Abstract
Naringin is a naturally occurring flavonoid found in plants of the Citrus genus that has historically been used in traditional Chinese medical regimens for the treatment of osteoporosis. Naringin modulates signaling through numerous molecular pathways critical to musculoskeletal development, cellular differentiation, and inflammation. Administration of naringin increases in vitro expression of bone morphogenetic proteins (BMPs) and activation of the Wnt/β-catenin and extracellular signal-related kinase (Erk) pathways, thereby promoting osteoblastic proliferation and differentiation from stem cell precursors for bone formation. Naringin also inhibits osteoclastogenesis by both modifying RANK/RANKL interactions and inducing apoptosis in osteoclasts in vitro. In addition, naringin acts on the estrogen receptor in bone to mimic the native bone-preserving effects of estrogen, with few systemic side effects on other estrogen-sensitive tissues. The efficacy of naringin therapy in reducing the osteolysis characteristic of common musculoskeletal pathologies such as osteoporosis, degenerative joint disease, and osteomyelitis, as well as inflammatory conditions affecting bone such as diabetes mellitus, has been extensively demonstrated in vitro and in animal models. Naringin thus represents a naturally abundant, cost-efficient agent whose potential for use in novel musculoskeletal biotherapies warrants re-visiting and further exploration through human studies. Here, we review the cellular mechanisms of action that have been elucidated regarding the action of naringin on bone resident cells and the bone microenvironment, in vivo evidence of naringin’s osteostimulative and chondroprotective properties in the setting of osteolytic bone disease, and current limitations in the development of naringin-containing translational therapies for common musculoskeletal conditions.
Keywords: diabetes mellitus, naringin, osteoarthritis, osteoclastogenesis, osteomyelitis, osteoporosis
Introduction
The anti-inflammatory, antioxidant, and anti-apoptotic properties of natural flavanones and their glycosides have been established in preclinical in vitro and in vivo models of atherosclerosis, cardiovascular disease, various cancers, diabetes mellitus, neurodegenerative conditions, osteoporosis, and rheumatic disease. Flavonoids are phenolic compounds widely distributed among vascular plants; there are approximately 4000 known natural products within the flavonoid family.1 The flavanones and their metabolites are highly lipophilic, with preferential accumulation in the stomach and reduced accumulation in the brain due to the blood–brain barrier.2,3 Naringin (naringenin 7-O-neohesperidose), a naturally occurring flavonoid commonly found in tomatoes, grapefruits, and other members of the Citrus genus, has been found to protect against retinoid acid-induced osteolytic bone diseases such as osteoporosis.3–5 Naringin naturally exists as a mixture of chiral isomers of varying proportions associated with the state of fruit maturation and purification.3 Naringin is the primary active component of Drynaria fortune, a traditional Chinese medicine used for osteoporosis.5 Plant-based derivatives such as naringin constitute a major class of widely available, cost-effective, and biologically active materials for the treatment of a number of skeletal pathologies due to their pro-osteogenic potential, effects on multiple signaling pathways, and actions on numerous cellular targets.
Here, we describe the signaling pathways through which naringin exerts its myriad of effects upon bone and the translational therapeutic potential of naringin for common musculoskeletal pathologies such as osteoporosis, osteoarthritis, avascular necrosis, and osteomyelitis (Table 1). We also briefly describe translational research and pharmacologic challenges that must be overcome to successfully harness the bone-promoting/preserving effects of naringin.
Table 1.
Effects of naringin on the musculoskeletal system.
| Pathology | Effects |
|---|---|
| Osteolytic diseases (e.g. osteoporosis secondary to age, estrogen deficiency, diabetes mellitus, or glucocorticoid use) |
• Directly increases BMP-2 expression • Increases BMP-2 expression through Wnt/β-catenin • Increases BMP-4 and RUNX2 expression and MSC differentiation down osteoblastic lineage • Decreases MSC differentiation down adipogenic lineage • Decreases RANK/RANKL interactions • Increases OPG expression • Decreases osteoclastogenic, proinflammatory cytokine expression • Directly acts on estrogen receptor α, and • Upregulates osteoclast death via Fas/FasL |
| Degenerative joint diseases (e.g. osteoarthritis) |
• Reduces the expression of cartilage-degrading cytokines • Increases the expression of reactive oxygen species-neutralizing enzymes • Decreases the production of cartilage-degrading enzymes • Increases the production of cartilage-protective IL-4 and recruits Treg cells, and • Decreases pannus formation |
| Bone and joint infections (e.g. osteomyelitis) |
• Direct antibiotic effects on Gram-positive and Gram-negative bacteria • Direct inhibition of bacterial quorum sensing system |
BMP, bone morphogenetic protein; IL, interleukin; MSC, mesenchymal stem cell; OPG, osteoprotegerin; Treg, regulatory T-cell.
In vitro characterization of the effects of naringin on bone resident cells
Bone morphogenetic proteins regulate skeletal formation through osteoblast activation
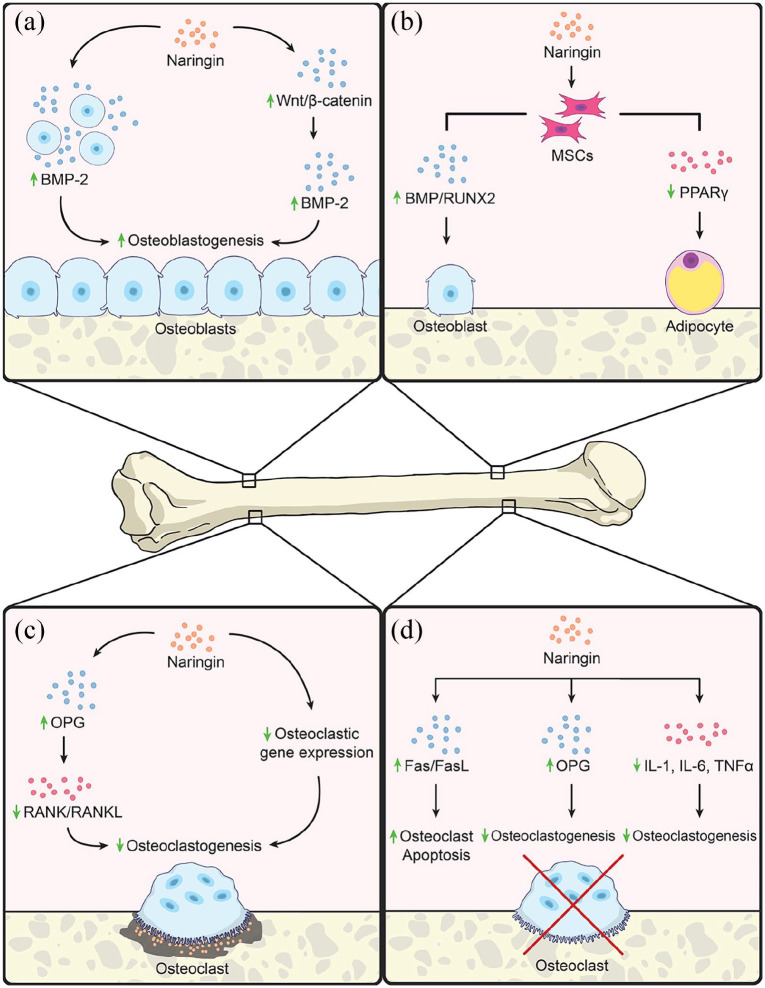
The positive effects of naringin upon bone health are many. Naringin enhances the production of cell survival proteins and reduces the expression of genes involved in the inflammatory response.3 In the musculoskeletal system, bone morphogenetic proteins (BMPs) have been shown to be important in skeletogenesis,6 limb and digit formation,7 and fracture repair.8 BMP-2 is responsible for the differentiation of multipotent stem cells to an osteoblast-like lineage.9 This osteoblastic differentiation yields increased bone formation, with clinical potential to restore bone mineral density (BMD) in osteopenia and osteoporosis and enhance fracture healing. Multiple groups have demonstrated that hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors used to treat hypercholesterolemia upregulate BMP-2 expression.10–13 At concentrations between 0.001 and 0.1 µmol/l, naringin increased osteoblast activity by inhibiting HMG-CoA reductase in a dose-dependent fashion in rat osteoblast-like UMR-106 cells.14 Given these effects on HMG-CoA, it has been posited that naringin augments BMP-2 expression for osteoblastogenesis and bone formation. Indeed, primary osteoblasts cultured with naringin displayed increased BMP-2 protein and mRNA levels.5 Yin et al. found that naringin upregulated osteoblastogenesis in vitro through a BMP-2-mediated mechanism15 [Figure 1(a)]. Osteoblast activity increased in the presence of naringin in UMR-106 cells, as demonstrated by the colorimetric tetrazolium assay, which evaluates cellular viability, metabolism, and proliferation via oxidoreductases, and increased total protein and alkaline phosphatase (ALP) activity.14 Together, these findings indicate that naringin activates BMP-2 for increased osteoblast and bone production.
Figure 1.
In vitro effects of naringin. (a) Naringin has been shown to increase osteoblastogenesis and thus bone anabolism. Naringin increases osteoblastogenesis through upregulating BMP-2, a protein responsible for the differentiation of MSCs down an osteoblastic lineage, directly and by stimulating the Wnt/β-catenin pathway which converges on further upregulation of BMP-2. (b) MSCs grown in the presence of naringin demonstrate increased osteoblastogenesis and decreased adipogenesis. MSCs in the presence of naringin express increased levels of osteoblastic genes such as BMPs and RUNX2 and decreased gene expression of the adipogenic PPARγ. (c) Naringin has been shown to decrease osteoclastogenesis and thus osteolysis in osteoclastic pathologies, such as osteoporosis in menopause and diabetic osteoporosis. Naringin decreases osteoclastogenesis by inhibiting RANK/RANKL interaction by inducing expression of the receptor decoy OPG as well as directly decreasing expression of osteoclastic genes. (d) Physiological levels of estrogen maintain bone stock in females by inducing osteoclast apoptosis and decreasing inflammatory cytokines that are osteoclastogenic; therefore, the loss of estrogen results in osteoporosis due to increased osteoclastogenesis. Through interactions with estrogen receptor α, naringin increases interactions between the death receptor Fas and its ligand FasL which increases osteoclast apoptosis, which tips bone homeostasis from a catabolic to an anabolic state, like endogenous estrogen. Naringin also decreases the inflammatory cytokines IL-1, IL-6, and TNF-α that stimulate osteoclastogenesis.
BMP-2, bone morphogenic protein 2; IL, interleukin; MSC, mesenchymal stem cell; OPG, osteoprotegerin; TNF, tumor necrosis factor;
Upstream effects of naringin on BMP activation via the Wnt/β-catenin pathway
Wnt signaling through the canonical Wnt/β-catenin pathway crucially regulates musculoskeletal development.16,17 Wnt and β-catenin serve as important upstream regulators of BMP signaling, thereby influencing osteoblastogenesis and bone formation.18 Wang et al. showed that naringin upregulated β-catenin mRNA and protein expression in osteoblast-like UMR-106 cells19 [Figure 1(a)]. Gene expression of the Wnt-related regulators β-catenin and cyclin D1 in human amniotic fluid-derived stem cells (hAFSCs) increased in the presence of naringin.20 Application of the Wnt-inhibitor DKK-1 reduced ALP activity, which is suggestive of the critical role of Wnt/β-catenin signaling in osteogenesis.20 Modulation of the Wnt/β-catenin pathway by naringin in this way contributes to bone anabolism.
Differentiation of bone resident cells and osteogenesis
Naringin increases ALP activity, osteocalcin (OCN) and osteopontin synthesis, and cell proliferation in primary cultured osteoblasts, in addition to promoting human bone mesenchymal stem cell (BMSC) proliferation and osteogenic differentiation.21–23 hAFSCs exposed to naringin increasingly underwent osteogenic proliferation and differentiation, as evidenced by increased ALP activity, a marker for early osteogenic differentiation, and calcium deposition, a marker for late osteogenic differentiation.20 Similar effects upon osteogenic proliferation and differentiation have been observed with naringin administration in human BMSCs,23 periodontal ligament stem cells (PDLSCs),24 and murine pre-osteoblastic MC3T3-E1 cells.5,25,26 Moreover, hAFSCs expressed increased levels of BMP-4 and RUNX2 mRNA in the presence of naringin, suggesting that the activation of BMP signaling by naringin promotes osteogenesis20 [Figure 1(b)]. Inhibition of BMP-4 and RUNX2 using the BMP-inhibitor noggin reduced ALP activity, which further suggests the necessity of BMP signaling pathways for osteogenesis.20 Moreover, it has been established that the extracellular signal-regulated kinase (ERK) MAPK pathway plays a vital role in osteoblastic differentiation.27 Naringin has been shown to increase Erk expression and phosphorylation in osteoblasts, promoting osteogenesis. Naringin doses of 10 µg/ml demonstrated the greatest pro-osteogenic effects upon rat bone marrow-derived mesenchymal stem cells (BM-MSCs), which persisted for 9 days.5 The pro-osteogenic potential of naringin may be harnessed or enhanced through novel formulations and drug delivering biomaterial constructs. Lai et al. loaded TiO2 (titanium) nanotubes with naringin via chitosan layers and observed that these naringin-eluting nanotubes enhanced osteoblast spreading and proliferation, ALP activity, and bone mineralization.28 The application of naringin-loaded polymeric micelles to human adipose-derived stem cells yielded greater osteogenic differentiation, as characterized by increased osteopontin expression and bony matrix mineralization, than was observed in the presence of free naringin.29 Naringin additionally enhances osteoblast survival by increasing 1α,25-dihydroxyvitamin D3 signaling in osteoblasts in vitro.30
The pro-osteogenic effects of naringin may also be linked to its ability to downregulate peroxisome proliferator-activated receptor (PPAR)γ (a key regulator of adipogenesis) expression in BMSCs, as well as its activation of the neurogenic locus Notch homolog protein (Notch) signaling pathway31,32 [Figure 1(b)]. This downregulation of PPARγ was also associated with increased microRNA (miRNA)-20a expression.31 Naringin thus concurrently reduces adipogenesis in bone and promotes osteogenic differentiation via Notch and miRNA transcriptional regulation, augmenting bone formation. These findings suggest that naringin is a potent natural promoter of osteogenesis with the potential for use in stem cell therapies, which traditionally use dexamethasone for lineage differentiation.5 Such treatments are often limited by adipogenesis secondary to glucocorticoid exposure, even when conducted in osteogenic media.
Regulation of osteoclastogenesis via RANK activation
Bone homeostasis is mediated by the delicate balance between osteoblast-mediated anabolism and osteoclast-mediated catabolism.33 Increased osteoclastogenesis is the hallmark of many bone pathologies, including senile osteoporosis due to increased adipogenesis, post-menopausal osteoporosis secondary to the loss of the osteoblastic and anti-osteoclastic effects of estrogen,34 and diabetic osteoporosis induced by the proinflammatory state and resultant macroangiopathies and microangiopathies characteristic of diabetes mellitus.35 Osteoclastogenesis is mediated by interactions between RANK/RANKL and osteoprotegrin (OPG);36 pathologic states increase the RANK/RANKL ratio present in bone and decrease production of OPG. Moreover, under proinflammatory conditions, increased expression of cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α promote osteoclastogenesis.37
Naringin has been shown to successfully disrupt osteoclastogenesis via the RANK/RANKL pathway [Figure 1(c)]. Incubation of RAW264.7 cells with medium containing naringin for 5 days inhibited osteoclast differentiation and proliferation, bone resorption, and RANK, TRAP, matrix metalloproteinases (MMP)-9 and NFATc1 mRNA expression critical to osteoclast differentiation, while upregulating C-fos expression.38 Naringin decreases degradation of RANKL-mediated IκB and enhances RANKL-mediated phosphorylation of Erk.23 Furthermore, naringin enhances OPG release from osteoblasts, reducing interactions between RANKL and its receptor and preventing osteoclast differentiation.22 The combination of these mechanisms suppresses the expression of genes critical to osteoclast development.
Estrogen and estrogen receptor signaling
The sex hormone estrogen plays a central role in bone development and metabolism. In childhood and adolescence, estrogen drives progression to skeletal maturity and the maturation and fusion of the epiphyseal growth plates.39 In adults, estrogen maintains bone stock and influences bone turnover by inhibiting bone resorption. Estrogen mediates its bone-protective effects through estrogen receptor α.40 Estrogen also induces apoptosis in osteoclasts by upregulating interactions between the death receptor and its ligand (Fas/FasL).40 Furthermore, estrogen inhibits osteoblast-mediated osteoclastogenesis by decreasing the production of IL-1, IL-6, and TNF-α, which induce osteoclast differentiation from progenitor cells.41 Estrogen also modulates the synthesis of OPG, which serves as a decoy receptor for RANKL, by osteoblasts, thus hindering osteoclastogenesis.42 The loss of estrogen production in menopause is the primary causative factor of diminished BMD and increased predisposition to fractures among post-menopausal women.43 While hormone replacement is effective against bone loss in menopause,44–46 estrogen replacement therapy is not without negative sequalae.47,48 For these reasons, alternative bone-preserving therapies for post-menopausal osteoporosis and other estrogen-depleting conditions without multisystemic side effects are desired.
Naringin has been shown to have estrogen-like effects in vitro on UMR-106 cells49 and is known to bind to the estrogen receptor50 [Figure 1(d)]. Naringin also downregulates expression of BCL-2 while upregulating expression of BAX, caspase-3, and cytochrome C, suggesting that naringin may induce osteoclast apoptosis through regulation of the mitochondrial apoptosis pathway.51 The ability of naringin to induce apoptosis in exposed osteoclasts51 and upregulate OPG mRNA levels in MC3T3-E1 cells hinders osteoclastogenesis in ways that mirror the native effects of estrogen on bone.30 By targeting pathways vital to the pathogenesis of menopause-induced bone loss, naringin may stem the musculoskeletal consequences of reduced estrogen production for the restoration of bone stock.
In vivo modeling of the restorative effects of naringin in pathologic conditions of bone
The dual actions of naringin in the treatment of osteoporotic bone
Naringin has shown promise as a treatment for osteolytic bone diseases such as osteoporosis, which is characterized by increased osteoclast activity and bone resorption.3–5 Daily oral naringin administration increased femoral bone mass in healthy mice by enhancing trabecular and cortical bone content and quality5 [Figure 2(a,b)]. The majority of osteoporosis animal models have employed ovariectomy (OVX) to reduce circulating estrogen levels and induce osteoclast-mediated bone resorption. This model simulates the loss of estrogen production characteristic of menopause, after which the cortical and trabecular BMD and interconnections between bone cells become drastically diminished and the risk of pathologic fracture significantly increases, resulting in osteopenia and osteoporosis [Figure 2(a)]. Orchidectomized,52 retinoic acid-induced,4 and disuse-induced animal models of osteoporosis have also been generated to study disease physiology and therapeutic avenues.53 Li et al. demonstrated that naringin decreased bone loss in OVX-induced osteoporosis Sprague–Dawley rats.51 Naringin has been found to mitigate the loss of bone calcium and phosphorus content,52 BMD,19,21,49,51,54–57 bone architecture quality (as measured by bone volume, trabecular thickness, and trabecula count19,49,51,54,55) and bone mechanical strength across all murine models of osteoporosis, particularly in OVX mice and rats19,51,55 [Figure 2(b)]. Treatment of OVX mice with naringin for 6 weeks significantly improved distal femur, proximal tibia, and lumbar spine bone quality, while suppressing the urinary excretion of calcium and loss of bone mass and strength characteristic of OVX mice.49,55 However, the benefits of naringin in bone health are not limited to bone structure and microarchitecture, but also extend to biomechanical strength. A combined 60-day regimen of oral naringin and treadmill exercise in OVX mice more effectively reduced the structural effects of osteoporosis on bone mass and increased bone strength than either monotherapy.55 Importantly, naringin administration in OVX mice has not been associated with increases in uterus weight, suggesting that the off-target estrogen-modulating effects of estrogen are limited.49 This represents a significant advantage over traditional post-menopausal hormone replacement therapy, which nonselectively acts upon bone, breast, ovarian, and endometrial tissue.
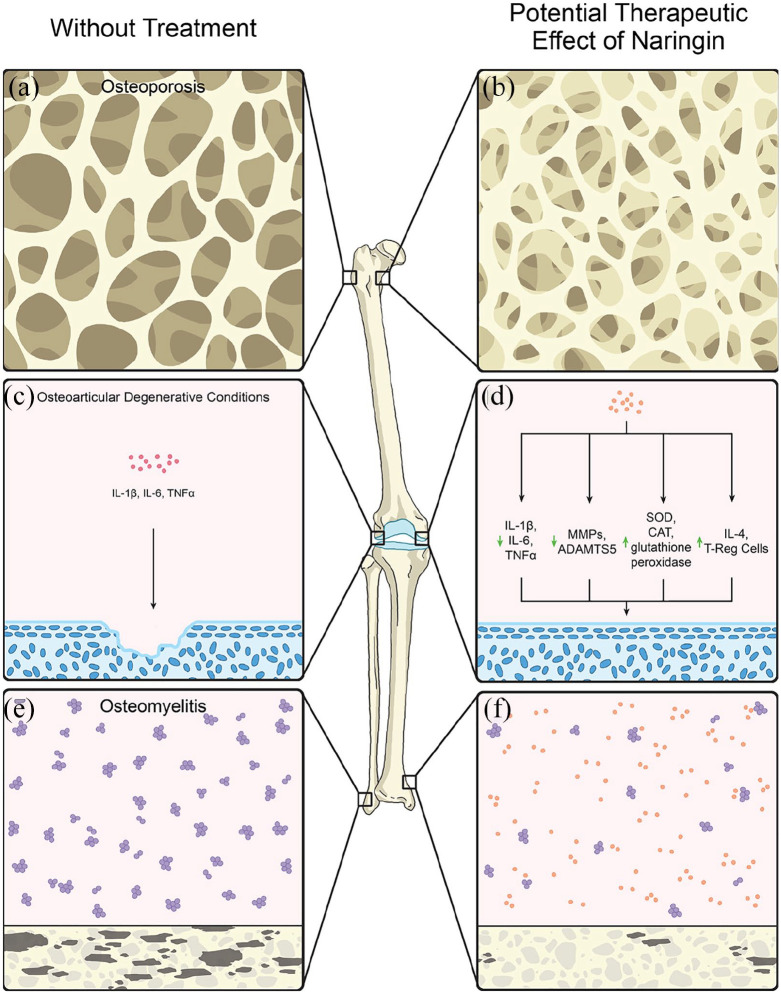
Figure 2.
In vivo effects of naringin. (a) Osteoporosis secondary to the loss of estrogen’s anabolic effects during menopause, the proinflammatory state of diabetes that promotes osteoclastogenesis, or the loss of p-ERK due to glucocorticoid use leads to decreased trabeculae volume, yielding an increased risk of fracture. (b) Naringin prevents osteoporotic bone fractures by increasing the number and thickness of trabeculae. (c) Osteoarticular degenerative conditions such as osteoarthritis, ankylosis spondylitis, and rheumatoid arthritis are characterized by increases in the expression of inflammatory cytokines such as IL-1β, IL-6, and TNF-α, decreases in reactive oxygen species-neutralizing enzymes such as SOD, CAT, and glutathione peroxidase, and increases in cartilage-degrading enzymes, including MMPs and ADAMTS5. (d) Naringin has been shown to be cartilage-protective by decreasing the expression of these inflammatory cytokines, reactive oxygen species, and destructive enzymes. Naringin has also been shown to increase the cartilage-protective effects of IL-4 and Tregs. (e) Osteomyelitis is characterized by the proliferation of bacteria that degrade bone both directly via bacterial products and indirectly by inducing inflammation productive of osteoclastic cytokines. (f) Naringin demonstrates antibacterial effects against common Gram-positive and Gram-negative organisms commonly implicated in the pathogenesis of osteomyelitis.
CAT, catalase; IL, interleukin; MMP, matrix metalloproteinase; SOD, superoxide dismutase; TNF, tumor necrosis factor; Treg, regulatory T-cell;
Perhaps the greatest therapeutic benefit of naringin in the treatment of osteoporosis has been demonstrated in models of disuse-induced osteoporosis. These conditions replicate the loss of bone mechanical loading seen in limited mobility and bed-ridden patients and astronauts in zero-gravity environments.5 Ma et al. created a model of disuse-induced osteoporosis in rats by denervating bone through sciatic neurectomy, which resulted in the loss of BMD, disruption of native trabecular microarchitecture in the distal femurs of experimental animals, and increase in urinary deoxypyridoline excretion.53 Dose-dependent recovery of trabecular microarchitecture and bone formation rates comparable to the sham group were observed with naringin treatment.
Several theories have been postulated regarding the etiology of naringin’s bone-preserving effects in osteoporosis in in vivo models. Naringin is believed to limit the upregulation of osteoclast activity and differentiation seen in osteoporosis. Naringin attenuates increases in circulating CTX-1, a biomarker that correlates with osteoclast-mediated resorption in osteoporosis.51–53,55,57 These reductions in osteoclastogenesis were confirmed by TRAP staining of bone tissue isolated from OVX mice, which revealed reduced osteoclast invasion of and activity within bone.51,53 This has been attributed to the induction of apoptosis in osteoclasts by naringin51 and the inhibition of osteoclast differentiation.53 In addition to mediating osteoclastogenesis, naringin has the potential to induce new bone formation in osteoporotic animals by upregulating the differentiation and activity of osteoblasts, as described previously. Biomarkers thought to correlate with osteoblast activity such as OCN and total procollagen type 1 N-terminal propeptide (P1NP) are increased in both naringin-treated osteoporotic mice and wild-type mice.51,53,55 Naringin treatment is histologically marked by increased osteoblast infiltration of bone and increased osteoid formation in in vivo models.4,19,51,53,55 These effects upon osteoblasts are thought to be mediated by the induction of Akt-mediated BMP-2 expression21,57 and β-catenin signaling through AMPK/Akt and semaphorin-3A (SEM3A) by naringin.19,53
Habauzit et al. hypothesized that naringin’s anti-inflammatory properties may be partly responsible for its bone-protective effects in the setting of osteoporosis, particularly in the setting of age-related bone inflammation.57 The authors observed that naringin lowered circulating IL-6 and nitric oxide (NO) levels in osteoporotic animal models.57 Finally, naringin may also prevent microvascular damage to osteopenic and osteoporotic bone. Naringin preserves bone microvascularity in OVX mice and reduces the perturbations of NO and endothelin levels, two key regulators of vasoactivity, seen in osteoporosis.56 In addition, naringin promotes angiogenesis and neovascularization during fracture callus formation in murine osteoporotic models, likely by way of vascular endothelial growth factor (VEGF) expression changes in osteocytes.50 The effects of naringin upon the Notch signaling pathway, as described previously, may thus have dual implications for bone health, as endothelial Notch activity simultaneously regulates both bone angiogenesis and osteogenesis, conferring both fracture healing and osteoprotective benefits.5 These effects resulted in the dose-dependent acceleration of fracture healing in murine models. More work is needed to further elucidate the mechanisms by which naringin exerts its effects upon bone resident cells in osteoporosis.
Pharmacological therapy for osteoporosis targets both osteoclastogenesis and new bone formation with agents such as bisphosphonates and intermittent parathyroid hormone, respectively. However, the reduced responsivity of protracted disuse osteoporosis to bisphosphonate treatment and transition from bone formation to adipogenesis in bone marrow with age necessitate the adoption of new osteostimulative and osteoprotective therapies.5
Modification of metabolic pathways and inflammation in diabetes mellitus
Diabetes mellitus (DM) is a chronic metabolic disorder associated with impaired new bone formation and fracture healing and increased risk of pathologic fracture secondary to systemic hyperglycemia, advanced glycation end-product and reactive oxidative species (ROS) formation, and inflammation.35 On the cellular level, these processes increase osteoclastogenesis and reduce osteoblast levels and activity. Consequently, diabetic rats display reduced trabecular volume and bone remodeling and formation, with increased trabecular spacing in the femur and lumbar spine,58,59 similar to the findings clinically observed in various forms of osteoporosis [Figure 2(a)]. In vivo models of diabetes typically utilize streptozocin injections, which damage the β-cells of the pancreas, to recreate the pathology seen in type 1 DM or prolonged feeding of high fat diets to simulate type 2 DM. Increased levels of superoxide dismutase (SOD) and catalase (CAT) activity in bone marrow are indicative of higher levels of inflammation and oxidative stress,60 which have been implicated in the pathogenesis of diabetes.61 Naringin has been found to normalize both SOD and CAT levels in diabetic rats. Naringin administration to diabetic rodents resulted in similar oxidative stress attenuation in the skin,62 liver,63–66 pancreas,64,66 and kidney.66 As such, naringin has also been shown to slow both structural and compositional trabecular bone quality decline in the long bones and calvaria of streptozocin-induced diabetic rodents60,67 [Figure 2(b)]. These findings were corroborated by observed increases in serum OCN levels, osteoid production, and osteoblast populations, as well as reduced osteoclastic infiltration of bone, in naringin-treated diabetic rats.60,67 Furthermore, enhanced bone formation was observed upon subcutaneous naringin administration in a titanium particle-induced diabetic murine calvarial model of osteolysis.67
Naringin has demonstrated strong reversing effects of the primary pathologic hallmarks of both type 1 and 2 DM by improving insulin resistance and production, glucose tolerance, and fasting glucose and HbA1c levels in vivo.68 These effects are postulated to stem from the positive regulation of glucose metabolism and GLUT-4 mobilization by naringin.68 In addition, the effects of naringin upon serum lipid regulation have been extensively investigated in vivo. By upregulating PPARγ expression in tissues, particularly the liver and kidney, naringin mollifies the increases in serum low-density lipoprotein, triglyceride, and total cholesterol concentrations and reduced high-density lipoprotein levels characteristic of DM and other metabolic syndromes.64,66,68 Naringin may also upregulate heat shock proteins HSP-72 and HSP-27 to influence PPARγ expression and suppress inflammation via the nuclear factor (NF)-κB pathway.66 However, it is possible that the bone-protective effects of naringin in diabetes is independent of these metabolic processes, as naringin has been shown to reduce bone resorption in the absence of changes to these metabolic pathways.60
The favorable lipid regulatory profile of naringin may be linked to its positive effects on bone secondary to its promotion of vascular and microvascular health. The relationship between hyperlipidemia and diabetic microangiopathy, resulting in reduced vascular delivery to bone and local hypoxia, inflammation, and oxidative stress responses, is well established.62 Naringin administration reduces the expression of various serum markers of inflammation in in vivo models of DM. These modulatory effects include reductions in serum TNF-α, IL-6, and C-reactive protein, NO,65,66,69 IL-6, TNF-α, and NF-κB expression in nervous tissue,70 hepatic expression of NF-κB, IL-6 and cyclooxygenase (COX)-2,63,66 and TNF-α, IL-6, and IL-1B expression in wound granulation tissue.62 In the context of diabetic wound healing, naringin treatment improved microvasculature to granulation tissue via localized increases in angiogenesis driven by higher local levels of VEGF-c, angiopoeitin, and insulin-like growth factor (IGF)-1.62 The aortae of diabetic mice treated with naringin displayed less endothelial damage, as visualized on transmission electron microscopy, as well as greater vascular functionality, as measured by responsivity to exogenously administered epinephrine, acetylcholine, and insulin.71 Moreover, naringin has been observed to induce SEM3A, a local bone micro-environmental factor that both promotes new bone formation and reduces bone resorption, expression in vivo.5 SEM3A deficiency has been directly implicated in the perturbation of bone remodeling and bone loss in diabetic rats.53,60
Finally, IGF-1 levels were found to be elevated in nervous tissue in the presence of naringin.70 Further research clarifying changes in the expression of IGF-1, a potent regulator of anabolism and new bone formation, in bone in the presence of naringin is needed to elucidate the mechanisms by which naringin treatment promotes bone health in osteoporosis, DM, and other inflammatory conditions.
Steroid-induced bone loss and its mollification by naringin
Synthetic glucocorticoids are routinely used in the management of inflammatory and autoimmune disorders such as rheumatoid arthritis, asthma, multiple sclerosis, systemic lupus erythematosus, and inflammatory bowel disease.72 Although these medications are effective in their ability to suppress the immune system, their deleterious effects are many, particularly upon bone. Chronic exogenous steroid administration contributes to osteopenia, osteoporosis, pathologic fracture, poor fracture healing, and avascular necrosis. In vivo models of glucocorticoid-treated mice have demonstrated that glucocorticoids not only reduce trabecular bone volume and whole bone strength, but also alter the osteocyte lacunae microenvironment, thereby decreasing the bone mineral to matrix ratio and increasing bone fragility73 [Figure 2(a,b)]. In this way, high dose and/or chronic glucocorticoid administration begets osteopenia and osteoporosis. In a rabbit model of steroid-induced avascular necrosis of the femoral head (SANFH), naringin treatment significantly mitigated steroid-associated reductions in serum OCN levels and observed rates of osteonecrosis.32
Multiple signaling pathways have been implicated in the pathogenesis of glucocorticoid-induced osteoporosis (GIO). Decreases in phosphorylated ERK (p-ERK) expression have been observed in osteoporotic mice. Chen et al. recapitulated these findings in animal models of GIO, as osteoblasts exposed to dexamethasone demonstrated lower levels of p-ERK expression.74 Jing et al. investigated the activation of the Wnt/β-catenin pathway in GIO rats and found that expression of Erk, Lrp-5, β-catenin mRNA levels, as well as p-GSK-3β protein expression, were reduced in GIO.27 GIO rats demonstrated decreased expression of BMP-2 and RUNX2, thus impairing osteoblastic differentiation.75 Phosphoinositide 3-kinase (PI3K) and its downstream effector protein kinase B (Akt) are also disrupted in GIO, resulting in perturbed bone growth and formation.76 Pan et al. observed significant reductions in BMD and ALP, OCN, and phosphorylated Akt expression in the bones of GIO animals.77 Naringin therapy was associated with lower total cholesterol levels and low-density lipoprotein/high-density lipoprotein ratios in SANFH animals.32 These effects have been attributed to upregulated caspase-3 and ALP activity and RUNX2, transcription factor sp7, PPARγ2, Notch, β-catenin, and phosphorylated Rac expression with the application of naringin.32 Together, these findings suggest that naringin is protective against SANFH through Notch signaling and upregulated PPARγ2 expression. The modulation of these signaling pathways in bone by naringin, as detailed previously, are indicative of naringin’s manifold local and systemic inflammatory effects. Additional in vivo research of the effects of naringin in models of GIO, fracture healing, and avascular necrosis are needed to more effectively prevent and combat glucocorticoid-induced conditions of bone.
Downregulation of the inflammasome by naringin in degenerative joint disease
Naringin has demonstrated potential in the treatment of osteoarticular degenerative conditions such as osteoarthritis, ankylosis spondylitis, and rheumatoid arthritis [Figure 2(c,d)]. In a mouse model of ankylosis spondylitis, intraperitoneal flavonoid therapy reduced the expression of TNF-α, IL-1β, IL-6, signal transducer and activator of transcription (STAT)-3, and Janus kinase (JAK)2 in a dose-dependent fashion.78 Naringin concomitantly increased the generation of ROS-neutralizing enzymes such as SOD, CAT, and glutathione peroxidase in vivo.78 Moreover, naringin has been shown to reduce the production of prostaglandin E2 (PGE2) and NO in a dose-dependent manner in aqueous humor and RAW 264.6 cells in a lipopolysaccharide-induced model of osteoarthritis.79,80 Through these mechanisms, oral naringin administration lessens cartilage destruction, intercondylar knee joint damage, pannus formation, and synovial infiltration by leukocytes in surgical and monosodium iodoacetate-induced models of osteoarthritis.80,81 Naringin therapy also improved weight bearing in osteoarthritic rats by inhibiting the expression of MMP, ADAMTS5, and proinflammatory cytokines, while increasing regulatory T-cell activity and IL-4 expression, thereby preserving cartilage proteoglycan content.5,82,83 The benefits of naringin in degenerative joint disorders may manifest not only through moderation of the inflammasome, but also the effects of naringin upon estrogen signaling. These effects on estrogen receptors have been described extensively with respect to osteoporosis both in vitro and in vivo but require further elucidation in the setting of osteoarthritis.
The antibacterial and anti-inflammatory potential of naringin in osteomyelitis treatment
Osteomyelitis is an infection of the bone associated with high morbidity and mortality.84,85 The mainstay of osteomyelitis treatment is the administration of an aggressive antibiotic regimen. Osteomyelitis is the product of coexistent infected, nonviable tissue and insufficient host immune responses, resulting in progressive inflammatory bone breakdown.86,87 The most common response of host cells to infection is necrosis. Established infections are characterized by the formation of fibrous tissue and chronic inflammation around granulation tissue and dead bone, reducing vascular flow to the infection site and the efficacy of the inflammatory response.86 The spread of infection from bone to surrounding soft tissue reduces vascular supply to bone, allowing for the formation of a sequestrum, or area of dead bone, constraining the medullary and periosteal blood supplies.86 Given the persistence, recurrence, and severe morbidity and mortality of osteomyelitis despite antibiotic treatment, new and improved therapeutic options are being sought. Though the flavonones promote bone health by reducing inflammatory injury and osteolysis and increasing new bone formation, their effects in osteomyelitis have yet to be thoroughly characterized.
The primary causative agent of osteomyelitis in the United States is Staphylococcus aureus, a hardy organism capable of surviving within phagocytes, neutrophils, and osteoblasts, small colony variant and biofilm formation, and antibiotic resistance.88 Infection of bone by S. aureus invokes host immune responses that contribute to progressive and chronic inflammatory bone destruction and the activation of Toll-like receptors and their inflammatory signaling pathways.87,89,90 S. aureus-mediated osteoblast apoptosis is associated with upregulation of TLR2 and the pro-apoptotic JNK pathway and may be a mechanism of inflammation-derived osteomyelitis-associated bone loss.91 Putnam et al. sought to investigate whether S. aureus osteomyelitis-induced bone destruction is mediated by proinflammatory cytokine production and signaling.92 Osteomyelitis is associated with higher levels of circulating proinflammatory cytokines, including TNF-α, IL-1β, and IL-6.93,94 Johansen et al. noted that COX-2 expression was upregulated in osteoblasts within 2 days of S. aureus inoculation, as well as significant osteoclastogenesis.95 S. aureus infection of human and murine osteoblasts increases phosphorylation of ERK1 and ERK2, which then promote the phosphorylation of substrates such as the transcription factors ATF-2, Elk-1, and c-Jun. The Erk pathway is a significant target in inflammation-mediated osteolysis.96 Furthermore, the production of proinflammatory cytokines, chemokines, prostaglandins, and MMPs by macrophages and fibroblasts in the setting of infection directly degrades bone matrix and activates osteoclasts and indirectly increases RANKL and NF-κB signaling, thus promoting osteolysis.97 Osteoclast differentiation and bone resorption are upregulated in the setting of S. aureus infection and osteomyelitis, with a concomitant decrease in osteoblast viability and differentiation.97
S. aureus is capable of inducing autophagy in infected cells by escaping from autophagosomes and increasing the expression of tumor necrosis factor-related apoptosis-inducing ligand, which activates caspase-8 to initiate apoptosis in bacterial-challenged osteoblasts.89 S. aureus protein A binding to TNF-1 on pre-osteoblasts initiates the proteolytic apoptotic cascade mediated by caspase-3 and caspase-6.98,99 S. aureus protein A binding to osteoblasts also increases the secretion of RANKL, thus promoting osteoclastogenesis.98 Furthermore, S. aureus reduces the expression of markers of osteoblast growth and replication such as ALP, collagen type I, OCN, and osteopontin, thereby inhibiting de novo bone matrix deposition.98,99 Pyroptosis is the gasdermin-mediated process of programmed cell death that transpires in response to bacterial insult and is characterized by cell swelling, plasma membrane rupture, and the release of proinflammatory contents, including IL-1β, IL-6 and IL-18.100,101 Mice with S. aureus osteomyelitis displayed higher levels of pyroptosis-associated proteins, and inhibition of these proteins reversed bone injury in vivo.102 Through these mechanisms, S. aureus infection both exacerbates bone death and inhibits new bone formation.
The flavonones possess varying levels of antibiotic potency against common Gram-positive and Gram-negative organisms [Figure 2(e,f)]. The addition of lipophilic groups to compounds is commonly associated with an increase in antimicrobial activity.103 The primary site of flavonone action is the cytoplasmic membrane. The cell wall of Gram-positive bacteria permits fatty acid circulation towards the nuclear membrane, imbuing free fatty acids with greater antimicrobial activity against Gram-positive over Gram-negative bacteria.103 Crude extracts containing flavonoids, triterpenes, and steroids have demonstrated significant activity against S. aureus, Streptococcus faecalis, and Escherichia coli.1 At a dose of 128 mg/l, the flavonoids rutin, naringin, and baicalin were found to inhibit 25% or less of Pseudomonas aeruginosa growth in vitro.104 Naringin has been observed to act upon the bacterial quorum sensing system of interactions between bacteria and hosts related to bacterial population cell density that mediates gene expression and virulence factor expression.105 Naringin, naringenin, kaempferol, quercetin, rutin, and neoeriocitrin, all isolated from Citrus sinesis, were found to inhibit quorum sensing signaling pathways in vitro.105 This may allude to the potential of these compounds to disrupt the formation of biofilms and antibiotic resistance.
Baicalin is one of the few flavonones whose effects upon osteomyelitis-induced bone resorption has been investigated. In the setting of S. aureus-induced osteomyelitis in a murine model, baicalin reduced TLR2, ALP, OCN, and collagen type 1 expression, serum levels of IL-1β, IL-6, and C-reactive protein, and activity in the MAPK pathway.106 This altered inflammatory response profile bears semblance to the already elucidated mechanisms of naringin signaling in reducing osteolysis in various models of bone loss. Moreover, the demonstrated ability of naringin to inhibit osteoclastogenesis and bone resident cell apoptosis and promote osteogenic differentiation in other models of osteolytic and inflammatory bone loss is suggestive of its potential to mitigate the osteolytic effects of entrenched bony infection. Further in vitro and in vivo studies investigating the anti-inflammatory effects of naringin therapy, and with a variety of drug delivery systems, in the setting of musculoskeletal infection are needed.
Given the rise in prevalence of antibiotic infection, particularly that of methicillin-resistant S. aureus, there is great need for the development of new treatment regimens and agents to prevent the occurrence, recurrence, and exacerbation of osteomyelitic infection. The demonstrated efficacy of flavonones in preventing osteoclastic bone resorption and promoting osteoblastic differentiation and activity in other osteolytic diseases may carry over to the progressive destruction of bone characteristic of osteomyelitis. The lipophilic nature of the flavonones may make them viable in the treatment of intracellular S. aureus infection. In addition, it may be possible to augment their potency through structural and biochemical modifications and alternate methods of compound preparation and administration. Furthermore, the natural occurrence, widespread availability, and low cost of isoflavonoids make them attractive in the treatment of osteomyelitis and in addressing the unequal burden of chronic osteomyelitis in low- and middle-income countries.107
Pharmacological profile of naringin and obstacles to translational therapy
Naringin has yet to obtain approval for clinical therapeutic use as a single and combinatorial agent. The extensive in vivo metabolism, low oral bioavailability, and poor water solubility, resulting in poor bodily absorption, of flavonoids significantly limit the therapeutic efficacy of naringin and related flavanones.5,108,109 Naringin is further degraded in acidic environments such as that of the stomach and is extensively acted upon by intestinal β-glucosidases.5,109 As a result of these factors, naringin is absorbed slowly and irregularly when administered orally, with significant individual variation due to the heterogeneity of the intestinal microbiome. In order to improve dietary flavonoid bioavailability, solubility, and dissolution, in vitro assessment of nanoparticle, microparticle, and water-soluble fiber naringin delivery systems are being increasingly explored.5,110 Naringin is also significantly degraded when administered intravenously; flavonoids undergo oxidation in circulation and within the liver, are acted upon by hepatic β-glucosidases, and are bound by serum albumin, facilitating excretion and reducing bioavailability.5,109,111 Nano-carriers for the transport of naringin to skeletal sites have yet to be established, but would confer improved solubility, bioavailability, and pharmacokinetic properties for sustained flavonoid release to bone.
Numerous biomaterial-based platforms have been investigated with the goal of optimizing the therapeutic effects of naringin by reducing its degradation and sustaining its spatiotemporal release within the body. Ji et al. embedded naringin within an electrospun nanoscaffold comprised of polycaprolactone (PCL) and poly(ethylene glycol)-b-polycaprolactone (PEG-b-PCL) nanofibers to study its effects upon bone regeneration.112 In the presence of these naringin nanoscaffolds, increases in MC3T3-E1 proliferation, osteogenic differentiation, and calcium mineralization by Alizarin Red S staining were observed under culture conditions that lacked additional osteogenic supplementation.112 This naringin nanoscaffold also suppressed osteoclastogenesis in a critical size defect of mouse calvarial bone, as demonstrated by a significant reduction in TRAP staining compared to blank PCL nanoscaffold controls. Together, these findings are suggestive not only of the successful delivery and persistence of naringin in bone via a scaffold construct, but also the potential utility of naringin in bone tissue engineering.112 Chen et al. developed a porous biodegradable construct from genepin-crosslinked gelatin and β-Ca3(PO4)2 ceramic microparticles mixed with 10 mg/ml naringin with the goal of facilitating in vivo bone repair in a rabbit calvarial defect model.113 Significant new bone deposition was observed within 8 weeks of implantation of these naringin-loaded gelatin and β-Ca3(PO4)2 ceramic microparticle composites. Similar results have been seen upon introduction of porous poly(l-lactide) scaffolds comprised of spray-dried naringin-loaded chitosan microspheres and parthenolide,114 an 0.85% naringin-loaded pH-responsive hydrogel composed of carboxymethyl-hexanoyl chitosan, β-glycerophosphate and glycerol,115 and a mineralized collagen coating with embedded naringin-containing metal organic frameworks116 with respect to new bone formation and bone resident cell differentiation from stem cell precursors. Together, these studies are indicative of the potential of naringin-loaded biomaterial constructs to improve the pharmacokinetic properties of naringin within the body and facilitate its actions upon bone.5 Further research is needed to characterize the in vivo performance of these novel naringin-loaded delivery systems, their effects upon the bioactivity of naringin in various bodily tissues, and their safety and utility in the treatment of diverse musculoskeletal conditions.
In a single ascending-dose randomized controlled trial of 18 adults by Rebello et al., no adverse effects or changes in blood safety biomarkers were observed after oral consumption of 150–900 mg naringenin, the flavonone form of naringin, relative to placebo.117 Measured serum biomarkers included alanine aminotransferase, creatinine phosphokinase, and potassium, as well as total white blood cell and eosinophil counts as markers of immune system activation. At these doses, naringin metabolites freely circulate and are effectively cleared from the body within 24 h of ingestion. The authors concluded that oral doses of 150–900 mg of naringinen are safe and tolerable in healthy adults, with serum concentrations proportional to the oral doses received.117 One randomized controlled trial of excessive grapefruit consumption reported constipation and diarrhea as adverse effects, though it is not clear whether this effect can be solely or primarily attributed to naringin.118 Several other toxicity studies failed to demonstrate the presence of toxic side effects following naringin administration.119,120
Clinical evaluation of common “nutraceuticals” and the supplemental potential of naringin
Natural chondroprotective agents such as glucosamine sulfate, chondroitin sulfate, collagen hydrolysate, hyaluronic acid, antioxidants, and fatty acids are commonly used in the treatment or prevention of degenerative musculoskeletal conditions such as osteoarthritis.121–124 Within North America, these natural pharmaceutical compounds, “nutraceuticals”, are limited to the designation of “safe dietary supplements.” Supplementation with these natural compounds has been shown to be more clinically and cost-effective than continued nonsteroidal anti-inflammatory drug (NSAID) prescription and use, while increasing patient quality of life.125–127
Glucosamine, also known as 2-amino-2-deoxy-D-glucose (C6H13NO5), is an amino acid synthesized from glucose in all bodily tissues that is most prevalent in connective tissue and cartilage.121 It may also be extracted from the chitinous exoskeletons of crustaceans and cell membranes of mushrooms. Glucosamine is a precursor of glycoprotein and glycosaminoglycan synthesis and is vital for the production of hyaluronic acid, chondroitin sulfate, and keratan sulfate, all components of the extracellular matrices of articular cartilage and synovial fluid.121 The utility of glucosamine supplementation in the forms of glucosamine sulfate and glucosamine hydrochloride has been explored in multiple randomized clinical trials of osteoarthritis patients.
Exogenous glucosamine undergoes significant first-pass metabolism, such that oral administration is associated with 25% bioavailability.128,129 The most commonly administered and evaluated dosage of glucosamine sulfate is 1500 mg/day.121 At this dose, glucosamine sulfate appears to slows the progression of hip osteoarthritis.130 A systematic review of symptomatic slow-acting drugs in osteoarthritis (SYSADOA) determined that there exists moderate- to high-quality evidence that glucosamine sulfate, among other natural compounds, reduces pain, disease progression, and the risk of future total joint replacement and improves physical function in osteoarthritis patients with few toxic side effects.131,132 Meta-analyses of all glucosamine studies conducted prior to 2014 determined that glucosamine significantly reduced joint space narrowing, with a modest effect size.123,133,134 However, the Glucosamine/chondroitin Arthritis Intervention Trial (GAIT) trial determined that glucosamine and chondroitin sulfate administered both alone and in combination failed to significantly reduce the pain of knee osteoarthritis relative to placebo.135,136 Several studies have suggested that glucosamine sulfate is superior to placebo and NSAIDs such as ibuprofen,137 though inconsistent trends between studies and study design effects such as industry funding bias have been reported.121 Gregory and Fellner critically appraised nutraceuticals such as glucosamine as disease-modifying treatments in osteoarthritis and determined that the strongest evidence of glucosamine’s efficacy exists with respect to delaying disease progression.123 However, the authors classified these data as “inconsistent and unclear” with a likely modest effect size, if any.123 Due to methodological considerations, the American College of Rheumatology recommended against the use of glucosamine in its 2000 guidelines and has continued to advise against its use in osteoarthritis.138,139 These considerations included a paucity of standardized case definitions and assessments and insufficient information on study designs. Similarly, the UK’s National Institute for Health and Clinical Excellence has also advised against the prescription of glucosamine in osteoarthritis due to a lack of evidence of its clinical utility.123 According to PJ Gregory, these recommendations were largely based on the results of the GAIT study.123 Regardless, glucosamine supplements are widely used and anecdotally associated with actual or perceived benefits.123,140
Chondroitin sulfate is the most common glycoprotein and glycosaminoglycan in cartilaginous aggrecan molecules.121 Its negative charge facilitates water retention within cartilage for pressure resistance and joint cushioning. Chondroitin sulfate increases synovial cell production of hyaluronan, which maintains the viscosity of synovial fluid.141 It is also believed to stimulate chondrocyte metabolism, thereby increasing collagen and proteoglycan synthesis, and inhibit the activity of leukocyte esterase and hyaluronidase, which are often upregulated in rheumatic disease.121,142 Due to the large size of this macromolecule, it is poorly absorbed and has an estimated bioavailability of 10–13%.143,144 This SYSADOA lessens pain, inflammation, and the rate of disease progression and serves as a structure-modifying drug in osteoarthritis.121 In a clinical trial of 162 patients given 800 mg of chondroitin sulfate or placebo for 6 months, chondroitin sulfate demonstrated superiority to placebo with respect to global hand pain, hand function, and morning stiffness reduction.145 Other clinical trials have demonstrated the beneficial effects of chondroitin sulfate in reducing disease progression and severity in patients with knee osteoarthritis over placebo.146–152 No significant local or systemic side effects were observed following intramuscular, intra-articular, or oral administration.153,154 The European League Against Rheumatism bestowed chondroitin sulfate with the highest level of evidence (1A) and recommendation strength (A) in 2003.155 However, as of 2014, American College of Rheumatology guidelines continued to recommend against the use of chondroitin sulfate.139
A randomized, double-blind, placebo-controlled study of a supplement containing glucosamine hydrochloride, chondroitin sulfate, and quercetin glycosides, natural flavonoid compounds similar to naringin, in patients with symptomatic knee osteoarthritis demonstrated improvement in two of four disease-related symptom and function subscales in patients treated with supplements relative to placebo.156,157 Patients who received the combined supplement also experienced improvement in the ratio of type II collagen synthesis to degradation relative to placebo-treated controls.156,157 Combined glucosamine and chondroitin sulfate supplementation was also observed to be effective in hindering the progression of spinal disc degeneration in a single patient treated over a 2-year period, though more extensive studies are needed to corroborate this observation.158
Four open-label and three double-blind trials have been conducted on the effects of collagen hydrolysate in osteoarthritis.159 A 24-week multinational double-blind randomized controlled trial of patients with knee osteoarthritis indicated that treatment with 10 g/day of collagen hydrolysate elicited no significant changes in the WOMAC index.160,161 Though collagen hydrolysate appears to demonstrate equivocal efficacy in reducing osteoarthritis-associated pain, its use is accepted in the treatment of osteoarthritis.160 Collagen hydrolysates are considered highly safe, with minimal adverse effects such as gastrointestinal fullness and unpleasant taste.162
The aforementioned common nutraceutical compounds have been associated with modest improvements in various parameters of degenerative joint disease severity and progression, though inconsistencies exist between clinical trial results and study designs. Moreover, these natural substances are plagued by bioavailability limitations, necessitating the oral consumption of higher doses. Regardless, these nutraceuticals are considered safe and tolerable due to their improved side-effect profile relative to NSAIDs. Though the described nutraceuticals are commonly used in the prevention and/or treatment of osteoarthritis due to their chondroprotective potential, recommendations regarding their use are conflicting. As previously described, the ability of naringin to improve numerous aspects of musculoskeletal health and protect against degenerative cartilaginous and bone pathology in preclinical studies merits clinical investigation. The favorable chondroprotective effects reported by Kanzaki et al. and Tiku et al. upon regular oral consumption of a combined supplement containing glucosamine, chondroitin sulfate, and a related flavonoid compound156,157 hints toward the potential of naringin as a combined or adjunctive supplement, which warrants further evaluation in formal trials. Naringin’s mechanisms of action are also distinct from those of the aforementioned nutraceuticals and may contribute to more consistent clinical improvements based on preclinical findings. Despite its inherent pharmacokinetic limitations similar to those of the other natural chondroprotective compounds, the enhanced delivery, release, and action of naringin on bone and connective tissue via novel bioformulations may address existent shortcomings in the clinical use and evaluation of nutritional supplements in musculoskeletal pathology. All of these potential therapeutic avenues of naringin have yet to be thoroughly clinically interrogated.
Conclusion
The multifarious effects of naringin in bone are indicative of its potential in the treatment of many common orthopedic conditions and fracture treatment and prevention. Naringin effectively reduces osteoclastogenesis, inflammation, and adipogenesis and induces osteoblastic differentiation from progenitor cells for the maintenance and preservation of both cartilage and bone. Though naringin demonstrates promise as a therapeutic agent for several forms of osteoporosis, DM, degenerative joint disease, and osteomyelitis, many limitations must be addressed before these early findings can be translated into viable therapies. Additional research is needed into the ways in which the pharmacokinetic properties of naringin can be improved, be it through the use of constructs such as nano-carriers and hydrogels, to optimize naringin delivery, action, and persistence at sites of action. Regardless, naringin represents a ubiquitous, cost-efficient, biologically active, natural compound whose potential can be harnessed to develop novel treatments for common musculoskeletal conditions.
Footnotes
Author contributions: Conceptualization: KEY, KDA, and FYL. Data Curation: KEY, KDA, MTM, AMM, and IL. Formal Analysis: KEY and KDA. Investigation: KEY, KDA, MTM, AMM, IL, SVC, H-KK, and JB. Methodology: KEY and KDA. Project Administration: KEY, KDA, JB, and FYL. Resources: FYL. Software: FYL. Supervision: H-KK, JB, and FYL. Validation: KEY, KDA, MTM, AMM, IL, SVC, H-KK, JB, and FYL. Visualization: KEY, SVC, H-KK, and JB. Writing, Original Draft: KEY, KDA, MTM, and AMM. Writing, Review & Editing: KEY, KDA, MTM, AMM, IL, SVC, H-KK, JB, and FYL. All authors approved the final version of this manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kristin E. Yu  https://orcid.org/0000-0002-8500-5200
https://orcid.org/0000-0002-8500-5200
Francis Y. Lee  https://orcid.org/0000-0003-2275-2441
https://orcid.org/0000-0003-2275-2441
Contributor Information
Kristin E. Yu, Department of Orthopaedics and Rehabilitation, Yale University School of Medicine, 330 Cedar St, TMP 523 PO Box 208071, New Haven, CT 06520-8071, USA.
Kareme D. Alder, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA
Montana T. Morris, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA
Alana M. Munger, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA
Inkyu Lee, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA; Department of Life Science, Chung-Ang University, Seoul, Republic of Korea.
Sean V. Cahill, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA
Hyuk-Kwon Kwon, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA.
JungHo Back, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA.
Francis Y. Lee, Department of Orthopædics & Rehabilitation, Yale University, School of Medicine, New Haven, CT, USA
References
- 1. Taleb-Contini SH, Salvador MJ, Balanco JM, et al. Antiprotozoal effect of crude extracts and flavonoids isolated from Chromolaena hirsuta (asteraceae). Phytother Res 2004; 18: 250–254. [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez R, Ballester I, Lopez-Posadas R, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr 2011; 51: 331–362. [DOI] [PubMed] [Google Scholar]
- 3. Bharti S, Rani N, Krishnamurthy B, et al. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med 2014; 80: 437–451. [DOI] [PubMed] [Google Scholar]
- 4. Wei M, Yang Z, Li P, et al. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med 2007; 35: 663–667. [DOI] [PubMed] [Google Scholar]
- 5. Lavrador P, Gaspar VM, Mano JF. Bioinspired bone therapies using naringin: applications and advances. Drug Discov Today 2018; 23: 1293–1304. [DOI] [PubMed] [Google Scholar]
- 6. Nifuji A, Noda M. Coordinated expression of noggin and bone morphogenetic proteins (BMPs) during early skeletogenesis and induction of noggin expression by BMP-7. J Bone Miner Res 1999; 14: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 7. Francis PH, Richardson MK, Brickell PM, et al. Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development 1994; 120: 209–218. [DOI] [PubMed] [Google Scholar]
- 8. Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, et al. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop 2011; 35: 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang E, Israel DI, Kelly S, et al. Bone morphogenetic protein-2 causes commitment and differentiation in C3Hl0T1/2 and 3T3 cells. Growth Factors 1993; 9: 57–71. [DOI] [PubMed] [Google Scholar]
- 10. Sugiyama M, Kodama T, Konishi K, et al. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun 2000; 271: 688–692. [DOI] [PubMed] [Google Scholar]
- 11. Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 286: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 12. Wang PS, Solomon DH, Mogun H, et al. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA 2000; 283: 3211–3216. [DOI] [PubMed] [Google Scholar]
- 13. Skoglund B, Forslund C, Aspenberg P. Simvastatin improves fracture healing in mice. J Bone Miner Res 2002; 17: 2004–2008. [DOI] [PubMed] [Google Scholar]
- 14. Wong RW, Rabie AB. Effect of naringin on bone cells. J Orthop Res 2006; 24: 2045–2050. [DOI] [PubMed] [Google Scholar]
- 15. Yin F, Xiao L, Zhang Y. Research progress on Drynaria fortunei naringin on inflammation and bone activity. Zhongguo Gu Shang 2015; 28: 182–186. [PubMed] [Google Scholar]
- 16. Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene 2004; 341: 19–39. [DOI] [PubMed] [Google Scholar]
- 17. Rawadi G, Roman-Roman S. Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets 2005; 9: 1063–1077. [DOI] [PubMed] [Google Scholar]
- 18. Zhang R, Oyajobi BO, Harris SE, et al. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013; 52: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Ma W, Wang F, et al. Stimulation of Wnt/beta-Catenin signaling to improve bone development by naringin via interacting with AMPK and Akt. Cell Physiol Biochem 2015; 36: 1563–1576. [DOI] [PubMed] [Google Scholar]
- 20. Liu M, Li Y, Yang S-T. Effects of naringin on the proliferation and osteogenic differentiation of human amniotic fluid-derived stem cells. J Tissue Eng Regen Med 2017; 11: 276–284. [DOI] [PubMed] [Google Scholar]
- 21. Wu JB, Fong YC, Tsai HY, et al. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol 2008; 588: 333–341. [DOI] [PubMed] [Google Scholar]
- 22. Zhang P, Dai K-R, Yan S-G, et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol 2009; 607: 1–5. [DOI] [PubMed] [Google Scholar]
- 23. Wang H, Li C, Li J, et al. Naringin enhances osteogenic differentiation through the activation of ERK signaling in human bone marrow mesenchymal stem cells. Iran J Basic Med Sci 2017; 20: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei K, Xie Y, Chen T, et al. ERK1/2 signaling mediated naringin-induced osteogenic differentiation of immortalized human periodontal ligament stem cells. Biochem Biophys Res Commun 2017; 489: 319–325. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Zeng Z, Cai G. Comparison of neoeriocitrin and naringin on proliferation and osteogenic differentiation in MC3T3-E1. Phytomedicine 2011; 18: 985–989. [DOI] [PubMed] [Google Scholar]
- 26. Ding P, Tang Q, Chen L. [Effects of naringin on proliferation, differentiation and matrix mineralization of MC3T3-E1 cells]. Zhongguo Zhong Yao Za Zhi 2009; 34: 1712–1716. [PubMed] [Google Scholar]
- 27. Jing Z, Wang C, Yang Q, et al. Luteolin attenuates glucocorticoid-induced osteoporosis by regulating ERK/Lrp-5/GSK-3β signaling pathway in vivo and in vitro. J Cell Physiol 2019; 234: 4472–4490. [DOI] [PubMed] [Google Scholar]
- 28. Lai M, Jin Z, Yan M, et al. The controlled naringin release from TiO2 nanotubes to regulate osteoblast differentiation. J Biomater Appl 2018; 33: 673–680. [DOI] [PubMed] [Google Scholar]
- 29. Lavrador P, Gaspar VM, Mano JF. Bioinstructive naringin-loaded micelles for guiding stem cell osteodifferentiation. Adv Healthc Mater 2019; 8: e1901188. [DOI] [PubMed] [Google Scholar]
- 30. Xu T, Wang L, Tao Y, et al. The function of naringin in inducing secretion of osteoprotegerin and inhibiting formation of osteoclasts. Evid Based Complement Alternat Med 2016; 2016: 8981650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan J, Li J, Fan Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARγ. Mol Med Rep 2015; 12: 4759–4765. [DOI] [PubMed] [Google Scholar]
- 32. Huang D, Li Z, Chen B, et al. Naringin protects against steroid-induced avascular necrosis of the femoral head through upregulation of PPARγ and activation of the Notch signaling pathway. Mol Med Rep 2018; 17: 3328–3335. [DOI] [PubMed] [Google Scholar]
- 33. Phan TC, Xu J, Zheng MH. Interaction between osteoblast and osteoclast: impact in bone disease. Histol Histopathol 2004; 19: 1325–1344. [DOI] [PubMed] [Google Scholar]
- 34. D’Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008; 43: 92–100. [DOI] [PubMed] [Google Scholar]
- 35. Jiao H, Xiao E, Graves DT. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep 2015; 13: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(Suppl. 1): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwan Tat S, Padrines M, Théoleyre S, et al. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 2004; 15: 49–60. [DOI] [PubMed] [Google Scholar]
- 38. Li F, Sun X, Ma J, et al. Effect of naringin on osteoclast differentiation. Zhongguo Zhong Yao Za Zhi 2015; 40: 308–312. [PubMed] [Google Scholar]
- 39. Weise M, De-Levi S, Barnes KM, et al. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A 2001; 98: 6871–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 2007; 130: 811–823. [DOI] [PubMed] [Google Scholar]
- 41. Qu Q, Härkönen P, Mönkkönen J, et al. Conditioned medium of estrogen-treated osteoblasts inhibits osteoclast maturation and function in vitro. Bone 1999; 25: 211–215. [DOI] [PubMed] [Google Scholar]
- 42. Hofbauer LC, Khosla S, Dunstan CR, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999; 140: 4367–4370. [DOI] [PubMed] [Google Scholar]
- 43. Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 1996; 11: 337–349. [DOI] [PubMed] [Google Scholar]
- 44. Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 1985; 102: 319–324. [DOI] [PubMed] [Google Scholar]
- 45. Ettinger B, Genant HK, Cann CE. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann Intern Med 1987; 106: 40–45. [DOI] [PubMed] [Google Scholar]
- 46. Quigley ME, Martin PL, Burnier AM, et al. Estrogen therapy arrests bone loss in elderly women. Am J Obstet Gynecol 1987; 156: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 47. Judd HL, Cleary RE, Creasman WT, et al. Estrogen replacement therapy. Obstet Gynecol 1981; 58: 267–275. [PubMed] [Google Scholar]
- 48. Lobo RA. Benefits and risks of estrogen replacement therapy. Am J Obstet Gynecol 1995; 173: 982–989. [DOI] [PubMed] [Google Scholar]
- 49. Pang WY, Wang XL, Mok SK, et al. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br J Pharmacol 2010; 159: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song N, Zhao Z, Ma X, et al. Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signaling pathway in osteoporotic rats. Chem Biol Interact 2017; 261: 11–17. [DOI] [PubMed] [Google Scholar]
- 51. Li F, Sun X, Ma J, et al. Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem Biophys Res Commun 2014; 452: 629–635. [DOI] [PubMed] [Google Scholar]
- 52. Mandadi K, Ramirez M, Jayaprakasha GK, et al. Citrus bioactive compounds improve bone quality and plasma antioxidant activity in orchidectomized rats. Phytomedicine 2009; 16: 513–520. [DOI] [PubMed] [Google Scholar]
- 53. Ma X, Lv J, Sun X, et al. Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep 2016; 6: 24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li N, Jiang Y, Wooley PH, et al. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J Orthop Sci 2013; 18: 478–485. [DOI] [PubMed] [Google Scholar]
- 55. Sun X, Li F, Ma X, et al. The effects of combined treatment with naringin and treadmill exercise on osteoporosis in ovariectomized rats. Sci Rep 2015; 5: 13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shangguan WJ, Zhang YH, Li ZC, et al. Naringin inhibits vascular endothelial cell apoptosis via endoplasmic reticulum stress and mitochondrialmediated pathways and promotes intraosseous angiogenesis in ovariectomized rats. Int J Mol Med 2017; 40: 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Habauzit V, Sacco SM, Gil-Izquierdo A, et al. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011; 49: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 58. Ma R, Wang L, Zhao B, et al. Diabetes perturbs bone microarchitecture and bone strength through regulation of Sema3A/IGF-1/β-Catenin in rats. Cell Physiol Biochem 2017; 41: 55–66. [DOI] [PubMed] [Google Scholar]
- 59. Alder KD, White AH, Chung Y-H, et al. Systemic parathyroid hormone enhances fracture healing in multiple murine models of type 2 diabetes mellitus. JBMR Plus 2020; 4: e10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rivoira M, Rodriguez V, Picotto G, et al. Naringin prevents bone loss in a rat model of type 1 diabetes mellitus. Arch Biochem Biophys 2018; 637: 56–63. [DOI] [PubMed] [Google Scholar]
- 61. Saito M, Kida Y, Kato S, et al. Diabetes, collagen, and bone quality. Curr Osteoporos Rep 2014; 12: 181–188. [DOI] [PubMed] [Google Scholar]
- 62. Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact 2014; 219: 101–112. [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez V, Plavnik L, Tolosa de Talamoni N. Naringin attenuates liver damage in streptozotocin-induced diabetic rats. Biomed Pharmacother 2018; 105: 95–102. [DOI] [PubMed] [Google Scholar]
- 64. Singh AK, Raj V, Keshari AK, et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARγ/GLUT4 dual agonistic action with strong metabolic regulation. Chem Biol Interact 2018; 280: 33–44. [DOI] [PubMed] [Google Scholar]
- 65. Mahmoud AM, Ashour MB, Abdel-Moneim A, et al. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications 2012; 26: 483–490. [DOI] [PubMed] [Google Scholar]
- 66. Sharma AK, Bharti S, Ojha S, et al. Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr 2011; 106: 1713–1723. [DOI] [PubMed] [Google Scholar]
- 67. Zhou X, Zhang P, Zhang C, et al. Promotion of bone formation by naringin in a titanium particle-induced diabetic murine calvarial osteolysis model. J Orthop Res 2010; 28: 451–456. [DOI] [PubMed] [Google Scholar]
- 68. Den Hartogh DJ, Tsiani E. Antidiabetic properties of naringenin: a citrus fruit polyphenol. Biomolecules 2019; 9: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Assini JM, Mulvihill EE, Burke AC, et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015; 156: 2087–2102. [DOI] [PubMed] [Google Scholar]
- 70. Al-Rejaie SS, Aleisa AM, Abuohashish HM, et al. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res 2015; 37: 924–933. [DOI] [PubMed] [Google Scholar]
- 71. Ren B, Qin W, Wu F, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol 2016; 773: 13–23. [DOI] [PubMed] [Google Scholar]
- 72. Frenkel B, White W, Tuckermann J. Glucocorticoid-induced osteoporosis. Adv Exp Med Biol 2015; 872: 179–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 2006; 21: 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen Z, Xue J, Shen T, et al. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin Exp Pharmacol Physiol 2016; 43: 268–276. [DOI] [PubMed] [Google Scholar]
- 75. Zhang X, Chen K, Wei B, et al. Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem Biol Interact 2016; 256: 188–197. [DOI] [PubMed] [Google Scholar]
- 76. Gu Y-X, Du J, Si M-S, et al. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J Biomed Mater Res A 2013; 101: 748–754. [DOI] [PubMed] [Google Scholar]
- 77. Pan JM, Wu LG, Cai JW, et al. Dexamethasone suppresses osteogenesis of osteoblast via the PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal Transduct Res 2019; 39: 80–86. [DOI] [PubMed] [Google Scholar]
- 78. Liu K, Wu L, Shi X, et al. Protective effect of naringin against ankylosing spondylitis via ossification, inflammation and oxidative stress in mice. Exp Ther Med 2016; 12: 1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shiratori K, Ohgami K, Ilieva I, et al. The effects of naringin and naringenin on endotoxin-induced uveitis in rats. J Ocul Pharmacol Ther 2005; 21: 298–304. [DOI] [PubMed] [Google Scholar]
- 80. Xu Q, Zhang ZF, Sun WX. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med Sci Monit 2017; 23: 3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao Y, Li Z, Wang W, et al. Naringin protects against cartilage destruction in osteoarthritis through repression of NF-κB signaling pathway. Inflammation 2016; 39: 385–392. [DOI] [PubMed] [Google Scholar]
- 82. Ahmad SF, Zoheir KM, Abdel-Hamied HE, et al. Amelioration of autoimmune arthritis by naringin through modulation of T regulatory cells and Th1/Th2 cytokines. Cell Immunol 2014; 287: 112–120. [DOI] [PubMed] [Google Scholar]
- 83. Kawaguchi K, Maruyama H, Hasunuma R, et al. Suppression of inflammatory responses after onset of collagen-induced arthritis in mice by oral administration of the Citrus flavanone naringin. Immunopharmacol Immunotoxicol 2011; 33: 723–729. [DOI] [PubMed] [Google Scholar]
- 84. Jones HW, Beckles VL, Akinola B, et al. Chronic haematogenous osteomyelitis in children: an unsolved problem. J Bone Joint Surg Br 2011; 93: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 85. Li GQ, Guo FF, Ou Y, et al. Epidemiology and outcomes of surgical site infections following orthopedic surgery. Am J Infect Control 2013; 41: 1268–1271. [DOI] [PubMed] [Google Scholar]
- 86. Calhoun JH, Manring MM, Shirtliff M. Osteomyelitis of the long bones. Semin Plast Surg 2009; 23: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fischer CR, Mikami M, Minematsu H, et al. Calreticulin inhibits inflammation-induced osteoclastogenesis and bone resorption. J Orthop Res 2017; 35: 2658–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ciampolini J, Harding KG. Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgrad Med J 2000; 76: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res 2004; 30: 291–308. [DOI] [PubMed] [Google Scholar]
- 90. Marriott I, Gray DL, Rati DM, et al. Osteoblasts produce monocyte chemoattractant protein-1 in a murine model of staphylococcus aureus osteomyelitis and infected human bone tissue. Bone 2005; 37: 504–512. [DOI] [PubMed] [Google Scholar]
- 91. Chen Q, Hou T, Luo F, et al. Involvement of toll-like receptor 2 and pro-apoptotic signaling pathways in bone remodeling in osteomyelitis. Cell Physiol Biochem 2014; 34: 1890–1900. [DOI] [PubMed] [Google Scholar]
- 92. Putnam NE, Fulbright LE, Curry JM, et al. MyD88 and IL-1R signaling drive antibacterial immunity and osteoclast-driven bone loss during staphylococcus aureus osteomyelitis. PLoS Pathog 2019; 15: e1007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 2006; 24: 33–63. [DOI] [PubMed] [Google Scholar]
- 94. Yoshii T, Magara S, Miyai D, et al. Local levels of interleukin-1β, -4, -6 and tumor necrosis factor α in an experimental model of murine osteomyelitis due to staphylococcus aureus. Cytokine 2002; 19: 59–65. [DOI] [PubMed] [Google Scholar]
- 95. Johansen LK, Iburg TM, Nielsen OL, et al. Local osteogenic expression of cyclooxygenase-2 and systemic response in porcine models of osteomyelitis. Prostaglandins Other Lipid Mediat 2012; 97: 103–108. [DOI] [PubMed] [Google Scholar]
- 96. Seo SW, Lee D, Minematsu H, et al. Targeting extracellular signal-regulated kinase (ERK) signaling has therapeutic implications for inflammatory osteolysis. Bone 2010; 46: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tucker KA, Reilly SS, Leslie CS, et al. Intracellular staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol Lett 2000; 186: 151–156. [DOI] [PubMed] [Google Scholar]
- 98. Claro T, Widaa A, O’Seaghdha M, et al. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS One 2011; 6: e18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Widaa A, Claro T, Foster TJ, et al. Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS One 2012; 7: e40586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016; 535: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 2017; 42: 245–254. [DOI] [PubMed] [Google Scholar]
- 102. Zhu X, Zhang K, Lu K, et al. Inhibition of pyroptosis attenuates staphylococcus aureus-induced bone injury in traumatic osteomyelitis. Ann Transl Med 2019; 7: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Celiz G, Daz M, Audisio MC. Antibacterial activity of naringin derivatives against pathogenic strains. J Appl Microbiol 2011; 111: 731–738. [DOI] [PubMed] [Google Scholar]
- 104. Ng TB, Ling JM, Wang ZT, et al. Examination of coumarins, flavonoids and polysaccharopeptide for antibacterial activity. Gen Pharmacol 1996; 27: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 105. Bouyahya A, Dakka N, Et-Touys A, et al. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac J Trop Med 2017; 10: 729–743. [DOI] [PubMed] [Google Scholar]
- 106. Wu T, Weng Z, Xu J, et al. Baicalin alleviates osteomyelitis by regulating TLR2 in the murine model. Pathog Dis 2018; 76: ftx123. [DOI] [PubMed] [Google Scholar]
- 107. Panteli M, Giannoudis PV. Chronic osteomyelitis: what the surgeon needs to know. EFORT Open Rev 2017; 1: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr 2017; 105: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81(Suppl. 1): 230S–242S. [DOI] [PubMed] [Google Scholar]
- 110. Kanaze FI, Kokkalou E, Niopas I, et al. Dissolution rate and stability study of flavanone aglycones, naringenin and hesperetin, by drug delivery systems based on polyvinylpyrrolidone (PVP) nanodispersions. Drug Dev Ind Pharm 2010; 36: 292–301. [DOI] [PubMed] [Google Scholar]
- 111. Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med 2004; 36: 829–837. [DOI] [PubMed] [Google Scholar]
- 112. Ji Y, Wang L, Watts DC, et al. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent Mater 2014; 30: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 113. Chen KY, Lin KC, Chen YS, et al. A novel porous gelatin composite containing naringin for bone repair. Evid Based Complement Alternat Med 2013; 2013: 283941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Guo Z, Bo D, He P, et al. Sequential controlled-released dual-drug loaded scaffold for guided bone regeneration in a rat fenestration defect model. J Mater Chem B 2017; 5: 7701–7710. [DOI] [PubMed] [Google Scholar]
- 115. Chang PC, Chao YC, Hsiao MH, et al. Inhibition of periodontitis induction using a stimuli-responsive hydrogel carrying naringin. J Periodontol 2017; 88: 190–196. [DOI] [PubMed] [Google Scholar]
- 116. Yu M, You D, Zhuang J, et al. Controlled release of naringin in metal-organic framework-loaded mineralized collagen coating to simultaneously enhance osseointegration and antibacterial activity. ACS Appl Mater Interfaces 2017; 9: 19698–19705. [DOI] [PubMed] [Google Scholar]
- 117. Rebello CJ, Beyl RA, Lertora JJL, et al. Safety and pharmacokinetics of naringenin: a randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes Metab 2020; 22: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fujioka K, Greenway F, Sheard J, et al. The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food 2006; 9: 49–54. [DOI] [PubMed] [Google Scholar]
- 119. Li P, Wang S, Guan X, et al. Six months chronic toxicological evaluation of naringin in Sprague–Dawley rats. Food Chem Toxicol 2014; 66: 65–75. [DOI] [PubMed] [Google Scholar]
- 120. Liu M, Zou W, Yang C, et al. Metabolism and excretion studies of oral administered naringin, a putative antitussive, in rats and dogs. Biopharm Drug Dispos 2012; 33: 123–134. [DOI] [PubMed] [Google Scholar]
- 121. Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids. Int J Rheumatol 2011; 2011: 969012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. De Silva V, El-Metwally A, Ernst E, et al. ; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford) 2011; 50: 911–920. [DOI] [PubMed] [Google Scholar]
- 123. Gregory PJ, Fellner C. Dietary supplements as disease-modifying treatments in osteoarthritis: a critical appraisal. P T 2014; 39: 436–452. [PMC free article] [PubMed] [Google Scholar]
- 124. Bottegoni C, Muzzarelli RA, Giovannini F, et al. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr Polym 2014; 109: 126–138. [DOI] [PubMed] [Google Scholar]
- 125. Bruyere O, Scholtissen S, Neuprez A, et al. Impact of chondroitin sulphate on health utility in patients with knee osteoarthritis: towards economic analysis. J Med Econ 2009; 12: 356–360. [DOI] [PubMed] [Google Scholar]
- 126. Conrozier T. [Chondroitin sulfates (CS 4&6): practical applications and economic impact]. Presse Med 1998; 27: 1866–1868. [PubMed] [Google Scholar]
- 127. Rubio-Terrés C. Grupo del estudio VECTRA. [An economic evaluation of chondroitin sulfate and non-steroidal anti-inflammatory drugs for the treatment of osteoarthritis. Data from the VECTRA study]. Reumatol Clin 2010; 6: 187–195. [DOI] [PubMed] [Google Scholar]
- 128. Barclay TS, Tsourounis C, McCart GM. Glucosamine. Ann Pharmacother 1998; 32: 574–579. [DOI] [PubMed] [Google Scholar]
- 129. Setnikar I, Rovati LC. Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review. Arzneimittelforschung 2001; 51: 699–725. [DOI] [PubMed] [Google Scholar]
- 130. Rozendaal RM, Koes BW, van Osch GJ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med 2008; 148: 268–277. [DOI] [PubMed] [Google Scholar]
- 131. Towheed TE, Anastassiades TP, Shea B, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001; CD002946. [DOI] [PubMed] [Google Scholar]
- 132. Bruyere O, Burlet N, Delmas PD, et al. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet Disord 2008; 9: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee YH, Woo JH, Choi SJ, et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int 2010; 30: 357–363. [DOI] [PubMed] [Google Scholar]
- 134. Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341: c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Reginster JY, Bruyere O, Neuprez A. Current role of glucosamine in the treatment of osteoarthritis. Rheumatology (Oxford) 2007; 46: 731–735. [DOI] [PubMed] [Google Scholar]
- 136. Distler J, Anguelouch A. Evidence-based practice: review of clinical evidence on the efficacy of glucosamine and chondroitin in the treatment of osteoarthritis. J Am Acad Nurse Pract 2006; 18: 487–493. [DOI] [PubMed] [Google Scholar]
- 137. Qiu GX, Gao SN, Giacovelli G, et al. Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung 1998; 48: 469–474. [PubMed] [Google Scholar]
- 138. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43: 1905–1915. [DOI] [PubMed] [Google Scholar]
- 139. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64: 465–474. [DOI] [PubMed] [Google Scholar]
- 140. Kwoh CK, Roemer FW, Hannon MJ, et al. Effect of oral glucosamine on joint structure in individuals with chronic knee pain: a randomized, placebo-controlled clinical trial. Arthritis Rheumatol 2014; 66: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. David-Raoudi M, Deschrevel B, Leclercq S, et al. Chondroitin sulfate increases hyaluronan production by human synoviocytes through differential regulation of hyaluronan synthases: role of p38 and Akt. Arthritis Rheum 2009; 60: 760–770. [DOI] [PubMed] [Google Scholar]
- 142. Huskisson EC. Glucosamine and chondroitin for osteoarthritis. J Int Med Res 2008; 36: 1161–1179. [DOI] [PubMed] [Google Scholar]
- 143. Conte A, de Bernardi M, Palmieri L, et al. Metabolic fate of exogenous chondroitin sulfate in man. Arzneimittelforschung 1991; 41: 768–772. [PubMed] [Google Scholar]
- 144. Ronca F, Palmieri L, Panicucci P, et al. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage 1998; 6(Suppl. A): 14–21. [DOI] [PubMed] [Google Scholar]
- 145. Gabay C, Medinger-Sadowski C, Gascon D, et al. Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial at a single center. Arthritis Rheum 2011; 63: 3383–3391. [DOI] [PubMed] [Google Scholar]
- 146. Uebelhart D, Knols R, de Bruin ED, et al. Chondroitin sulfate as a structure-modifying agent. Adv Pharmacol 2006; 53: 475–488. [DOI] [PubMed] [Google Scholar]
- 147. Zegels B, Crozes P, Uebelhart D, et al. Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis. Results of a randomized double blind placebo-controlled study. Osteoarthritis Cartilage 2013; 21: 22–27. [DOI] [PubMed] [Google Scholar]
- 148. Selvan T, Rajiah K, Nainar MS, et al. A clinical study on glucosamine sulfate versus combination of glucosamine sulfate and NSAIDs in mild to moderate knee osteoarthritis. ScientificWorldJournal 2012; 2012: 902676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Durmus D, Alayli G, Aliyazicioglu Y, et al. Effects of glucosamine sulfate and exercise therapy on serum leptin levels in patients with knee osteoarthritis: preliminary results of randomized controlled clinical trial. Rheumatol Int 2013; 33: 593–599. [DOI] [PubMed] [Google Scholar]
- 150. Morreale P, Manopulo R, Galati M, et al. Comparison of the anti-inflammatory efficacy of chondroitin sulfate and diclofenac sodium in patients with knee osteoarthritis. J Rheumatol 1996; 23: 1385–1391. [PubMed] [Google Scholar]
- 151. Bucsi L, Poór G. Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow-acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis. Osteoarthritis Cartilage 1998; 6(Suppl. A): 31–36. [DOI] [PubMed] [Google Scholar]
- 152. Mazieres B, Combe B, Phan Van A, et al. Chondroitin sulfate in osteoarthritis of the knee: a prospective, double blind, placebo-controlled multicenter clinical study. J Rheumatol 2001; 28: 173–181. [PubMed] [Google Scholar]
- 153. Oliviero U, Sorrentino GP, De Paola P, et al. Effects of the treatment with matrix on elderly people with chronic articular degeneration. Drugs Exp Clin Res 1991; 17: 45–51. [PubMed] [Google Scholar]
- 154. Conrozier T. [Anti-arthrosis treatments: efficacy and tolerance of chondroitin sulfates (CS 4&6)]. Presse Med 1998; 27: 1862–1865. [PubMed] [Google Scholar]
- 155. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence-based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003; 62: 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kanzaki N, Saito K, Maeda A, et al. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled study. J Sci Food Agric 2012; 92: 862–869. [DOI] [PubMed] [Google Scholar]
- 157. Tiku ML, Narla H, Jain M, et al. Glucosamine prevents in vitro collagen degradation in chondrocytes by inhibiting advanced lipoxidation reactions and protein oxidation. Arthritis Res Ther 2007; 9: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. van Blitterswijk WJ, van de Nes JC, Wuisman PI. Glucosamine and chondroitin sulfate supplementation to treat symptomatic disc degeneration: biochemical rationale and case report. BMC Complement Altern Med 2003; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vitetta L, Cicuttini F, Sali A. Alternative therapies for musculoskeletal conditions. Best Pract Res Clin Rheumatol 2008; 22: 499–522. [DOI] [PubMed] [Google Scholar]
- 160. Bello AE, Oesser S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature. Curr Med Res Opin 2006; 22: 2221–2232. [DOI] [PubMed] [Google Scholar]
- 161. Moskowitz RW. Role of collagen hydrolysate in bone and joint disease. Semin Arthritis Rheum 2000; 30: 87–99. [DOI] [PubMed] [Google Scholar]
- 162. Vaishya R, Pariyo GB, Agarwal AK, et al. Non-operative management of osteoarthritis of the knee joint. J Clin Orthop Trauma 2016; 7: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]