801D121337), and Startup Foundation for Doctors of Shanxi Medical University (03201553).
Disclosures
None.
Supporting information
Table S1
Acknowledgments
We give special thanks for the technical support from the Institute of Radiation Protection in China.
(J Am Heart Assoc. 2020;9:e016875 DOI: 10.1161/JAHA.120.016875.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016875
For Sources of Funding and Disclosures, see page 10.
References
- 1. Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Rosenblatt E, Izewska J, Anacak Y, Pynda Y, Scalliet P, Boniol M, Autier P. Radiotherapy capacity in European countries: an analysis of the directory of radiotherapy centres (dirac) database. Lancet Oncol. 2013;14:e79–e86. [DOI] [PubMed] [Google Scholar]
- 3. Recht A. Radiation‐induced heart disease after breast cancer treatment: How big a problem, and how much can‐and should‐we try to reduce it? J Clin Oncol. 2017;35:1146–1148. [DOI] [PubMed] [Google Scholar]
- 4. Aleman B, van den Belt‐Dusebout A, De Bruin M, van't Veer MB, Baaijens M, Boer J, Hart A, Klokman WJ, Kuenen MA, Ouwens G, et al. Late cardiotoxicity after treatment for hodgkin lymphoma. Blood. 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 5. Dent S, Liu P, Brezden‐Masley C, Lenihan D. Cancer and cardiovascular disease: the complex labyrinth. J Oncol. 2015;2015:516450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu H, Xiong M, Xia Y, Cui N, Lu R, Deng L, Lin Y, Rong T. Studies on pentoxifylline and tocopherol combination for radiation‐induced heart disease in rats. Int J Radiat Oncol Biol Phys. 2009;73:1552–1559. [DOI] [PubMed] [Google Scholar]
- 7. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. [DOI] [PubMed] [Google Scholar]
- 8. Azimzadeh O, Sievert W, Sarioglu H, Yentrapalli R, Barjaktarovic Z, Sriharshan A, Ueffing M, Janik D, Aichler M, Atkinson MJ, et al. Ppar alpha: a novel radiation target in locally exposed mus musculus heart revealed by quantitative proteomics. J Proteome Res. 2013;12:2700–2714. [DOI] [PubMed] [Google Scholar]
- 9. Subramanian V, Seemann I, Merl‐Pham J, Hauck SM, Stewart FA, Atkinson MJ, Tapio S, Azimzadeh O. Role of TGF beta and PPAR alpha signaling pathways in radiation response of locally exposed heart: Integrated global transcriptomics and proteomics analysis. J Proteome Res. 2017;16:307–318. [DOI] [PubMed] [Google Scholar]
- 10. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla C. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. [DOI] [PubMed] [Google Scholar]
- 11. Awadalla M, Hassan MZO, Alvi RM, Neilan TG. Advanced imaging modalities to detect cardiotoxicity. Curr Probl Cancer. 2018;42:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan R, Song J, Wu Z, Guo M, Liu J, Li J, Hao X, Li S. Detection of myocardial metabolic abnormalities by 18F‐FDG PET/CT and corresponding pathological changes in beagles with local heart irradiation. Korean J Radiol. 2015;16:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jingu K, Kaneta T, Nemoto K, Ichinose A, Oikawa M, Takai Y, Ogawa Y, Nakata E, Sakayauchi T, Takai K, et al. The utility of 18F‐fluorodeoxyglucose positron emission tomography for early diagnosis of radiation‐induced myocardial damage. Int J Radiat Oncol Biol Phys. 2006;66:845–851. [DOI] [PubMed] [Google Scholar]
- 14. Unal K, Unlu M, Akdemir O, Akmansu M. 18F‐FDG PET/CT findings of radiotherapy‐related myocardial changes in patients with thoracic malignancies. Nucl Med Commun. 2013;34:855–859. [DOI] [PubMed] [Google Scholar]
- 15. Zöphel K, Hölzel C, Dawel M, Hölscher T, Evers C, Kotzerke J. PET/CT demonstrates increased myocardial FDG uptake following irradiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1322–1323. [DOI] [PubMed] [Google Scholar]
- 16. Evans J, Gomez D, Chang J, Gladish G, Erasmus J, Rebueno N, Banchs J, Komaki R, Welsh J. Cardiac 18F‐fluorodeoxyglucose uptake on positron emission tomography after thoracic stereotactic body radiation therapy. Radiother Oncol. 2013;109:82–88. [DOI] [PubMed] [Google Scholar]
- 17. Inglese E, Leva L, Matheoud R, Sacchetti G, Secco C, Gandolfo P, Brambilla M, Sambuceti G. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole‐body 18F‐FDG PET/CT. J Nucl Med. 2007;48:1662–1669. [DOI] [PubMed] [Google Scholar]
- 18. Giorgetti A, Marras G, Genovesi D, Filidei E, Bottoni A, Mangione M, Emdin M, Marzullo P. Effect of prolonged fasting and low molecular weight heparin or warfarin therapies on 2-deoxy-2-[18f]-fluoro‐d-glucose pet cardiac uptake. J Nucl Cardiol. 2018;25:1364–1371. [DOI] [PubMed] [Google Scholar]
- 19. Fajardo LF, Stewart JR. Experimental radiation‐induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 20. Boerma M. Experimental radiation‐induced heart disease: past, present, and future. Radiat Res. 2012;178:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steiner I. Pathology of radiation induced heart disease. Rep Pract Oncol Radiother. 2020;25:178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davey K, Garlick P, Warley A, Southworth R. Immunogold labeling study of the distribution of glut-1 and glut-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H2009–H2019. [DOI] [PubMed] [Google Scholar]
- 23. Umezawa R, Takase K, Jingu K, Takanami K, Ota H, Kaneta T, Takeda K, Matsushita H, Ariga H, Takahashi S, et al. Evaluation of radiation‐induced myocardial damage using iodine-123 beta‐methyl‐iodophenyl pentadecanoic acid scintigraphy. J Radiat Res. 2013;54:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song J, Yan R, Wu Z, Li J, Yan M, Hao X, Liu J, Li S. (13)n‐ammonia pet/ct detection of myocardial perfusion abnormalities in beagle dogs after local heart irradiation. J Nucl Med. 2017;58:605–610. [DOI] [PubMed] [Google Scholar]
- 25. Sioka C, Exarchopoulos T, Tasiou I, Tzima E, Fotou N, Capizzello A, Ragos V, Tsekeris P, Fotopoulos A. Myocardial perfusion imaging with (99 m)tc‐tetrofosmin spect in breast cancer patients that received postoperative radiotherapy: a case‐control study. Radiat Oncol. 2011;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, Goddu SM, Lang A, Cooper DH, Faddis M, et al. Phase i/ii trial of electrophysiology‐guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malik D, Basher R, Vadi S, Mittal B, Bhattacharya A. Cardiac metastasis from lung cancer mimicking as perfusion defect on n-13 ammonia and fdg myocardial viability pet/ct scan. J Nucl Cardiol. 2017;24:1442–1444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


