Abstract
Most patients diagnosed with hepatocellular carcinoma (HCC) have advanced diseases, and many are not eligible for curative therapies. There is growing evidence suggesting that the combination treatment of PD-1/PD-L1 inhibitors and tyrosine kinase inhibitors (TKIs) is becoming a prospective trend for advanced HCC. For those HCC patients with sorafenib resistance, the efficacy of regorafenib combined with PD-1/PD-L1 inhibitors remains unclear. Herein, we represent a case of HCC with lung metastasis in the setting of Hepatitis B virus (HBV)-induced liver cirrhosis responding dramatically to the sequential treatment with regorafenib followed by PD-1 inhibitor after initial liver resection. A 51-year-old man diagnosed with alpha fetoprotein (AFP)-negative HCC underwent liver resection in September 2015 and was found to have solitary liver recurrence and multiple lung metastases in March 2017. He received microwave coagulation therapy (MCT) and trans-arterial chemoembolization (TACE) for liver tumor and treatment was started with sorafenib 400 mg twice daily for controlling lung metastases. In December 2018, an abdominal computerized tomography (CT) scan showed two new lesions in the liver. In March 2019, disease progression of lung metastases was measured and he received 160 mg regorafenib once daily. After a short period of partial response, in December 2019, due to the progression of the disease, he started treatment with regorafenib 160 mg in combination with sintilimab (PD-1 inhibitor) (200 mg, 3 weeks as a cycle). Surprisingly, after five cycles of sintilimab injection, he showed complete response in target lesions. There was no clinical evidence of disease progression, and the side-effects were mild. The current overall survival (OS) is 58 months. Data from this clinical case report suggest that sequential treatment with regorafenib followed by PD-1 inhibitor is a promising therapeutic option for sorafenib-refractory cases of HCCs.
Keywords: regorafenib, sorafenib, PD-1 inhibitor, hepatocellular carcinoma, sorafenib-refractory
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death in the world and its incidence rate has been rising in recent years.1 In China, HCC accounts for nearly half of the world’s new cases.2 Both the incidence and mortality rates are 2–3 times higher in China than those in the majority of other countries.3 Liver resection, liver transplantation, and local ablation are curative treatments for earlier stages of HCC, which achieve good surgical outcomes. However, HCC frequently relapses and most patients have locally advanced or extrahepatic metastasis when curative treatments are no longer available. For these patients, evidence for highly effective therapies on overall survival (OS) is still lacking.
Sorafenib, a small-molecule multi-kinase inhibitor, had been widely used as the only systemic therapeutic agent for patients with advanced HCC for about 10 years after a landmark study revealed improvements in time to progression (TTP) and OS.4,5 However, the therapeutic impact of sorafenib remains limited, and patients often acquire resistance soon after treatment. Thus, for HCCs who are refractory or intolerant to sorafenib, regorafenib treatment showed a significant improvement in OS compared with placebo. Regorafenib inhibits the activity of several protein kinases involved in angiogenesis, oncogenesis, metastasis, and tumor immunity.6 It has a distinct molecular target profile and had more potent pharmacological activity than sorafenib in previous studies.6,7 Nevertheless, this therapy remains unsatisfactory. Therefore, there is an urgent need for more effective systemic therapies for HCC, particularly after treatment with sorafenib and regorafenib.
Growing evidence suggested that HCC was an immunogenic tumor stemming from an immunosuppressive environment. In the past 3 years, breakthroughs in the inhibitors of the programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) have emerged as a promising therapeutic strategy in a variety of solid tumors.8 HCC takes advantage of the immune tolerance to initiate and promotes carcinogenesis and progression which may guide immunotherapeutic strategies to those that prevent immune suppressive mechanisms, rather than directly increase the immune ability of HCC patients. Overexpression of vascular endothelial growth factor (VEGF), which is an active intrinsic immune evasion pathway molecule, has been linked to the development and progression of HCC.9 Anti-VEGF therapies reduce immunosuppression within the tumor microenvironment and may enhance anti–PD-1 and PD-L1 efficacy by reversing VEGF-mediated immunosuppression and promoting T-cell infiltration in tumors.10,11 These results provide evidence for the synergistic effect of immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) amalgamation in treating advanced HCC, indicating that combination therapy might become a prospective trend for HCC. The recently published open-label, Phase 3 (IMbrave150) trial reported atezolizumab combined with bevacizumab resulted in better OS and progression-free survival (PFS) outcomes than sorafenib, with an objective response rate (ORR) of 33.2% vs 13.3% in patients with advanced HCC, which was approved as a first-line treatment for patients with advanced or metastatic HCC by the Food and Drug Administration (FDA).12
To evaluate the clinical efficacy of the combination therapy of ICIs and TKIs, we present a case of sorafenib-refractory HCC with lung metastasis responding dramatically to the sequential treatment with regorafenib followed by PD-1 inhibitor after initial liver resection and we hope to explore further studies for combined therapy of regorafenib and PD-1/PD-L1 inhibitor for HCC in the future.
Case Report
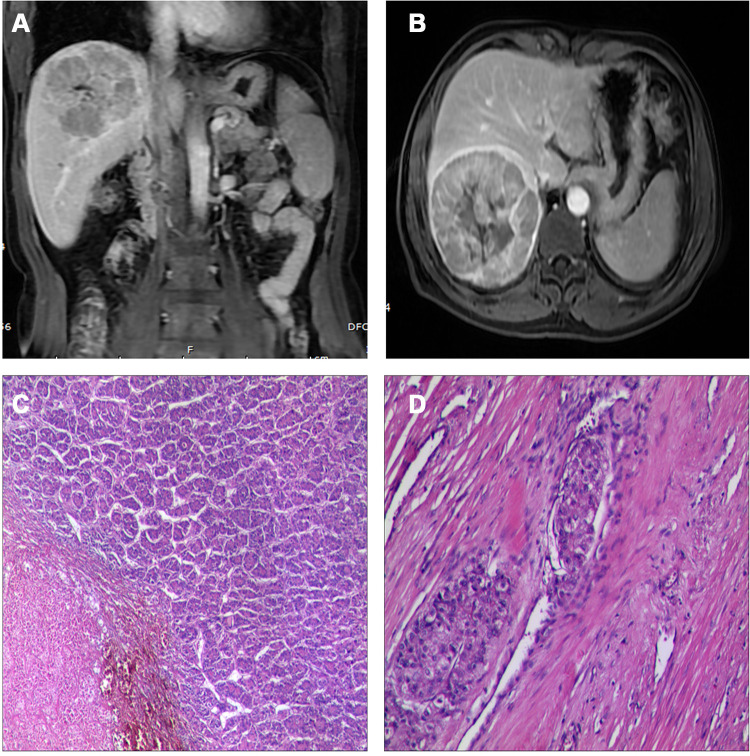
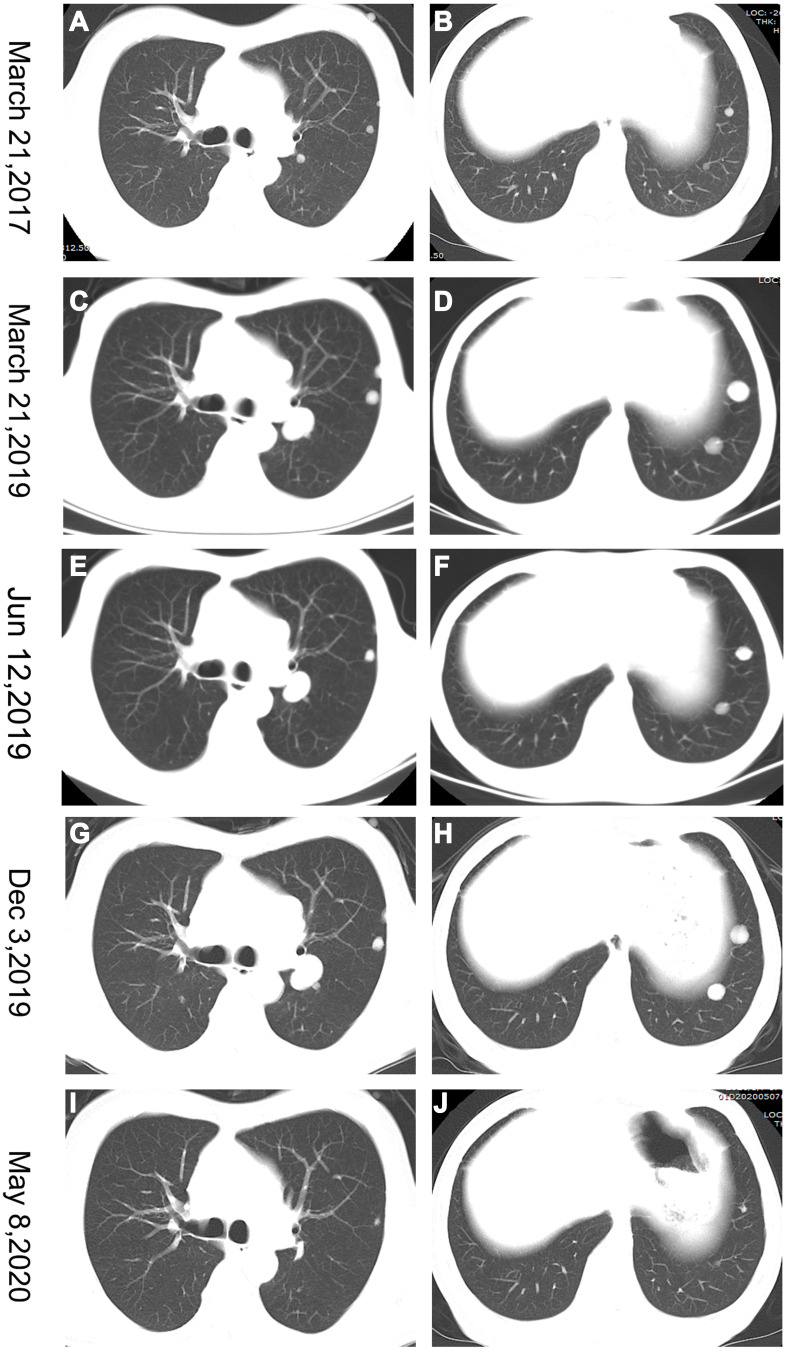
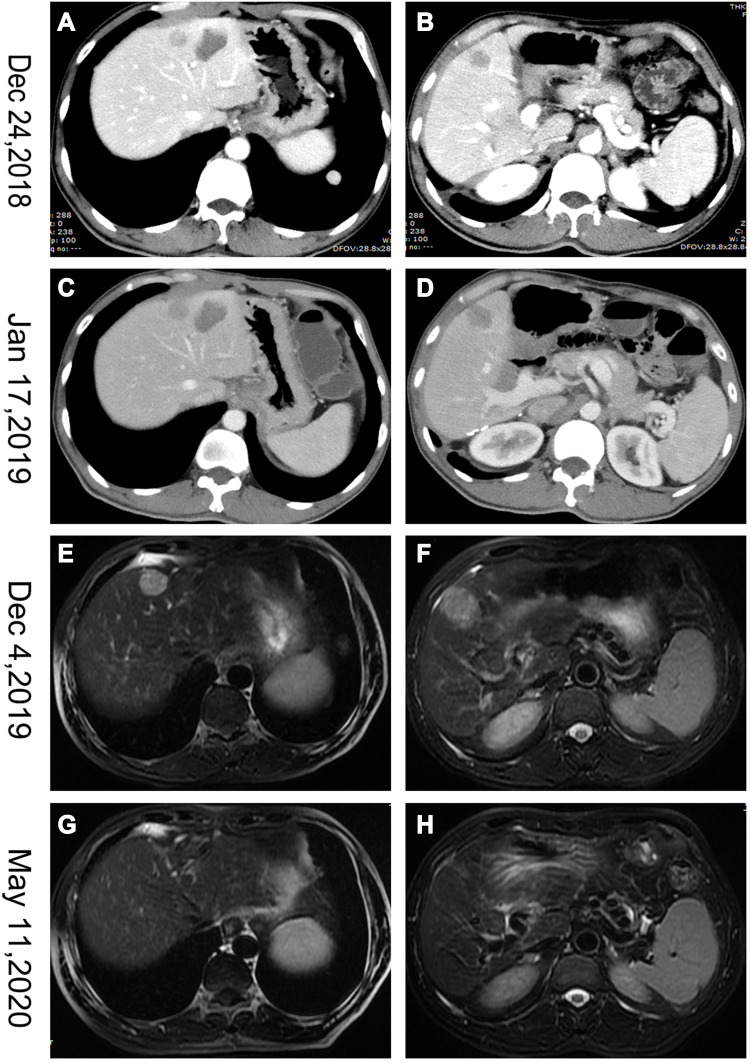
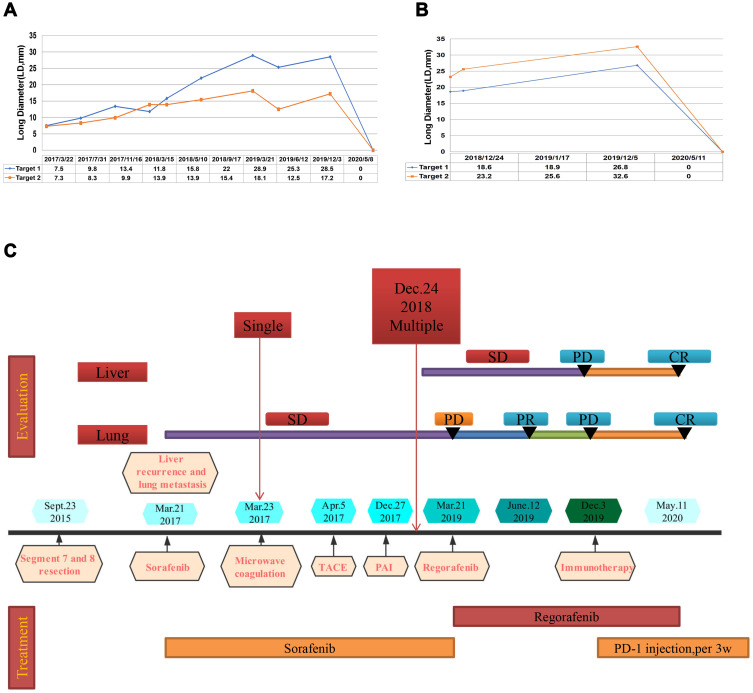
A 51-year-old man with a history of chronic HBV infection for more than 30 years presented to the Hepatic Surgery Center (Tongji Hospital, TongjiMedical College, Huazhong University of Science and Technology, Wuhan, China) with abdominal discomfort in September 2015. Enhanced abdominal magnetic resonance imaging (MRI) revealed a mass with the largest diameter measuring up to 11.0*9.5cm in size in segments 7 and 8 within the right lobe of the liver (Figure 1A and B). The patient was diagnosed with HCC with the background of cirrhosis secondary to HBV infection based on imaging studies and confirmed with the clinical diagnosis of Barcelona Clinic Liver Cancer (BCLC) A and Child-Pugh class A. Entecavir treatment was routinely used once per day from that time. His alpha-fetoprotein (AFP) level was in the normal range. On September 23, 2015, he underwent segment 7 and 8 liver resection and cholecystectomy. The postoperative pathological examination showed hemorrhage with necrosis in the middle of the tumor, with moderate differentiation and vascular cancer embolus. The incisal edge was negative and did not invade the hepatic capsule (Figure 1C and D). He recovered well and discharged from hospital. With regular examination, unfortunately in March, 2017, he had liver mass recurrence and lung metastases (BCLC C). According to the guidelines, he started systemic treatment with sorafenib (400 mg, twice per day). Enhanced abdominal computerized tomography (CT) scan showed a heterogeneous irregular mass measuring up to 2.3*2.2cm with arterial phase enhancement and venous phase washout in the left lobe of liver (Supplementary Figure S1). Chest CT scan revealed that multiple pulmonary nodules on both sides of the lung which were diagnosed as lung metastases, the largest up to 8-mm in diameter (Figure 2A and B). The patient received percutaneous microwave coagulation for the liver tumor on March 23, 2017. On April 5, 2017, he proceeded with TACE and then pulmonary arterial infusion (PAI) on December 27, 2017. On December 24, 2018, enhanced abdominal CT scan showed two lesions with the largest diameter greater than 3 cm in both sides of the liver (Figure 3A–D). He had radiographic progression in lung metastases after 3 months (Figure 2C and D). Disease progression was measured using Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v 1.1) or modified RECIST for HCC (mRECIST). Under this circumstance, he received 160 mg regorafenib orally once daily for 3 weeks in each 4-week cycle. The patient had no dose reductions in the period of medical treatment. On June 12, 2019, restaging chest CT scan showed partial response (PR) in lung metastases (Figure 2E and F). Despite good tolerability of regorafenib, repeat MRI scans of both chest and abdominal on December 3, 2019 revealed obvious tumor progression in liver and lung (Figures 2G and H and 3E and F). The patient started treatment with regorafenib 160 mg in combination with sintilimab (PD-1 inhibitor) on December 4, 2019. Sintilimab was given 200 mg over a period of 30–60 minutes for every 3 weeks as a cycle. The patient tolerated well the treatment, except potentially treatment associated general pruritus grade 2, with mild skin changes occurring but without rash. After five cycles of sintilimab injection, a follow-up abdominal MRI scan showed complete response (CR) in target lesions of liver without any tumor activity, as assessed by mRECIST (Figure 3G and H), while chest CT scan revealed CR in target lesions of lung, as evaluated by RECIST v 1.1 (Figure 2I and J). Figure 4 showed local and systemic treatment and a summary diameter of target lesions according to RECIST v 1.1 or mRECIST criteria. During the process of treatment, the patient had no severe complications and showed good liver functions (Child-Pugh A) (Table 1). At his last follow-up, nearly 5 years have elapsed since the diagnosis of HCC, and it is up to 40 months since lung metastases have been diagnosed. He was in a very good condition, without evidence of disease progression till now. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. This study was approved to publish the case details by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Figure 1.
Abdominal magnetic resonance imaging (MRI) showed a mass with the largest diameter measuring up to 11.0*9.5 cm in size in segments 7 and 8 in the right lobe of liver before surgical resection (A and B). Post-operative pathology revealed moderate differentiation and vascular cancer embolus (C and D).
Figure 2.
Chest computerized tomography (CT) scans showing the course of lung metastases. (A and B) Baseline tumor situation of the targets at the start of sorafenib systemic treatment. (C and D) Progressive disease upon treatment with sorafenib. (E and F) Partial response (PR) in lung metastases after treatment with regorafenib for 3 months. (G and H) Marked tumor progression in the lung metastases. (I and J) Complete response (CR) in target lesions of lungs after treatment with sintilimab for five cycles.
Figure 3.
Abdominal CT and MRI scans showed the course of hepatocellular cancer in the liver. (A–D) Two masses in both sides of liver. (E and F) Marked tumor progression in the liver. (G and H) Complete response (CR) in target lesions of liver.
Figure 4.
Local and systemic treatment methods (C) and tumor volume changes (A and B), summary diameter of target lesions according to RECIST v.1.1.
Table 1.
The Liver Function and Full Blood Test Analysis Corresponding to the Treatment
| Date | Child-Pugh | Ascites | T-BIL (µmol/L) |
ALB (g/L) |
PT (s) |
WBC (*10^9) |
HB (g/L) |
PLT (*10^9) |
|---|---|---|---|---|---|---|---|---|
| March 21, 2017 | A | N | 11 | 41.3 | 13.7 | 3.97 | 138 | 104 |
| May 14, 2017 | A | N | 35.8 | 39.7 | 14.7 | 3.66 | 128 | 96 |
| December 27, 2017 | A | N | 33.8 | 41.2 | 14.1 | 5.26 | 129 | 88 |
| December 24, 2018 | A | N | 19.3 | 39.8 | 13.5 | 5.03 | 146 | 103 |
| March 21, 2019 | A | N | 14.2 | 44.1 | 12.4 | 4.77 | 148 | 100 |
| June 12, 2019 | A | N | 36.0 | 36.7 | 14.8 | 7.38 | 140 | 127 |
| December 5, 2019 | A | N | 16.2 | 38.8 | 11.9 | 5.49 | 132 | 172 |
| May 11, 2020 | A | N | 24.2 | 39.6 | 12.1 | 5.67 | 138 | 166 |
Discussion
Liver resection is recommended as the first line treatment for patients with early stage HCC with well-preserved liver function.13 However, resection of even tumors less than 2 cm and without vascular invasion or satellites (BCLC stage 0), is associated with a recurrence rate of nearly 60% in 5 years.13,14 HCC patients are often diagnosed at advanced stages when recurrences occur, and curative therapy options are limited. The prognosis is extremely poor, leading to a 5-year OS rate of 2%.3
The systemic treatment options available for HCC patients with advanced stages are limited. Overall, more than 50% of HCC patients receive systemic therapies at some point during the disease progression.15 Sorafenib, a molecular kinase inhibitor, was thought to be a breakthrough in treating unresectable HCC, although only 3 months longer OS was observed in the SHARP trial.4 For nearly a decade, sorafenib was the only FDA-approved therapy and the benefit was limited for lack of either a therapeutic alternative or second-line treatment for those who are intolerant or resistant. However, since 2017, treatment for patients with advanced HCC has dramatically changed by new approved multi-target inhibitors, such as regorafenib, lenvatinib, and cabozantinib, or immune checkpoint inhibitors, such as nivolumab and pembrolizumab. Regorafenib is an oral multi-kinase inhibitor that blocks the activity of protein kinases, involved in angiogenesis, oncogenesis, metastasis, and tumor immunity.6,7 Moreover, regorafenib is the only systemic treatment shown to provide a survival benefit in advanced HCC patients progressing on sorafenib treatment. The RESORCE trial indicated that regorafenib improved OS with a hazard ratio of 0.63 (95% CI=0.50–0.79; one-sided P<0.0001); median survival was 10.6 months (95% CI=9.1–12.1) for regorafenib vs 7.8 months (6.3–8.8) for placebo.6 Although regorafenib has been approved as a second-line treatment for patients with advanced HCC who showed progression after sorafenib therapy, the treatment efficacy remains insufficient. With the development of immune therapy, the treatment effects on HCC need to be urgently explored.
PD-1 is expressed by activated T lymphocytes and is a key immune checkpoint receptor that mediates immunosuppression via binding to the PD-L1 expressed by tumor cells.16 In recent years, immune checkpoint blockade has brought a paradigm shift in the treatment of a number of solid tumors. Various immune checkpoint blocking inhibitors are being verified for their efficacy in HCC. Furthermore, PD-1 inhibitors had been considered as second-line treatment for HCC and demonstrated promising clinical activity, however, they did not significantly improve OS despite being associated with response rates of 15~20%.17,18 Recently, the IMbrave150 trial indicated better overall and progression-free survival (PFS) outcomes of atezolizumab plus bevacizumab as compared with sorafenib in patients with unresectable hepatocellular carcinoma who had not previously received systemic therapy, which inspired us to administer this combination therapy in advanced or metastatic HCC.12 Sintilimab (Innovent Biologics, Suzhou, China) is a highly selective, humanized, monoclonal antibody that blocks interactions between PD-1 and its ligands and has been tested regarding the safety and activity in patients with advanced-stage solid tumors and was approved for lymphoma by the Chinese Center for Drug Evaluation in China in 2018.19–21 Findings from a Phase 1 study of sintilimab (200 mg injection every 3 weeks) in advanced solid tumors showed clinical benefit.20,22
For those HCC patients with sorafenib resistance, the efficacy of regorafenib combined with PD-1/PD-L1 inhibitors remains unclear. Regorafenib was confirmed to be an immune-modulator in tumor microenvironment while PD-1 inhibitor blocks the co-inhibitory signals and unlocks the negative regulation of the immune response.23,24 Therefore, further investigations for combining regorafenib and PD-1/PD-L1 inhibitors in the treatment of HCC are urgently needed to provide their efficacy data in clinical trials. However, there are no published data from randomized controlled trials (RCTs) in HCC, which were registered on clinicaltrial.gov currently (summarized in Table 2). Standard combination and sequencing of the therapy need to be established with deeper insight into the rationale of combined action and further RCTs. Also, for most of the patients enrolled in, present with preserved liver function, while the advanced HCC patients in real clinical phase may have a much worse performance. Whether they can tolerate the combination therapy remains unclear and the clinical trials will not take the risk to enroll these patients. A multicenter retrospective study in Korea analyzed real-world data to assess the clinical effectiveness of regorafenib compared to the RESORCE trial and showed similar efficacy to the RESORCE trial. Although three patients (2.7%) showed a CR and 11 (9.8%) had a PR, their median PFS was only 2.7 months (95% CI=2.5–2.9 months) in this study.25 Another real-world study compared the effectiveness of regorafenib (n=223) and nivolumab (n=150) after sorafenib failure and demonstrated that only one patient (0.7%) in the nivolumab group but none in the regorafenib group achieved a CR.26 Joerger et al27 presented the case of a sorafenib-refractory patient probably experiencing progressive disease during ICI combination treatment with the anti-PD-1 monoclonal antibody nivolumab and the anti-Glucocorticoid Induced Tumor Necrosis Factor Receptor (anti-GITR) monoclonal antibody BMS-986156 within a clinical phase-1 trial followed by a prolonged tumor response according to RECIST v 1.1 during third-line treatment with regorafenib. In this case, sorafenib-immunotherapy-regorafenib sequential treatment was applied and the last evaluation was documented as PR and the patient started taking regorafenib at a reduced dose of 80mg and later further reduced to 40mg per day.27 However, despite good tolerability of regorafenib with a standard dose in the patient we reported, radiological examination of both the chest and abdomen revealed obvious tumor progression in the liver and lung near 9 months after regorafenib treatment. Therefore, the patient started treatment with regorafenib in combination with sintilimab. After five cycles of sintilimab injection, he was evaluated as CR. At up to 44 months since lung metastases was diagnosed, the PFS is 11 months after sintilimab treatment. He is in a very good condition without evidence of disease progression till now. The common adverse events of TKIs included hand–foot syndrome, diarrhea, loss of appetite, hypertension, general fatigue, and proteinuria. It is important that these adverse events are managed well to increase the therapeutic efficacy.28 No life-threatening adverse events were found in our patient. The combination therapy of regorafenib and PD-1/PD-L1 inhibitor will be a novel therapy method for the future gold standard in the systemic treatment of sorafenib-refractory HCC.
Table 2.
Ongoing Clinical Trials with Regorafenib and PD-1/PD-L1 Inhibitors in Hepatocellular Carcinoma
| NCT Number | Study Title | N | Interventions | Trial Phase | Primary Endpoint | Current Status |
|---|---|---|---|---|---|---|
| NCT 04170556 |
Regorafenib Followed by Nivolumab in Patients With Hepatocellular Carcinoma | 60 | Regorafenib+ Nivolumab | 1 and 2 | AE TTP |
Recruiting |
| NCT 04183088 |
Regorafenib Plus Tislelizumab as First-line Systemic Therapy for Patients With Advanced Hepatocellular Carcinoma | 125 | Regorafenib+ Tislelizumab |
2 | AE ORR |
Not yet recruiting |
| NCT 03347292 |
Regorafenib Plus Pembrolizumab in First Line Systemic Treatment of HCC | 57 | Regorafenib+ Pembrolizumab |
1 | AE | Recruiting |
| NCT 03475953 |
A Phase I/II Study of Regorafenib Plus Avelumab in Solid Tumors | 362 | Regorafenib+ Avelumab |
1 and 2 | Phase 1: Recommended phase 2 Dose Phase 2: Assessment of the antitumor activity of regorafenib |
Recruiting |
| NCT 04310709 |
Combination of Regorafenib and Nivolumab in Unresectable Hepatocellular Carcinoma | 42 | Regorafenib+ Nivolumab |
2 | RR | Not yet recruiting |
Abbreviations: AE, adverse events; TTP, time to progression; ORR, objective response rate; RR, response rate.
It’s worth noting that tumor response is often inconsistent according to the different evaluation criteria. In general, the classical RECIST radiologic assessment only considers tumor shrinkage in a single diameter as the sole measure of response, hampering the proper assessment of response in complex tumors, such as HCC.29 Actually, it does not reflect tumor necrosis caused by targeted drugs. mRECIST criteria which needs enhanced image assessment (CT or MRI scans), has served its purpose since being applied or included in clinical practice guidelines (European, American and Asian) for the management of HCC, it has also been instrumental for assessing tumor response and time-to-event endpoints in Phase II and III studies.30 For the past few years, mRECIST has become the standard criteria for measurement of radiological endpoints at early/intermediate stages of HCC, whereas guidelines recommend both methods at advanced stages. mRECIST has been proven to capture higher objective response rates in tumors treated with molecular therapies and those responses have shown to be independently associated with better survival rates.29,30 In the present case, after five cycles of PD-1 injection, a follow-up enhanced abdominal MRI scan showed complete response (CR) in target lesions of liver without any tumor activity, as assessed by mRECIST. According to RECIST v 1.1, it was evaluated as almost CR due to little tumor debris (Figure 3G and H). There are still several limitations in the present case. First, the mRECIST radiologic assessment cannot completely ascertain tumor inactivation due to no pathological results. Second, this is a retrospective case report, so we did not test the PD-L1 expression of tumor tissues and could not present the initial pathological immuno-staining results of TAM and T cells due to the long time after liver resection. Therefore, we cannot prove that the initial tumor was immunogenic.
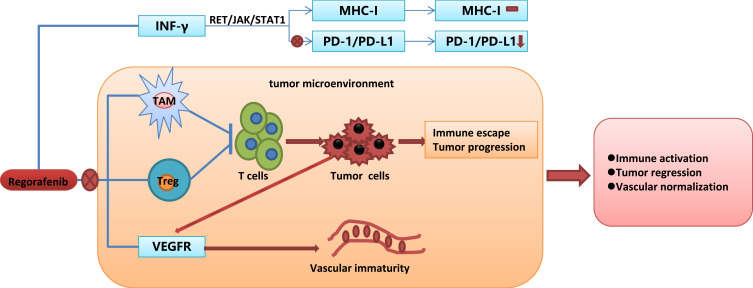
Recently, PD-1/PD-L1inhibitors may be promising for combination therapy with antiangiogenic drugs because the major toxicity effects of TKIs and ICIs do not overlap. There may be synergistic biological effects between TKIs and ICI agents.31 However, the precise mechanism of synergistic effects between regorafenib and PD-1/PD-L1 inhibitors remains unclear. Over-expression of VEGF in the tumor exerts an immunosuppression microenvironment by accumulation of regulatory T cells (Tregs) which inhibits dendritic cell (DC) maturation,32,33 inhibits T cell infiltration, and upregulates the expression of immune checkpoints on CD8+ T cells.34,35 Anti-VEGF therapy was found to inhibit an immune-suppressive environment that suppressed tumor progression and provided a rationale for combination therapy of ICI and TKIs.36 A previous study indicated that inhibition of the JAK1/2-STAT1 signaling pathway blocked MHC-I expression while suppressing the MAPK pathway increased MHC-I expression.37 Through inhibiting both the RET-Src axis/JAK1/2-STAT1 and MAPK pathway, regorafenib had little influence on MHC-I expression. Taken together, through blocking the IFN-γ induced PD-L1 expression and little influence on MHC-I expression, regorafenib reduced PD-L1 expression without compromising the antigen presentation processes. In addition, tumor-associated macrophage (TAM) is the major population of immune cells which has a crucial role in tumor development. One of the reasons described for the failure of anti-angiogenic therapy and tumor evasion is the strong infiltration of TAMs. TAM functions as an important driving factor in the immunosuppressive tumor microenvironment (TME) and, for instance, the secreted TGF-β inhibits CD8+ T-cell responses to kill the cancer cells.38,39 Regorafenib modulates TAM re-polarization and relieved the immunosuppressive effect of TAM via binding to CSF-1R.39 Terme et al40 demonstrated that regorafenib reduced the proportion of regulatory T-cell and inhibited tumor-induced Tregs proliferation via targeting the vascular endothelial growth factor A (VEGFA)/VEGF receptor 2 (VEGFR2) signaling pathway. The probable mechanism of synergistic effects of regorafenib and PD-1/PD-L1 inhibitors are shown in Figure 5. Eventually, it will be quite important to determine the effects of these combination therapies and their timing in order to establish and validate useful biomarkers of response and improve the prognosis of HCC patients.
Figure 5.
The probable mechanism of synergistic effect of regorafenib and PD-1/PD-L1 inhibitors.
Conclusion
In summary, this study provided the first case of complete tumor response to the combination of regorafenib and sintilimab as second line treatment for a patient with pulmonary metastatic, advanced HCC which was refractory to first-line sorafenib. Data from this clinical case report support future exploration of combination treatment of the oral multi-kinase inhibitor regorafenib with PD-1/PD-L1 inhibitors in sorafenib-refractory HCC patients. Regorafenib plus PD-1 or PD-L1 inhibitors may present a new potential treatment option for the advanced HCC patients with sorafenib resistance. Nevertheless, their synergistic effects and safety need further investigation in a randomized phase 3 study.
Acknowledgments
This work was supported by the grants from National Natural Science Foundation of China (81902839) and National Science and Technology Major Project of China (2017ZX10203207-002)
Informed Consent
Informed consent was obtained from the patient for being included in the study.
Disclosure
The authors declare that they have no conflicts of interest related to this report.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 7.Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12(7):1322–1331. doi: 10.1158/1535-7163.MCT-12-1162 [DOI] [PubMed] [Google Scholar]
- 8.Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313–1325. doi: 10.1002/jcp.27172 [DOI] [PubMed] [Google Scholar]
- 9.Morse MA, Sun W, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–920. doi: 10.1158/1078-0432.CCR-18-1254 [DOI] [PubMed] [Google Scholar]
- 10.Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018;24(4):193–204. doi: 10.1097/PPO.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 11.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117–124. doi: 10.1016/j.semcancer.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 13.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 14.Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer</=2 cm: results from two Western centers. Hepatology. 2013;57(4):1426–1435. doi: 10.1002/hep.25832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4 [DOI] [PubMed] [Google Scholar]
- 16.Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death −1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84–106. doi: 10.1016/j.pharmthera.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 18.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30(2):250–258. doi: 10.1093/annonc/mdy540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6(1):e12–e9. doi: 10.1016/S2352-3026(18)30192-3 [DOI] [PubMed] [Google Scholar]
- 22.Duan X, Zhang H, Zhou L, Jiang B, Mao X. Complete response to the combination of sintilimab and IBI305 for a patient with HBV-associated hepatocellular carcinoma with multiple lung metastasis. Dig Liver Dis. 2020. doi: 10.1016/j.dld.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 23.Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl 1):147–159. doi: 10.1159/000481245 [DOI] [PubMed] [Google Scholar]
- 24.Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy. Semin Liver Dis. 2018;38(4):379–388. doi: 10.1055/s-0038-1673621 [DOI] [PubMed] [Google Scholar]
- 25.Lee MJ, Chang SW, Kim JH, et al. Real-world systemic sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Korea. Invest New Drugs. 2020. doi: 10.1007/s10637-020-00977-4 [DOI] [PubMed] [Google Scholar]
- 26.Choi WM, Choi J, Lee D, et al. Regorafenib versus nivolumab after sorafenib failure: real-world data in patients with hepatocellular carcinoma. Hepatol Commun. 2020;4(7):1073–1086. doi: 10.1002/hep4.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joerger M, Guller U, Bastian S, Driessen C, von Moos R. Prolonged tumor response associated with sequential immune checkpoint inhibitor combination treatment and regorafenib in a patient with advanced pretreated hepatocellular carcinoma. J Gastrointest Oncol. 2019;10(2):373–378. doi: 10.21037/jgo.2018.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotani K, Enomoto M, Okada M, et al. Interstitial pneumonia suspected during regorafenib administration and exacerbated by subsequent therapy with lenvatinib for unresectable hepatocellular carcinoma. Clin J Gastroenterol. 2019;12(4):355–360. doi: 10.1007/s12328-019-00983-x [DOI] [PubMed] [Google Scholar]
- 29.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 30.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi: 10.1016/j.jhep.2019.09.026 [DOI] [PubMed] [Google Scholar]
- 31.Zhu XD, Tang ZY, Sun HC. Targeting angiogenesis for liver cancer: past, present, and future. Genes Dis. 2020;7(3):328–335. doi: 10.1016/j.gendis.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012;2012:492920. doi: 10.1155/2012/492920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi: 10.2217/imt.15.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. doi: 10.1182/blood-2002-07-1956 [DOI] [PubMed] [Google Scholar]
- 35.Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L, Hernandez MO, Zhao Y, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418–30 e6. doi: 10.1016/j.ccell.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu RY, Kong PF, Xia LP, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–4541. doi: 10.1158/1078-0432.CCR-18-2840 [DOI] [PubMed] [Google Scholar]
- 38.Zhao P, Wang Y, Kang X, et al. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci. 2018;9(10):2674–2689. doi: 10.1039/c7sc04853j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810–1820. doi: 10.1182/blood-2011-09-379214 [DOI] [PubMed] [Google Scholar]
- 40.Terme M, Tartour E, Taieb J. VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer. Oncoimmunology. 2013;2(8):e25156. doi: 10.4161/onci.25156 [DOI] [PMC free article] [PubMed] [Google Scholar]