Abstract
Aims:
Line immune-assays (LIA) for the detection of myositis-specific antibodies (MSA) are used widely for characterization of idiopathic inflammatory myopathies (IIM). Their current use and significance for the diagnosis of IIM remains unclear.
Methods:
In this retrospective analysis, we retrieved clinical diagnoses of patients tested for MSA and myositis-associated antibodies (MAA) Jo-1, Mi-2α, Mi-2β, TIF1γ, SRP, MDA-5, NXP-2, SAE, PL-7, PL-12, EJ, OJ, PM-Scl100, PM-Scl75 and Ku. We calculated clinical specificity, clinical sensitivity, negative- and positive predictive values (PPV) as well as positive and negative likelihood ratios.
Results:
In total, we analyzed 3167 samples. After exclusion of repeated measurements and patients with insufficient clinical information, data of 1118 patients were available for analysis. A total of 242 patients tested positive for at least one antibody, of which 45 patients had a diagnosis of IIM; 25 IIM patients were negative for all MSA/MAA. Clinical specificity of MSA/MAA for the diagnosis of IIM ranged between 94.2% and 99.9%. Clinical sensitivity and PPV across all antibodies tested ranged from 0.0% to 12.9% and 0.0% to 72.7%, respectively.
Conclusion:
In clinical practice MSA/MAA are used widely for diagnostic work-up of IIM, resulting in a low pre-test probability. Clinicians should be aware that PPVs for most MSA/MAA are low.
Keywords: auto-antibody, idiopathic inflammatory myopathy, line-immune assay, myositis-specific antibody
Introduction
Idiopathic inflammatory myopathies (IIM) are rare autoimmune disorders characterized by immune-mediated muscle destruction resulting in muscle weakness. In addition, systemic involvement of the skin, joints, lungs, and heart is common, contributing to the high morbidity and mortality of these diseases. IIM includes dermatomyositis (DM), inclusion-body myositis (IBM), immune-mediated necrotizing myopathy, and the increasingly doubted polymyositis (PM).1 IIM can overlap with other systemic autoimmune disorders or develop in the context of malignancy. Although the spectrum of IIM is highly heterogeneous, myositis-specific antibodies (MSA) and myositis-associated autoantibodies (MAA) can help to identify subsets of patients with relatively homogeneous clinical features.2 MSA like melanoma-derived antigen (MDA)-5 identify patients at risk for interstitial lung disease (ILD), whereas antibodies against transcriptional intermediary factor (TIF)-1γ indicate DM-associated malignancy.1,2 Furthermore, in IIM with complicated features refractory to conventional treatment, the presence of MSA/MAA may predict a better response to rituximab.3 Identification and characterization of single MSA was initially performed by immunoprecipitation (IP) resulting in a very high clinical specificity for IIM. However, individual MSA have low clinical sensitivity occurring only in a subset of IIM patients. Simultaneous testing for multiple MSA using a single line immuno-assay (LIA) was developed as a potential alternative to IP,4 and moderate-to-high agreement of both methods was demonstrated.5,6 Furthermore, LIA are fast and easier to perform, allowing detection of MSA/MAA in standard diagnostic laboratories.5
Most previous studies investigating the diagnostic accuracy MSA/MAA LIAs were performed in relatively small and homogenous cohorts of IIM patients. We hypothesize that this does not reflect the current clinical situation where MSA/MAA may be used increasingly as biomarkers in the diagnostic work-up of suspected IIM. The diagnostic use of MSA/MAA LIAs in this setting is unclear and has not been assessed so far.7,8
This study aims to investigate the use and diagnostic value of MSA/MAA LIA in a large cohort of patients.
Patients and methods
Patients
All results of the Euroline Autoimmune Inflammatory Myopathies LIA (Euroimmun, Lübeck, Germany) obtained in our diagnostic laboratory between October 2014 and October 2017 were included in this retrospective analysis. In addition, a second cohort of 104 patients with an established diagnosis of metastatic or locally advanced pancreatic, breast or lung cancer without signs of IIM was tested for the prevalence of MSA and MAA. This group was compared to 88 age- and sex-matched healthy controls (general population). Our study was approved by the institutional review board of the Medical University of Graz (EK 30-239ex17/18). An informed consent was not sought for this study, and the need for informed consent was waived by the ethic committee because of the retrospective study design.
MSA/MAA line immunoassay
The following 15 MSA and MAA were assessed: anti-Jo-1, anti-Mi-2α, anti-Mi-2β, anti-TIF1γ, anti-SRP, anti-MDA-5, anti-NXP-2, anti-SAE, anti-PL-7, anti-PL-12, anti-EJ, anti-OJ, anti-PM-Scl100, anti-PM-Scl75 and anti-Ku (Euroline Autoimmune Inflammatory Myopathies LIA, Euroimmun). Although included on the LIA, we did not evaluate the results of anti-Ro-52 as it is tested routinely by an enzyme-linked immunoassay (ELISA) in our laboratory. LIAs were performed according to the instructions provided by the manufacturer, using EUROBlotOne (Euroimmun). Intensities of the LIA strips were analyzed using the EUROLineScan system (Euroimmun) resulting in semi-quantitative units. According to the manufacturer, results greater than 10 units were considered as positive.
Patient data
The electronic medical records of the tested patients were reviewed for clinical diagnoses of IIM, other autoimmune disorders, ILD or malignancy from the time of the LIA test until present. Patients categorized as ‘other autoimmune disorder’ had a clinical diagnosis of an autoimmune disease other than IIM and no signs of IIM in their electronic records. Patients without sufficient clinical data were excluded from analysis. In case of repeated test results, the most recent result was used for analysis.
Statistical analysis
Clinical specificity, clinical sensitivity, negative predictive values (NPV) and positive predictive values (PPV), the positive and negative likelihood ratio (LR) were calculated for the presence of IIM and ILD. Additionally, PPV was calculated for the presence of autoimmune diseases other than IIM and malignancy. Correlations of MSA/MAA results and age were calculated using Spearman’s correlation coefficient. Fisher’s exact test was performed to compare MSA/MAA results of cancer and control patients. The p value was adjusted for multiple testing using the Sidàk correction (corrected p value was p ⩽ 0.003). Receiver operating characteristic curve (ROC) analysis was performed to identify possible new cut-offs for individual MSA/MAAs. Calculations were performed using MS Excel 2010 (Microsoft, Redmond, WA, USA) and SPSS V22.0 (IBM, Chicago, IL, USA).
Results
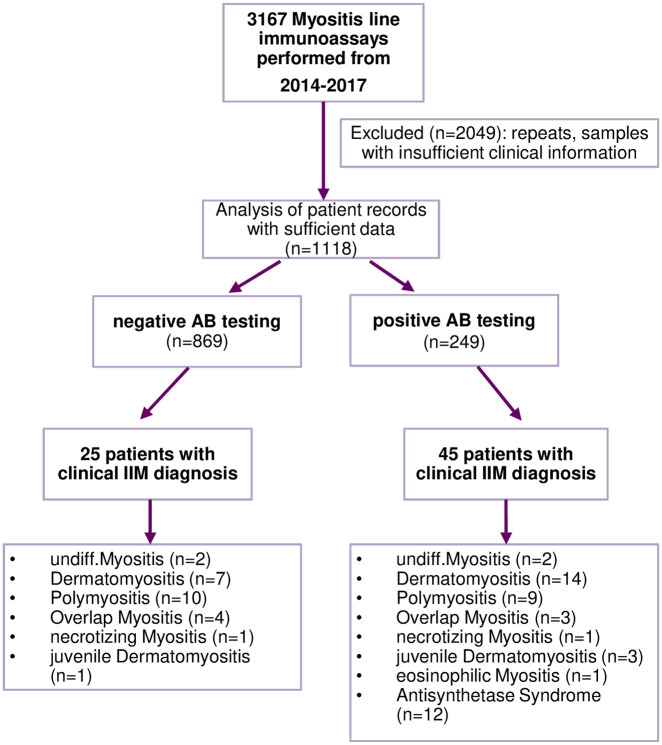
In total, we analyzed the LIA results of 3167 samples; 31% of the samples were sent from rheumatology out-patient clinics, 7% from dermatology departments, 62% from other departments and physicians. Reasons for ordering LIA tests were suspicion or presence of IIM (17%), muscle weakness or creatinine kinase elevation (8%), myalgia (7%), differential diagnosis of other autoimmune rheumatic disease, including Raynaud’s phenomenon (24%) and others (44%). Test results from 2049 samples were excluded because of insufficient clinical data or repeated testing of the same patient. In this case, the most recent result was used for analysis. For further analyses, clinical records of 1118 patients were reviewed (Figure 1); 55% of patients were female and the mean age [± standard deviation (SD)] of this cohort was 54.9 (±16.3) years of age. A total of 249 (22.3%) patients had at least one antibody tested positive, of which 45 patients had a clinical diagnosis of IIM (as documented by the treating physician). The frequency of IIM, ILD, AID and malignancy with positive MSA and MAA is depicted in Table 1.
Figure 1.
Flow chart of data analysis.
AB, antibody; IIM, idiopathic inflammatory myopathies; undiff. undifferentiated.
Table 1.
Frequency of diseases in MSA/MAA positive patients.*
| MSA/MAA | n, positive tested | IIM, n (%) | ILD, n (%) | Malignancy, n (%) | AID, n (%) | ND, n (%) |
|---|---|---|---|---|---|---|
| Jo-1 | 20 | 9 (45.0) | 4 (20.0) | 4 (20.0) | 4 (20.0) | 11 (55.0) |
| TIF1γ | 19 | 2 (10.5) | 0 (0) | 1 (5.3) | 9 (47.4) | 17 (89.5) |
| MDA-5 | 15 | 4 (26.7) | 0 (0) | 2 (13.3) | 3 (20.0) | 10 (66.7) |
| NXP-2 | 12 | 4 (33.3) | 1 (8.3) | 1 (8.3) | 1 (8.3) | 8 (66.7) |
| SAE | 4 | 2 (50.0) | 0 (0) | 1 (25.0) | 0 (0) | 2 (50.0) |
| PM-Scl100 | 26 | 5 (19.2) | 0 (0) | 5 (19.3) | 14 (53.9) | 21 (80.8) |
| PM-Scl75 | 66 | 3 (4.5) | 4 (6.1) | 4 (6.1) | 27 (40.9) | 62 (63.9) |
| PL-7 | 24 | 6 (25.0) | 6 (25.0) | 3 (12.5) | 11 (45.8) | 18 (75.0) |
| EJ | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| OJ | 3 | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 3 (100) |
| PL-12 | 27 | 1 (3.7) | 4 (14.8) | 2 (7.4) | 7 (25.9) | 26 (96.3) |
| SRP | 34 | 8 (23.5) | 4 (11.8) | 4 (11.8) | 7 (20.6) | 25 (73.5) |
| Mi-2α | 11 | 8 (72.7) | 1 (9.1) | 1 (9.1) | 3 (27.3) | 3 (27.3) |
| Mi-2β | 21 | 5 (23.8) | 2 (9.5) | 4 (19.1) | 9 (42.9) | 16 (76.2) |
| Ku | 25 | 5 (20.0) | 1 (4.0) | 3 (12.0) | 8 (32.0) | 20 (80.0) |
Positive auto-antibodies may belong to more than one disease category.
AB, antibody; AID, autoimmune-disorder; IIM, idiopathic inflammatory myopathies; ILD, interstitial lung disease; MAA, myositis-associated antibody; MSA, myositis-specific antibody; ND, not diagnosed as IIM, ILD, AID or malignancy.
The test characteristics of individual MSA/MAA is summarized in Table 2. We performed ROC analysis to identify more specific cut-offs for the individual MSA/MAAs. However, increasing the cut-offs did not lead to fewer false-positive results (data not shown). Considering only MSA/MAA positive patients with indirect immunofluorescence (IIF) pattern on HEp-2 cells compatible with the respective MSA/MAA (according to the International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns - ICAP) decreased clinical sensitivity but did not enhance the clinical specificity (supplemental Table S1).9,10 MSA/MAA levels and age or sex did not show any significant correlations. A clinical diagnosis of ILD was present in 9 MSA/MAA negative and in 12 MSA/MAA positive patients (Table 2).
Table 2.
Testing of the diagnostic value of myositis antibodies.
| MSA/MAA | n, negative tested | n, positive tested | IIM | posLR | negLR | ILD | posLR | negLR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical sensitivity | Clinical specificity | PPV | NPV | Clinical sensitivity | Clinical specificity | PPV | NPV | |||||||
| Jo-1 | 1098 | 20 | 12.86 | 98.99 | 45.00 | 94.63 | 12.69 | 0.88 | 23.81 | 98.64 | 25.00 | 98.55 | 17.49 | 0.77 |
| TIF1γ | 1099 | 19 | 2.86 | 98.43 | 10.53 | 94.02 | 1.83 | 0.99 | 0.00 | 98.28 | 0.00 | 98.10 | 0.00 | 1.02 |
| MDA-5 | 1103 | 15 | 4.29 | 98.90 | 20.00 | 94.13 | 3.88 | 0.97 | 9.52 | 98.82 | 13.33 | 98.29 | 8.07 | 0.92 |
| NXP-2 | 1106 | 12 | 5.71 | 99.26 | 33.33 | 94.23 | 7.76 | 0.95 | 9.52 | 99.09 | 16.67 | 98.29 | 10.50 | 0.91 |
| SAE | 1114 | 4 | 2.86 | 99.82 | 50.00 | 94.10 | 15.51 | 0.97 | 4.76 | 99.73 | 25.00 | 98.21 | 17.49 | 0.95 |
| PM-Scl100 | 1092 | 26 | 7.14 | 98.07 | 19.23 | 94.25 | 3.69 | 0.95 | 4.76 | 97.73 | 3.85 | 98.18 | 2.10 | 0.97 |
| PM-Scl75 | 1052 | 66 | 4.29 | 94.20 | 4.55 | 93.85 | 0.74 | 1.02 | 19.05 | 94.37 | 6.06 | 98.39 | 3.39 | 0.86 |
| PL-7 | 1094 | 24 | 8.57 | 98.34 | 25.00 | 94.35 | 5.17 | 0.93 | 28.57 | 98.37 | 25.00 | 98.64 | 17.49 | 0.73 |
| EJ | 1117 | 1 | 0.00 | 99.91 | 0.00 | 93.94 | 0.00 | 1.00 | 0.00 | 99.91 | 0.00 | 98.13 | 0.00 | 1.00 |
| OJ | 1115 | 3 | 0.00 | 99.72 | 0.00 | 93.93 | 0.00 | 1.00 | 0.00 | 99.73 | 0.00 | 98.13 | 0.00 | 1.00 |
| PL-12 | 1091 | 27 | 1.43 | 97.61 | 3.70 | 93.89 | 0.60 | 1.01 | 19.05 | 97.91 | 14.81 | 98.45 | 9.13 | 0.83 |
| SRP | 1084 | 34 | 11.43 | 97.61 | 23.53 | 94.47 | 4.77 | 0.91 | 19.05 | 97.28 | 11.76 | 98.44 | 7.00 | 0.83 |
| Mi-2α | 1107 | 11 | 11.43 | 99.72 | 72.73 | 94.59 | 41.37 | 0.89 | 4.76 | 99.09 | 9.09 | 98.20 | 5.25 | 0.96 |
| Mi-2β | 1097 | 21 | 7.14 | 98.53 | 23.81 | 94.27 | 4.85 | 0.94 | 9.52 | 98.28 | 9.52 | 98.28 | 5.52 | 0.92 |
| Ku | 1093 | 25 | 7.14 | 98.16 | 20.00 | 94.25 | 3.88 | 0.95 | 4.76 | 97.82 | 4.00 | 98.18 | 2.19 | 0.97 |
AB, antibody; IIM, idiopathic inflammatory myopathies; ILD, interstitial lung disease; LR, likelihood ratio; MAA, myositis-associated antibody; MSA, myositis-specific antibody; NPV, negative predictive value; PPV, positive predictive value.
The PPVs for autoimmune diseases other than IIM and malignancies are listed in Table 3. In total, 22 patients had a malignancy (n = 3 with ILD, n = 8 with AID, n = 11 without diagnosis of IIM, ILD, or AID); 39 patients had an AID other than IIM. Patients positive for anti-Ku were diagnosed more often with SLE than IIM (24% vs. 20%, Table 4), and 15% of anti-PL12 positive individuals had a diagnosis of systemic sclerosis (SSc) rather than IIM (4%). Unexpectedly, malignancy was present in 20% of anti-Jo-1 positive patients and only 5% of TIF-1γ positive patients (Table 3).
Table 4.
Frequency of diagnosis of autoimmune diseases in MSA/MAA positive patients (in percent of total antibody positive patients).
| MSA/MAA | SSc | CLE | Psor | SLE | PMR | PsA | pSS | TH | other |
|---|---|---|---|---|---|---|---|---|---|
| n*(%) | |||||||||
| Jo-1 | 1 (5.0) | 0 | 1 (5.0) | 0 | 1 (5.0) | 0 | 1 (5.0) | 1 (5.0) | 1 (5.0) |
| TIF 1y | 0 | 3 (15.8) | 0 | 1 (5.3) | 0 | 0 | 0 | 3 (15.8) | 3 (15.8) |
| MDA5 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 0 |
| NXP2 | 0 | 1 (8.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PM-SCL 100 | 3 (11.5) | 1 (3.8) | 1 (3.8) | 3 (11.5) | 3 (11.5) | 0 | 0 | 0 | 3 (11.5) |
| PM-SCL 75 | 3 (4.5) | 0 | 0 | 6 (9.1) | 3 (4.5) | 1 (4.5) | 1 (4.5) | 3 (4.5) | 13.6 |
| Pl-7 | 1 (4.2) | 0 | 1 (4.2) | 1 (4.2) | 1 (4.2) | 1 (4.2) | 1 (4.2) | 0 | 16.7 |
| EJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OJ | 0 | 0 | 0 | 33.3 | 0 | 0 | 0 | 0 | 0 |
| Pl-12 | 4 (14.8) | 0 | 0 | 1 (3.7) | 0 | 0 | 1 (3.7) | 0 | 1 (3.7) |
| SRP | 1 (2.9) | 0 | 0 | 1 (2.9) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Mi-2α | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (9.1) |
| Mi-2β | 0 | 0 | 2 (9.5) | 0 | 1 (4.8) | 0 | 0 | 2 (9.6) | 3 (14.3) |
| Ku | 3 (12.0) | 0 | 0 | 6 (24.0) | 0 | 0 | 0 | 0 | 0 |
A single patient may be diagnosed with more than one AID.
AID, autoimmune disease; CLE, cutaneous lupus erythematosus; IIM, idiopathic inflammatory myopathy; MAA, myositis-associated antibody; MSA, myositis-specific antibody; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; pSS, primary Sjögren syndrome; Psor, psoriasis vulgaris; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; TH, thyreoiditis Hashimoto.
Other: celiac disease, primary biliary cholangitis, antiphopspholipid syndrome, myasthenia gravis, giant cell arteritis, sarcoidosis, undiff arthritis, Lambert-Eaton syndrome, axial spondylitis, ulcerative colitis, adult Stills disease, microscopic polyangiitis, multiple sclerosis, reactive arthritis juvenile idiopathic arthritis, leukocytoclastic vasculitis.
Table 3.
Positive predictive values of myositis-blot antibodies in malignancies and autoimmune diseases other than IIM.
| MSA/MAA | AID (n = 39) | Malignancy (n = 22) | ||
|---|---|---|---|---|
| n * | PPV (%) | n * | PPV (%) | |
| Jo-1 | 4 | 20.00 | 4 | 20,00 |
| TIF1γ | 9 | 47.37 | 1 | 5,26 |
| MDA-5 | 3 | 20.00 | 2 | 13.33 |
| NXP-2 | 1 | 8.33 | 1 | 8.33 |
| SAE | 0 | 0.00 | 1 | 25.00 |
| PM-Scl100 | 14 | 53.85 | 5 | 19.23 |
| PM-Scl75 | 27 | 40.91 | 4 | 6.06 |
| PL-7 | 11 | 45.83 | 3 | 12.50 |
| EJ | 0 | 0.00 | 0 | 0.00 |
| OJ | 1 | 33.33 | 0 | 0.00 |
| PL-12 | 7 | 25.93 | 2 | 7.41 |
| SRP | 7 | 20.59 | 4 | 11.76 |
| Mi-2α | 3 | 27.27 | 1 | 9.09 |
| Mi-2β | 9 | 42.86 | 4 | 19.05 |
| Ku | 8 | 32.00 | 3 | 12.00 |
A single patient may be positive for more than one MSA/MAA.
AID, autoimmune disease other than IIM; MAA, myositis-associated antibody; MSA, myositis-specific antibody; PPV, positive predictive value.
To assess if MSA/MAA were more frequent in cancer patients than in the general population, we analyzed a second cohort without signs of IIM consisting of 88 healthy controls and 104 patients with locally advanced or metastatic breast (33%), lung (32%), and pancreatic (35%) cancer. We found no significant differences in MSA/MAA between cancer patients and controls (supplemental Table S2).
Discussion
Although MSA are considered to be highly specific for IIM, their value in the diagnostic work-up of patients suspected of IIM is unclear.1 Despite the lack of evidence, physicians in daily clinical practice may be tempted to use MSA/MAA for diagnostic purposes rather than for stratification and prognosis of established IMM. Analyzing the reasons for ordering MSA/MAA LIA in our real-world cohort revealed that physicians ordered the test in the setting of suspected or established IIM in only a minority of cases. Differential diagnosis of suspected autoimmune rheumatic diseases, creatinine kinase elevation, muscle weakness and myalgia were frequent reasons. In addition, recommendations to screen for the presence of IIM using MSA/MAA in the setting of idiopathic interstitial pneumonia may explain the number of tests ordered by pulmonologists.11 Despite the large number of MSA/MAA tests performed, we identified only 70 IIM patients in our cohort. This indicates that clinicians in daily routine are using MSA/MAA mainly as a tool for diagnostic work up. The number of LIA tests performed in Spain (~250 per 106 inhabitants/year), and New Zealand (~200 per 106 inhabitants/year) is comparable with the number performed in our laboratory (~260 per 106 inhabitants/year) suggesting that diagnostic use of MSA/MAA is global practice.12,13 Naturally, this results in a low pre-test likelihood as IIMs are rare diseases. A recent Swedish study estimated the incidence of IIM to be 11 cases per 106 inhabitants/year.14 Previous studies on MSA/MAA have been performed in well-defined IIM cohorts with a high pre-test likelihood, thus not reflecting the clinical reality of MSA/MAA use.4 Studies reporting results of larger sample sizes did not have access to clinical diagnoses.12,13 Our study reveals unexpectedly low PPVs and positive likelihood ratios for all MSA/MAA, except for Mi-2α. In line with our results, Zampeli et al. recently reported that only 32% of the patients positive for anti-tRNA synthetases by LIA satisfied the classification criteria for antisynthetase syndrome.15 Montagnese et al. analysed the utility of LIA-based MSA in the diagnostic work up of patients with neuromuscular disease.16 While MSA where the most useful parameter to identify IIM among patients with undifferentiated myopathy, MSA were of limited use in diagnosing IIM in general. In summary, these data suggest that MSA/MAA testing by LIA may not qualify as tool for the diagnostic work up of suspected IIM.
To increase clinical specificity and PPV, a recent study suggested excluding MSA with a low-positive signal intensity.15,17,18 However, ROC analysis of our data revealed that higher cut-offs had a lower clinical sensitivity without increasing clinical specificity. This could be due to the low number of MSA/MAA positive IIM patients identified in our cohort. Infantino et al. proposed to improve clinical specificity of MSA detection by additionally considering the corresponding HEp-2 IIF pattern.9 Applying this approach to our cohort did not result in increased clinical specificity. The different assays used to detect MSA may explain the discrepant results. Furthermore, both studies were not sufficiently powered to answer this question. Therefore, larger multi-center analyses are needed to improve clinical specificity of MSA/MAA testing by LIA.
MSA like TIF-1γ indicate risk of IIM-associated malignancy.2 TIF-1γ is highly expressed in cancer and regenerating muscle.19,20 TIF-1γ may arise as a result of an anti-tumor response and then react with similar antigen in regenerating muscle tissue.19 This would imply that some cancer patients could develop MSA like TIF-1γ before, or even without, symptoms of IIM. This might be of clinical relevance as anti-cancer therapy with immune checkpoint inhibitors in patients with pre-existing MSA/MAA may be a risk factor to develop rare immune checkpoint inhibitor-related myositis.21 We found a high frequency of past or current cancer when reviewing the medical history of patients positive for MSA/MAA. However, MSA/MAA were not increased significantly in an independent cohort of cancer patients compared with age-matched controls. However, the statistical power of our approach was not sufficient to detect a small increase in the frequency of MSA/MAA in cancer patients. Furthermore, analysis of anti-TIF-1γ by LIA showed only moderate agreement with IP hindering conclusions from these results.5 In line with our findings, anti-TIF-1γ was rarely present in solid cancer or paraneoplastic rheumatic syndromes.22
Our study has limitations due to its design analyzing clinical diagnosis by chart review retrospectively. Therefore, we did not have sufficiently detailed clinical data to apply the 2019 ACR/EULAR criteria to our cohort. Analyzing a subgroup with sufficient data and positive MSA/MAA revealed that IIM was not underdiagnosed in these patients.
Another limitation is the lack of validation of MSA/MAA-positive samples by other methods such as immunoprecipitation. Other studies comparing LIA with immunoprecipitation resulted in a variable degree of agreement.5,23 We therefore cannot determine if positive test results of non-IIM patients were related to the low clinical specificity of the LIA method, or the natural occurrence of MSA/MAA in non-IIM patients.
In summary, assessment of MSA/MAA by LIA is used widely in the diagnostic work-up of patients with suspicion of IIM. This practice results in low PPVs for most MSA/MAA limiting the diagnostic value of the test.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X20975907 for The use and diagnostic value of testing myositis-specific and myositis-associated autoantibodies by line immuno-assay: a retrospective study by Angelika Lackner, Viktoria Tiefenthaler, Jalia Mirzayeva, Florian Posch, Christopher Rossmann, Kastriot Kastrati, Helga Radner, Ulrike Demel, Judith Gretler, Michael Stotz, Winfried B Graninger and Martin H Stradner in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We thank Sonja Scherling and Michael Stidl for their support in data analysis.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Angelika Lackner  https://orcid.org/0000-0002-2036-9564
https://orcid.org/0000-0002-2036-9564
Martin H Stradner  https://orcid.org/0000-0002-7884-6626
https://orcid.org/0000-0002-7884-6626
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Angelika Lackner, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Viktoria Tiefenthaler, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Jalia Mirzayeva, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Florian Posch, Division of Clinical Oncology, Department of Internal Medicine, Medical University Graz, Graz, Steiermark, Austria.
Christopher Rossmann, Division of Clinical Oncology, Department of Internal Medicine, Medical University Graz, Graz, Steiermark, Austria.
Kastriot Kastrati, Division of Rheumatology, Department of Medicine III, Medical University of Vienna, Vienna, Austria.
Helga Radner, Division of Rheumatology, Department of Medicine III, Medical University of Vienna, Vienna, Austria.
Ulrike Demel, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Judith Gretler, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Michael Stotz, Division of Clinical Oncology, Department of Internal Medicine, Medical University Graz, Graz, Steiermark, Austria.
Winfried B Graninger, Division of Rheumatology and Immunology, Department of Internal Medicine, Medical University of Graz, Graz, Steiermark, Austria.
Martin H Stradner, Department of Rheumatology & Immunology, Medical University of Graz, Auenbruggerplatz 15, Graz, Steiermark 8036, Austria.
References
- 1. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017; 76: 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol 2018; 14: 290–302. [DOI] [PubMed] [Google Scholar]
- 3. Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology (Oxford). Epub ahead of print 1 January 2017. DOI: 10.1093/rheumatology/kew146. [DOI] [PubMed] [Google Scholar]
- 4. Tan TC, Wienholt L, Adelstein S. TEST performance of a myositis panel in a clinical immunology laboratory in New South Wales, Australia. Int J Rheum Dis 2016; 19: 996–1001. [DOI] [PubMed] [Google Scholar]
- 5. Espinosa-Ortega F, Holmqvist M, Alexanderson H, et al. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann Rheum Dis 2019; 78: 858–860. [DOI] [PubMed] [Google Scholar]
- 6. Cavazzana I, Fredi M, Ceribelli A, et al. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J Immunol Methods 2016; 433: 1–5. [DOI] [PubMed] [Google Scholar]
- 7. Mahler M, Fritzler MJ. Detection of myositis-specific antibodies: additional notes. Ann Rheum Dis. Epub ahead of print 13 March 2018. DOI: 10.1136/annrheumdis-2018-213320. [DOI] [PubMed] [Google Scholar]
- 8. Vulsteke J-B, De Langhe E, Claeys KG, et al. Detection of myositis-specific antibodies. Ann Rheum Dis. Epub ahead of print 25 January 2018. DOI: 10.1136/annrheumdis-2017-212915. [DOI] [PubMed] [Google Scholar]
- 9. Infantino M, Tampoia M, Fabris M, et al. Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology (Oxford) 2019; 58: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 10. Chan EKL, Damoiseaux J, Carballo OG, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 Cell Patterns (ICAP) 2014-2015. Front Immunol. Epub ahead of print 20 August 2015. DOI: 10.3389/fimmu.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; 46: 976–987. [DOI] [PubMed] [Google Scholar]
- 12. Martinez Becerra MJ, Romero F, Sánchez O, et al. AB0736 Myositis specific autoantibodies (MSA) and myositis associated autoantibodies (MAA). Experience in a Spanish cohort. Ann Rheum Dis 2013; 72(Suppl. 3): A1013. [Google Scholar]
- 13. O’donnell J, Keating P, van Voorthuizen M, et al. FRI0395 Incidence of Myositis-Specific Autoantibody (MSA) specificities in sera referred to New Zealand (NZ) medical laboratories. Ann Rheum Dis 2017; 76(Suppl. 2): 637. [Google Scholar]
- 14. Svensson J, Arkema EV, Lundberg IE, et al. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford) 2017; 56: 802–810. [DOI] [PubMed] [Google Scholar]
- 15. Zampeli E, Cinoku II, Mavragani CP, et al. Clinical significance of higher cutoffs for myositis autoantibody positivity using the euroimmun research line blot: comment on the article by Mecoli et al. Arthritis Rheumatol 2020; 72: 1042–1044. [DOI] [PubMed] [Google Scholar]
- 16. Montagnese F, Babačić H, Eichhorn P, et al. Evaluating the diagnostic utility of new line immunoassays for myositis antibodies in clinical practice: a retrospective study. J Neurol 2019; 266: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 17. Piette Y, De Sloovere M, Vandendriessche S, et al. Pitfalls in the detection of myositis specific antibodies by lineblot in clinically suspected idiopathic inflammatory myopathy. Clin Exp Rheumatol 2020; 38: 212–219. [PubMed] [Google Scholar]
- 18. Mecoli CA, Albayda J, Tiniakou E, et al. Myositis autoantibodies: a comparison of results from the oklahoma medical research foundation myositis panel to the euroimmun research line blot. Arthritis Rheumatol 2020; 72: 192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohassel P, Rosen P, Casciola-Rosen L, et al. Expression of the dermatomyositis autoantigen transcription intermediary factor 1γ in regenerating muscle. Arthritis Rheumatol 2015; 67: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain S, Singhal S, Francis F, et al. Association of overexpression of TIF1γ with colorectal carcinogenesis and advanced colorectal adenocarcinoma. World J Gastroenterol 2011; 17: 3994–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solimando AG, Crudele L, Leone P, et al. Immune checkpoint inhibitor-related myositis: from biology to bedside. Int J Mol Sci. Epub ahead of print 1 May 2020. DOI: 10.3390/ijms21093054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venalis P, Selickaja S, Lundberg K, et al. Association of anti-transcription intermediary factor 1γ antibodies with paraneoplastic rheumatic syndromes other than dermatomyositis. Arthritis Care Res (Hoboken) 2018; 70: 648–651. [DOI] [PubMed] [Google Scholar]
- 23. Ghirardello A, Rampudda M, Ekholm L, et al. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology 2010; 49: 2370–2374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X20975907 for The use and diagnostic value of testing myositis-specific and myositis-associated autoantibodies by line immuno-assay: a retrospective study by Angelika Lackner, Viktoria Tiefenthaler, Jalia Mirzayeva, Florian Posch, Christopher Rossmann, Kastriot Kastrati, Helga Radner, Ulrike Demel, Judith Gretler, Michael Stotz, Winfried B Graninger and Martin H Stradner in Therapeutic Advances in Musculoskeletal Disease