Abstract
Background and Aims:
Interferon beta (IFNb) is a safe first-line drug commonly used for relapsing-remitting (RR)-MS. Nevertheless, a considerable proportion of patients do not respond to IFNb treatment. Therefore, until now, a number of studies have investigated various markers that could predict the patients who would respond to IFNb therapy. The objective of this study was to identify reliable biomarkers to predict the efficacy of IFNb treatment in MS.
Methods:
In a group of 116 patients with clinically isolated syndrome (CIS) and RR-MS, we explored the association between CSF detectability of a large set of proinflammatory and anti-inflammatory molecules at the time of diagnosis and response to IFNb after the first year of treatment. The absence of clinical relapses, radiological activity and disability progression (NEDA-3) was assessed at the end of 1-year follow up. The results were compared with those obtained in additional groups of CIS and RR-MS patients treated with other first-line drugs (dimethyl fumarate and glatiramer acetate).
Results:
CSF undetectability of macrophage inflammatory protein (MIP)-1α was the main predictor of reaching NEDA-3 status after 1 year of IFNb treatment. Moreover, detectable platelet-derived growth factor (PDGF) was associated with higher probability of reaching NEDA-3. Conversely, no associations with the CSF molecules were found in the two other groups of patients treated either with dimethyl fumarate or with glatiramer acetate.
Conclusion:
MIP-1α and PDGF could potentially represent suitable CSF biomarkers able to predict response to IFNb in MS.
Keywords: cerebrospinal fluid (CSF), clinically isolated syndrome (CIS), interferon beta (IFNb), macrophage inflammatory protein (MIP)-1α, NEDA-3, platelet-derived growth factor (PDGF), relapsing-remitting (RR)-MS
Introduction
Interferon beta (IFNb) is one of the most frequently prescribed first-line disease-modifying therapies (DMTs) in patients with clinically isolated syndrome (CIS) and relapsing-remitting (RR)-multiple sclerosis (MS). IFNb treatment is used commonly in newly diagnosed patients due to beneficial risk–benefit profile, since IFNb administration slightly improves the course of MS, moderately slowing the long-term progression of disability associated with a 30% reduction of relapse rate.1,2 Nevertheless, a significant proportion of patients do not respond to IFNb treatment,3 partly due to the pathogenetic differences between patients, resulting in both non-responders and good clinical responders among them. Consequently, numerous attempts have been made to identify possible markers to predict response to IFNb therapy. It has been reported that some disease demographic characteristics, including clinical disability, disease duration, and pretreatment relapse rate could be associated with IFNb response; particularly, higher age before starting IFNb therapy,4 and lower annual relapse rate before treatment initiation.5 Furthermore, it has been proposed that the expression of various genes involved in inflammatory response and its modulation during IFN treatment could contribute to explain inter-individual variability in IFNb response.6,7 However, clear associations have not yet been established, due mainly to differences in the definition of response to treatment, different populations studied, and different statistical power linked to number of patients included in the studies. Therefore, the identification of biomarkers capable to predict the response to IFNb treatment might be extremely useful.
It has been demonstrated that inflammation negatively influences the disease course of RR-MS. Accordingly, elevated cerebrospinal fluid (CSF) concentrations of various proinflammatory molecules at the time of diagnosis have been associated with enhanced disease activity, increased long-term disability and shift to the second-line therapies.8 Notably, previous studies have demonstrated that IFNb treatment has immunomodulatory properties and regulates the expression of different proinflammatory molecules. IFNb reduces antigen presentation by microglia and monocytes, decreases T-cell responses,9 inhibits T cells migration across the blood-brain barrier, prevents Th17 differentiation reducing the expression of IL-17 and IFN gamma (IFNg), and enhances the production of IL-10.10
It is not clear whether pre-treatment expression of CSF inflammatory and anti-inflammatory molecules could play a role in predicting the individual variability of the response to IFNb therapy. We therefore explored in a group of CIS and RR-MS patients, the association between CSF detectability of numerous molecules at the time of diagnosis, and response to IFNb after the first year of treatment. Moreover, the same association was explored in two different groups of patients treated with other first-line DMTs [glatiramer acetate (GA) and dimethyl fumarate (DF)].
Methods
Study protocol and MS patients
A group of 226 patients admitted to the neurological clinics of University Tor Vergata Hospital and Neuromed Institute, diagnosed as CIS or RR-MS, between 2009 and 2019, were enrolled in this retrospective study. The study was approved by the Ethics Committees of the University Tor Vergata Hospital in Rome and Neuromed Research Institute in Pozzilli, Italy, according to the Declaration of Helsinki. All patients gave written informed consent to participate in the study.
In all patients recruited in this investigation, the diagnosis of CIS or RR-MS was made based on the clinical, laboratory, and magnetic resonance imaging (MRI) findings.11 Clinical evaluation, brain and spinal cord MRI, and CSF collection, were performed during hospitalization.
The mean follow-up period was 20.3 ± 82.7 months, median 19.7 month (IQR = 29.6). Follow-up consisted of neurological examination and MRI scans performed every 6 months after diagnosis. Additional clinical evaluation and MRI were performed, when clinical relapses had occurred. DMTs were initiated after the diagnosis. The IFNb group (n = 116) included people with MS treated with different IFNb preparations (Table 1). Two groups of CIS and RR-MS patients, treated either with DF (n = 59) or GA (n = 51) were also included in the study.
Table 1.
Interferon-beta preparations in patients with CIS and RR-MS treated with interferon beta.
| Interferon-beta preparations | Number (%) |
|---|---|
| Avonex | 11 (9.5) |
| Rebif 22 mcg | 11 (9.5) |
| Rebif 44 mcg | 62 (53.5) |
| Betaferon | 28 (24.1) |
| Plegridy | 4 (3.4) |
| Total | 116 (100) |
Treatment duration with interferon-beta ranged from 1 to 12 months (mean 10.4 months); 85/116 (73.3%) were treated for 12 months.
CIS, clinically isolated syndrome; RR-MS, relapsing-remitting multiple sclerosis.
Clinical evaluation and MRI parameters
Disability was measured using the Expanded Disability Status Scale (EDSS).12 Disease duration was calculated as the time interval between the first episode of neurological dysfunction suggestive of MS and the time of confirmed diagnosis during hospitalization at above-mentioned neurological clinics. Clinical relapses have been defined as the development of new or recurrent neurological symptoms persisting at least 24 h and not associated with fever or infection. The number of clinical relapses that occurred before lumbar puncture (LP) was recorded for each patient. Disease activity at the time of LP was defined as the presence of clinical relapse or active MRI lesions at that time.
In all patients, MRI of the brain and spinal cord (cervical, thoracic and lumbar) were performed. Patients were examined by 1.5 or 3.0 T MRI, including the following sequences: dual-echo proton density, fluid-attenuated inversion recovery, T1-weighted spin-echo (SE), T2-weighted fast SE, and contrast-enhanced T1-weighted SE after intravenous gadolinium (Gd) infusion (0.2 ml/kg). A Gd-enhancing (Gd+) lesion was defined as an area of hyperintense signaling on contrast-enhanced T1-weighted images. An active MRI was defined as one showing new or enlarging T2-weighted lesions and/or contrast-enhanced T1-weighted lesions.
No evidence of disease activity (NEDA) status was assessed for each patient at the end of 1-year follow up. In the present study, NEDA was defined as the absence of radiological and clinical disease activity and the absence of disability progression (NEDA-3).13
CSF collection and analysis
CSF was collected by LP. Immediately after LP, CSF was centrifuged and stored at −80°C until being analyzed using a Bio-Plex multiplex cytokine assay (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions. CSF detectability of the following molecules has been examined: interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL17, tumor necrosis factor alpha (TNFa), IFNg, macrophage inflammatory protein (MIP)-1α/CCL3, MIP-1β/CCL4, monocyte chemoattractant protein (MCP)-1/CCL2, interferon gamma-induced protein 10 (IP-10)/CXCL10, eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), vascular-endothelial growth factor (VEGF), and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES)/CCL5. CSF delectability of all molecules tested in our CIS and RRMS patients, separated in three groups according to DMTs (IFN-beta, GA, DF) used, is presented in the Supplemental Table.
Statistical analysis
To determine the significance of the different proportions, a chi square test was used, and the mean values compared by using ANOVA, if the data distribution was normal. In case of not normal distribution, non-parametric tests were used for testing statistical significance. Predictive value of investigated variables was assessed by logistic regression analysis, with NEDA-3 after 1 year (yes/no) as the dependent variable. All variables with significance levels of 0.05 by univariate analysis were included in the multivariate models (method: enter). The statistical significance of CSF chemokine (MIP-1α and PDGF) detectability pattern was assessed by a chi square test for trend. Sensitivity, specificity, and accuracy, were calculated. Two-tailed p values less than 0.05 were considered as significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (Advanced Statistics, version 21.0, Chicago, IL, USA).
Results
IFNb cohort
Demographic and clinical characteristics of CIS and RR-MS patients treated with IFNb are shown in Table 2.
Table 2.
Demographic and clinical characteristic of CIS and RR-MS patients.
| Interferon-beta | GA | DF | |
|---|---|---|---|
| Variable | Value | Value | Value |
| Number | 116 | 51 | 59 |
| Duration of MS, years (mean ± SD) | 2.7 ± 5.6 | 2.7 ± 4.8 | 4.0 ± 6.8 |
| Gender | |||
| -male (n, %) | 41 (35.3) | 13 (25.5) | 18 (30.5) |
| -female (n, %) | 75 (64.7) | 38 (74.5) | 41 (69.5) |
| Age, years (mean ± SD) | 34.1 ± 11.1 | 38.1 ± 11.8 | 36.8 ± 12.9 |
| EDSS score (median, IQR) | 1.5 (1.0) | 2.0 (1.9) | 2.0 (1.8) |
| Unique CSF OCB | * | ** | *** |
| -positive (n, %) | 83 (73.5) | 39 (84.8) | 38 (66.7) |
| -negative (n, %) | 30 (26.5) | 7 (15.2) | 19 (33.3) |
| Previous number of relapses before LP (mean ± SD) | 1.6 ± 0.9 | 1.5 ± 0.9 | 1.4 ± 0.7 |
missing data for three patients (2.6%); **missing data for five patients (9.8%); ***missing data for two patients (3.4%).
CIS, clinically isolated syndrome; CSF, cerebrospinal fluid; DF, dimethyl fumarate; EDSS, expanded disability status scale; GA, glatiramer acetate; IQR, interquartile range; LP, lumbar puncture; MS, multiple sclerosis; OCB, oligoclonal bands; RR-MS, relapsing remitting multiple sclerosis; SD standard deviation.
In the IFNb group, the proportion of NEDA-3 patients at the end of a 1-year follow up was 61/116 (52.6%). Treatment duration with IFNb ranged from 1 to 12 months (mean 10.4 months), 85/116 patients (73.3%) were treated during 12 months.
Predictors of reaching NEDA-3 in the IFNb group by univariate logistic regression analysis are presented in Table 3. We found that only PDGF and MIP-1α CSF detectability were associated significantly with NEDA-3 status. MIP-1α detectability was associated significantly with reduced probability of reaching NEDA-3 status, 1 year after diagnosis [odds ratio (OR) = 0.24; 95% confidence interval (CI) = 0.08–0.74; p = 0.013]. Conversely, PDGF detectability was associated significantly with higher probability of reaching NEDA-3 at a 1-year follow up (OR = 3.15; 95% CI = 1.22–8.14; p = 0.018). No significant association emerged between other CSF molecules detectability and NEDA-3. After including both variables significant in univariate analysis at the level of p < 0.05, in the multivariate model, MIP-1α CSF detectability was independent predictor for NEDA-3 status in the IFNb group [OR = 0.24 (95% CI = 0.08–0.74); p = 0.013)].
Table 3.
Predictors of reaching NEDA-3 in patients treated with interferon-beta by univariate logistic regression analysis.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Disease duration | 1.02 | 0.95–1.10 | 0.538 |
| Gender | 0.69 | 0.32–1.49 | 0.344 |
| Age | 1.00 | 0.97–1.04 | 0.847 |
| Baseline EDSS score | 0.73 | 0.50–1.07 | 0.110 |
| Unique CSF OCB | 0.66 | 0.28–1.52 | 0.321 |
| Number of relapses before LP | 1.05 | 0.68–1.61 | 0.834 |
| CSF detectability of PDGF | 3.15 | 1.22–8.14 | 0.018 |
| CSF detectability of MIP-1α | 0.24 | 0.08–0.74 | 0.013 |
Non-significant correlations with CSF molecules are not reported; bold values denote statistical significance.
CI, confidence interval; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; LP, lumbar puncture; MIP-1α, macrophage inflammatory protein-1α; NEDA, no evidence of disease activity; OCB, oligoclonal bands; OR, odds ratio; PDGF, platelet-derived growth factor.
In order to assess the trend of relative risk to reach NEDA-3 after a 1-year follow-up, we combined three variables: presence/absence of NEDA-3, CSF detectability/undetectability of both MIP-1α, and PDGF. The analysis was performed in a group 55 of patients in which both MIP-1α and PDGF had been analyzed. We considered patients who reached NEDA-3 with detectable PDGF and not detectable MIP-1α, as a reference group (OR = 1.00). Patients who had one of the following settings: a) detectable, both MIP-1α and PDGF, and b) detectable neither MIP-1α, nor PDGF, had lower probability to reach NEDA-3 (OR = 0.35) in comparison with reference group. Finally, those patients who had detectable CSF MIP-1α and undetectable PDGF had the lowest probability to reach NEDA-3 (OR = 0.16). This trend is statistically highly significant (χ2 = 7.045, p = 0.008) (Figure 1).
Figure 1.
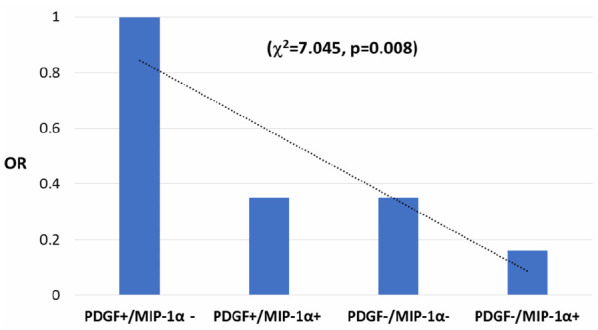
Probability to reach NEDA-3 based on CSF MIP-1α and PDGF detectability in patients treated with interferon beta after a 1-year follow-up.
CSF, cerebrospinal fluid; MIP-1α, macrophage inflammatory protein-1α; NEDA, no evidence of disease activity; OR, odds ratio; PDGF, platelet-derived growth factor.
We have assessed the statistical validity of these biomarkers. The sensitivity of non-detectability of MIP-1α was high (98.2%), and specificity was 56.9%. The accuracy of non-detectability of MIP-1α to correctly classify patients with CIS and MS treated with IFNb according to reaching NEDA-3 after 1-year follow up was high (77.0%). In addition, the sensitivity of detectability of PDGF was 56%, and specificity was high (81.8%). The accuracy of detectability of PDGF to correctly classify patients with CIS and MS treated with interferon-beta according to reaching NEDA-3 after 1-year follow up was also high (68.6%).
GA and DF cohorts
The association between CSF inflammatory molecules detectability and response to treatment was explored in two additional cohorts of patients treated with other first-line DMTs (GA and DF).
Demographic and clinical characteristics of CIS and RR-MS patients treated with GA or DF are shown in Table 2.
In the GA group, NEDA-3 status at the end of a 1-year follow up was achieved in 20/51 patients (40%). We explored predictors of reaching NEDA-3 in patients treated with GA by univariate logistic regression analysis. No significant associations emerged between NEDA-3 status and CSF molecules explored.
In the DF group, 42/59 patients (75%) reached NEDA-3 at the end of a 1-year follow up. Predictors of reaching NEDA-3 in patients treated with DF were explored by univariate logistic regression analysis. Number of clinical relapses before LP was the only variable significantly associated with NEDA-3 in this group: OR = 0.27 (95% CI = 0.08–0.92), p = 0.036. No significant associations emerged between CSF molecules and NEDA-3.
We applied the univariate regression model on patients on GA and DF, which showed that none of variables examined was predictive for reaching NEDA-3 after 1-year follow up (Table 4). Therefore, it was not possible to perform multivariate regression analysis.
Table 4.
Predictors of reaching NEDA-3 in patients treated with glatiramer acetate or dimethyl fumarate by univariate logistic regression analysis.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Disease duration | 0.96 | 0.89–1.04 | 0.311 |
| Gender | 1.62 | 0.67–3.92 | 0.285 |
| Age | 1.02 | 0.99–1.05 | 0.249 |
| Baseline EDSS score | 1.19 | 0.84–1.70 | 0.321 |
| Unique CSF OCB | 1.42 | 0.68–2.92 | 0.521 |
| Number of relapses before LP | 0.97 | 0.59–1.58 | 0.894 |
| CSF detectability of PDGF | 0.57 | 0.22–1.46 | 0.243 |
| CSF detectability of MIP-1α | 2.48 | 0.21–8.61 | 0.268 |
Other non-significant correlations with CSF molecules are not reported.
CI, confidence interval; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; LP, lumbar puncture; MIP-1α, macrophage inflammatory protein-1α; NEDA, no evidence of disease activity; OCB, oligoclonal bands; OR, odds ratio; PDGF, platelet-derived growth factor.
Discussion
The increasing number of available DMTs makes clinical management of MS patients more complex. One of the common strategies today for the significant number of RR-MS patients worldwide is still to start MS treatment as soon as possible with first-line drugs, including IFNb, GA and DF. However, a considerable proportion of these patients continue to exhibit disease activity during such treatments. For them, second-line therapies are available, such as alemtuzumab, natalizumab, or ocrelizumab, have proven to be more effective, but potentially associated with serious adverse events.14 Therefore, the identification of reliable biomarkers able to predict the efficacy of generally safe IFNb treatment in individual patients with MS might be extremely useful.
In the present study, we explored in a group of newly diagnosed CIS and RR-MS patients treated with IFNb, the association between a large set of proinflammatory and anti-inflammatory molecules at the time of diagnosis, and NEDA-3 status 1-year after treatment. Our results showed that CSF undetectability of the proinflammatory molecule MIP-1α was the main factor associated with increased probability of reaching NEDA-3 status after 1 year of IFNb treatment. However, also detectable PDGF in CSF was found to predict higher probability of reaching NEDA-3. Therefore, patients who had detectable CSF MIP-1α and undetectable PDGF had 84% lower probability to reach NEDA-3, and this trend was statistically highly significant.
It has been demonstrated that proinflammatory molecules play a crucial role in the pathogenesis of MS, influencing the disease course. For example, elevated CSF levels of the proinflammatory molecules IL-6 and IL-8, have been associated with increased disease activity and disability in RR-MS and CIS patients.8 In addition, CSF expression of IL-1β, has been also associated with worse disease course in MS.15 Thus, it has been shown that detectable CSF IL-1β in RR-MS patients during remission at the time of diagnosis was associated with increased midterm disease progression.15
MIP-1α/CCL3 is a proinflammatory molecule released by several immune cells, including monocytes/macrophages, T and B lymphocytes, neutrophils, dendritic, and glial cells.16,17 MIP-1 proteins bind to various receptors, including CCR1, CCR3, and CCR5, influencing the activity of numerous immune cells and regulating cell differentiation, chemotaxis, and synthesis of inflammatory mediators.18 MIP-1α interacts with G-protein-coupled receptors promoting Th1 differentiation; accordingly, CCR5-deficient mice show Th2 polarization.19 Therefore, MIP-1 proteins represent key players involved in the pathogenesis of many autoimmune inflammatory diseases including EAE and MS.
Previous studies have demonstrated that MIP-1α participates in the CNS inflammatory process observed in EAE and MS. Studies in animal models of MS have shown that expression of MIP-1α was enhanced during acute inflammation and correlated with the degree of inflammation.20 Experimental data support a role of MIP-1α in the pathogenesis of EAE. Accordingly, it has been shown that the administration of anti-MIP-1α antibodies prevented the transfer of EAE.21 Moreover, also selective blocking of MIP-1α receptors strongly reduced clinical and pathological manifestations.22 In MS patients, enhanced expression of MIP-1α has been evidenced in peripheral T lymphocytes, in the CSF and in postmortem brain samples.17,23 MIP-1α and its receptors CCR3 and CCR5 are expressed by astrocytes and macrophages within the plaque.17 These receptors are crucially involved in the pathogenesis of MS regulating the migration of immune cells.24 Accordingly, it has been demonstrated that peripheral T cells from MS patients showed increased migration toward RANTES and MIP-1α compared with control subjects, which is associated with increased expression of CCR5.25 These findings are in line with our results, which have demonstrated that undetectability of MIP-1α in CSF is associated with higher probability of reaching NEDA-3 in patients treated with IFNb.
Conversely, anti-inflammatory molecules and neurotrophins could exert beneficial effect, decreasing T cell infiltration, reducing neurodegeneration, and promoting better disease course in EAE and MS. Previous findings have shown that PDGF promotes neuronal growth and remyelination,26,27 and may play a protective role in CIS and RR-MS patients.28 It has been proposed that the beneficial effects of PDGF in MS could be related to increased synaptic plasticity expression, as reported in vitro and in RR-MS patients.29,30 Accordingly, this molecule could enhance the compensation of clinical deficit caused by newly appearing demyelinating lesions, promoting stable clinical course.28
It has been consistently demonstrated that IFNb has immunomodulatory properties, reducing Th1 cytokines and inducing a shift toward the production of anti-inflammatory molecules.31 Accordingly, it has been evidenced that IFNb reduces the expression of inflammatory cytokines, and enhances expression of several anti-inflammatory molecules and growth factors, including IL-10, BDNF, and PDGF.32–34 Studies in EAE have shown that IFNb administration decreases the expression of several chemokines including MIP-1α and RANTES and their receptors by Th1/Th17 cells, reducing CNS infiltration.35 In addition, it has been reported that IFNb inhibits signaling via Toll-like receptor 9 in plasmacytoid dendritic cells from MS patients, reducing the release of immune mediators including MIP-1α, MIP-1β, and RANTES.36 Overall, these results could indicate that the immunomodulatory effects of IFNb imply reduced activation of the innate immune system and that down-regulation of MIP-1α expression may decrease the inflammatory response and lymphocytic infiltration into the CNS.
Potential inflammatory biomarkers associated with IFNb response in MS have been previously investigated. Some studies explored the role of inter-individual genetic variability investigating whether the expression of certain genes could be associated to response to IFNb therapy.6,7 In one study, exploring gene expression in peripheral blood mononuclear cells from RR-MS patients, and its modulation during IFNb treatment, it has been shown that the expression of type I IFN-regulated genes could predict 2-year response to IFNb.7 In particular, the basal expression of various type I IFN-regulated genes was found to be increased in non-responders; conversely, in responders low basal expression of these genes increased during treatment. Therefore, it has been hypothesized that non-responder patients to IFNb treatment may represent a pathogenetically different MS phenotype, characterized by intrinsically activated innate immunity and by monocytic dysfunction.7
In the present study, after analyzing an ample array of CSF proinflammatory and anti-inflammatory molecules, we found that MIP-1α and PDGF detectability profile predicted NEDA-3 status in patients treated with IFNb. Short follow-up duration represents a limitation of the present study. Therefore, assessing NEDA-3 status after longer follow up would strongly support the potential clinical application of these biomarkers. Moreover, whether the actual CSF levels of these molecules could also be useful to predict the response to IFNb should be investigated in a larger cohort of MS patients considering the extremely high variability of CSF cytokine levels. These results suggest that MIP-1α detectability/PDGF undetectability could represent suitable biomarkers able to predict response to IFNb therapy.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286420970833 for Cerebrospinal fluid inflammatory biomarkers predicting interferon-beta response in MS patients by Mario Stampanoni Bassi, Jelena Drulovic, Tatjana Pekmezovic, Ennio Iezzi, Francesco Sica, Luana Gilio, Antonietta Gentile, Alessandra Musella, Georgia Mandolesi, Roberto Furlan, Annamaria Finardi, Girolama Alessandra Marfia, Paolo Bellantonio, Roberta Fantozzi, Diego Centonze and Fabio Buttari in Therapeutic Advances in Neurological Disorders
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F.S. acted as Advisory Board members of Novartis, Biogen and Merck Serono. R.Fu. has received honoraria as speaker or for research support from Biogen, Novartis, Merck, Roche, Genzyme. G.A.M. received honoraria for speaking, consultation fees and travel funding from Roche, Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Mylan and Teva. She is the principal investigator in clinical trials for Actelion, Biogen Idec, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, Teva. D.C. is an Advisory Board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. F.B. acted as Advisory Board members of Teva and Roche and received honoraria for speaking or consultation fees from Merck Serono, Teva, Biogen Idec, Sanofi, and Novartis and non-financial support from Merck Serono, Teva, Biogen Idec, and Sanofi.
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
M.S.B., E.I., J.D., T.P., L.G., A.F., A.G., A.M., G.M., R.Fa., P.B. declare no conflict of interest.
Data Availability Statement: Datasets are available on request. Please contact corresponding author.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by FISM-Fondazione Italiana Sclerosi Multipla- cod. 2019/S/1 to DC; by the Italian Ministry of Health (Ricerca corrente-IRCCS Neuromed to DC; Ricerca Finalizzata 2018, RF-2018- 12366144 to DC and GM; Ricerca Finalizzata 2018, GR-2018-12366154 to AG and FB) and by 5 × 1000 grant to IRCCS Neuromed.
ORCID iD: Diego Centonze  https://orcid.org/0000-0002-8390-8545
https://orcid.org/0000-0002-8390-8545
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mario Stampanoni Bassi, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Jelena Drulovic, Clinic of Neurology, Clinical Center of Serbia, Belgrade, Serbia; Faculty of Medicine, University of Belgrade, Serbia.
Tatjana Pekmezovic, Institute of Epidemiology, Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
Ennio Iezzi, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Francesco Sica, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Luana Gilio, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Antonietta Gentile, Synaptic Immunopathology Lab, IRCCS San Raffaele Pisana, Rome, Italy; Synaptic Immunopathology Lab, Department of Systems Medicine, Tor Vergata University, Rome, Italy.
Alessandra Musella, Synaptic Immunopathology Lab, IRCCS San Raffaele Pisana, Rome, Italy; San Raffaele University of Rome, Rome, Italy.
Georgia Mandolesi, Synaptic Immunopathology Lab, IRCCS San Raffaele Pisana, Rome, Italy; San Raffaele University of Rome, Rome, Italy.
Roberto Furlan, Clinical Neuroimmunology Unit, Institute of Experimental Neurology, Division of Neuroscience, San Raffaele Scientific Institute, Milano, Italy.
Annamaria Finardi, Clinical Neuroimmunology Unit, Institute of Experimental Neurology, Division of Neuroscience, San Raffaele Scientific Institute, Milano, Italy.
Girolama Alessandra Marfia, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy; Multiple Sclerosis Clinical and Research Unit, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Paolo Bellantonio, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Roberta Fantozzi, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Diego Centonze, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy; Synaptic Immunopathology Lab, Department of Systems Medicine, Tor Vergata University, Rome, Italy.
Fabio Buttari, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
References
- 1. Drulovic J, Ivanovic J, Mesaros S, et al. Long-term disability outcomes in relapsing-remitting multiple sclerosis: a 10-year follow-up study. Neurol Sci 2019; 40: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 2. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple. Lancet 1998; 352: 1498–1504. [PubMed] [Google Scholar]
- 3. Río J, Nos C, Tintoré M, et al. Assessment of different treatment failure criteria in a cohort of relapsing-remitting multiple sclerosis patients treated with interferon beta: implications for clinical trials. Ann Neurol 2002; 52: 400–406. [DOI] [PubMed] [Google Scholar]
- 4. Portaccio E, Zipoli V, Siracusa G, et al. Response to interferon-beta therapy in relapsing-remitting multiple sclerosis: a comparison of different clinical criteria. Mult Scler 2006; 12: 281–286. [DOI] [PubMed] [Google Scholar]
- 5. Sellebjerg F, Søndergaard HB, Koch-Henriksen N, et al. Prediction of response to interferon therapy in multiple sclerosis. Acta Neurol Scand 2014; 130: 268–275. [DOI] [PubMed] [Google Scholar]
- 6. van Baarsen LG, Vosslamber S, Tijssen M, et al. Pharmacogenomics of interferon-beta therapy in multiple sclerosis: baseline IFN signature determines pharmacological differences between patients. PLoS One 2008; 3: e1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comabella M, Lünemann JD, Río J, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain 2009; 132: 3353–3365. [DOI] [PubMed] [Google Scholar]
- 8. Stampanoni Bassi M, Iezzi E, Landi D, et al. Delayed treatment of MS is associated with high CSF levels of IL-6 and IL-8 and worse future disease course. J Neurol 2018; 265: 2540–2547. [DOI] [PubMed] [Google Scholar]
- 9. McKay FC, Hoe E, Parnell G, et al. IL7Rα expression and upregulation by IFNβ in dendritic cell subsets is haplotype-dependent. PLoS One 2013; 8: e77508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology 2010; 74(Suppl. 1): S17–S24. [DOI] [PubMed] [Google Scholar]
- 11. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 12. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 13. Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8: 254–260. [DOI] [PubMed] [Google Scholar]
- 14. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 15. Rossi S, Studer V, Motta C, et al. Cerebrospinal fluid detection of interleukin-1β in phase of remission predicts disease progression in multiple sclerosis. J Neuroinflammation 2014; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conlon K, Lloyd A, Chattopadhyay U, et al. CD8+ and CD45RA+ human peripheral blood lymphocytes are potent sources of macrophage inflammatory protein 1 alpha, interleukin-8 and RANTES. Eur J Immunol 1995; 25: 751–756. [DOI] [PubMed] [Google Scholar]
- 17. Simpson J, Newcombe J, Cuzner M, et al. Expression of monocyte chemoattractant protein-1 and other b-chemokines by resident and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol 1998; 84: 238–249. [DOI] [PubMed] [Google Scholar]
- 18. Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol 2004; 36: 1882–1886. [DOI] [PubMed] [Google Scholar]
- 19. Andres PG, Beck PL, Mizoguchi E, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 2000; 164: 6303–6312. [DOI] [PubMed] [Google Scholar]
- 20. Glabinski A, Tani M, Strieter R, et al. Synchronous synthesis of a- and b-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of experimental autoimmune encephalomyelitis. Am J Pathol 1997; 150: 617–630. [PMC free article] [PubMed] [Google Scholar]
- 21. Karpus WJ, Lukacs NW, McRae BL, et al. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 1995; 155: 5003–5010. [PubMed] [Google Scholar]
- 22. Eltayeb S, Sunnemark D, Berg AL, et al. Effector stage CC chemokine receptor-1 selective antagonism reduces multiple sclerosis-like rat disease. J Neuroimmunol 2003; 142: 75–85. [DOI] [PubMed] [Google Scholar]
- 23. Balashov KE, Rottman JB, Weiner HL, et al. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 1999; 96: 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartosik-Psujek H, Stelmasiak Z. The levels of chemokines CXCL8, CCL2 and CCL5 in multiple sclerosis patients are linked to the activity of the disease. Eur J Neurol 2005; 12: 49–54. [DOI] [PubMed] [Google Scholar]
- 25. Zang YC, Samanta AK, Halder JB, et al. Aberrant T cell migration toward RANTES and MIP-1 alpha in patients with multiple sclerosis. Overexpression of chemokine receptor CCR5. Brain 2000; 123: 1874–1882. [DOI] [PubMed] [Google Scholar]
- 26. Erlandsson A, Enarsson M, Forsberg-Nilsson K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J Neurosci 2001; 21: 3483–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woodruff RH, Fruttiger M, Richardson WD, et al. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci 2004; 25: 252–262. [DOI] [PubMed] [Google Scholar]
- 28. Stampanoni Bassi M, Iezzi E, Marfia GA, et al. Platelet-derived growth factor predicts prolonged relapse-free period in multiple sclerosis. J Neuroinflammation 2018; 15: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng F, Yao H, Bai X, et al. Platelet-derived growth factor-mediated induction of the synaptic plasticity gene Arc/Arg3. 1. J Biol Chem 2010; 285: 21615–21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mori F, Nicoletti CG, Rossi S, et al. Growth factors and synaptic plasticity in relapsing-remitting multiple sclerosis. Neuromolecular Med 2014; 1: 490–498. [DOI] [PubMed] [Google Scholar]
- 31. Kozovska ME, Hong J, Zang YC, et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology 1999; 53: 1692–1697. [DOI] [PubMed] [Google Scholar]
- 32. Rudick RA, Ransahoff RM, Peppler R, et al. Interferon beta induces interluekin-10 expression: relevance to multiple sclerosis. Ann Neurol 1996; 40: 618–627. [DOI] [PubMed] [Google Scholar]
- 33. Graber JJ, Ford D, Zhan M, et al. Cytokine changes during interferon-beta therapy in multiple sclerosis: correlations with interferon dose and MRI response. J Neuroimmunol 2007; 185: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makar TK, Trisler D, Bever CT, et al. Stem cell based delivery of IFN-beta reduces relapses in experimental autoimmune encephalomyelitis. J Neuroimmunol 2008; 196: 67–81. [DOI] [PubMed] [Google Scholar]
- 35. Cheng W, Zhao Q, Xi Y, et al. IFN-β inhibits T cells accumulation in the central nervous system by reducing the expression and activity of chemokines in experimental autoimmune encephalomyelitis. Mol Immunol 2015; 64: 152–162. [DOI] [PubMed] [Google Scholar]
- 36. Balashov KE, Aung LL, Vaknin-Dembinsky A, et al. Interferon-β inhibits toll-like receptor 9 processing in multiple sclerosis. Ann Neurol 2010; 68: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286420970833 for Cerebrospinal fluid inflammatory biomarkers predicting interferon-beta response in MS patients by Mario Stampanoni Bassi, Jelena Drulovic, Tatjana Pekmezovic, Ennio Iezzi, Francesco Sica, Luana Gilio, Antonietta Gentile, Alessandra Musella, Georgia Mandolesi, Roberto Furlan, Annamaria Finardi, Girolama Alessandra Marfia, Paolo Bellantonio, Roberta Fantozzi, Diego Centonze and Fabio Buttari in Therapeutic Advances in Neurological Disorders


