Abstract
One of the most consistent findings in the depression literature is that stressful life events predict the onset and course of depressive episodes. Cognitive and biological responses to life stressors have both been identified, albeit largely independently, as central to understanding the association between stress and depression. I maintain that the largest advances in the understanding of depression will come from examining the ways that cognitive and biological responses to stressors reciprocally influence one another and, in doing so, contribute to the onset and maintenance of depression. I summarize the cognitive and biological stress responses implicated in depression and then describe the reciprocal ways that they are associated with each other. Finally, I discuss the broader implications of taking this integrated approach and suggest directions and considerations for future research.
Keywords: stress, depression, cognition, biology
One of the most consistent findings in the depression literature is that stressful life events—particularly those that involve the loss of interpersonal relationships or self-esteem—predict the onset and course of depressive episodes. Mazure (1998), for example, found that a stressful life event preceded more than 80% of community cases of depression. Importantly, however, most people do not develop depression following exposure to a stressor, even when they encounter a major negative life event. Thus, a critical question is why some people, in response to stressors, become trapped in a downward spiral that precipitates or exacerbates a depressive episode.
Cognitive theories of depression have been instrumental in answering this question (Beck, 1968; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Consistent with these theories, findings have shown that some people think about stressful events in ways that increase depressed mood and risk for depressive episodes (for a review, see LeMoult & Gotlib, 2019). In other words, cognitive responses to stressors play an important role in determining risk for depression. However, in the last several decades, there has been an explosion of research showing that biological stress reactivity is also central to depression, and there is growing recognition that cognitive and biological responses do not operate in isolation (e.g., Beck & Bredemeier, 2016; Disner, Beevers, Haigh, & Beck, 2011; Hyde, Mezulis, & Abramson, 2008; Koster, De Lissnyder, Derakshan, & De Raedt, 2011; Slavich & Irwin, 2014). Instead, they reciprocally influence one another. Thus, not only do cognitive and biological responses to stressful life events independently increase risk for depression, but also the bidirectional associations between them compound the consequences of either alone (Fig. 1).
Fig. 1.
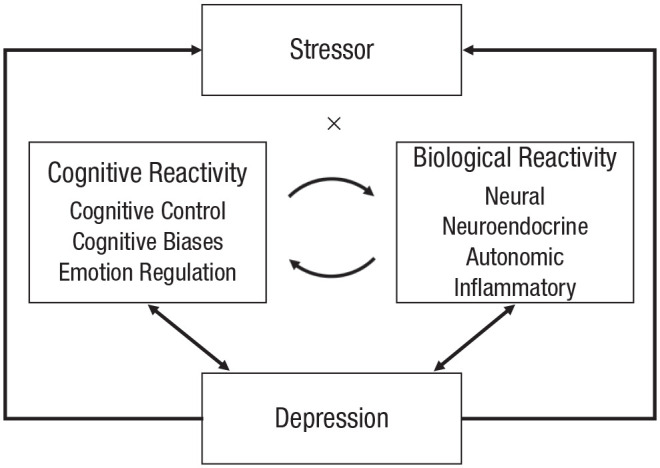
The pathways through which exposure to stressful life events leads to the onset or intensification of depression. In the context of stressor exposure, some individuals show cognitive and biological stress responses that contribute to symptoms of depression. Cognitive stress reactivity and biological stress reactivity contribute independently to symptoms of depression. In addition, cognitive and biological stress reactivity are reciprocally associated with one another, which over time, creates a downward spiral of increasingly maladaptive stress reactivity and greater depression. Further, as per the stress-generation hypothesis, depression leads to higher rates of stressful life events, thereby perpetuating the stress–depression cycle.
In this article, I integrate cognitive and biological science to illuminate the connection between stress and depression. I propose that some individuals show cognitive and biological stress responses that lead to the onset or intensification of depression following stressor exposure. Importantly, cognitive and biological stress reactivity both contribute independently to symptoms of depression and are also reciprocally associated with one another in ways that over time increase maladaptive stress reactivity and depression. Integrating cognitive and biological responses to stress has advantages from both clinical-science and applied perspectives. From a clinical-science perspective, it allows for a better understanding of each stress-response system itself, and it can improve prediction of depression onset and course by offering a more comprehensive understanding of the maladaptive stress response that precipitates or exacerbates depression. From an applied perspective, an integrated approach can identify additional avenues for prevention and intervention, and it can encourage the use of other outcomes to measure change. I first summarize the independent bodies of literature on cognitive stress reactivity and on biological stress reactivity. I then describe how cognitive and biological factors reciprocally influence one another and, in doing so, contribute to the link between stress and depression. Finally, I conclude by briefly discussing the clinical implications and future possibilities for this research.
Independent Bodies of Literature on Cognitive and Biological Stress Reactivity
Cognitive stress reactivity
Cognitive theories of depression often focus on two aspects of cognition: (a) the way people think about, or process, stressful events (e.g., what they look at, what they remember, and how they interpret information) and (b) the way people regulate their emotions in response to stressful events (Beck, 1968; Nolen-Hoeksema et al., 2008). People with depression or at risk for depression process stressful events with a negative bias; for example, they look longer at negative than positive information, recall more negative than positive information, and negatively interpret ambiguous information (for a review, see LeMoult & Gotlib, 2019). Importantly, negatively biased thinking is associated with greater emotional reactivity. Specifically, individuals who demonstrate more negative biases in attention, memory, or interpretation exhibit greater or prolonged distress in response to acute laboratory stressors. Negative cognitive biases also predict a more pernicious course of depression and increase the likelihood that individuals will experience or reexperience a depressive episode, particularly during times of stress (as reviewed in LeMoult & Gotlib, 2019).
The way people regulate their emotions in response to environmental stressors also predicts the onset and severity of depressive episodes (Nolen-Hoeksema et al., 2008). For example, more frequent use of some strategies (e.g., rumination, suppression) and less frequent use of other strategies (e.g., reappraisal) are associated with depressive symptoms (Garnefski & Kraaij, 2006; Nolen-Hoeksema et al., 2008). One emotion-regulation strategy studied commonly in relation to depression is rumination, which is typified by repetitively and passively thinking about negative events and symptoms (Nolen-Hoeksema et al., 2008). Rumination, and particularly the brooding subtype of rumination, precipitates the onset of depressive episodes and increases the severity of current episodes (Nolen-Hoeksema et al., 2008).
Recent research in this area has shown that difficulty controlling the contents of working memory (i.e., cognitive-control deficits) influences the emotion-regulation strategies that people use (Joormann & Tanovic, 2015). In the context of depression, cognitive-control deficits are evidenced by difficulty inhibiting, disengaging from, or manipulating negative material in working memory. As a result, negative material dominates the contents of working memory when stressors are encountered. Cognitive-control deficits have been linked to greater use of some emotion-regulation strategies (e.g., brooding) and less use of other emotion-regulation strategies (e.g., reappraisal). They are also associated with the negative biases in attention and memory described above (Joormann & Tanovic, 2015). Together, these cognitive processes increase emotional reactivity to acute stressors and are a driving force behind the onset of depressive episodes (Joormann & Tanovic, 2015; LeMoult & Gotlib, 2019).
Biological stress response
A substantial, albeit largely independent, literature focuses on the role that the biological stress response plays in depression (Burke, Davis, Otte, & Mohr, 2005; Gotlib & Hamilton, 2008; Rottenberg, Clift, Bolden, & Salomon, 2007; Slavich & Irwin, 2014). In general, stressful life events lead to a host of interconnected and bidirectional biological changes designed to prepare an individual to respond to a stressor (for excellent reviews, see Rottenberg et al., 2007; Slavich & Irwin, 2014; Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012). Here I focus on four of the most commonly studied systems in depression: neural activation, the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nervous system, and the immune system. Mobilization of these interconnected systems is necessary for adaptive response to and recovery from stress. However, chronic or repeated activation of the stress-response system can lead to aberrant biological responses to stressors and, in such instances, can potentiate risk for depression.
Indeed, neural abnormalities are observed in people with depression and those at risk for depression (Gotlib & Hamilton, 2008; Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015). Depression is associated with greater activation in areas of the brain associated with generating emotional responses to stressors (e.g., amygdala) and less activation in areas of the brain associated with regulating emotional responses to stressors (e.g., dorsolateral prefrontal cortex). Depression is also associated with reduced connectivity between emotion-generating and emotion-regulating areas, which researchers suggest hinders people’s ability to regulate depressed mood. Structural changes in this circuitry are also evidenced in depression and risk for depression. Not only do these neural abnormalities typify depressed and at-risk individuals, but also there is increasing evidence that they prolong distress and increase risk for the onset of depressive episodes.
People with depression and who are at risk for depression also show aberrant HPA-axis, autonomic, and immune responses to stress. For example, individuals at risk for depression show elevations in cortisol, a primary stress hormone and marker of HPA-axis activity (LeMoult, Chen, Foland-Ross, Burley, & Gotlib, 2015; although evidence of cortisol dysregulation in depression has been mixed; see Burke et al., 2005; Lopez-Duran, Kovacs, & George, 2009). In turn, cortisol elevations among individuals at risk for depression predict the onset of depressive episodes and increases in depressive symptoms, particularly following a stressful life event (Adam et al., 2010; Halligan, Herbert, Goodyer, & Murray, 2007; LeMoult, Ordaz, Kircanski, Singh, & Gotlib, 2015). Similarly, depression is associated with stressor-induced dysregulation of the autonomic nervous system, measured, for example, by heart rate and respiratory sinus arrhythmia (Rottenberg et al., 2007) and elevations in markers of inflammation, including the proinflammatory cytokines interleukin-1β and interleukin-6 (Slavich & Irwin, 2014). Evidence from experimental studies also indicates that increases in inflammation induce symptoms of sickness, including the emotional and physical symptoms of depression (e.g., sad mood, fatigue), and over the course of months, predict clinically significant depressive episodes (for a review, see Slavich & Irwin, 2014). Thus, taken together, this work suggests a general pattern in which markers of stress dysregulation—often in interaction with acute negative life events—predict the onset of depressive episodes.
The Association Between Cognitive and Biological Stress Reactivity
In recent years, researchers have increasingly focused on examining the interplay between cognitive and biological responses to stress (e.g., Beck & Bredemeier, 2016; De Raedt, Vanderhasselt, & Baeken, 2015; Disner et al., 2011; Gibb, Beevers, & McGeary, 2012; Hyde et al., 2008; Slavich & Irwin, 2014). Integrating these previously separate bodies of literature has been critical in documenting the reciprocal and compounding effect that cognitive and biological responses to stress have on one another and on symptoms of depression. Historically, most research on the association between cognitive and biological stress reactivity focused on brain systems that subserve cognition and emotion (e.g., Ledoux, 1989). Consistent with this original proposition, more recent theories posit that dysfunction in brain regions that promote emotional processing and regulation may underlie maladaptive cognitive responses to stress (for a review, see Disner et al., 2011; Koster et al., 2011).
However, there is reason to focus on the reciprocal associations between cognitive and biological stress reactivity and to consider a wider range of biological systems (e.g., the endocrine, autonomic, and immune systems) that increase risk for depression in the context of environmental stressors. Some of the strongest evidence for the influence of cognition on the biological stress response comes from experimental studies testing whether changes in cognition lead to changes in biology. Toward this goal, researchers have trained individuals to have less negative and more positive biases or to use more adaptive emotion-regulation strategies; they then have examined changes in participants’ biological stress response (e.g., Beevers, Clasen, Enock, & Schnyer, 2015; LeMoult & Joormann, 2014). For example, my colleagues and I documented that training individuals at risk for depression to attend toward positive and away from negative facial expressions was associated 1 week later with less autonomic reactivity to an acute laboratory stressor, evidenced by lower heart rate in anticipation of the stressor (LeMoult, Joormann, Kircanski, & Gotlib, 2016). There are similar benefits of working memory training (Jopling, Gotlib, & LeMoult, 2020). Depressed individuals who received 2 weeks of training to retain positive information and expel negative information from working memory exhibited an attenuated autonomic and neuroendocrine response to an acute laboratory stressor compared with individuals who did not receive the training. Moreover, LeMoult and Joormann (2014) provided experimental evidence that rumination was associated with a prolonged neuroendocrine response to a laboratory stressor, evidenced via cortisol elevations that continued for approximately 60 min after stressor offset. Benefits of cognitive training were also reported by other groups, with training benefits found in the form of reduced inflammation (lower proinflammatory cytokines) and autonomic stress responses (Beevers et al., 2015; Slavich & Irwin, 2014).
Consistent with the formulation that aberrant cognitive and biological stress responses have reciprocal effects on one another, evidence has been found that chronic autonomic, neuroendocrine, and inflammatory dysregulation further alters cognition over time. Specifically, chronic biological dysregulation can alter the brain in ways that disrupt learning, memory, and emotion regulation (e.g., reduced synaptic plasticity, reduced neurogenesis, accelerated cellular aging, neural atrophy; Kim & Diamond, 2002). Consistent with this formulation, findings have shown that biological stress responses and, in particular, chronic cortisol production can adversely alter regions of the brain responsible for regulating emotions and coping with life stress (McEwen, 2006). Similarly, as outlined in Slavich and Irwin’s (2014) social-signal-transduction theory of depression, chronic stress-related increases in inflammation disrupt these same brain regions and, over time, can lead to numerous cognitive and biological consequences as well as increases in depressive symptoms. Thus, taken together, there is evidence that cognitive and biological responses to stressors reciprocally influence one another in ways that, over time, increase maladaptive stress reactivity and risk for depression.
Clinical Implications and Future Directions
The interconnected nature of cognitive and biological stress reactivity allows for multiple opportunities to break the stress–depression link. For example, stress responses may be modulated by neurostimulation (as reviewed by De Raedt et al., 2015), which targets neurocircuits associated with emotion and stress regulation. Alternatively, interventions that target negative cognitive biases (e.g., cognitive therapy) or teach more flexible and adaptive emotion regulation (e.g., rumination-focused cognitive behavioral therapy) might attenuate biological stress responses and improve depressive symptoms (Beck, 1979; Watkins, 2016). However, few studies have tested whether or why biological markers of stress change as a result of treatment. Additional research is needed to understand the course and extent of biological change following a cognitive intervention. Research is also needed to clarify the mechanisms through which biological changes take place, particularly in light of noncognitive factors (e.g., behavioral, interpersonal) that can improve with treatment and thus could be driving changes in biology.
With this in mind, several important future directions could be considered. First, additional longitudinal and experimental research is needed to better understand the reciprocal effects of cognitive and biological stress reactivity on one another and on symptoms of depression. In this work, attention should be given to identifying the precise time interval over which changes unfold, as many questions remain unanswered about the time course of cognitive and biological changes. Prospective studies of at-risk youths could allow researchers to investigate the iterations of cognitive and biological changes that take place between stressor exposure and depression onset. Alternatively, treatment-outcome studies might provide a useful format to explore the reciprocal associations between cognitive and biological improvements by examining dynamic changes in cognitive stress reactivity, biological stress reactivity, and depressive symptoms. On one hand, initial changes in cognition may improve biological stress reactivity. It is also possible, however, that a milder biological response could facilitate more adaptive cognitive processing of environmental stressors.
Second, it is critical that we better disentangle the cognitive and biological responses that predict the onset of depression from those that predict the course or recurrence of depression. There is reason to believe that onset, maintenance, and recurrence factors differ from one another (Monroe & Harkness, 2005). For example, with each additional episode, individuals become more sensitive to developing depression following stress (Monroe, Anderson, & Harkness, 2019). Previous depressive episodes may leave cognitive and biological “scars” that change the association between risk factors and depression onset (De Raedt & Koster, 2010; Slavich & Irwin, 2014). Relatedly, exposure to chronic stressors and early life stress, particularly during sensitive periods of development, alters biological and cognitive processes (Heim et al., 2000; Jopling, Tracy, & LeMoult, 2020). Thus, there is reason to examine the extent to which associations between cognitive and biological stress reactivity change on the basis of depression history, stressor timing, and duration of stressor exposure.
Finally, many of the findings reported here have been obtained through rigorous designs, and we need to continue prioritizing multimethod studies that focus on best-practice assessment methods. By including multiple measures of cognition and biology, researchers would be positioned to add specificity to integrated models of depression and to more precisely identify the most critical targets for interventions. Additionally, as we move forward with answering these and other questions in the field, it is critical to use assessment tools with the highest psychometric properties that are minimally influenced by reporting biases, including interview-based assessments of life stress and objective indices of cognition (Harkness & Monroe, 2016; LeMoult & Gotlib, 2019).
Conclusions
Taken together, evidence shows that cognitive and biological systems do not operate in isolation. Rather, data support the formulation that cognitive and biological stress reactivity contribute to one another in ways that thwart adaptive emotional responses to life stress and increase risk for depression. Understanding the dynamic, complex, and compounding interplay between cognitive and biological stress reactivity has the potential to provide valuable insights into the mechanisms through which stressful life events contribute to the onset and maintenance of depression. Many questions, however, remain unanswered, and thus there are exciting opportunities in this area of research. It is also important to acknowledge that other factors—including exposure to early life stress, genetic risk, pubertal development, sex, and gender—influence the likelihood of maladaptive cognitive or biological stress reactivity and increase risk for depression following exposure to stressful life events (Gibb et al., 2012; Hankin & Abramson, 2001; LeMoult et al., 2020). Although a full review of these factors is outside the scope of the current article, they are important considerations for future research. With strong methods and a focus on understanding the way cognitive and biological stress reactivity contribute to one another and to depression, we have the potential to build on the work that has been done and further reduce the prevalence and costs of this debilitating disorder.
Recommended Reading
Disner, S. G., Beevers, C. G., Haigh, E. A. P., & Beck, A. T. (2011). (See References). Describes the neural mechanisms associated with the cognitive model of depression.
Gotlib, I. H., & Hammen, C. L. (Eds.). (2015). Handbook of depression (3rd ed.). New York, NY: Guilford Press. A comprehensive overview of theory and research on depression, including chapters on cognitive and biological risk factors.
Hammen, C. L. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293–319. An overview of when and why stress leads to depression.
Harkness, K. L., & Monroe, S. M. (2016). (See References). Evaluation of strategies to assess acute life events, chronic stressors, and daily hassles, as well as best-practice guidelines on stress assessment.
LeMoult, J., & Gotlib, I. H. (2019). (See References). A review and model of the cognitive mechanisms that contribute to depression.
Acknowledgments
I thank Sheila Woody, Frances Chen, Daniela Palombo, and Katerina Rnic for providing helpful feedback on a draft of this article.
Footnotes
ORCID iD: Joelle LeMoult  https://orcid.org/0000-0003-0931-2855
https://orcid.org/0000-0003-0931-2855
Transparency
Action Editor: Robert L. Goldstone
Editor: Robert L. Goldstone
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: Writing of this article was supported by grants from the Michael Smith Foundation for Health Research (17713), Natural Sciences and Engineering Research Council (RGPIN-2018-04837), and Canadian Institutes of Health Research (12R12134) awarded to J. LeMoult.
References
- Adam E. K., Doane L. D., Zinbarg R. E., Mineka S., Craske M. G., Griffith J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35, 921–931. doi: 10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T. (1968). Depression: Clinical, experimental, and theoretical aspects. JAMA, 203, 1144–1145. doi: 10.1001/jama.1968.03140130056023 [DOI] [Google Scholar]
- Beck A. T. (1979). Cognitive therapy of depression. New York, NY: Guilford Press. [Google Scholar]
- Beck A. T., Bredemeier K. (2016). A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science, 4, 596–619. doi: 10.1177/2167702616628523 [DOI] [Google Scholar]
- Beevers C. G., Clasen P. C., Enock P. M., Schnyer D. M. (2015). Attention bias modification for major depressive disorder: Effects on attention bias, resting state connectivity, and symptom change. Journal of Abnormal Psychology, 124, 463–475. doi: 10.1037/abn0000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H. M., Davis M. C., Otte C., Mohr D. C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30, 846–856. doi: 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- De Raedt R., Koster E. H. W. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, & Behavioral Neuroscience, 10, 50–70. doi: 10.3758/CABN.10.1.50 [DOI] [PubMed] [Google Scholar]
- De Raedt R., Vanderhasselt M. A., Baeken C. (2015). Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clinical Psychology Review, 41, 61–69. doi: 10.1016/j.cpr.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Disner S. G., Beevers C. G., Haigh E. A. P., Beck A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12, 467–477. doi: 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. (2006). Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences, 40, 1659–1669. [Google Scholar]
- Gibb B. E., Beevers C. G., McGeary J. E. (2012). Toward an integration of cognitive and genetic models of risk for depression. Cognition & Emotion, 27, 193–216. doi: 10.1080/02699931.2012.712950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I. H., Hamilton J. P. (2008). Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science, 17, 159–163. doi: 10.1111/j.1467-8721.2008.00567.x [DOI] [Google Scholar]
- Halligan S. L., Herbert J., Goodyer I., Murray L. (2007). Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry, 62, 40–46. doi: 10.1016/j.biopsych.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Hankin B. L., Abramson L. L. Y. (2001). Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin, 127, 773–796. doi: 10.1037/0033-2909.127.6.773 [DOI] [PubMed] [Google Scholar]
- Harkness K. L., Monroe S. M. (2016). The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. Journal of Abnormal Psychology, 125, 727–745. doi: 10.1037/abn0000178 [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D. J., Heit S., Graham Y. P., Wilcox M., Bonsall R., . . . Nemeroff C. B. (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA, 284, 592–597. doi: 10.1001/jama.284.5.592 [DOI] [PubMed] [Google Scholar]
- Hyde J. S., Mezulis A. H., Abramson L. Y. (2008). The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review, 115, 291–313. doi: 10.1037/0033-295X.115.2.291 [DOI] [PubMed] [Google Scholar]
- Joormann J., Tanovic E. (2015). Cognitive vulnerability to depression: Examining cognitive control and emotion regulation. Current Opinion in Psychology, 4, 86–92. doi: 10.1016/j.copsyc.2014.12.006 [DOI] [Google Scholar]
- Jopling E., Gotlib I. H., LeMoult J. (2020). Effects of working memory training on cognitive, affective, and biological responses to stress in major depression: A novel cognitive bias modification protocol. Journal of Affective Disorders, 265, 45–51. doi: 10.1016/j.jad.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling E., Tracy A., LeMoult J. (2020). Childhood maltreatment, negative self-referential processing, and depressive symptoms during stress. Psychology Research and Behavior Management, 13, 79–87. doi: 10.2147/PRBM.S231505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R. H., Andrews-Hanna J. R., Wager T. D., Pizzagalli D. A. (2015). Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72, 603–611. doi: 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Diamond D. M. (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience, 3, 453–462. doi: 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- Koster E. H. W., De Lissnyder E., Derakshan N., De Raedt R. (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31, 138–145. doi: 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- LeDoux J. E. (1989). Cognitive-emotional interactions in the brain. Cognition & Emotion, 3, 267–289. [Google Scholar]
- LeMoult J., Chen M. C., Foland-Ross L. C., Burley H. W., Gotlib I. H. (2015). Concordance of mother-daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biological Psychology, 108, 98–104. doi: 10.1016/j.biopsycho.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Gotlib I. H. (2019). Depression: A cognitive perspective. Clinical Psychology Review, 69, 51–66. doi: 10.1016/j.cpr.2018.06.008 [DOI] [PubMed] [Google Scholar]
- LeMoult J., Humphreys K. L., Tracy A., Hoffmeister J. -A., Ip E., Gotlib I. H. (2020). Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 59, 842–855. doi: 10.1016/j.jaac.2019.10.011 [DOI] [PubMed] [Google Scholar]
- LeMoult J., Joormann J. (2014). Depressive rumination alters cortisol decline in major depressive disorder. Biological Psychology, 100, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Joormann J., Kircanski K., Gotlib I. H. (2016). Attentional bias training in girls at risk for depression. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57, 1326–1333. doi: 10.1111/jcpp.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Ordaz S. J., Kircanski K., Singh M. K., Gotlib I. H. (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124, 850–859. doi: 10.1037/abn0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran N. L., Kovacs M., George C. J. (2009). Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34, 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure C. M. (1998). Life stressors as risk factors in depression. Clinical Psychology: Science and Practice, 5, 291–313. doi: 10.1111/j.1468-2850.1998.tb00151.x [DOI] [Google Scholar]
- McEwen B. S. (2006). Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience, 8, 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. M., Anderson S. F., Harkness K. L. (2019). Life stress and major depression: The mysteries of recurrences. Psychological Review, 126, 791–816. doi: 10.1037/rev0000157 [DOI] [PubMed] [Google Scholar]
- Monroe S. M., Harkness K. L. (2005). Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review, 112, 417–445. doi: 10.1037/0033-295X.112.2.417 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B. E., Lyubomirsky S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. [DOI] [PubMed] [Google Scholar]
- Rottenberg J., Clift A., Bolden S., Salomon K. (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44, 450–458. doi: 10.1111/j.1469-8986.2007.00509.x [DOI] [PubMed] [Google Scholar]
- Slavich G. M., Irwin M. R. (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140, 774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. F., Åhs F., Fredrikson M., Sollers J. J., III, Wager T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36, 747–756. [DOI] [PubMed] [Google Scholar]
- Watkins E. R. (2016). Rumination-focused cognitive-behavioral therapy for depression. New York, NY: Guilford Press. [Google Scholar]


