Abstract
Context
Lean body mass is essential for health, yet consensus regarding the effectiveness of protein interventions in increasing lean body mass is lacking.
Objective
The aim of this systematic review was to evaluate the dose–response relationship of the effects of protein intake on lean body mass.
Data Sources
The PubMed and Ichushi-Web databases were searched electronically, and reference lists of the literature included here and in other meta-analyses were searched manually.
Study Selection
Randomized controlled trials evaluating the effects of protein intake on lean body mass were included.
Data Extraction
Two authors independently screened the abstracts; 5 reviewed the full texts.
Results
A total of 5402 study participants from 105 articles were included. In the multivariate spline model, the mean increase in lean body mass associated with an increase in protein intake of 0.1 g/kg of body weight per day was 0.39 kg (95%CI, 0.36–0.41) and 0.12 kg (95%CI, 0.11–0.14) below and above the total protein intake of 1.3 g/kg/d, respectively.
Conclusions
These findings suggest that slightly increasing current protein intake for several months by 0.1 g/kg/d in a dose-dependent manner over a range of doses from 0.5 to 3.5 g/kg/d may increase or maintain lean body mass.
Systematic Review Registration
UMIN registration number UMIN000039285.
Keywords: protein, body composition, diet, supplement
INTRODUCTION
Skeletal muscle, which is responsible for movement and activity, is the largest organ in the human body, accounting for 40% of total body weight (BW). Among young and middle-aged adults, decreased muscle mass increases the risk of chronic metabolic diseases such as type 2 diabetes and obesity.1,2 Moreover, among the elderly, sarcopenia, a progressive decrease in muscle mass with age, is a risk factor for fractures, physical disabilities, and frailty.3 Accordingly, sustaining and increasing muscle mass is extremely important for the promotion and maintenance of health across all populations.4
Protein, an energy-producing nutrient, is a major component of skeletal muscle in living organisms and is involved in the regulation of metabolism.5,6 A decrease in muscle mass may be accelerated by a decline in the assimilation response to insufficient protein intake.7 According to a meta-analysis of nitrogen delivery tests to evaluate the amount of protein required in healthy adults, the average protein requirement was estimated to be 0.66 g/kg BW/d.8 However, although some randomized controlled trials (RCTs) reported increased skeletal muscle mass following intake of more than the required amount of protein,9–15 no consistent results have been demonstrated.12
A recent meta-analysis of RCTs reported the dose–response relationship between protein intake and increased muscle mass in healthy adults.14 However, this report mainly demonstrates the magnitude of the effect in the dose–response curve, and thus the dose–response relationship between increased muscle mass and protein intake cannot be estimated from confidence intervals. Furthermore, these analyses only included studies that examined the effect of protein supplementation in conjunction with resistance training; thus, the effect of protein supplementation without resistance training was not considered.
Ingestion of protein and amino acids strongly stimulates muscle protein synthesis,16 and the digested and absorbed proteins and amino acids also act as structural components of muscle hypertrophy.5 Additionally, resistance training facilitates muscle protein synthesis and subsequent increases in muscle mass. The aims of the present meta-analysis were as follows: (1) to evaluate the dose–response relationship between protein intake and the increase in lean body mass (LBM); and (2) to assess this relationship within the context of the presence or absence of resistance training. The hypothesis in this study was that increased protein intake would result in an increase in muscle mass in a dose-dependent manner and that ingestion of small amounts of protein, especially among a resistance-trained population, would be effective in increasing muscle mass. This study is the first meta-analysis to examine the dose–response relationship between a wide range of protein intakes and an increase in muscle mass in the presence or absence of resistance training. On the basis of the findings of this study, recommendations are provided for appropriate amounts of protein required to sustain and improve muscle mass in a diverse population.
METHODS
Study protocol
This study was registered in UMIN Clinical Trials Registry (registration no. UMIN000039285).
Reporting
This systematic review and meta-analysis was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.17
Data sources
A systematic review of published literature was conducted using the PubMed and Ichushi-Web (online database of academic articles in Japan) databases (last accessed on May 27, 2019). Results were limited to English- and Japanese-language RCTs. The combinations of search terms and search parameters are shown in Table S1 in the Supporting Information online. Additionally, the reference lists of the literature included in this review and in other meta-analyses were searched manually.
Study identification and data extraction
Two authors (R.T. and K.N.) independently screened titles and abstracts of all the search results, and eligibility was judged on the basis of the criteria described below. Any disagreement regarding eligibility was resolved through deliberations. Articles judged to be potentially eligible in the primary screening, along with articles for which no such decision could be made, were subjected to secondary screening to determine eligibility using the full-text version. Data on attributes of participants, intervention conditions, and the target outcome were extracted from the articles judged to be eligible during secondary screening. If a trial included more than one intervention group, each group was treated as a separate trial. Measurements taken in the middle of the intervention period were excluded, and only one result before and one result after the full intervention were utilized. When data required for the creation of a forest plot could not be collected, the article's corresponding author was contacted. In cases in which numerical data were not available and a response could not be obtained from the corresponding author but the data were available as graphs, numerical values were obtained using the web-based tool WebPlotDigitizer, version 4.1 (Ankit Rohatgi; Pacifica, CA).18 Five authors (K.I., K.U., R.T., C.S., and K.N.) conducted the secondary screening and data extraction, and 2 authors (K.I. and K.U.) conducted the verification.
Eligibility
Randomized controlled trials that studied the effects of protein intake on LBM (or fat-free mass, if LBM was not available) and in which supplemental protein doses varied between study groups were selected for analysis. The PICOS (population, intervention, comparison, outcome, and study design) criteria were used to define the research questions (Table 1). The target population was limited to study participants who did not have any serious illness (eg, HIV infection, cancer, chronic renal failure, terminal illness, or diseases that seriously affect physical activity). The protein intervention period was set as 2 weeks or more, which was considered a sufficient length of time for protein supplementation to enhance LBM,19 so that data from all potentially eligible RCTs could be collected. The supplemental protein dose (g/d or g/kg BW/d) was set in advance of the intervention. Trials with intergroup differences in the amounts for interventions with muscle hypertrophy promoters (leucine, β-hydroxy-β-methyl butyrate, creatine, etc) or vitamin D were excluded. When there was more than one control group, priority was given to the group with equal energy intake and with larger differences in the supplemental protein dose. Control groups with different conditions other than nutrition (such as exercise) were excluded.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Inclusion criterion |
|---|---|
| Population | Adult participants (not critically ill) |
| Intervention | Supplementary protein intake for ≥ 2 weeks |
| Comparator | Placebo or no intervention |
| Outcome | Lean body mass or fat-free mass |
| Study design | Randomized controlled trial |
Outcomes
When extracting data for muscle mass, muscle strength, and body fat mass as outcomes, the target of analysis in this systematic review and meta-analysis was LBM. For LBM, 2 values were recorded: LBM change in each group, and the difference in LBM changes between an intervention group and a control group. The former was used to evaluate the effect of total protein intake as calculated by the sum of the supplemental protein dose and the dietary protein intake in each group, and the latter was used to evaluate the effect of the difference in supplemental protein doses between groups.
Quality assessment
Two authors (K.I. and K.N.) independently evaluated the quality of the selected articles, using the Cochrane risk-of-bias tool.20 Disagreements about quality were resolved through discussions with a third author (R.T.). The articles identified as containing high-risk items all contained sufficient descriptions to allow inclusion, and thus, to avoid bias, all articles were evaluated.
Statistical analysis
A meta-analysis of the effect of protein intake on LBM was conducted using the mean change in LBM and the standard deviation (SD) of change (SDchange). In cases where SDchange was not reported, it was calculated using the equations shown below.20 In cases where all data for SD before the intervention (SDbaseline), SD after the intervention (SDfinal), and SDchange were available, the correlation coefficient (Corr) was calculated using the following equation:
In cases where SDchange was unknown, but SDbaseline and SDfinal were available, SDchange was calculated using the following equation:
In cases where none of the above data were available, SDchange was obtained by contacting the corresponding author.
The effects of differences in supplemental doses on differences in LBM changes between groups were analyzed by point estimation and displayed as a forest plot of the point estimates of the mean difference and 95%CI. To investigate the effect of resistance training, subgroup analyses were conducted. The analyses were performed using a random-effects model in which it was assumed that trial errors were included, since the trials selected used a wide range of conditions and were not limited by sex, age, or exercise conditions. Statistical heterogeneity was evaluated using the inconsistency index (I2) and χ2 test, but the entire analysis was performed even when heterogeneity was high, since it was assumed that corrections for confounding factors would be incorporated later. Publication bias was evaluated visually using a funnel plot.
Moreover, unadjusted or multivariate-adjusted spline models were used to evaluate the dose–response relationship between protein intake (total protein intake in each group or the difference in supplemental protein doses between groups) and change in LBM (a value in each group or the difference between groups). Multivariate analysis was verified in 2 models. Model 1 adjusted for age (continuous), sex (percent male), intervention period (continuous), and resistance training (yes or no), while model 2 adjusted for the factors in model 1 and also for weight change (continuous). The missing values of these covariates (from all 105 selected trials, sex was missing in 8 cases, age in 1 case, and weight change in 14 cases) were substituted with the average values (for weight change only, missing values were substituted with 0) of all included trials. Model 1 was adjusted for variables that were used in a Dietary Reference Intake as confounders.21 Weight change was the selected mediator of these relationships in model 2. Additionally, the stratified-models analysis was performed according to the presence or absence of resistance training, and the results were expressed as the effect size and 95%CI, with the former being calculated relative to the control group. The mean effect size, along with the corresponding 95%CI for an increase in LBM, was estimated for a 0.1 g/kg BW/d increment in protein intake stratified by approximately 1.3 g/kg BW/d (< 1.3 kg or ≥ 1.3 kg), which was the inflection point with the association between protein intake and fat-free mass using a multivariate spline model. In these analyses, when the 95%CI of the magnitude of effect did not straddle 0, it was estimated that P < 0.05. When the 95%CI of the magnitude of effect straddled 0, it was estimated that P ≥ 0.05.
Statistical significance was considered when both sides were less than 5%. Analyses were conducted using Review Manager (RevMan), version 5.3 (Nordic Cochrane Centre; Cochrane Collaboration, Copenhagen, Denmark) and Stata/MP, version 15.0 (StataCorp LP; College Station, TX).
RESULTS
Study selection
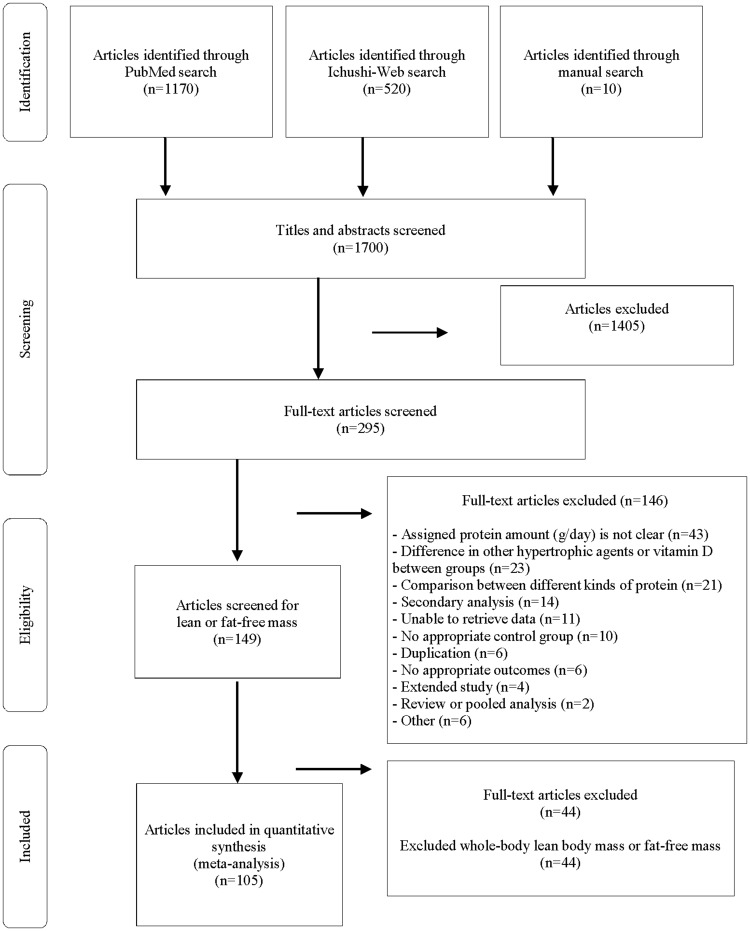
Figure 1 shows the results of the literature search. In the search conducted through May 27, 2019, 1700 potentially relevant articles were identified. Primary screening of titles and abstracts identified 295 articles that were potentially eligible or for which no clear judgment could be made. Secondary screening using the full-text versions identified 149 eligible articles. The search was narrowed according to the target outcome specified for analysis in this meta-analysis, and data for 105 articles, 138 intervention groups, and 5402 individuals were obtained. The data for interventions with resistance training were extracted from 53 articles that described 72 intervention groups and 2325 individuals. The data for interventions without resistance training were extracted from 56 articles that described 66 intervention groups and 3077 individuals. Four of the included articles reported on interventions both with and without resistance training. All 105 articles were used to create a forest plot and to create spline models to evaluate the relationship between differences in supplemental protein doses and LBM change between groups; 92 articles were also used to create spline models to evaluate the relationship between total protein intake and change in LBM in each group.
Figure 1.
Flow diagram of the literature search process.
Study characteristics
Tables S2 through S5 in the Supporting Information online summarize the features of the 105 selected articles. Total protein intake ranged from 0.64 to 3.50 g/kg BW/d (mean ± SD, 1.58 ± 0.59 g/kg BW/d) in intervention groups and from 0.52 to 2.00 g/kg BW/d (mean ± SD, 1.04 ± 0.35 g/kg BW/d) in control groups. There was a significant increase in total protein intake in the intervention groups (mean ± SD, 31 ± 27 g/d; range, −13 to 135 g/d; P < 0.01) and a significant decrease in the control groups (mean ± SD; −5 ± 15 g/d; range, −55 to 47 g/d; P < 0.01) such that the change in total protein intake was significantly greater in the intervention groups (P < 0.01). Relative total protein intake (g/kg BW/d) significantly increased in the intervention groups (before, 1.13 ± 0.33 g/kg BW/d; after, 1.52 ± 0.51 g/kg BW/d; change, 0.38 ± 0.33 g/kg BW/d; P < 0.01) and significantly decreased in the control groups (before, 1.12 ± 0.31 g/kg BW/d; after, 1.06 ± 0.33 g/kg BW/d; change, −0.05 ± 0.19 g/kg BW/d; P < 0.01), such that there was a greater change in the intervention groups (P < 0.01). Differences in supplemental protein doses between an intervention group and a control group ranged from 0.06 to 2.38 g/kg BW/d (0.51 ± 0.37 g/kg BW/d). There were 68 RCTs evaluating LBM only, 35 evaluating fat-free mass only, and 2 evaluating both LBM and fat-free mass. There were 66 trials in which protein supplementation was added to regular meals and 39 trials in which the meal content itself was changed. The intervention period spanned a wide range, from 2 weeks to 18 months, with a mean of 19.8 weeks. Concerning energy balance, 41 trials used interventions for aggressive weight loss; 2 trials, aggressive weight gain; and 62 trials, neither. With regard to sex, 2459 study participants were female and 2422 male; the sex of the remaining 530 participants was unknown (data not available). The mean age of the study participants ranged from 19 to 81 years, with an overall mean of 47.2 years.
Risk of bias
Assessment of risk of bias is summarized in Figure S1 in the Supporting Information online. Conditions indicative of high risk of bias included the following: blinding of participants and personnel in 58 trials, incomplete outcome data in 7 trials, random sequence generation in 3 trials, and allocation concealment in 3 trials. Publication bias was not observed in the funnel plot (see Figure S2 in the Supporting Information online).
Meta-analysis
Figure S3 in the Supporting Information online shows the forest plot that consolidates the results of the trials for 138 intervention conditions stratified by the presence or absence of resistance training. The results of subgroup analyses by supplemental doses with or without resistance training are summarized in Table 2. Protein supplementation in a wide range of doses was significantly effective in improving LBM, with or without resistance training. As a whole, the weighted average difference in supplemental protein doses was 0.51 kg (95%CI, 0.36–0.65 kg) (P < 0.01). For statistical heterogeneity, I2 = 72%, and χ2 tests demonstrated statistical significance (P < 0.01).
Table 2.
Summary of the effect of protein supplementation on change in lean body mass (LBM), stratified by the supplemental protein dose or by the presence or absence of resistance training
| Subgroup | Mean difference in LBM (kg) | 95%CI (kg) | No. of trials | Total no. of individuals |
|---|---|---|---|---|
| All trials | 0.51 | 0.36–0.65 | 138 | 5866 |
| Protein dose < 0.3 g/kg BW/d | 0.38 | 0.20–0.55 | 40 | 1815 |
| Protein dose 0.3–0.6 g/kg BW/d | 0.41 | 0.19–0.63 | 56 | 2641 |
| Protein dose ≥ 0.6 g/kg BW/d | 0.80 | 0.45–1.14 | 42 | 1410 |
| With resistance training | 0.48 | 0.31–0.65 | 72 | 2686 |
| Without resistance training | 0.53 | 0.36–0.76 | 66 | 3180 |
Abbreviations: BW, body weight.
Dose–response analyses with multivariate-adjusted spline models
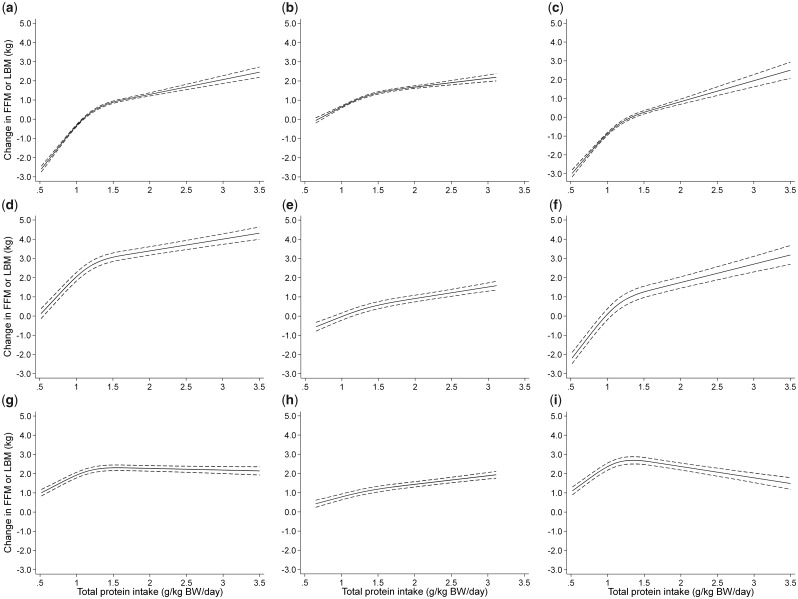
The effect of total protein intake on change in LBM in each group was analyzed with 3 spline models: an unadjusted model, the multivariate-adjusted model 1, or the multivariate-adjusted model 2 (Figure 2). In the analyses conducted with the unadjusted model or with multivariate-adjusted model 1 (which did not include weight change), the change in LBM became incrementally greater with total protein intake across a wide range of intakes, with or without resistance training. When multivariate-adjusted model 1 was used, the mean increase in LBM (and the corresponding 95%CI) associated with a protein intake increment of 0.1 g/kg BW/d was 0.39 kg (95%CI, 0.36–0.41) below and 0.12 kg (95%CI, 0.11–0.14) above a total protein intake of 1.3 g/kg BW/d. Positive correlations across a range of total protein intakes were also observed when the sample was stratified according to resistance training groups: a protein intake of 0.52 to 1.30 g/kg BW/d was associated with a mean increase in LBM of 0.06 kg (95%CI, 0.03–0.08) with resistance training and 0.40 kg (95%CI, 0.37–0.43) without resistance training, and a protein intake of 1.31 to 3.50/kg BW/d was associated with a mean increase in LBM of 0.08 kg (95%CI, 0.06–0.09) with resistance training and 0.26 kg (95%CI, 0.23–0.29) without resistance training. In model 2, in which change in BW was added as a mediator to those variables already present in model 1, after a total protein intake of 1.3 g/kg BW/d was exceeded, the effect on LBM change continued to rise with resistance training and declined without resistance training. The effect of differences in supplemental protein doses was analyzed in the same manner as before (see Figure S4 in the Supporting Information online). Before and after the differences in supplemental protein doses reached approximately 0.5 g/kg BW/d, the effects on LBM change declined and rose, respectively.
Figure 2.
Dose–response relationship between total protein intake and change in lean body mass in each group. Spline curves illustrating the associations between total protein intake and change in lean body mass in each group in an unadjusted model (a, b, and c for all trials, trials with resistance training, and trials without resistance training, respectively), in multivariate-adjusted model 1 (d, e, and f for all trials, trials with resistance training, and trials without resistance training, respectively), or multivariate-adjusted model 2 (g, h, and i for all trials, trials with resistance training, and trials without resistance training, respectively). The solid line and dashed line represent the mean change in LBM and 95%CIs, respectively. Covariates of multivariate-adjusted model 1 are age, sex, intervention period, and resistance training. Covariates of multivariate-adjusted model 2 are weight change in addition to the covariates of multivariate-adjusted model 1. Abbreviations: BW, body weight; FFM, fat-free mass; LBM, lean body mass.
DISCUSSION
Primary findings
The purpose of this study was to elucidate the dose–response relationship using a meta-analysis of RCTs investigating the effect of protein intake on LBM increase, considering the presence or absence of resistance training. The primary findings include the following: (1) The subgroup analyses, displayed as forest plots, indicated that protein supplementation was significantly effective for increasing LBM with or without resistance training; (2) Dose–response analyses with the multivariate-adjusted spline model indicated that total protein intake over a wide range of doses (from 0.5 to 3.5 g/kg BW/d) was positively correlated with an increase in LBM. Slightly increasing the current protein intake by 0.1 g/kg BW/d may potentially increase or maintain current muscle mass; (3) The rate of increase in the effect of protein supplementation rapidly diminished after 1.3 g/kg BW/d was exceeded, and resistance training markedly suppressed this decline.
Effect of protein supplementation with or without resistance training
Several previous systematic reviews and meta-analyses of RCTs that included resistance training report that protein supplementation has a significant positive effect on muscle mass, 9,10,14 but this meta-analysis demonstrates for the first time that protein supplementation is significantly effective without resistance training in a diverse population without specific serious health conditions. Subgroup analysis by the presence or absence of resistance training demonstrated no superior effects of protein supplementation with resistance training (Table 2). It seems that resistance training has no synergistic effects, but it may have a simple additive effect.22,23
Effective dose of protein
Subgroup analyses by supplemental protein doses demonstrated that protein supplementation of less than 0.3 g/kg BW/d (0.17 g/kg BW/d, on average) was sufficient to significantly increase LBM. Furthermore, the multivariate-adjusted spline model revealed that total protein intake positively correlated with LBM change over a wide range of protein intakes (0.5–3.5 g/kg BW). Lean body mass increased by 0.39 kg (95%CI, 0.36–0.41) and 0.12 kg (95%CI, 0.11–0.14) per 0.1 g/kg BW/d increment in total protein intake below and above 1.3 g/kg BW/d, respectively. These results suggest that a small amount of protein supplementation promotes an increase in LBM. Daily addition of a high-protein food item such as an egg (6–8 g protein) or 1 cup (200 mL) of milk (6.8 g protein) to usual meals may increase muscle mass. These findings may be helpful for managing nutrition in people who have difficulty eating sufficient amounts of food, such the elderly or people with dysphagia, as well as in low-income, food-insufficient populations.
Correlation between total protein intake and change in lean body mass
Multivariate-adjusted spline model 1 (which did not include weight change) indicated that total protein intake and change in LBM were positively correlated over a wide range of protein intakes (from 0.5 to 3.5 g/kg BW/d); model 2 (which included weight change) revealed that the effect of total protein intake was especially pronounced at protein intakes below 1.3 g/kg BW/d, indicating that increasing total protein intake within the range recommended by the Dietary Reference Intakes in Japan21 leads to linear increases in LBM relative to change in BW. Considering that differences between supplementation doses and differences in LBM increases were negatively associated with low doses, total protein intake may be essential to accurately estimate the dose–response relationship of protein intake. Indeed, several previous reports indicate positive correlations between total protein intake and LBM in a diverse population.24–29
Effect of higher protein intake with or without resistance training
The rate of increase in the effect of protein supplementation rapidly diminished when total protein intake exceeded 1.3 g/kg BW/d in the multivariate-adjusted spline model 2 (which included weight change). This result suggests that the efficiency of conversion of ingested protein into LBM declines when protein is ingested in sufficient amounts or more. This finding is consistent with previous research using meta-regression between total protein intake and change in fat-free mass.14 Interestingly, this decline was markedly suppressed by resistance training, suggesting that resistance training may contribute to maintaining or improving the efficiency of protein anabolism. According to the National Health and Nutrition Survey in Japan,30 roughly 33% of Japanese adults may have a total protein intake that exceeds 1.3 g/kg BW/d. Although resistance training has become increasingly popular in Japan, currently only approximately 10% of Japanese adults perform resistance training once or more per week.31 For health-oriented people who consume high amounts of protein in daily meals, resistance training is strongly recommended to increase LBM.
Adverse effects of excessive protein intake
While the efficacy of protein intake for maintaining or increasing muscle mass is well known, it is important to highlight potential adverse effects related to excess protein intake. High protein intake during pregnancy has been reported to increase the risk of small-for-gestational-age infants32 and neonatal death.33 While inconsistent results regarding renal function34 and cancer mortality35 have been reported, some studies report that high protein intake is associated with lower renal function in individuals with mild renal insufficiency36 and that high consumption of animal proteins is associated with a higher risk of cancer mortality in middle-aged individuals.37 Thus, consuming moderate amounts of protein to maintain overall nitrogen balance is important in these populations, particularly because of the risks associated with excessive protein intake.
Strengths and limitations
This study has several strengths. First, a large number of selected studies (trials) and individuals were included, approximately 2 to 3 times more than those in previous meta-analyses.9–15 Second, dose reactivity was described using a multivariate-adjusted spline model. Surprisingly, although numerous meta-analyses have been published to date, only a few analyzed dose–response relationships, despite major interest and concerns about the amount of protein intervention and its effect size. Third, this study is the first meta-analysis to examine the dose–response relationship between a wide range of protein intakes and the increase in LBM while also considering the presence or absence of resistance training.
This study also has several limitations. First, included studies were limited to those published in English or Japanese. Second, for articles in which the magnitude of effect on LBM was not mentioned in either the text or tables, the corresponding authors were contacted, but the response rate was low (7 responses of 36 requests). However, 18 the 29 articles for which no response was received contained related graphs, and the WebPlotDigitizer tool was used to extract data from these graphs. Third, bias related to blinding was high. Double-blind trials are difficult to perform in dietary protein interventions, since meals with different protein content must be provided to study participants. However, since most protein intervention studies are not double-blind, excluding non–double-blind studies may result in large deviations from the current status of protein intervention studies. Consequently, studies that were not double-blind were also included in this meta-analysis.
Perspectives
A future large-scale RCT is necessary to examine the dose–response relationship of multiple amounts of supplemental protein under the same conditions to more accurately elucidate the relationship between the amount of supplemental protein and the increase in muscle mass. Only 4 of the 105 articles included in this meta-analysis examined the dose–response relationship under identical conditions. Moreover, additional intervention studies that include individuals with conditions caused by severely insufficient protein intake (such as frailty and sarcopenia) are also warranted. Sarcopenia and frailty in the elderly and kwashiorkor in young children in developed and developing countries, respectively, are important global health issues that require well-designed and multifaceted studies to further clarify the relationship between protein intake and muscle mass.
Conclusion
This meta-analysis revealed that total protein intake enhances the increase in LBM in a dose-dependent manner over a wide range of doses (0.5–3.5 g/kg BW). These results suggest that slightly increasing protein intake for several months, even by as little as 0.1 g/kg BW/d, may increase or help maintain muscle mass. The effect of protein intake on LBM increase relative to weight change rapidly diminishes after the intake of 1.3 g/kg BW/d is exceeded, and resistance training markedly suppresses this decline. Therefore, both increasing protein intake and performing resistance training is recommended to optimally augment LBM. The findings of this study indicate the appropriate protein intakes required to sustain and improve muscle mass in diverse populations and provide a better understanding of the influence of resistance training on the effect of protein intake.
Supplementary Material
Acknowledgments
The authors would like to thank Aya Yoshimura, Yuri Saito, and Kae Yamazaki, researchers for Meiji Co, Ltd, Tokyo, Japan, for their support in data extraction.
Author contributions. R.T. and D.W. are co-first authors. M.M., R.T., and C.S. contributed to the conception of this research. M.M., R.T., and D.W. designed the research. R.T. and K.N. screened the literature. K.I., K.U., R.T., C.S., and K.N. selected and extracted the data. K.I. and K.N. performed quality assessment. M.M., D.W., and R.T. conducted the analyses and drafted the manuscript. All authors contributed to the critical review of the manuscript.
Funding/support. No external funds supported this work.
Declaration of interest. R.T., K.I., K.U., K.N., and C.S. are employees of Meiji Co, Ltd, Tokyo, Japan.
Supporting Information
The following Supporting Information is available through the online version of this article on the publisher's website.
Appendix S1 PRISMA checklist
Table S1 Search strategy for PubMed and Ichushi-Web databases
Table S2 Summary of characteristics of the included studies
Table S3 Summary of nutrition surveys
Table S4 Summary of assigned protein amounts and differences between groups
Table S5 Summary of conditions of the interventions in the included studies
Figure S1 Risk-of-bias assessment
Figure S2 Funnel plot of all included studies for changes in lean body mass
Figure S3 Forest plot assessing the effect of protein supplementation on changes in lean body mass
Figure S4 Dose–response relationship between difference in supplemental protein doses and difference in lean body mass changes between groups
References
- 1. Park SW, Goodpaster BH, Strotmeyer ES, et al. ; Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes Care. 2007;30:1507–1512. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. [DOI] [PubMed] [Google Scholar]
- 3. Walrand S, Boirie Y.. Optimizing protein intake in aging. Curr Opin Clin Nutr Metab Care. 2005;8:89–94. [DOI] [PubMed] [Google Scholar]
- 4. Tieland M, Trouwborst I, Clark BC.. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makovický P, Makovický P, Jílek F.. Short review of some properties of muscular proteins. Cesk Fysiol 2008;57:10–14. PMID: 18630139 [PubMed] [Google Scholar]
- 6. Peyrollier K, Hajduch E, Blair AS, et al. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350(pt 2):361–368. [PMC free article] [PubMed] [Google Scholar]
- 7. Breen L, Phillips SM.. Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond). 2011;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M, Sun F, Piao JH, et al. Protein requirements in healthy adults: a meta-analysis of nitrogen balance studies. Biomed Environ Sci 2014;27:606–613. doi:10.3967/bes2014.093 [DOI] [PubMed] [Google Scholar]
- 9. Hou L, Lei Y, Li X, et al. Effect of protein supplementation combined with resistance training on muscle mass, strength and function in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2019;23:451–458. [DOI] [PubMed] [Google Scholar]
- 10. O’Bryan KR, Doering TM, Morton RW, et al. Do multi-ingredient protein supplements augment resistance training-induced gains in skeletal muscle mass and strength? A systematic review and meta-analysis of 35 trials. Br J Sports Med. 2020;54:573–581. [DOI] [PubMed] [Google Scholar]
- 11. Hanach NI, McCullough F, Avery A.. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr. 2019;10:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ten Haaf DSM, Nuijten MAH, Maessen MFH, et al. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108:1043–1059. [DOI] [PubMed] [Google Scholar]
- 13. Tieland M, Franssen R, Dullemeijer C, et al. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: individual participant data and meta-analysis of RCT's. J Nutr Health Aging. 2017;21:994–1001. [DOI] [PubMed] [Google Scholar]
- 14. Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu ZR, Tan ZJ, Zhang Q, et al. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr. 2015;113:25–34. [DOI] [PubMed] [Google Scholar]
- 16. Holwerda AM, Paulussen KJM, Overkamp M, et al. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J Nutr. 2019;149:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drevon D, Fursa SR, Malcolm AL.. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41:323–339. [DOI] [PubMed] [Google Scholar]
- 19. Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Updated March 2011. Accessed December 7, 2019.
- 21.Ministry of Health, Labour and Welfare of Japan. Dietary Reference Intakes for Japanese (2020) [in Japanese]. https://www.mhlw.go.jp/content/10904750/000586553.pdf. Published March 23, 2013. Accessed September 13, 2020.
- 22. Verreijen AM, Engberink MF, Memelink RG, et al. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr J. 2017;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Layman DK, Evans E, Baum JI, et al. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. [DOI] [PubMed] [Google Scholar]
- 24. Mangano KM, Sahni S, Kiel DP, et al. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am J Clin Nutr. 2017;105:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexandrov NV, Eelderink C, Singh-Povel CM, et al. Dietary protein sources and muscle mass over the life course: the Lifelines Cohort Study. Nutrients. 2018;10:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Switkowski KM, Jacques PF, Must A, et al. Associations of protein intake in early childhood with body composition, height, and insulin-like growth factor I in mid-childhood and early adolescence. Am J Clin Nutr. 2019;109:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colonetti T, Grande AJ, Milton K, et al. Effects of whey protein supplement in the elderly submitted to resistance training: systematic review and meta-analysis. Int J Food Sci Nutr. 2017;68:257–264. [DOI] [PubMed] [Google Scholar]
- 28. Mori H, Tokuda Y.. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac J Clin Nutr. 2019;28:157–165. [DOI] [PubMed] [Google Scholar]
- 29. Otsuka R, Kato Y, Tange C, et al. Protein intake per day and at each daily meal and skeletal muscle mass declines among older community dwellers in Japan. Public Health Nutr. 2020;23:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health, Labour and Welfare of Japan. The National Health and Nutrition Survey in Japan, 2018 [in Japanese]. e-Stat website. https://www.e-stat.go.jp/stat-search/files? page=1&layout=datalist&toukei=00450171&tstat=000001041744&cycle=7&tclass1=000001139646&cycle_facet=tclass1. Published April 17, 2020. Accessed September 13, 2020.
- 31. Sasakawa Sports Foundation. The: 2018 SSF National Sports-Life Survey [in Japanese]. https://www.ssf.or.jp/report/sldata/tabid/1739/Default.aspx. Published February 19, 2019 Accessed September 13, 2020. [Google Scholar]
- 32. Ota E, Tobe-Gai R, Mori R, et al. Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database Syst Rev 2012;(9):CD000032. doi:10.1002/14651858.CD000032.pub2 [DOI] [PubMed] [Google Scholar]
- 33. Rush D, Stein Z, Susser M.. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics. 1980;65:683–697. [PubMed] [Google Scholar]
- 34. Halbesma N, Bakker SJ, Jansen DF, et al. ; PREVEND Study Group. High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol. 2009;20:1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naghshi S, Sadeghi O, Willett WC, et al. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2020;370:m2412. doi:10.1136/bmj.m2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. [DOI] [PubMed] [Google Scholar]
- 37. Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.