Abstract
Background:
Fibromyalgia and functional gastrointestinal disorders (FGID) including irritable bowel syndrome (IBS) are common conditions presenting in clinical settings and are more prevalent in women. While the relationship between IBS and fibromyalgia has been demonstrated, a review of the prevalence of the broader group of FGID in adults with fibromyalgia has not been undertaken. The aim of this review was to systematically review the published literature, identifying the comorbidity of FGID in people with fibromyalgia, and to discuss the clinical implications, limitations of current research and areas of interest for future research
Methods:
Medline, Embase, CINAHL and Web of Science were searched during June 2019. Results were screened for original research articles meeting established criteria for identification of FGID in adults diagnosed with fibromyalgia.
Results:
A total of 14 studies involving 1340 adults with fibromyalgia, 363 healthy controls and 441 adults with other pathologies were included in this review. Only 1 of the 14 studies included surveyed the full range of FGID . Functional gut disorders were matched to Rome II criteria for reporting and comparison. In addition to increased abdominal pain and functional bloating or gas, IBS of mixed-pattern and constipation-types appear to be more prevalent than diarrhoea-predominant IBS in adults with fibromyalgia.
Conclusion:
This review confirms previous reports that IBS is common in people living with fibromyalgia and suggests that IBS-mixed and constipation types predominate. An association with a range of FGID other than IBS is suggested, but data are limited. Research exploring the association between fibromyalgia and functional gastrointestinal dysfunction beyond IBS are warranted.
Keywords: comorbidity, diagnostic criteria, FGID, fibromyalgia, functional gastrointestinal disorders, IBS, irritable bowel syndrome, women’s health
Background
Both functional gastrointestinal disorders (FGID) and fibromyalgia are poorly understood syndromes significantly impacting quality of life and contributing to high levels of work absenteeism.1 The two conditions are associated with increased visits to physicians and impose a substantial financial burden,2–4 with healthcare-associated costs reaching up to quadruple that of a reference population.5
The FGID were defined in 1989 as ‘a variable combination of chronic or recurrent gastrointestinal symptoms not explained by structural or biochemical abnormalities. This may include syndromes attributed to the oesophagus, stomach, biliary tree, small or large intestines, or anus’.6 Both fibromyalgia and FGID are more commonly identified in women, with fibromyalgia estimated to affect 3.98% (95% CI: 2.8–5.2) of women and 0.01 (95% CI: 0.04–0.06) of men.7 With few exceptions, the broad range of FGID are more prevalent in women,2,8 with medically diagnosed IBS affecting approximately four times as many women as men.9
It is thought that both fibromyalgia and FGID are attributable to sympathetic dysfunction with resultant central sensitisation.10,11 Both conditions were recently categorised as ‘Central Sensitivity Syndromes’.12,13 While a common etiology has been proposed,14 there is currently no compelling evidence to support a targeted intervention.
Fibromyalgia accounts for about 15% of all referrals to rheumatology clinics and has a global prevalence of around 2% with wide variation (0.2–6.6%) depending on the country and assessment tool used.7,15–17 Reports consistently concur a higher prevalence for females – up to 10-fold that of males.7,16,18,19 The higher prevalence rates in women have been contested as being driven primarily by selection bias and that rates in men and women are possibly quite similar.15
The major feature of fibromyalgia is chronic widespread pain (CWP) and is typically accompanied by a range of symptoms including fatigue, headache, sleep and cognitive disturbances, as well as digestive disorders, including IBS.20
It is estimated that about 50% of all digestive disorders presented in primary and secondary care are functional in nature,21 with IBS being the most common FGID diagnosed.22 The high comorbidity of IBS with other somatic disorders including fibromyalgia has previously been established,23 but results of co-prevalence studies vary greatly. Triadafilopoulos et al. reported that 73% of patients with fibromyalgia had altered bowel patterns.24 Lubrano and colleagues, using Rome Foundation criteria for IBS and ACR 1990 for fibromyalgia, reported fibromyalgia in 20% of patients with IBS (n = 130).25 A 2002 systematic review of comorbid conditions in IBS conveyed that 28–59% of subjects with fibromyalgia had IBS, and 32–77% of those with IBS have fibromyalgia, suggesting a bi-directional relationship.23 A recent systematic review by Heidari et al. calculated the prevalence of fibromyalgia in those with IBS at 12.9% (95% CI 12.70–13.10).7 These variations in prevalence reports are likely associated with the heterogeneous study designs, including the diagnostic criteria employed, as well as cultural and socioeconomic factors that influence whether a person seeks medical care for either their pain or the accompanying condition.
Other disorders not typically related to IBS, such as functional nausea, vomiting and belching, are also commonly reported in patients with fibromyalgia.26 While the relationship between IBS and fibromyalgia has been demonstrated, a review of the prevalence of the broader group of the FGID in adults with fibromyalgia has not been undertaken.
This paper presents a systematic review of the published literature reporting the relationship between the spectrum of FGID and fibromyalgia in adults. Clinical implications, limitations and areas for future research, are discussed.
Methods
To identify literature relevant to the research question: ‘What is the prevalence of any of the FGID in adults with fibromyalgia?’, a search was conducted in Medline, CINAHL, Embase and Web of Science using the search terms in Appendix 1. Citation chaining was undertaken to identify additional studies that may have been relevant to our objectives.
For inclusion, the following criteria were applied: published in English, available as full-text article, reported the prevalence of any FGID in subjects with fibromyalgia, included adults (⩾18 years of age), cohort studies, (prospective or retrospective), published since 1978. Searches of the literature took place over June 23–24, 2019. Where diagnostic or demographic data were unclear, attempts were made to contact the authors by email. If no response was obtained, that specific paper was removed from the analysis. For inclusion, fibromyalgia diagnosis based on specifications as outlined by Yunus (1981)27 Smythe (1985)28 or American College of Rheumatology (ACR) criteria (199029, 201030 or 201131) were required. Studies detailing a diagnosis of FGID based on Manning,32 Drossman,33 Kruis34 or any of the Rome criteria (Table 1) were retained.
Table 1.
Comparative classifications of irritable bowel syndrome.
| Manning32 | Drossman33 | Rome I35 | Rome II36 | Rome III37 | Rome IV38 |
|---|---|---|---|---|---|
| Two or more of: | A. Irregular or varying bowel pattern > 25% of the time | A minimum 3 months of: Continuous or recurrent abdominal pain; | Minimum 12 weeks out of the previous 12 months | Minimum 3 of previous 6 months: | Minimum of last 3 months, onset at least 6 months before diagnosis: |
| - Abdominal distension | - Relieved by passing stool or | Abdominal discomfort or pain plus 2 of the following: | Recurrent abdominal pain or discomfort in 3 days/month in the last 3 months, plus at least 2 of: | Recurrent abdominal pain, on average, at least 1 day/week in the last 3 months, plus at least 2 of: | |
| - Pain improved with passing stool | B. Abdominal pain: minimum six episodes in the last year and at least three of: | - Associated with change in frequency or consistency of stool | - Relieved by passing stool | - Improvement with defaecation | - Related to defaecation |
| - Frequent stool, with onset of pain | AND at least 3 of: (for at least 25% of days) | - Onset associated with a change in frequency of stool | - Onset associated with change in stool frequency | - Associated with a change in stool frequency | |
| - Pain relieved by bowel movement | - Altered stool frequency | - Onset associated with a change in form (appearance) of stool. | - Onset associated with a change in form (appearance) of stool | - Associated with a change in stool form (appearance) | |
| - Looser stool, with onset of pain | - Altered stool form | Supportive symptoms include: | Supportive symptoms as for Rome II | ||
| - Loose stools associated with the pain | - Altered stool passage (straining, urgency, sense of incomplete evacuation) | - Abnormal stool frequency (>3/day and < 3/week); | |||
| - Passing mucous | - More frequent stools associated with the pain | - Passing mucous | - Abnormal stool form (lumpy/hard or loose/ watery stool); | ||
| - Sensation of incomplete evacuation | - Abdominal distension- Mucous in the stool- Sense of incomplete evacuationC. Constipation- Straining at the stools > 25% of the time OR- Two or fewer stools per weekD. Diarrhoea:- Loose or watery stools > 25% of the time, or | - Bloating or feeling of abdominal distension | - Abnormal stool passage (straining, urgency, or feeling of incomplete evacuation)- Passage of mucous- Bloating or feeling of abdominal distension | ||
| - More than 21 stools per week |
Papers meeting the search criteria were downloaded into EndNote X8 citation software (Clarivate Analytics), and the following data tabulated in a Microsoft Excel spreadsheet: first author, publication year, country in which conducted, study type, age, gender, fibromyalgia diagnostic criteria, FGID criteria used, specific FGID/s identified.
As we set out to evaluate correlations reported in epidemiological data rather than results of clinical trials and their validity, the Joanna Briggs Critical Appraisal Checklist for Studies Reporting Prevalence Data was used to assess all included papers.39 This was carried out independently by two authors (SE and JEH), and any disagreements were resolved by consensus. Average scores were calculated as percentages and are shown in Table 2. This systematic review was registered with PROSPERO (Reg no: CRD42019139878).
Table 2.
Summary of 14 Studies reporting FGID in fibromyalgia.
| Author (Year) | Country | Fibromyalgia cases |
Controls | Diagnostic criteria |
Quality score | ||
|---|---|---|---|---|---|---|---|
| n = (% female) | AGE mean SD (range) | FMS | FGID | ||||
| Triadafilopoulos (1991) 24 | US | 123 (921.9) | 47 (24–64) | 46 NC, 54 DJD | ACR1990 | Drossman* | 83% |
| Veale (1991) 44 | Ireland | 20 (75) | (33–36) | 20 NC, 20 IBD, 20 IA | Smythe | Manning | 83% |
| Sivri (1996)45 | Turkey | 75 (87) | 36.3 ± 11 | 50 NC | ACR1990 | Drossman* | 83% |
| Sperber (1999) 42 | Israel | 100 (100) | 48.6 ± 10.8 – ± 14.9 | 0 | ACR1990 | Rome I | 92% |
| Yunus (2000)43 | US | ♀469 (100) | 46.3 ± 13.2 | 0 | ACR1990 | Manning | 79% |
| ♂67 (0) | 47.1 ± 13.3 | 36 NC | |||||
| Choudhury (2001)46 | Bangladesh | 30 (90) | 28.6 ± 10 | 30 NC, 30 RA | ACR1990 | Manning | 75% |
| Pace (2001)47 | Italy | 27 (92.6) | 51(25–73) | 25 NC, 32 IBS | ACR1990 | Rome I | 83% |
| Pimentel (2004)48 | US | 42 (86) | 46.6 ± 0.3 | 15 NC, 111 IBS | ACR1990 | Rome I | 92% |
| Kurland (2006)49 | US | 105 (93) | 52.9 ± 11.3 | 62 OR | ACR1990 | Rome II* | 92% |
| Zoppi (2008)50 | Italy | 67 (97) | 55.6 ± 11.01 | 0 | ACR1990 | Rome II | 50% |
| Almansa (2009)51 | Spain | 100 (93) | 50.5 ± 9.6 | 100 NC | ACR1990 | Rome II* | 92% |
| Akkaya (2011)40 | Turkey | 65 (100) | 36.21 ± 7.42 | 41 NC | ACR1990 | Rome Ia | 92% |
| Okumus (2011)52 | Turkey | 12 (54.8) | 40.1 ± 4.4 | 112 PD | ACR1990 | Rome II | 75% |
| Marum (2017)41 | Portugal | 38 (100) | 51 (>18) | 0 | ACR2011 | Rome III*,a | 88% |
| Total | 1340 (90.7) | NC 363, Other 441 | |||||
Information provided by email.
Separated IBS sub-types.
ACR, American College of Rheumatology; DJD, degenerative joint disease; FGID, functional gastrointestinal disorders; FMS, fibromyalgia; IA, inflammatory arthritis; IBD, inflammatory bowel disease; NC, normal controls; OR, other rheumatic diseases; PD, peritoneal dialysis; RA, rheumatoid arthritis; SD, standard deviation; US, United States.
Results
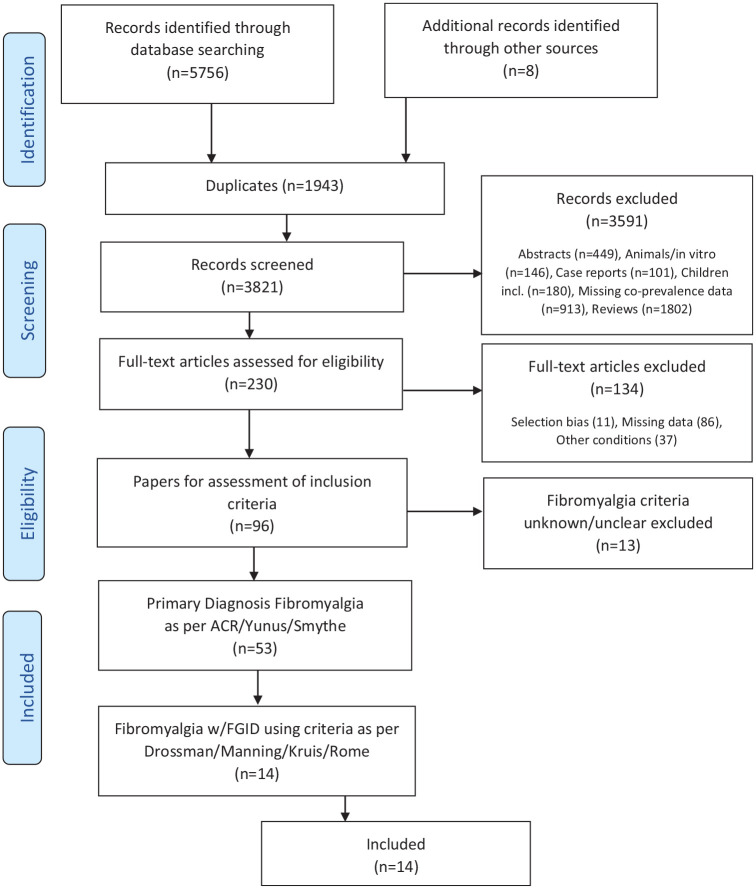
The search retrieved 5765 records. A diagrammatic representation of the selection process is shown in Figure 1.
Figure 1.
PRISMA flow diagram showing study selection process.
ACR, American College of Rheumatology; FGID, functional gastrointestinal disorders; PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Of 53 studies meeting accepted diagnostic criteria for fibromyalgia, 39 (73.5%) were discarded due to inadequate or unclear criteria for identification of the FGID. The remaining 14 studies reported data on 1340 subjects with fibromyalgia. Of these 14 studies, 12 employed the ACR 1990 criteria (Table 2), representing 96% of the total fibromyalgia cohort in this systematic review. Nine used a healthy control population (n = 363). Other comparison populations variously comprised other rheumatological conditions (n = 166), IBS or inflammatory bowel disease (IBD) (n = 162) and other control subjects with a range of pathologies (n = 112). The mean age ranged from 28.6–55.6 years, [standard deviation (SD) ±0.3 to ±14.9].
Three studies exclusively included women,40–42 one provided age data for males and females separately and is reflected accordingly in Table 2.43 The pooled population across all studies was 90.7% female, which decreases marginally to 89.1% when female-only studies are removed. Table 2 outlines key information from the included studies.
The prevalence of FGIDs varied widely, from 13.8% in an all-male cohort, to 98% in a primarily female cohort. All studies reported IBS in subjects, which varied from 13.8% to 95%, and all except one41 utilised diagnostic criteria according to Rome II, or earlier. Table 3 details the classification reported, as matched to Rome II criteria. Pooled data from all 14 studies reveals an overall prevalence of FGID in subjects of 50.8%, and 46.2% for IBS. It should be noted that 11 of the 14 studies reported only IBS, hence FGID and IBS prevalence in these does not differ. Sivri et al. reported abdominal pain in 38.2% of those with fibromyalgia.45 No data are provided that confirm whether these are independent of, or included in, any other group.
Table 3.
Summary of FGID types and prevalence reported in 14 included studies.
| Author | FGID Type | Fibromyalgia |
Normal controls |
Other controls |
|||
|---|---|---|---|---|---|---|---|
| (Rome II) | FGID % | IBS% | IBS % | p value | IBS % | p value | |
| Triadafilopoulos24 | C1, C2, C3, C4, D1 | 74.0 | 60.0 | 0 | nr | DJD 13 | nr |
| Veale44 | C1 | 70.0 | 70.0 | 10 | nr | IBD 5 | nr |
| IA 15 | nr | ||||||
| Sivri45 | C1, C*, C2, C3, C4, D1 | 41.8 | 41.8 | 16 | <0.05 | - | - |
| Sperber42 | C1 | 32.0 | 32.0 | - | - | - | - |
| Yunus43 | C1 | ♀38.9 | 38.9 | 0 | <0.03 | - | - |
| ♂13.8 | 13.8 | - | - | - | - | ||
| Choudhury46 | C1 | 30.0 | 30.0 | 7 | 0.02 | RA 3 | < 0.02 |
| Pace47 | C1 | 66.7 | 66.7 | 0 | nr | - | - |
| Pimentel48 | C1 | 52.4 | 52.4 | nr | - | - | - |
| Kurland49 | C1, C*, C3, C4 | 81.0 | 81.0 | - | - | OR 24 | <0.001 |
| Zoppi50 | C1 | 14.9 | 14.9 | - | - | - | - |
| Almansa51 | A1, A2, A3, A4, A5, B1, B2, B3, C1, C2, C3, C4, D1, D2, E1, E2, F2a, F2b, F3 | 98.0 | 39.0 | 3 | <0.001 | - | - |
| Akkaya40 | C1 | 61.5 | 61.5 | nr | - | - | - |
| Okumus52 | C1 | 16.7 | 16.7 | - | - | PD 7 | 0.25 |
| Marum41 | C1, C*, C3, C4 | 95.0 | 95.0 | - | - | - | - |
| Pooled data | 50.8 | 46.2 | 4.9 | 11.7 | |||
A1, Globus; A2, Rumination syndrome; A3, Fx chest pain; A4, Fx heartburn; A5, Fx dysphagia; B1, Fx dyspepsia; B2, Aerophagia; B3, Fx vomiting; C*, Alternating constipation and diarrhoea; C1, IBS; C2, Fx bloating; C3, Fx constipation; C4, Fx diarrhoea; D1, abdominal pain; D2, unspecified functional pain; DJD, degenerative joint disease; E1, gallbladder dysfunction; E2, sphincter of Oddi dysfunction; F1, Fx incontinence; F2a, levator ani syndrome; F2b, proctalgia fugax; F3, pelvic floor dysfunction; FGID, functional gastrointestinal disorder; Fx, functional; IA, inflammatory arthritis; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; NC, normal controls; nr, not reported; OR, other rheumatic diseases; PD, peritoneal dialysis; RA, rheumatoid arthritis.
One study set out to examine the prevalence of the range of FGIDs in fibromyalgia,51 five focused on IBS.24,42,44,45,49 In four studies,41,43,46,50 IBS was recorded as part of a bigger research question, and in the remaining four studies IBS data was reported as a secondary not primary outcome measure.40,47,48,52 Four studies included additional cohorts of patients with IBS42,44,47,48; these subjects are excluded from control data presented in Table 2. Appendix B contains information related to the aim of each of the included studies.
Two studies included controls with non-rheumatic diseases: IBD and chronic kidney failure.44,52 Separation of control groups revealed that IBS occurred in those with other pathologies at more than double the rate of healthy controls.
While not detailed, it appears the control group in the study by Pimentel et al. was selected based on absence of both fibromyalgia and IBS.48 Yunus et al. did not include a female control group as their purpose was comparing fibromyalgia in men and women42; a small group of male controls were included, for whom data was not compared with females.
While all studies reported IBS, further detail allowing separation of IBS sub-types was reported by five.24,41,45,49,51 Three studies compared prevalence of sub-types of IBS with that of normal controls24,45,51; these are detailed in Table 4.
Table 4.
Prevalence of sub-types of FGID in fibromyalgia and healthy controls from five studies (%).
| Author | IBS (C1) |
Alternating constipation and diarrhoea (C*) |
Bloating (or ‘gas’) (C2) |
Constipation (C3) |
Diarrhoea (C4) |
Abdominal pain (D1) |
Unspecified functional abdominal pain (D2) |
Faecal incontinence (F1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FMS | NC | FMS | NC | FMS | NC | FMS | NC | FMS | NC | FMS | NC | FMS | NC | FMS | NC | |
| Triadafilopoulos24 | 60.0 | 0 | 62.6 | 0 | 59.0 | 13.0 | 12.0 | 13.0 | 9.0 | 2.0a | 54.0 | 4.0 | - | - | - | - |
| Sivri45 | 41.8 | 16.0 | 41.8 | 20.0 | 45.5 | - | 30.7 | 8.0 | 7.0 | 1.0 | 38.2 | 8.0 | - | - | - | - |
| Kurland49 | 81.0 | - | 61.0 | - | - | - | 15.2 | - | 4.8 | - | - | - | - | - | - | - |
| Almansa51 | 39.0 | 3.0 | - | - | 34 | 12.0 | 15.0 | 5.0 | 2.0 | 0 | 75.0 | 0.0 | 19.0 | 1.0 | 56.0 | 25.0 |
| Marum41 | 95.0 | - | 22.2 | - | - | - | 69.4 | - | 8.3 | - | - | - | - | - | - | - |
| FGID average b | 67.2 | 52.9 | 65.4 | 21.3 | 6.0 | 57.1 | 19.0 | 56.0 | ||||||||
Estimated from Triadafilopoulos Figure 2 (data not provided).
Calculated from studies reporting that FGID.
FGID, functional gastrointestinal disorder; FMS, fibromyalgia patients; NC, normal controls.
Almansa et al. reported at least one FGID in 98% of patients and 39% of controls.51 High rates of oesophageal and gastroduodenal conditions were also reported, with all symptoms except vomiting present at significantly higher rates in patients than in controls (p < 0.05) (data not shown). IBS had the strongest association with fibromyalgia, followed by functional bloating and functional faecal incontinence, as outlined in Table 4.
The overall prevalence of IBS in the study by Triadafilopoulos et al. presented in Tables 3 and 4 above is extracted from data presented in Table 2 of their report.24 Notably, this is at odds with their in-text report, which stated that 81% of patients had ‘normal alternating with irregular bowel pattern’, meeting their Category 1 ‘alternating bowel pattern’. The figure in column C* in Table 4 is for those with IBS-alternating/mixed (IBS-M) by Rome II criteria.
None of the included studies provided detail regarding coexistence of more than one FGID in subjects. For example, Almansa’s group reported functional constipation in 15% of subjects,51 39% had IBS, and while at least one FGID was found in 98% of subjects, data on comorbidity of multiple FGIDs are not provided.
Discussion
The relationship between fibromyalgia and the group of gastrointestinal disorders that are collectively grouped as ‘functional’ beyond IBS is underexplored. Of the studies included in our review, just one sought to answer this question. This systematic review found that half of people with fibromyalgia have at least one FGID, with a heavy weighting of IBS prevalence data. Without further investigation, the totality of FGID in subjects with fibromyalgia and, therefore, the true comorbidity, remains undetermined.
The odds ratio (OR) of fibromyalgia in subjects with IBS is 1.8 and some of the earliest studies examining associations between fibromyalgia and IBS revealed a strong bidirectional relationship.42,44 This was confirmed in Whitehead’s 2002 systematic review, with 32.5% (range 28–65%) of IBS patients having fibromyalgia, and 48% of patients with fibromyalgia having IBS (range 32–77%).23 The overall prevalence of IBS in people with fibromyalgia identified in our review is 46.2%, closely aligning with Whitehead findings, and is about four times the 12.9% [95% confidence interval (CI) 12.7–13.1] co-prevalence reported in the 2017 meta-analysis by Heidari et al., and the 11% estimated global prevalence of IBS.7,54 The variation in reported prevalence identified in our results ranged from 13% to 95%, which is somewhat broader than Whitehead’s 28–65%.23 Inclusion criteria for fibromyalgia in Heidari’s report were less stringent than ours and the prevalence they determined ranged from 0.71% to 4.82%, depending on the diagnostic tool used.7
Some of the earliest studies examining associations between fibromyalgia and IBS revealed a strong bidirectional relationship.42,44 The overall prevalence of IBS in people with fibromyalgia identified in our review is 46.2%, closely aligning with the 49.2% found in Whitehead’s systematic review,23 and is about four times the 12.9% co-prevalence reported by Heidari et al. and the 11% estimated global prevalence of IBS.7,54 The variation in reported prevalence identified in our results ranged from 13% to 95%, which is somewhat broader than Whitehead’s 28% to 65%.23
Of the 14 studies in our analysis, 2 reported fibromyalgia-FGID comorbidity rates of less than 20%. Okumus et al. investigated IBS in patients with end-stage renal disease (ESRD),52 and found that 16.7% of patients with both ESRD and fibromyalgia had IBS, compared with 7.1% of those with ESRD but without fibromyalgia. Given the known alterations to the gut microbiome in patients with renal disease,55 the low comorbidity suggests the small patient group (n = 12 with ESRD plus fibromyalgia versus those with ESRD only n = 112 ) may underlie the non-significant difference reported. Zoppi et al. acknowledged that the 14.9% IBS rate in their cohort was the only condition occurring at lower rates than either the general population or other fibromyalgia studies at that time.50 A control group was not included and there is insufficient information provided in their report to inform further discussion.
Our review included research in which data on the presence of any FGID and fibromyalgia was reported. In some cases, this was not an objective of the included study, rather part of the description of the cohort. In the papers reviewed, only one provided detailed FGID data. Generally, investigators tended to regard IBS as a single entity, rather than a condition with several subtypes, a shortcoming which has been reported previously.56 This lack of differentiation limits a more comprehensive interpretation of results in the current era where an altered microbiome has been identified in patients with IBS,57 is considered to play a role in FGIDs,58 and where the role the human gut microbiota on gastrointestinal functions (such as gas production and dynamics, bloating and motility) is evolving.
The five studies that provided details beyond the generic classification of IBS (Table 4), reported that IBS-M was the most common subtype, followed by IBS-C, which affected subjects at more than three times the rate of diarrhoea-predominant IBS (IBS-D). In other studies excluded from this review, due to not meeting our criteria for identification of FGID, varying rates of constipation were noted: 12% by Garcia-Leiva et al. (alternating pattern in 63%),59 55.5% (double the rate of diarrhoea) by Rios’ group and 56.5% in Zanetti’s cohort.60,61 Conversely, Buchwald et al. reported diarrhoea in 62% of their fibromyalgia cohort and provided no information regarding constipation or alternating bowel habit.62 Constipation is estimated to occur in 12–19% of the general population and, where not secondary to other conditions, may be associated with dehydration and other dietary and lifestyle factors as well as medications.63 Technically, these and primary causes should be ruled out before a diagnosis of IBS-C is made, but this is not consistent amongst clinicians.59 Also associated with an increase in methanogenic bacteria, IBS-C may be predictable by methane gas production as measured in human breath, with 91.7% sensitivity and 81.3% specificity.64 Pimentel et al. conducted hydrogen-methane testing in subjects with fibromyalgia but did not report either methane levels or IBS sub-types.48
Our review also concludes a high prevalence of functional bloating and/or gas (65.4%), similar to Zanetti et al.’s reported prevalence of 56.5% in women with fibromyalgia,61 although in their report a ‘functional’ classification was not specified. Bloating is associated with bacterial degradation of fermentable compounds and increased hydrogen and methane gas production.65–67 The small number of studies (n = 3) in our results reporting gas/bloating precludes generalisability but significantly higher rates of fibromyalgia have been reported in people with IBS with bloating compared with those with IBS without bloating.68 The significant correlation between peak breath hydrogen production and fibromyalgia pain (p < 0.01) as described in Pimentel’s study,48 irrespective of IBS status, also suggests that an altered microbiome may play an important role in fibromyalgia.
Marum et al. recruited subjects evaluated by a rheumatologist with a dietitian in attendance with the purpose of assessing the effect of a diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) on fibromyalgia symptoms.41 Hence, a selection bias towards patients with gastrointestinal symptoms undoubtedly underlies the high rate of IBS (95%) reported. The cohort was small (n = 38) and removal of this study from our analysis reduced the overall prevalence of IBS from 46.2% to 44.8% and IBS-constipation (IBS-C) from 21.3% to 17.1%. A significant reduction in a range of symptoms and disease scores related to both fibromyalgia and IBS was noted after 4 weeks of adherence to the low FODMAP diet (all p < 0.05). Consuming a diet that is low in fermentable compounds has been shown to be effective in the management of IBS,69,70 including in subjects with fibromyalgia.71 While lowering the intake of fermentable foods is known to alter gut bacterial populations,72 the effect of such a diet on global symptoms of fibromyalgia is unexplored as yet.
IBS and functional dyspepsia are the most common FGIDs, with prevalence ranging from 5.3 to 20.4%.8 Researchers sometimes include dyspepsia under the category of gastro-oesophageal reflux disease (GERD).73 As GERD can be secondary to organic disease, including infection and as a side effect of medication, we excluded cases of GERD or dyspepsia where the aetiology was unclear. While we screened papers that reported dyspepsia, reflux and GERD, only one study reported appropriately defined functional dyspepsia, and found it occurred in people with fibromyalgia at seven times the rate of controls.51
A survey evaluating the relationship between body mass index (BMI) and functional dyspepsia, IBS, functional constipation and functional diarrhoea in over 35,000 subjects found that 10.4% had more than one FGID: for example, those presenting with functional dyspepsia also presented with IBS (23.5%) and functional constipation (15.1%).74 While a significant limitation of that study is the exclusion of the broader range of FGIDs, it nevertheless serves to highlight the possibility of missing coexisting FGIDs. The authors did not report mixed-pattern IBS, yet the higher rates of IBS and functional constipation in women mirrors our finding in those with fibromyalgia – also primarily women. Functional dyspepsia was also more prevalent in women 4.5% compared with 2.0% in men (p < 0.001).74
Three controlled studies reporting the prevalence of IBS in fibromyalgics included control subjects with other musculoskeletal pathologies.44,46,49 Both healthy controls and those with rheumatoid arthritis (RA) had low rates of IBS in Choudhury’s small study (n = 30 in each group),46 using the Manning criteria. These criteria report a much higher rate of IBS diagnosis compared with the Rome II criteria9,75 (see Table 1). Kurland’s control group comprised patients with a range of conditions, including RA, osteoarthritis and systemic lupus erythematosus (SLE), in whom the overall prevalence of IBS was 24%.49 This is higher than the estimated prevalence in the United States (US) of 14.1%,9 suggesting a possible gut-musculoskeletal relationship. SLE is an autoimmune condition more common in women and also associated with a higher incidence of IBS; where this was the case, subjects were more likely to have fibromyalgia (OR 2.87, 95% CI 1.11–7.43, p = 0.02).76 There were four comparison groups in Veale’s study: IBD, inflammatory arthritis (IA), normal controls (NC) and a group with IBS for bidirectional comparison.44 We detected an error in data reporting IBS prevalence, whereby the text states ‘IBD=1, IA=2, NC=2’ (i.e. n = 5), yet ‘all control patients. . . six had IBS’. The latter matches data in their Figure 1b, where the bar for IA appears to represent n = 3 (not n = 2). While numbers are small, this difference means 15% of the group with IA had IBS, not 10%.
Evidence suggests that the gut bacterial community may be altered in IBS,77 constipation and fibromyalgia.58,78–80 Increasingly, research reports indicate an important role of the gastrointestinal milieu in health and a wide range of conditions, from chronic kidney disease to allergies and diseases of connective tissue and in fibromyalgia.55,79–82
The identification of commensal gut bacteria at sub-infective levels in synovial fluid and joint cartilage in people with arthritis adds credence to what currently is a hypothetical gut microbiota-musculoskeletal interaction.83–85 While we did not seek to explore FGIDs in other musculoskeletal conditions, these groups are often used in comparison with fibromyalgia. Our review found higher rates of IBS in subjects with other musculoskeletal conditions compared with healthy controls, further supporting an interaction between the gut microbiota and the musculoskeletal system.86,87
Sperber et al. reported that fibromyalgics with IBS had worse symptoms of pain, fatigue and morning tiredness compared with those without IBS.42 This is consistent with other reports linking aggravation of digestive symptoms during periods of exacerbations of fibromyalgia.24 Iovino et al. demonstrated that patients with mild IBS were invariably negative for fibromyalgia,88 whereas moderate-to-severe symptoms of IBS were associated significantly with fibromyalgia. Lubrano et al. also found a significant association between fibromyalgia and the severity of IBS symptoms (p = 0.002),25 supporting the notion that by-products associated with a dysbiotic gut environment may be drivers of exacerbations in actual pain, or pain perception. However, measurements of markers of gut-derived inflammation, such as calprotectin, lipopolysaccharide (LPS), intestinal fatty acid binding protein (iFABP), monocyte chemo-attractant protein-1 (MCP-1) and co-receptor soluble cluster of differentiation (sCD) have failed to demonstrate a relationship between these markers and the severity of symptoms associated with IBS or musculoskeletal pain.89
Recent advances have also expanded understanding of the possible role of the gut ecological environment on pain signalling and peripheral sensitisation,90 with data suggesting a relationship between chronic pain and the intestinal microbiome exists in CRPS,80,91 endometriosis,92 interstitial cystitis,93 restless leg syndrome (RLS) and migraine.94,95
An altered microbiota in patients with fibromyalgia is further supported by pain reduction subsequent to antibiotic treatment and following Marum’s dietary intervention.41,96 While a low FODMAP diet may improve IBS symptoms associated with changes to the gut microbiome and intestinal gas production,65,69,97 the effect on fibromyalgia symptoms had not been investigated previously. Other dietary interventions that have been studied in fibromyalgia are also associated with clinical improvements,98 which may be due to diet-driven alterations in gut microbial populations.
The ever-expanding research exploring the gut–brain axis has emerged as a possible candidate for unexplained pain syndromes.99 Recent advances in understanding this axis and the intricate interplay of various hormones, chemokines, neurotransmitters and gut-derived metabolites are gaining traction,100 including in fibromyalgia research.101,102 As the technology enabling isolation and identification of not only organisms that previously eluded detection, but their metabolic processes and by-products evolves, so too does the understanding of the possible role these might play in extra-gastrointestinal pathologies with elusive aetiologies.
A proposed explanation for the pain in fibromyalgia is sensitisation of the central nervous system,18 but an underlying mechanism remains elusive. Neuropathic pain syndrome is associated with pain, insomnia and fatigue as well as hyperalgaesia. Women with fibromyalgia tend to present with higher rates of these symptoms compared with healthy controls (p < 0.001).103 Other conditions associated with central sensitisation and sympathetic nervous system predominance include temporomandibular disorder, IBS, dyspepsia, RLS, chronic pelvic pain, interstitial cystitis, headache, migraine, chronic fatigue syndrome and vulvodynia all of which present at higher rates in women, and commonly coexist with other conditions from within the same classification.11,53,104–109 The interplay of genetics and epigenetics relevant to each syndrome adds an additional layer of complexity to the determination of factors that may form a basis for comorbidity.8,110
Our search terms were crafted to capture the spectrum of FGIDs, yet results did not identify any co-prevalence data capturing the range until 2009,51 with the exception of functional abdominal pain in two papers (1991 and 1996).24,45 This may reflect the emergence of the first detailed guideline for identification and classification of the FGIDs in 1990 and highlights a significant gap in the research.35
The large variance in the prevalence of FGID in our studies may be explained – at least in part – by changes in the understanding of functional gut disorders over time and the various diagnostic criteria employed. The timespan of the included studies is some 26 years, over which time diagnostic criteria for IBS, FGID and fibromyalgia underwent several changes, each of which has the potential to alter results. This has been demonstrated with application of Rome II criteria in a population survey resulting in lower rates of IBS than Rome I,9 although Kurland et al. found the opposite to be true in fibromyalgia.49
The earliest diagnostic criteria for fibromyalgia employed by the included studies was that proposed by Smythe (n = 1), and 12 of the 14 studies utilised the ACR 1990 criteria, based on widespread pain and the determination of at least 11 of 18 specific tender points.111 Just one paper used ACR 2011, which was a modification of the 2010 criteria, and more suited for use in survey-based screening. These criteria and the modification are detailed in Wolfe.112
Including studies employing established diagnostic criteria for both fibromyalgia and FGID is a strength of this review, and the rigour of our process ensures minimal likelihood of non-fibromyalgia groups being captured. For purposes of analysis, we utilised Rome II criteria as 9 of the 14 studies employed pre-Rome II tools, 4 used Rome II, 1 used Rome III and none used the most recent Rome IV. While attempts to match symptoms as reported in earlier studies to Rome II were made, the potential for misclassification exists. It was observed that terms such as constipation, diarrhoea, abdominal pain, etc., are seldom described as ‘functional’. We hypothesised that organic causes of these conditions would have meant any affected subjects were removed from the initial cohort, as was stated by some authors and was previously a requirement for IBS diagnosis, as in Rome I.35
While we aimed to exclude known cause-and-effect reported FGID (such as narcotic bowel syndrome), few studies provided sufficient detail for certainty in this regard. For example, it is possible that constipation rates reported may be associated with factors such as diet, fluid intake, exercise or drug use (such as analgaesics), which we were unable to determine.
Limitations in our study relate directly to the inherent quality of the studies included. Stringent inclusion criteria resulted in a smaller cohort for evaluation. In calculating the prevalence of FGID, we relied on author’s reports; yet overlaps in the data are possible. For example, Sivri et al. using Drossman’s criteria (Table 1),33 pooled the data for altered bowel patterns to arrive at an overall prevalence of IBS of 41.8%,45 which is the same as their figure for alternating pattern, but less than the reported rate of bloating of 45.5%.
Our data is strongly representative of females with fibromyalgia even when all-female cohorts were excluded from the analysis. This may not necessarily reflect a true epidemiological disparity; the possibility of gender-bias in fibromyalgia and FGID sample populations is a consideration that has been discussed in a review by Houghton et al.113 Additionally, epidemiological data from community-dwelling subjects in Europe indicates the prevalence in women is just double that of men,16 suggesting women are more likely to seek healthcare for their condition.
Approximately 70% of people with IBS do not seek medical input for their gastrointestinal complaint and up to 13% of a population may have undiagnosed fibromyalgia, exposing an opportunity to evaluate the coexistence of these disorders in patients presenting to primary or secondary practice, possibly with coexisting disorders associated with heightened pain sensitivity.16,54 While the causes of any FGID and how these may influence the clinical features of fibromyalgia is undetermined, strategies to address contributing factors and thus improve gastrointestinal function have the potential to contribute to disease modification of both conditions.
Conclusion
This review confirms previous reports that IBS is common in people living with fibromyalgia and demonstrates that this appears to be predominantly mixed and constipation types. However, a significant gap exists in the research regarding the relationship with other FGIDs. Indications that reductions in gastrointestinal symptoms correlate with improvements in fibromyalgia suggests patients may benefit from identification of the wider range of gastrointestinal disorders and implementation of clinical strategies to address contributing factors. Future studies evaluating the full range of FGIDs in subjects with fibromyalgia using established criteria for both conditions should include adequate detail related to confounders such as medication use, diet and lifestyle. A comprehensive examination of the FGID-gut ecology relationship would provide further insights in this patient population and help elucidate its role in people with both FGID and fibromyalgia.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820977402 for A systematic review of the association between fibromyalgia and functional gastrointestinal disorders by Sharon Erdrich, Jason A. Hawrelak, Stephen P. Myers and Joanna E. Harnett in Therapeutic Advances in Gastroenterology
Acknowledgments
Thanks to Yulia Ulyannikova for guidance in methodology for structuring and conducting the systematic review.
Footnotes
Author contributions: SE co-designed the study, conducted the searches, sorted the results and created the initial draft of the manuscript.
JAH co-designed the study, assisted in interpretation of the data, and edited the manuscript. SPM assisted in interpreting the data and editing the manuscript.
JEH co-designed the study, contributed to interpretation of data, and was a major contributor in writing the manuscript.
All authors read and approved the final manuscript.
Authors’ note: An earlier version of this manuscript was uploaded to Research Square, DOI: 10.21203/rs.2.21966/v1
Availability of data and materials: Data sharing not applicable to this article as no datasets were generated. All datasets reviewed in this article are cited in the Results section.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Sharon Erdrich  https://orcid.org/0000-0002-8448-5644
https://orcid.org/0000-0002-8448-5644
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sharon Erdrich, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Science Road, Camperdown, Sydney, New South Wales 2006, Australia.
Jason A. Hawrelak, College of Health and Medicine, University of Tasmania, Hobart, Tasmania, Australia
Stephen P. Myers, NatMed Research Unit, Office of the Deputy Vice Chancellor (Research), Southern Cross University, Lismore, New South Wales, Australia
Joanna E. Harnett, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, New South Wales, Australia
References
- 1. Corsetti M, Tack J, Attara G, et al. IBS Global Impact Report 2018. Uncovering the true burden of irritable bowel syndrome (IBS) on people’s lives. Allergan & Gastrointestinal Society, 2018. [Google Scholar]
- 2. Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993; 38: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 3. Buono JL, Mathur K, Averitt AJ, et al. Economic burden of irritable bowel syndrome with diarrhea: retrospective analysis of a U.S. commercially insured population. J Manag Care Spec Pharm 2017; 23: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tack J, Stanghellini V, Mearin F, et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol 2019; 19: 69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger A, Dukes E, Martin S, et al. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J Clin Pract 2007; 61: 1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson WG, Dotevall G, Drossman DA, et al. Irritable bowel syndrome: Guidelines for the diagnosis. Gastroenterol Int 1989; 2: 92–95. [Google Scholar]
- 7. Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017; 37: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 8. Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil 2015; 21: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005; 21: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 10. Sarzi-Puttini P, Atzeni F, Di Franco M, et al. Dysfunctional syndromes and fibromyalgia: a 2012 critical digest. Clin Exp Rheumatol 2012; 30: 143–151. [PubMed] [Google Scholar]
- 11. Martínez-Martínez L-A, Mora T, Vargas A, et al. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol 2014; 20: 146–150. [DOI] [PubMed] [Google Scholar]
- 12. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008; 37: 339–352. [DOI] [PubMed] [Google Scholar]
- 13. Slim M, Calandre EP, Rico-Villademoros F. An insight into the gastrointestinal component of fibromyalgia: clinical manifestations and potential underlying mechanisms. Rheumatol Int 2015; 35: 433–444. [DOI] [PubMed] [Google Scholar]
- 14. Chang L. The association of functional gastrointestinal disorders and fibromyalgia. Eur J Surg 1998: 32–36. [DOI] [PubMed] [Google Scholar]
- 15. Wolfe F, Walitt B, Perrot S, et al. Fibromyalgia diagnosis and biased assessment: sex, prevalence and bias. PLoS One 2018; 13: e0203755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 2010; 39: 448–453. [DOI] [PubMed] [Google Scholar]
- 17. Marques AP, Adriana de Sousa do ES, Berssaneti AA, et al. Prevalence of fibromyalgia: literature review update. Rev Bras Reumatol Engl Ed 2017; 57: 356–363. [DOI] [PubMed] [Google Scholar]
- 18. Queiroz LP. Worldwide epidemiology of fibromyalgia topical collection on fibromyalgia. Curr Pain Headache Rep 2013; 17: 356. [DOI] [PubMed] [Google Scholar]
- 19. Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on international classification of diseases, 9th revision codes. J Clin Rheumatol 2006; 12: 124–128. [DOI] [PubMed] [Google Scholar]
- 20. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46: 319–329. [DOI] [PubMed] [Google Scholar]
- 21. Thompson WG. The road to Rome. Gastroenterol 2006; 130: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 22. Shivaji UN, Ford AC. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterol 2014; 5: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterol 2002; 122: 1140–1156. [DOI] [PubMed] [Google Scholar]
- 24. Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci 1991; 36: 59–64. [DOI] [PubMed] [Google Scholar]
- 25. Lubrano E, Iovino P, Tremolaterra F, et al. Fibromyalgia in patients with irritable bowel syndrome: an association with the severity of the intestinal disorder. Int J Colorectal Dis 2001; 16: 211–215. [DOI] [PubMed] [Google Scholar]
- 26. Pamuk ON, Ümit H, Harmandar O. Increased frequency of gastrointestinal symptoms in patients with fibromyalgia and associated factors: a comparative study. J Rheumatol 2009; 36: 1720–1724. [DOI] [PubMed] [Google Scholar]
- 27. Yunus MB., Masi AT. Association of primary fibromyalgia syndrome (PFS) with stress-related syndromes. Clinical Research 1985; 33, A923–A923. [Google Scholar]
- 28. Smythe H. The clinical significance of tenderpoints. Br J Rheumatol 1985; 24, 212–213. [Google Scholar]
- 29. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33, 160–172. [DOI] [PubMed] [Google Scholar]
- 30. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010; 62, 600–610. [DOI] [PubMed] [Google Scholar]
- 31. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol 2011; 38, 1113–1122. [DOI] [PubMed] [Google Scholar]
- 32. Manning AP, Thompson WG, Heaton KW, et al. Towards positive diagnosis of the irritable bowel. Br Med J 1978; 2: 653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drossman DA, Sandler RS, McKee DC, et al. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterol 1982; 83: 529–534. [PubMed] [Google Scholar]
- 34. Kruis W, Thieme C, Weinzierl M, et al. A diagnostic score for the irritable bowel syndrome: its value in the exclusion of organic disease. Gastroenterol 1984; 87: 1–7. [PubMed] [Google Scholar]
- 35. Drossman DA, Thompson WG, Talley NJ, et al. Identification of sub-groups of functional gastrointestinal disorders. Gastroenterol Int 1990; 3: 159–172. [Google Scholar]
- 36. Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut 1999; 45: II43–II47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterol 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 38. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterol 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 39. Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc 2015; 13: 147–153. [DOI] [PubMed] [Google Scholar]
- 40. Akkaya N, Akkaya S, Polat Y, et al. Helicobacter pylori seropositivity in fibromyalgia syndrome. J Clin Rheumatol 2011; 30: 43–49. [DOI] [PubMed] [Google Scholar]
- 41. Marum AP, Moreira C, Tomas-Carus P, et al. A low fermentable oligo-di-mono-saccharides and polyols (FODMAP) diet is a balanced therapy for fibromyalgia with nutritional and symptomatic benefits. Nutr Hosp 2017; 34: 667–674. [DOI] [PubMed] [Google Scholar]
- 42. Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: Studies of prevalence and clinical implications. Am J Gastroenterol 1999; 94: 3541–3546. [DOI] [PubMed] [Google Scholar]
- 43. Yunus MB, Inanici F, Aldag JC, et al. Fibromyalgia in men: comparison of clinical features with women. J Rheumatol 2000; 27: 485–490. [PubMed] [Google Scholar]
- 44. Veale D, Kavanagh G, Fielding JF, et al. Primary fibromyalgia and the irritable bowel syndrome: Different expressions of a common pathogenetic process. Br J Rheumatol 1991; 30: 220–222. [DOI] [PubMed] [Google Scholar]
- 45. Sivri A, Cindas A, Dincer F, et al. Bowel dysfunction and irritable bowel syndrome in fibromyalgia patients. Clin Rheumatol 1996; 15: 283–286. [DOI] [PubMed] [Google Scholar]
- 46. Choudhury AK, Yunus MB, Haq SA, et al. Clinical features of fibromyalgia syndrome in a Bangladeshi population. J Musculoskelet Pain 2001; 9: 25–33. [Google Scholar]
- 47. Pace F, Sarzi-Puttini P, Manzionna G, et al. Visceral hypersensitivity is not a feature of fibromyalgia syndrome. J Musculoskelet Pain 2001; 9: 47–55. [Google Scholar]
- 48. Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis 2004; 63: 450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kurland JE, Coyle WJ, Winkler A, et al. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig Dis Sci 2006; 51: 454–460. [DOI] [PubMed] [Google Scholar]
- 50. Zoppi M, Maresca M. Symptoms accompanying fibromyalgia. Reumatismo 2008; 60: 217–220. [DOI] [PubMed] [Google Scholar]
- 51. Almansa C, Rey E, Sanchez RG, et al. Prevalence of functional gastrointestinal disorders in patients with fibromyalgia and the role of psychologic distress. Clin Gastroenterol Hepatol 2009; 7: 438–445. [DOI] [PubMed] [Google Scholar]
- 52. Okumus M, Parpucu H, Kocaoglu S, et al. The frequency of fibromyalgia syndrome and the quality of life in patients with peritoneal dialysis. Open J Rheumatol Autoimmune Dis 2011; 63: 88–93. [Google Scholar]
- 53. Cole JA, Rothman KJ, Cabral HJ, et al. Migraine, fibromyalgia, and depression among people with IBS: a prevalence study. BMC Gastroenterol 2006; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hobby GP, Karaduta O, Dusio GF, et al. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol 2019; 316: F1211–F1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013; 19: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. J Acad Nutr Diet 2019; 28: 22. [DOI] [PubMed] [Google Scholar]
- 58. Shin A, Preidis GA, Shulman R, et al. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol 2019; 17: 256–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garcia-Leiva JM, Carrasco JLO, Slim M, et al. Celiac symptoms in patients with fibromyalgia: a cross-sectional study. Rheumatol Int 2015; 35: 561–567. [DOI] [PubMed] [Google Scholar]
- 60. Rios G, Estrada M, Mayor AM, et al. Factors associated with tender point count in Puerto Ricans with fibromyalgia syndrome. P R Health Sci J 2014; 33: 112–116. [PMC free article] [PubMed] [Google Scholar]
- 61. Zanetti CB, Domiciano DS. Celiac symptoms in Brazilian patients with fibromyalgia from a tertiary hospital [Conf Abstr]. 35th Brazilian Congr Rheumatol (SBR 2018). Rio de Janeiro: Advances in Rheumatology, 2018. [Google Scholar]
- 62. Buchwald D, Goldenberg DL, Sullivan JL, et al. The “chronic, active Epstein-Barr virus infection” syndrome and primary fibromyalgia. Arthritis Rheum 1987; 30: 1132–1136. [DOI] [PubMed] [Google Scholar]
- 63. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol 2011; 25: 16B–21B. [PMC free article] [PubMed] [Google Scholar]
- 64. Hwang L, Low K, Khoshini R, et al. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci 2010; 55: 398–403. [DOI] [PubMed] [Google Scholar]
- 65. Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol 2014; 109: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilder-Smith CH, Olesen SS, Materna A, et al. Fermentable sugar ingestion, gas production, and gastrointestinal and central nervous system symptoms in patients with functional disorders. Gastroenterol 2018; 155: 1034–1044. [DOI] [PubMed] [Google Scholar]
- 67. Huang Y, Jia L, Liu Y. The relationships between hydrogen gas and methane gas and the symptoms of irritable bowel syndrome as determined by a breath test. Int J Clin Exp Med 2019; 12: 7356–7364. [Google Scholar]
- 68. Hod K, Ringel Y, van Tilburg MAL, et al. Bloating in irritable bowel syndrome is associated with symptoms severity, psychological factors, and comorbidities. Dig Dis Sci 2019; 64: 1288–1295. [DOI] [PubMed] [Google Scholar]
- 69. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil 2017; 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 70. Varju P, Farkas N, Hegyi P, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: a meta-analysis of clinical studies. PLoS One 2017; 12: e0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rossi A, Bellini M, Ricciardi A, et al. A low fermentable oligo-di-monosaccharides and polyols diet improves symptoms without affecting body composition and extracellular body water in fibromyalgic patients with irritable bowel syndrome. XVII Med Congr Rheumatol. Clin Exp Rheumatol 2018; S59. [Google Scholar]
- 72. Sloan TJ, Jalanka J, Major GAD, et al. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS One 2018; 13: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Silverman SL, Backonja M, Pauer L, et al. Effect of baseline characteristics on the pain response to pregabalin in fibromyalgia patients with comorbid depression. Pain Med 2018; 19: 419–428. [DOI] [PubMed] [Google Scholar]
- 74. Le Pluart D, Sabaté J-M, Bouchoucha M, et al. Functional gastrointestinal disorders in 35447 adults and their association with body mass index. Aliment Pharmacol Ther 2015; 41: 758–767. [DOI] [PubMed] [Google Scholar]
- 75. Ghoshal UC, Abraham P, Bhatia SJ, et al. Comparison of Manning, Rome I, II, and III, and Asian diagnostic criteria: report of the Multicentric Indian Irritable Bowel Syndrome (MIIBS) study. Indian J Gastroenterol 2013; 32: 369–375. [DOI] [PubMed] [Google Scholar]
- 76. Garcia Carrasco M, Mendoza Pinto C, Lopez Colombo A, et al. Irritable bowel syndrome-type symptoms in female patients with mild systemic lupus erythematosus: Frequency, related factors and quality of life. Neurogastroenterol Motil 2013; 25: 958–966. [DOI] [PubMed] [Google Scholar]
- 77. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol 2019; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ohkusa T, Koido S, Nishikawa Y, et al. Gut microbiota and chronic constipation: a review and update. Front Med 2019; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Minerbi A, Gonzalez E, Brereton NJB, et al. Altered microbiome composition in individuals with fibromyalgia. Pain 2019; 00: 1–14. [DOI] [PubMed] [Google Scholar]
- 80. Erdrich S, Hawrelak JA, Myers SP, et al. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet Disord 2020; 21: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Melli LC, do Carmo-Rodrigues MS, Araujo-Filho HB, et al. Intestinal microbiota and allergic diseases: a systematic review. Allergol Immunopathol (Madr) 2016; 44: 177–188. [DOI] [PubMed] [Google Scholar]
- 82. Talotta R, Atzeni F, Ditto MC, et al. The microbiome in connective tissue diseases and vasculitides: an updated narrative review. J Immunol Res 2017; 2017: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhao Y, Chen B, Li S, et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep 2018; 8: 14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dunn C, Velasco C, Rivas A, et al. Identification of a human cartilage microbial DNA signature and characterization of distinct microbiome profiles associated with osteoarthritis [abstract]. 2018 ACR/ARHP Annual Meeting. Arthritis Rheumatol, 2018. [Google Scholar]
- 85. Li Y, Luo W, Deng Z, et al. Diet-intestinal microbiota axis in osteoarthritis: a possible role Mediat Inflamm 2016; ID: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Berthelot J-M, Sellam J, Maugars Y, et al. Cartilage-gut-microbiome axis: a new paradigm for novel therapeutic opportunities in osteoarthritis RMD Open 2019; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Caminer AC, Haberman R, Scher JU. Human microbiome, infections, and rheumatic disease. Clin Rheumatol 2017; 36: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 88. Iovino P, Tremolaterra F, Consalvo D, et al. Perception of electrocutaneous stimuli in irritable bowel syndrome. Am J Gastroenterol 2006; 101: 596–603. [DOI] [PubMed] [Google Scholar]
- 89. Undseth R, Berstad A, Valeur J. Systemic symptoms in irritable bowel syndrome: an investigative study on the role of enterocyte disintegrity, endotoxemia and inflammation. Mol Med Rep 2016; 14: 5072–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guo R, Chen L-H, Xing C, et al. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. [DOI] [PubMed] [Google Scholar]
- 91. Reichenberger ER, Alexander GM, Perreault MJ, et al. Establishing a relationship between bacteria in the human gut and complex regional pain syndrome. Brain Behav Immun 2013; 29: 62–69. [DOI] [PubMed] [Google Scholar]
- 92. Leonardi M, Hicks C, El-Assaad F, et al. Endometriosis and the microbiome: a systematic review. BJOG 2020; 127: 239–249. [DOI] [PubMed] [Google Scholar]
- 93. Braundmeier-Fleming A, Russell NT, Yang W, et al. Stool-based biomarkers of interstitial cystitis/bladder pain syndrome. Sci Rep 2016; 6: 26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weinstock LB, Walters AS. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med 2011; 12: 610–613. [DOI] [PubMed] [Google Scholar]
- 95. Gasbarrini A, De Luca A, Fiore G, et al. Beneficial effects of Helicobacter pylori eradication on migraine. Hepatogastroenterology 1998; 45: 765–770. [PubMed] [Google Scholar]
- 96. Pimentel M, Chow EJ, Hallegua D, et al. Small intestinal bacterial overgrowth: a possible association with fibromyalgia. J Musculoskelet Pain 2001; 9: 107–113. [Google Scholar]
- 97. Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition 2018; 45: 24–31. [DOI] [PubMed] [Google Scholar]
- 98. Silva AR, Bernardo A, Costa J, et al. Dietary interventions in fibromyalgia: a systematic review. Ann Med 2019; 51: S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharon G, Sampson TR, Geschwind DlH, et al. The central nervous system and the gut microbiome. Cell 2016; 167: 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Neuman H, Debelius JW, Knight R, et al. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev 2015; 39: 509–521. [DOI] [PubMed] [Google Scholar]
- 101. Tomasello G, Mazzola M, Bosco V, et al. Intestinal dysbiosis and hormonal neuroendocrine secretion in the fibromyalgic patient. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018; 162: 258–262. [DOI] [PubMed] [Google Scholar]
- 102. Malatji BG, Mason S, Mienie LJ, et al. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019; 15: 54. [DOI] [PubMed] [Google Scholar]
- 103. Kosehasanogullari M, Erdinc Gunduz N, Akalin E. Is fibromyalgia syndrome a neuropathic pain syndrome? Arch Rheumatol 2019; 34: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. den Boer C, Dries L, Terluin B, et al. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalizations and measurement instruments. J Psychosomat Res 2019; 117: 32–40. [DOI] [PubMed] [Google Scholar]
- 105. Dahan H, Shir Y, Velly A, et al. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain 2015; 16: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yilmaz V, Aras B, Erturk FA, et al. Migraine in patients with fibromyalgia and outcomes of greater occipital nerve blockage. Clin Neurol Neurosurg 2019; 181: 54–57. [DOI] [PubMed] [Google Scholar]
- 107. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol 2010; 184: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 108. Lau CI, Lin CC, Chen WH, et al. Association between migraine and irritable bowel syndrome: a population-based retrospective cohort study. Eur J Neurol 2014; 21: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 109. Leusink P, Kaptheijns A, Laan E, et al. Comorbidities among women with vulvovaginal complaints in family practice. J Sex Med 2016; 13: 220–225. [DOI] [PubMed] [Google Scholar]
- 110. D’Agnelli S, Arendt-Nielsen L, Gerra MC, et al. Fibromyalgia: genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol Pain 2019; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 112. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol 2011; 38: 1113–1122. [DOI] [PubMed] [Google Scholar]
- 113. Houghton LA, Heitkemper M, Crowell M, et al. Age, gender and women’s health and the patient. Gastroenterol 2016; 150: 1132–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820977402 for A systematic review of the association between fibromyalgia and functional gastrointestinal disorders by Sharon Erdrich, Jason A. Hawrelak, Stephen P. Myers and Joanna E. Harnett in Therapeutic Advances in Gastroenterology