Abstract
Hostile microenvironment produced by abnormal blood vessels, which is characterized by hypoxia, low pH value and increasing interstitial fluid pressure, would facilitate tumor progression, metastasis, immunosuppression and anticancer treatments resistance. These abnormalities are the result of the imbalance of pro-angiogenic and anti-angiogenic factors (such as VEGF and angiopoietin 2, ANG2). Prudent use of anti-angiogenesis drugs would normalize these aberrant tumor vessels, resulting in a transient window of vessel normalization. In addition, use of cancer immunotherapy including immune checkpoint blockers when vessel normalization is achieved brings better outcomes. In this review, we sum up the advances in the field of understanding and application of the concept of tumor vessels normalization window to treat cancer. Moreover, we also outline some challenges and opportunities ahead to optimize the combination of anti-angiogenic agents and immunotherapy, leading to improve patients’ outcomes.
Keywords: abnormal blood vessels, tumor microenvironment, anti-angiogenesis, tumor vessels normalization, immune checkpoint blockers
Introduction
Cancer immunotherapy is emerging as a novel therapeutic modality capable of revolutionizing cancer treatment.1,2 Recent advances in immune checkpoint blockers (ICBs) have shifted immunotherapy from the bench to the frontline of clinical oncology.3 In contrast to conventional chemotherapy, which directly targets cancer cells, ICBs galvanizes immune cells in the tumor microenvironment (TME) by blocking immune checkpoint proteins to fight cancer cells.4 Infiltrating immune cells, stromal cells and abnormal vascular and lymphatic vessels compose the TME, which is characterized by a high interstitial fluid pressure, hypoxia and a low pH.5 The abnormal TME can eclipse the potency of all anticancer treatments, including immunotherapy.6-9 Therefore, normalizing the TME or at least 1 component of it could improve the efficacy and achieve better outcomes.10-12 Over the past decade, an astonishing outpouring of research has shown that judicious use of antiangiogenic therapy (AT) temporarily normalizes tumor blood vessels, relieves hypoxia and increases the delivery of drugs and antitumor immune cells, thus improving the results of various treatments.13-18 Therefore, combining ATs and immunotherapies should increase the effectiveness of tumor control and produce better outcomes in patients.
In this review, we focus on the crucial role of blood vessels in the TME and discuss how normalizing the vasculature by judicious AT application potentiates immunotherapy. In addition, we will also discuss the concept of the window of vessel normalization and how to widen it through judicious use of AT. Therefore, the interplay between tumor vessel normalization and immune activation creates a new approach to devise combination therapies for cancer patients. Finally, we propose a new paradigm of treatment that uses immunotherapy in the window of vessel normalization, which is a new avenue to enhance cancer immunotherapy.
Abnormal Tumor Vessels and Their Roles in the TME
Abnormal Tumor Vessels
Angiogenesis is a hallmark of cancer.19 Rapid proliferation of tumor cells consumes large amounts of oxygen and nutrients. Therefore, tumors rely on angiogenesis to support growth and progression.9 Tumor vessels are different from vessels that sustain normal rapidly growing organs or tissues.16 Specifically, tumor blood vessels are abnormal in structure and function. Unlike normal vessels, tumor blood vessels are tortuous, saccular and chaotic in organization. Moreover, the structures of the tumor blood vessels wall are aberrant with large gaps between endothelial cells, detached pericytes and abnormally basement membranes, leading to leakiness.13 In addition to structural abnormal, their functions are also different from normal. Through abnormal expression of a range of adhesion molecules in tumor blood vessels, certain immunosuppressive cells can be allowed into tumor tissues and the infiltration of certain effector cells into tumors are stymied. For examples, Clever-1/Stabilin-1 could facilitate T regulatory cells and pro-tumoral M2-phenotype macrophages trafficking20; Expression of FAS antigen ligand in the tumor vasculature was associated with scarce CD8(+) infiltration and a predominance of FoxP3(+) T regulatory (Treg) cells21; Overexpression of endothelin B receptor in ovarian cancer can block the infiltrating of T lymphocytes.22 Abnormal blood vessels serve just like a specific barrier that can block certain immune cells and allow certain immune cells. These abnormalities of tumor blood vessels promote tumor progression and metastasis by impairing perfusion, which results in a pro-metastatic niche characterized by hypoxia, acidity and immunosuppression.23 Moreover, blood perfusion in the TME is both spatially and temporally heterogeneous, which is caused by the aberrant tumor vessel network. This heterogeneity can impede the delivery of drugs and immune cells from the peripheral blood circulation into tumors, resulting in low anticancer efficacies.11
The abnormal TME can facilitate tumor progression, invasion and metastasis through myriad mechanisms. For example, hypoxia is a characteristic of the low perfusion caused by abnormal vessels, which is recognized as a key factor in facilitating the progression and metastasis of tumors through alterations in some aspects, such as genetic instability, angiogenesis, immunosuppression, and inflammation.23-26 Hypoxia in the TME induces excessive expression of proangiogenic factors, especially VEGF.27 The imbalance between the levels of pro- and antiangiogenic factors promotes rapid but abnormal formation of tumor vessels, which are tortuous, dilated and unevenly distributed.16 Apart from hypoxia, acidic or low pH TME has multiple consequences relevant to immune cells, resulting in an immunosuppressive TME.28 It has been shown that acidic pH profoundly induces an anergic state in tumor specific CD8+ T lymphocytes and negatively affects maturation and function of Th1 lymphocytes by inactivation of IFN-γ and suppression of tumor necrosis factor-α.29,30 In addition, acidic pH has been shown to stimulate the process of activation and transformation of the TAMs into a pro-tumoral M2-phenotype.31 Abnormal endothelial cells and lack of structural integrity in the tumor blood vessels wall lead to excessive leakiness.32 Vessel leakiness would also increase hematocrit and blood viscosity that reduce blood flow. In addition, leakiness reduces the intravascular pressure gradient, which is the force to flow. Moreover, vessel leakiness can cause interstitial fluid pressure up.24 In all, vessel leakiness impairs tumor blood flow and increases “intratumor fluid pressure,” which can hamper the distribution of anticancer drugs, including immunotherapeutic agents.18 The aberrant leakiness of vessels can also promote the intravasation and shedding of tumor cells into the peripheral circulation, resulting in dissemination to distal organs and formation of metastases.16,33,34
Effects on the Tumor Immune Microenvironment
The interplay of tumor angiogenesis and the tumor immune microenvironment is intimate, and the development of both can occur simultaneously. Antitumor immunity is rendered by immune cells that reside in tissues, the blood and the TME.35,36 Thus, an inflamed microenvironment is a prerequisite for effective immunotherapeutic strategies.37-39 Sufficient immune cell infiltration is critical for the efficacy of immunotherapy, and insufficiency lowers the effectiveness of immunotherapy.40,41 In the TME, various angiogenic factors, such as VEGF, angiopoietin 2 (ANG2), hypoxia inducible factors (HIFs), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor-beta (TGF-β) and other chemokines, can impact immune cell infiltration and the tumor immune microenvironment.26,42-45 These molecules work with tumor cells and stromal cells together to promote local immunosuppression, affecting immune cell infiltration and the tumor immune microenvironment.17
VEGF has various activities in the TME and has been extensively investigated in many tumor models. The prototypical members VEGFA, VEGFB, VEGFC, VEGFD and PIGF compose the VEGF family.46,47 The binding of VEGFA and VEGFR2 initiates the dominant pathway of angiogenesis.47 Additionally, excessive amounts of VEGF also induce the development of immunosuppression in the TME via at least 3 mechanisms. First, increases in VEGF levels directly hamper the trafficking, proliferation and effector function of T cells.48,49 VEGF binds to VEGFR2 on the surface of CD8+ T cells, resulting in upregulation of PD-1, CTLA-4, T cell immunoglobulin mucin receptor 3 (TIM3) and lymphocyte activation gene 3 (LAG3) protein expression, contributing to T cell exhaustion.48,50 Second, VEGF can also hinder the maturation and antigen presentation of dendritic cells (DCs) as a consequence of the impediment in T cell activation via the nuclear factor-κB (NF-κB) signaling pathway.51-53 An in vitro study demonstrated that the differentiation of monocytes into DCs was inhibited by VEGF.54 Overexpression of PD-L1 in DCs induced by VEGF suppresses the function and expansion of T cells.55 Third, a high level of VEGF can increase the density of a variety of immunosuppressive cells in the TME, including M2-phenotype macrophages, myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs).15,56,57
Another critical regulator of angiogenesis is angiopoietin.58,59 However, its roles in immunomodulation are still elusive. ANG1 produced by pericytes promotes vessel maturation. ANG1 overexpression stimulates pericyte coverage, resulting in a vasculature that is more mature in appearance. While ANG2 secreted by endothelial cells has been reported to possess antagonistic activity.60 Some studies have shown that ANG2 promotes immunosuppression in tumors through several mechanisms. On the one hand, ANG2 can facilitate the recruitment of MDSCs and Tregs.61-63 On the other hand, ANG2 can modulate monocytes by inhibiting the secretion of tumor necrosis factor (TNF).64
Increasing evidence has shown that VEGF and ANG2 can work together to enhance immunosuppression in the TME, depending on the relative levels of these cytokines.65-68 Some studies illustrated that optimal strategies for simultaneous blockade of VEGF and ANG2 alleviated immunosuppression in the TME, normalized the vessels and produced improved outcomes.65,66,69 A study showed that blockade of both VEGF and ANG2 induced tumor-associated macrophages (TAMs) to repolarize into antitumor M1-phenotype cells in a GL261 mouse glioblastoma (GBM) model.67 In parallel, in a mouse melanoma model, blockade of both VEGF and ANG2 also tilted the balance of macrophage phenotypes toward the M1 phenotype, leading to better outcomes.66
HIF-1 is a key transcriptional mediator of the response to hypoxic conditions in the TME. It participates in vasculature formation with other proangiogenic factors, such as VEGF and angiopoietin.70 Apart from its proangiogenic effects, HIF-1 also reprograms immune cells into a protumor phenotype. HIF-1 and cytokines can reduce the ability of effector immune cells to fight cancer. In particular, TGF-β reduces T lymphocyte activity and limits the ability of DCs to process tumor-associated antigens.26,71,72 Furthermore, HIF-1 signaling promotes increased levels of PD-L1 expression by MDSCs, TAMs, DCs, and cancer cells, exerting an immunosuppressive effect on the TME. This suggests that therapeutic strategies targeting HIF-1 signaling in the TME will affect anticancer therapy generally and immunotherapy specifically.26
HGF is a cognate ligand of the Met receptor tyrosine kinase. HGF/Met signaling contributes to tumorigenesis, tumor angiogenesis and metastasis.73 Some evidence from preclinical and clinical studies has shown that HGF expression is upregulated in situations of VEGF receptor blockade resistance, which indicates that HGF-mediated angiogenesis may act as an alternative angiogenic pathway74 This suggests that targeting HGF may be a potential strategy to circumvent resistance to VEGF receptor tyrosine kinase inhibitors in the clinic. Apart from its wide range of roles in angiogenesis, HGF also plays a role in the TME.75-77 HGF is a potent immunomodulatory factor that inhibits DC function, leading to defective antigen presentation.78 In addition, HGF can induce the differentiation of T cells into CD25+Foxp3+ Tregs with high levels of IL-10 and downregulation of the expression of surface markers of T cell activation.79 Due to the immunosuppressive function of HGF, targeting the HGF signaling pathway may be useful in combination with immunotherapy. Cabozantinib, a small-molecule inhibitor targeting the HGF/Met signaling pathway, was shown to increase the infiltration of CD8+ T cells into the TME through direct normalization of the tumor vasculature in mice.80 It was also demonstrated that cabozantinib treatment could not only modulate the immune landscape both peripherally and intratumorally but also sensitize the TME to immunotherapy.80
The PDGF family comprises PDGF-A to PDFG-D, which can function as either homodimers or heterodimers. Activation of PDGF signaling has wide effects on embryonic development and tumor vascularization.81 However, the effect of PDGF on immune cells is not well elucidated. An in vitro study showed that PDGF inhibited the maturation of human DCs and induced IL-10 secretion. Culture of PDGF-DCs with T cells induced the polarization of T cells into FoxP3-expressing Tregs that secreted IL-10.82 This suggests that PDGF may play an immunosuppressive role in the TME.
FGFs are ubiquitously expressed and have various functions, including the regulation of cell growth and angiogenesis.83 FGFs exert their proangiogenic activity by interacting with various endothelial cell surface receptors, including tyrosine kinase receptors, heparan sulfate proteoglycans, and integrins.84 Aside from their proangiogenic effect on the TME, FGFs also impact the tumor immune microenvironment. Experimental and clinical evidence has indicated that FGFs play roles in tumor-infiltrating myeloid cells, including monocytes/macrophages, neutrophils, eosinophils, mast cells, and DCs, to facilitate the formation of an immunosuppressive microenvironment.85
Window of Vessel Normalization
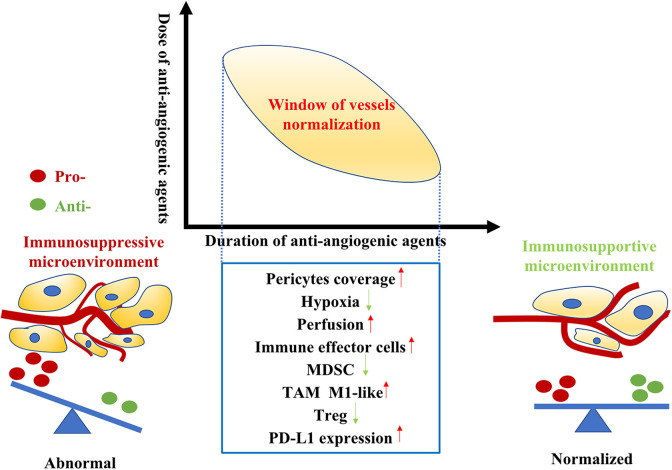
During treatment with pharmacological ATs, there is usually a transient time window in which the tumor vessels are normal in structure and function (Figure 1). Structural normalization is characterized by morphological changes in the tumor vascular network, wherein tumor vessel diameters, density, and tortuosity are drastically reduced.86 This leads to improved vessel perfusion and reduced tissue hypoxia.15 A preclinical study showed that radiotherapy given during the window of vessel normalization had better outcomes than radiotherapy given outside the window.87 During the normalization window, pericytes are recruited to existing tumor blood vessels, and hypoxia and the abnormally thick basement membrane of tumor vessels are greatly reduced.87 However, the window of vessel normalization is transient and hard to capture. In both human and mouse models, vascular normalization induced by AT occurs very quickly, sometimes within 1 day, and lasts a short time spanning from 1 week to a few months in humans, possibly based on the type of tumor.16,87-89
Figure 1.
The tumor vessels normalization window relies on judicious use of anti-angiogenic agents. The dose and duration should be well manipulated and tailored according to different types of tumor and parameters of tumor vessels. In tumor vessels normalization window, the tumor immune microenvironment switches from immunosuppressive to immunosupportive. Vessels normalization generates a homogeneous distribution of perfused tumor vessels, reducing hypoxia and increasing pericyte coverage. Moreover, the infiltration of immune effector cells into tumor microenvironment increase while immune regulatory cells including Treg cells and MDSC (myeloid-derived suppressor cell) accumulation reduce. In addition, improved vascular perfusion polarizes TAMs to an immunostimulatory M1-like phenotype. Furthermore, the PD-L1 expression in tumor microenvironment elevated. This supports the rationale for treatment of immunotherapy in the window of tumor vessels normalization.
In the vessel normalization window, the TME is reprogrammed. In particular, the tumor immune microenvironment shifts from an immunosuppressive state to an immunosupportive state, rendering antitumor therapy more effective.17,90 Normalized tumor vessels can directly alleviate TME hypoxia and facilitate CD4+ and CD8+ T cell infiltration.66 In addition, hypoxia alleviation preferentially induces the polarization of TAMs into an immunosupportive M1-like phenotype.15 Moreover, vessel normalization also decreases the recruitment of Tregs and MDSCs into the TME.91,92 In such an immune-active microenvironment formed during the tumor vessel normalization window, immunosuppressive signals may also emanate from activated adaptive immune cells through regulatory negative-feedback mechanisms, such as the induction of immune checkpoint molecules (for example, PD-L1). In a transgenic MMTV-PyMT model, RNA-seq analysis revealed that PD-L1 expression was upregulated in tumor-derived endothelial cells after antiangiogenic treatment. In addition, the study also showed that perivascular CD8+ T cell numbers correlated with the expression of PD-L1 in the corresponding vascular segments. Furthermore, PD-1 blockade improved tumor control in different cancer models during vessel normalization.66 Another study also found that the infiltration of CD4+ and CD8+ T cells increased in AT-treated renal cell carcinoma primary tumors. In concordance with the aforementioned results, specimens from patients treated with AT also showed enhanced expression of PD-L1.93 Collectively, these pieces of evidence demonstrate that antitumor immune responses are stimulated during vessel normalization but PD-L1 expression is increased. This supports the rationale for treatment with ICBs during the window of tumor vessel normalization.
Tactics for Normalization of Tumor Blood Vessels
Numerous molecular mechanisms lead to abnormal angiogenesis, the most important of which is the imbalance between pro- and antiangiogenic factors. Thus, restoring the balance would theoretically allow blood vessels to recover structurally and functionally. In the TME, proangiogenic cytokines induced by hypoxia or any other reason are pivotal culprits in vessel abnormality development.18 Therefore, removing redundant angiogenesis signals has become a mainstay strategy.
A number of preclinical studies illustrated that anti-VEGF therapy normalized tumor vessels transiently and converted the immunosuppressive TME.13,15,87 Furthermore, the dose and duration of anti-VEGF agent therapy exerted a great influence on efficacy. Notably, high-dosage antiangiogenic treatment could result in excessive tumor vessel pruning, leading to exacerbation of hypoxia in the TME, thus shortening the normalization window.15 Moreover, high doses of anti-VEGF drugs also facilitate extracellular matrix deposition, which promotes the infiltration of immunosuppressive immune cells such as MDSCs and Tregs.15 Notably, a long duration of anti-VEGF therapy can destroy blood vessels and inevitably increase tumor hypoxia.87 This phenomenon is known as overpruning of tumor blood vessels. In contrast, low doses of antiangiogenic agents prolong vessel normalization. A study showed that targeting tumor vessels with a lower dose of antiangiogenic agent rather than a higher dose made the distribution of functional tumor blood vessels more uniform.15 In addition, compared with high doses, lower doses perform better in transforming TAMs from an immune inhibitory M2-like phenotype into an immune-supporting M1-like phenotype and in facilitating CD8+ T cell infiltration.15 These results indicate that lower doses of antiangiogenic drugs can reprogram the TME. Two retrospective clinical studies showed that low-dose bevacizumab produced better outcomes than high-dose bevacizumab in patients with glioblastoma.94,95 Therefore, optimal doses and schedules of antiangiogenic agents should be personalized.
The ANG2-Tie2 axis, which confers resistance to anti-VEGF therapy, also plays a key role in tumor angiogenesis.96 ANG2 is an important proangiogenic cytokine that transmit signals through the ANG1 and ANG2 receptor (Tie2). Tie2 is expressed mainly on vascular endothelial cells. ANG1 expression in pericytes primarily sustains quiescent mature vessels. In contrast to ANG1, ANG2 functions in tumor progression and metastasis by inducing vascular destabilization. ANG1 acts as the stimulating, agonistic ligand of TIE2. By contrast, the effects of ANG2 on the vasculature seem to be contextual to enable agonistic and antagonistic effects on TIE2 signaling.60 Therefore, ANG2 may act as a context-dependent antagonist. For instance, inhibition of ANG2 prunes tumor blood vessels and improves vessel normalization through impairment of endothelial and smooth muscle cell contact loss.97,98 Inhibition of ANG1 has only a small effect on tumor blood vessels.87,98 Nevertheless, the combination of ANG1 and ANG2 blockade trims tumor blood vessels but does not achieve normalization.99 These results indicate that the normalization of tumor blood vessels depends on ANG1 and the inhibition of ANG2 can enhance the interaction of ANG-1/Tie2. Accordingly, targeting ANG2 would sustain the normal vascular phenotype and hamper vessel sprouting, leading to tumor vessel normalization. A study showed that inhibition of ANG2 by L1-7 significantly retarded tumor growth. In this experiment, more uniformity, more pericytes, fewer endothelial sprouts and more linear endothelial junctions were found in tumor blood vessels after treatment with L1-7.98 Simultaneous activation of Tie2 and blockade of ANG2 in mice with some types of cancers normalized tumor blood vessels more efficiently than activation or blockade alone. This approach favored blood perfusion and delivery of chemotherapeutic drugs. It also markedly alleviated lactate acidosis in the TME and inhibited tumor growth and metastasis.43 In addition, this study also found that simultaneous Tie2 activation and Ang2 inhibition could produce a favorable TME. Another study in mice model demonstrated that double inhibition of VEGFRs and ANG-2 inhibited tumor proliferation more efficiently than single inhibition and widened the normalization window.43
Another strategy is to disrupt the angiogenesis signaling pathway via gene modification. For example, G-protein signaling 5 (Rgs5) is key in the abnormal morphology of tumor vessels,100,101 Through changes in tumor blood vessels, gene disruption of Rgs5 could enhance the infiltration of effector immune cells into the tumor parenchyma, resulting in profoundly prolonged survival of tumor-bearing mice.102 RGS5-deficient mice exhibited a normalized tumor vasculature, which was characterized by the presence of mature pericytes, reduced vessel leakiness and improved oxygenation. Moreover, knocking out Rgs also influenced leukocyte attachment and facilitated transmigration into the tumor parenchyma.102 However, the exact mechanism underlying the vascular normalization induced by Rgs5 gene knockout remains elusive.
In addition to perturbation of proangiogenic signaling, treatment targeting angiostatin factors can also be considered a strategy. IFN-γ and TNF-α are well-known cytokines that can play various roles. Both cytokines act directly or indirectly on tumor blood vessels.103,104 Intra-tumoral low-dose TNF-α treatment stabilizes the vascular network and improves vessel perfusion, resulting in markedly improved penetration of anticancer drugs. Delivery of low-dose TNF-α also enhances the infiltration of effector immune cells into the tumor parenchyma and polarizes TAMs into pro-tumoral M2-phenotype macrophages.105 In contrast to TNF-α, IFN-γ maintains the tumor in an ischemic state and prevents proliferation.105-107 LIGHT, which is also known as TNF superfamily member 14 (TNFSF14), is a TNF superfamily member. In a study using the peptide CGKRK (a vascular homing peptide) to deliver LIGHT to angiogenic tumor vessels, the brain tumor vasculature was normalized by restoring pericyte contractility and re-establishing endothelial barrier integrity.108 Furthermore, CGKRK was fused to LIGHT/TNFSF14 and injected intravenously into murine orthotopic glioblastoma multiforme models.109 After treatment, the vascular system was less abnormal, the integrity of the endothelial barrier was improved, peripheral contractility was restored, and blood perfusion was recovered. Additionally, high endothelial venules (HEVs) appeared, and T cell infiltration also increased.109
Increasing studies have shown that immune cells also play roles in tumor vessel normalization. Thus, stimulation of such immune cells may be a new strategy to normalize the tumor vasculature. A recent study demonstrated that profound changes in the TME involving vessel normalization and reprogramming of TAMs could be found in MO4 tumor-bearing mice that received simultaneous intravenous injection of eosinophils and T cells.110 In this study, it was shown that vessel normalization could be induced solely by eosinophils. After intravenous infusion of eosinophils and T cells, the normalization of tumor blood vessels was indicated by various parameters, including reduced hypoxia, reduced blood vessel leakage, increased blood vessel perfusion, increased coverage of blood vessels with mature pericytes and low expression of Rgs5.110 The precise mechanism underlying vascular normalization induced by eosinophils is not clear, but it may be that these cells initiate TAM polarization into M1-like cells via eosinophil-derived TNF and IFN-γ, which eventually leads to a decrease in VEGF production.110 Another study identified the causal role of T helper 1 (TH1) cells in vascular normalization by using patient-derived xenograft models planted from an immunocompetent environment (original patients) into an immunodeficient (animals) environment.111 In this study, after TH1 cells were transferred into mouse models of breast cancer, hypoxia and vessel leakiness decreased, large and dilated vessels were pruned, and perfusion efficiency was improved. These parameters indicated that vessels were normalized. In addition, the vascular normalization process also depended on IFN-γ signaling.111 In mice treated with anti-PD-1 antibodies, TH1 cells improved vessel normalization. Hence, vascular normalization or regression probably occurs in a manner dependent on the activation of tumor-specific T cells and secretion of IFN-γ, and this phenomenon can boost immune cell infiltration to form a feedback cycle. Another study also showed that the activation of CD8+ T cells mediated by immune checkpoint therapy including anti-PD-1 or anti-CTLA4 could facilitate tumor vessel normalization via the IFN-γ signaling pathway.112 Based on these facts, the potential mechanism underlying normalization of tumor blood vessels is that effector T cells are activated through cancer immunotherapy, resulting in production and secretion of IFN-γ. Consequently, the IFN-γ interacts with the receptors in the surface of vascular cells, leading to normalization of the tumor vessels. In summary, cancer immunotherapy can promote the normalization of tumor blood vessels and form a virtuous circle with anti-angiogenesis treatments.
Monitoring of Vessel Normalization Window
Determining how to evaluate the vascular normalization window and the initial and final points is critical to tailoring treatment strategies. To date, tumor blood vessel perfusion, microvessel density, vascular morphology and permeability are still the gold standards for confirmation of the normalization of tumor blood vessels. Detection of parameters such as CD31, desmin, αSMA and so on mainly relies on histological staining. However, this invasive detection method has poor reproducibility and does not dynamically monitor the process of tumor vascular normalization. Therefore, finding a convenient and noninvasive method to monitor the time window accurately, which is a prerequisite for guiding drug administration accurately, is of great significance. Imaging and peripheral blood biopsy may provide simple and noninvasive ways to detect the vessel normalization window.
Computed tomography (CT) imaging is used to visualize the perfusion, vascular density, and bifurcation of tumors. CT includes 2 types of techniques, CT perfusion imaging and dynamic contrast-enhanced CT (DCE-CT). CT perfusion imaging is an emerging tool that provides both qualitative and quantitative information and can obtain hemodynamic parameters that are calculated by software using mathematical models.113 It has been suggested that CT perfusion parameters, including blood volume, blood flow, peak enhancement index and permeability surface area, can be used to evaluate tumor vessel normalization.113-115 With the development of functional imaging, DCE-CT, which is a noninvasive technique to image the intratumoral vascular physiological status, can offer more comprehensive information, such as perfusion (F), permeability surface area (PS), fractional intravascular plasma (fp), and interstitial space (fis).116-119 In a mouse study of human pancreatic tumor xenografts, DCE-CT was used to monitor the effects of AT through parametric maps of tumor perfusion and fractional plasma volume (Fp) that were calculated before and after antiangiogenic treatment. The results showed that a more homogeneous physiological window was observed 1 week after receiving low-dose antiangiogenic anti-VEGF receptor-2 antibody therapy.118 This result suggests that DCE-CT is capable of monitoring changes in the physiological parameters of tumor vessel perfusion in response to AT. Given this capability, DCE-CT is well suited to measure the vascular normalization window. More studies are needed to verify this conclusion, especially in the context of clinical practice.
Magnetic resonance imaging (MRI) is another promising noninvasive imaging technique to assess the response of the tumor vasculature to ATs and detect the normalization window.120-122 Dynamic contrast-enhanced MRI (DCE-MRI) can quantitatively measure tumor perfusion and vascular permeability through parameters including the volume transfer coefficient (Ktrans), volume fraction of extravascular extracellular space (Ve), and rate constant of backflux (Kep).121-123 Blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) is another advanced imaging sequence that can detect aerobic metabolism in the tumor microenvironment and is suitable to measure tumor hypoxia during AT.124 A study showed that Ktrans, a parameter related to microcirculation perfusion and microvascular permeability, gradually increased within 6 days of bevacizumab treatment and maintained elevated values during 3-12 days after bevacizumab administration.122 This indicates that DCE-MRI is capable of detecting the tumor vessel normalization window. Moreover, the value of R2*, a parameter of BOLD-MRI that reflects tumor hypoxia, gradually decreased from 0 to 9 days and then increased. Overall, DCE-MRI is superior for detecting vessel maturity and normalization, while BOLD-MRI may perform better in assessing hypoxia after AT administration. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging (IVIM DW-MRI) has emerged as a promising approach that can be used to predict the efficacy of ATs without the need for exogenous contrast agents.125 The blood pseudodiffusion coefficient (D*) and perfusion fraction (f), parameters of IVIM DW-MRI, can satisfactorily reflect tumor angiogenesis and microvessel density.126-128 In a study of CT26 colon carcinoma-bearing mice, IVIM DW-MRI was used to detect the process of tumor vascular normalization at different times after Endostar (recombinant human endostatin) treatment.129 The D* and f values were well correlated with pericyte coverage and relative perfusion. Furthermore, they were significantly higher on days 6 and 8 after treatment with Endostar than without treatment, indicating the vascular normalization window.129 These results suggest that IVIM DW-MRI has the potential to become a noninvasive imaging protocol for monitoring tumor vascular normalization after antiangiogenic treatment. Overall, MRI provides a very comprehensive and versatile platform to assess the function and morphology of tumor vessels. In the future, designing more functional MRI experimental paradigms and integrating different MRI paradigms to evaluate tumor vessels will warrant further investigation.
Aside from CT and MRI, Doppler ultrasound is another helpful noninvasive imaging technique to analyze tumor blood vessels. Dynamic contrast-enhanced ultrasonography (DCE-US) is a functional technique using Doppler ultrasound with contrast agents to evaluate microvessels and quantitatively assess solid tumor perfusion through mathematical models and calculations. Parameters derived from a global time-intensity curve describing contrast agent flow are surrogates for characterizing tumor perfusion and neovascular morphology and include the peak intensity, time-to-peak intensity, area under the curve, wash-in rate, and wash-out rateb.130 A clinical trial with 539 enrolled patients was conducted to assess the value of DCE-US in the evaluation of the tumor response to antiangiogenic treatments. DCE-US was carried out at the baseline time point and 7 days after treatment with bevacizumab. The results showed that DCE-US, as a functional imaging technique, provided a criterion at day 7, the mean transit time (MTT), that could be used as a biomarker for vascular normalization.131 A recent study provided evidence that the possible timing of the normalization window was 20-24 hours after the administration of bevacizumab in patients with breast cancer through 3-dimensional power Doppler ultrasonography.132 Overall, monitoring tumor vascular normalization by Doppler ultrasound is feasible. However, Doppler ultrasound has some limitations. First, it cannot be used for metastases in the lungs or brain. Second, it requires the selection of a single target tumor.
Emission CT is a computer imaging method. It includes positron emission tomography (PET) and single-photon emission computed tomography (SPECT). In mouse xenograft models, dynamic PET was used to detect changes in tumor perfusion following AT. The results showed that the perfusion-related rate parameter was significantly decreased at 24 hours and then increased at 72 hours after treatment. It was concluded that longitudinal dynamic PET is an imaging method that can be used to identify the time frame of potential tumor vasculature normalization.133 In another study, 99mTc-(CO)3 His-Annexin A5 Micro-SPECT was used to monitor the time window during which tumor vessels became normalized.134 Overall, MRI, CT, ultrasonography and PET have been used to evaluate the vessel normalization time window, but no consensus has yet been reached. To date, no single method has been proven to detect the sophisticated process of tumor vessel normalization.135
In addition, as a method for noninvasively monitoring the process of tumor normalization, liquid biopsy has already been implemented in clinical settings. ANG2 is a potential biomarker for prediction and prognosis. High levels of ANG2 in the circulation correlate with a poor prognosis across myriad types of cancer.136-139 For instance, high levels of circulating ANG2 are associated with poor outcomes in colorectal carcinoma patients treated with anti-VEGF therapy.140 In patients with ovarian carcinoma, a decrease in circulating ANG2 levels in response to anti-VEGF treatment.136 In addition, in patient with glioblastoma that treated with anti-VEGF drugs the levels of plasma ANG2 transiently decreased and subsequently elevated in line with treatment response.89 These results suggest that low plasma ANG2 level indicates a vessel normalization window. Convenient accessibility and sensitivity to alterations in tumor vessels render liquid biopsy a promising tool for monitoring tumor vessel normalization. However, there are still no validated predictive biomarkers that have been confirmed to accurately reflect the response to AT. In the future, combining imaging methods and liquid biopsy to establish a much more precise and effective evaluation system for delineating the landscape of tumor vascular normalization during AT deserves more attention.
Vessel Normalization Improves Cancer Immunotherapy
The window of tumor vessel normalization is a very special period during which the vessels in the TME are transiently normal in structure and function. Vasculature-normalizing therapies can reprogram the TME, especially reprogramming the immunosuppressive TME into an immunosupportive microenvironment.90 As discussed previously, judicious use of antiangiogenic agents can facilitate vessel normalization and thus improve the effect of immunotherapy. Therefore, emerging evidence suggests that the combination of antiangiogenic agents with immune checkpoint blockade can strengthen treatment by establishing positive feedback to improve the treatment effect.
In a preclinical study of mouse models of cancer, blocking VEGFA and ANGPT2 with the bispecific antibody A2 V simultaneously strengthened the antitumor treatment effect and the effects of each individual strategy.66 A2 V impaired tumor angiogenesis, increased tumor necrosis and normalized the residual blood vessels. Moreover, it also activated tumor-infiltrating CD8+ T cells, increased tumor antigen presentation and promoted perivascular T cell accumulation.66 Based on these results, it is rational to combine anti-PD-1/PD-L1 antibodies with A2 V, and study results illustrated the potential benefits of the addition of PD-1 blockade to dual ANGPT2 and VEGFA neutralization. However, the magnitude of the benefit achieved with the combination approach varied with the tumor model. A preclinical study showed that the combination of anti-VEGFR2 and anti-PD-L1 antibodies successfully induced polyoma middle T oncoprotein (PyMT) breast cancer and Rip1-Tag2 pancreatic neuroendocrine tumors (RT2-PNET) but not glioblastoma to form high endothelial venules. The induction activated lymphotoxin β receptor signaling (LTβR), resulting in the promotion of lymphocyte infiltration and activity.141 Further activation of LTβR could enhance cytotoxic T cell activity and sensitize tumors to anti-PD-L1 treatment. Overall, anti-PD-L1 therapy sensitized and prolonged the antiangiogenic effect, whereas AT improved the response to anti-PD-L1 therapy.141 The feedback between an ICB and AT strengthens itself and eventually drives immune-mediated eradication of tumor cells.
Recently, a growing number of clinical trials have tested the combination of antiangiogenic agents and ICBs in various types of solid tumors (Table 1). For example, in patients with metastatic melanoma receiving treatment with a combination of ipilimumab and bevacizumab, safety and good responses, including 8 partial responses, 22 stable disease responses and a disease-control rate of 67.4%, were observed.142 In this clinical trial, on-treatment biopsies revealed that the vessel endothelium was activated, and massive CD8+ T cell and macrophage infiltration occurred.142 Another study confirmed that the combination of ipilimumab and bevacizumab enhanced lymphocyte infiltration and antibody responses in patients with metastatic melanoma, leading to augmentation of immunological recognition.143 Impower150 (NCT02366143) proved that adding atezolizumab to bevacizumab-based chemotherapy profoundly improved progression-free survival and overall survival in patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), which ranks as the leading cause of cancer mortality worldwide.144 Pembrolizumab or Avelumab plus Axitinib were characterized by an improved progression-free-survival and a high response rate with a low rate of intrinsic resistance in patients with metastatic renal cell carcinoma.145 The combinations now have been approved as standard first-line therapies for advanced renal carcinoma.145 The combination of SHR-1210 (an anti-PD-1 antibody) and apatinib (a VEGFR2 inhibitor) was assessed in patients with advanced hepatocellular carcinoma, esophagogastric junction cancer or gastric carcinoma and showed encouraging clinical activity and manageable toxicity.146 In addition, a clinical trial assessed the safety and preliminary antitumor activity of a combination of ramucirumab (an IgG1 VEGFR-2 antagonist) and pembrolizumab (an IgG4 PD-1 antagonist) in patients with advanced gastric or gastroesophageal junction adenocarcinoma, NSCLC or urothelial carcinoma who had already received multiple lines of therapy.147 In addition to anti-tumor activity, combination treatments may increase the risk of side effects over either treatment alone. Based on previous clinical trials, the most common adverse reactions from the combination treatment include hematological, diarrhea and liver function compromising.145 In general, an increasing number of clinical trials are being performed to investigate the safety and efficacy of combinations of immune checkpoint inhibitors and VEGF pathway inhibitors. We believe that this combinatorial approach will change our clinical guidance and improve patient prognosis.
Table 1.
Some Ongoing Clinical Trials Evaluating Immune-Checkpoint Blockers in Combination With Anti-Angiogenic Agents (Data from https://clinicaltrials.gov up to July 5, 2020).
| Immune-checkpoint blockers and anti-angiogenic agents | Clinical trial ID | Study phase | Cancer types |
|---|---|---|---|
| Pembrolizumba+Bevacizumab | NCT02681549 | Phase 2 | Melanoma|Non-small Cell Lung Cancer|Brain Metastasis |
| Pembrolizumab plus Axitinib | NCT04197219 | Phase 2 | Recurrent Endometrial Cancer |
| NCT02636725 | Phase 2 | Alveolar Soft Part Sarcoma|Soft Tissue Sarcomas | |
| NCT02853331 | Phase 3 | Renal Cell Carcinoma | |
| Anti-PD-1 Combinations of D-CIK Immunotherapy and Axitinib | NCT03736330 | Phase 2 | Renal Cancer Metastatic |
| Pembrolizumab plus Lenvatinib | NCT03895970 | Phase 2 | Liver Neoplasm Malignant Primary|Cholangiocarcinoma |
| NCT03797326 | Phase 2 | Advanced Solid Tumors|Triple Negative Breast Cancer|Ovarian Cancer|Gastric Cancer|Colorectal Cancer|Glioblastoma|Biliary Tract Cancers | |
| NCT03898180 | Phase 3 | Urothelial Carcinoma | |
| NCT03820986 | Phase 3 | Malignant Melanoma | |
| NCT03829332 | Phase 3 | Non-small Cell Lung Cancer | |
| NCT02973997 | Phase 2 | Thyroid Carcinoma | |
| NCT03713593 | Phase 3 | Hepatocellular carcinoma | |
| NCT03517449 | Phase 3 | Endometrial Neoplasms | |
| NCT03321630 | Phase 2 | GastroEsophageal Cancer | |
| NCT03609359 | Phase 2 | Advanced Gastric Cancer | |
| Pembrolizumab plus Apatinib | NCT03407976 | Phase 1|Phase 2 | Advanced Malignancies|Urothelial Carcinoma|MSI-H or dMMR Solid Tumors|Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma |
| Pembrolizumab plus Sorafenib | NCT03211416 | Phase 1|Phase 2 | Advanced or Metastatic Liver Cancer |
| Atezolizumab plus Bevacizumab | NCT03836066 | Phase2 | Non Small Cell Lung Cancer |
| NCT03818061 | Phase2 | Head and Neck Neoplasms | |
| NCT02982694 | Phase2 | ColoRectal Cancer | |
| NCT03272217 | Phase2 | Urothelial Carcinoma | |
| NCT03175432 | Phase2 | Melanoma | |
| NCT04102098 | Phase3 | hepatocellular carcinoma | |
| NCT03526432 | Phase2 | Endometrial Cancer | |
| Atezolizumab plus Cabozantinib | NCT03170960 | Phase 1|Phase 2 | Urothelial Carcinoma|Renal Cell Carcinoma|Non-Small Cell Lung Cancer|Castration-resistant Prostate Cancer|Triple Negative Breast Cancer|Ovarian Cancer|Endometrial Cancer|Hepatocellular Carcinoma|Gastric Cancer|Gastroesophageal Junction Adenocarcinoma|Colorectal Cancer|Head and Neck Cancer|Differentiated Thyroid Cancer|Lower Esophageal Cancer |
| Atezolizumab plus Cabozantinib | NCT03755791 | Phase 3 | Hepatocellular Carcinoma |
| Atezolizumab plus Ramucirumab | NCT03689855 | Phase2 | Non-small Cell Lung Cancer |
| Durvalumab plus Bevacizumab | NCT03847428 | Phase 3 | Hepatocellular Carcinoma |
| NCT03778957 | Phase 3 | Hepatocellular Carcinoma | |
| Nivolumab plus Bevacizumab | NCT03452579 | Phase 2 | Glioblastoma |
| NCT03890952 | Phase2 | Recurrent Adult Brain Tumor | |
| Nivolumab plus Bevacizumab plus Rucaparib | NCT02873962 | Phase 2 | Peritoneal Cancer|Ovarian Cancer|Fallopian Tube Cancer |
| Nivolumab plus Sorafenib | NCT03439891 | Phase 2 | Hepatocellular Carcinoma |
| Nivolumab plus Axitinib | NCT03595124 | Phase 2 | Metastatic Renal Cell Carcinoma|Renal Cell Carcinoma Associated With Xp11.2 Translocations/TFE3 Gene Fusions|Stage III Renal Cell Cancer |
| NCT03172754 | Phase 1|Phase 2 | Renal Cell Carcinoma | |
| Nivolumab plus Lenvatinib | NCT04044651 | Phase 2|Phase 3 | Hepatocellular Carcinoma |
| NCT03418922 | Phase 1 | Hepatocellular Carcinoma | |
| NCT03841201 | Phase 2 | Hepatocellular Carcinoma | |
| Nivolumab and Ramucirumab | NCT03502746 | Phase 2 | Malignant mesothelioma |
| Ipilimumab plus Bevacizumab | NCT01950390 | Phase 2 | Melanoma |
Perspectives and Concluding Remarks
Abnormal tumor vessels have long been known to be a major feature of the TME, which is characterized by immunosuppression, hypoxia, a low pH and a high interstitial pressure. A large amount of data has shown that a reasonable dosage of AT can reduce vascular permeability and interstitial fluid pressure as well as improve blood flow perfusion, leading to normalization of tumor blood vessels. In turn, tumor blood vessel normalization can reduce tissue hypoxia and facilitate the delivery of antitumor drugs, including ICBs. An increasing number of studies, including the aforementioned preclinical and clinical studies, support the combination of AT and immunotherapy to produce improved patient outcomes. However, we still face many problems in the process to optimize the combination of AT and immunotherapy. Here, we outline several challenges and opportunities and identify some research priorities for the future.
First, the dosage and duration of AT are critical. Both preclinical and clinical data show that the dose of antiangiogenic drugs targeting the VEGF pathway is a pivotal consideration.13,17 Amassed data demonstrate that low doses but not high doses can facilitate the tumor vessel normalization.15,87 Moreover, the duration of anti-VEGF treatment is proportional to the levels of tumor blood vessel perfusion and hypoxia in the TME.87 Thus, determining how to optimally adjust the dosage and duration of AT based on the type of tumor and parameters of the tumor vasculature, such as tumor perfusion, microvessel density, vascular morphology and permeability, is critical to normalizing tumor vessels and extending the window of vessel normalization.
Second, determining how to detect the time window of vascular normalization and delineate this sophisticated process precisely and noninvasively is a substantial obstacle. Although CT, MRI, ultrasonography and PET perfusion imaging systems have been developed to monitor the therapeutic efficacy of AT and detect the time window of tumor vessel normalization, no consensus has yet been reached. More trials and data are needed to validate these imaging techniques. In the future, a platform comprising different imaging techniques and liquid biopsy information could be used to guide precision medicine.
Third, tumor vessel normalization offers a promising opportunity to identify new and more effective combinations of ATs and ICBs. However, the interplay between these approaches is extremely complicated and far from fully elucidated. As previously mentioned, AT can reprogram the TME from an immunosuppressive state into an immunosupportive state. In contrast, ICBs can also engage with the process of tumor vessel normalization.111,112,148 Therefore, bridging the gaps in our understanding of the molecular and cellular mechanisms underlying the interplay between vessel normalization and immunotherapy is a challenge that needs to be addressed.
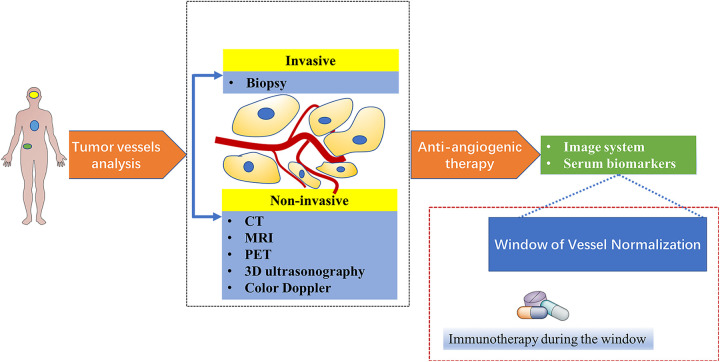
With the help of rapid advances in genomic, proteomic, bioinformatic and medical imaging technologies, much more efficient treatment strategies tailored to every patient with different types of cancer will become feasible (Figure 2).
Figure 2.
New proposed paradigm for combination of anti-angiogenesis and immunotherapy. Tumor vessels can be assessed through noninvasive and invasive methods. Judicious use of anti-angiogenic agents according to the information from assessments of tumor vessels analysis would facilitate tumor vessels normalization and prolong the normalization window. After confirming the normalization window by serum biomarkers and image system, immunotherapy is administrated in this window.
Abbreviations
- ANG2
angiopoietin 2
- AT
antiangiogenic therapy
- BOLD-MRI
blood oxygen level-dependent magnetic resonance imaging
- CT
computed tomography
- DCs
dendritic cells
- DCE-CT
dynamic contrast-enhanced CT
- DCE-MRI
dynamic contrast-enhanced MRI
- DCE-US
dynamic contrast-enhanced ultrasonography
- FGF
fibroblast growth factor
- GBM
glioblastoma
- HIFs
hypoxia inducible factors
- HGF
hepatocyte growth factor
- HEVs
high endothelial venules
- ICBs
immune checkpoint blockers
- IVIM DW-MRI
Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging
- LAG3
lymphocyte activation gene 3
- LTβR
lymphotoxin β receptor signaling
- MDSCs
myeloid-derived suppressor cells
- MRI
Magnetic resonance imaging
- MTT
mean transit time
- PDGF
platelet-derived growth factor
- PET
positron emission tomography
- PyMT
polyoma middle T oncoprotein
- Rgs5
G-protein signaling 5
- RT2-PNET
Rip1-Tag2 pancreatic neuroendocrine tumors
- SPECT
single-photon emission computed tomography
- TGF-β
transforming growth factor-beta
- TME
tumor microenvironment
- TIM3
T cell immunoglobulin mucin receptor 3
- Tregs
regulatory T cells
- TNF
tumor necrosis factor
- TAMs
tumor-associated macrophages
- TNFSF14
TNF superfamily member 14
Footnotes
Author Contributions: All authors developed the idea. Li collected data, drew the figures and drafted the manuscript together with Zhang. Hong revised and edited the final version of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by grants: The Natural Science Foundation of Zhejiang Province (LQ18H280006) and the Medical Science & Technology Program of Zhejiang Province, China (2019RC021).
ORCID iD: Qi Zhang  https://orcid.org/0000-0002-6096-0690
https://orcid.org/0000-0002-6096-0690
References
- 1. Dougan M, Dranoff G, Dougan SK. Cancer immunotherapy: beyond checkpoint blockade. Annu Rev Cancer Biol. 2019;3(1):55–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilkinson RW, Leishman AJ. Further advances in cancer immunotherapy: going beyond checkpoint blockade. Front Immunol. 2018;9:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lizée G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2013;64(1):71–90. [DOI] [PubMed] [Google Scholar]
- 5. Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18(6):359–376. [DOI] [PubMed] [Google Scholar]
- 6. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. [DOI] [PubMed] [Google Scholar]
- 7. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui L, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. [DOI] [PubMed] [Google Scholar]
- 9. Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. [DOI] [PubMed] [Google Scholar]
- 10. Datta M, Coussens LM, Nishikawa H, Hodi FS, Jain RK. Reprogramming the tumor microenvironment to improve immunotherapy: emerging strategies and combination therapies. Am Soc Clin Oncol Educ Book. 2019;39:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stylianopoulos T, Munn LL, Jain RK. Reengineering the tumor vasculature: improving drug delivery and efficacy. Trends Cancer. 2018;4(4):258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stambrook PJ, Maher J, Farzaneh F. Cancer immunotherapy: whence and whither. Mol Cancer Res. 2017;15(6):635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev Physiol. 2019;81:505–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 20. Karikoski M, Marttila-Ichihara F, Elima K, et al. Clever-1/Stabilin-1 controls cancer growth and metastasis. Clin Cancer Res. 2014;20(24):6452–6464. [DOI] [PubMed] [Google Scholar]
- 21. Motz GT, Santoro SP, Wang L-P, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. [DOI] [PubMed] [Google Scholar]
- 23. Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–770. [DOI] [PubMed] [Google Scholar]
- 24. Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer. 2018;4(4):292–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noman MZ, Hasmim M, Messai Y, et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309(9):C569–C579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palazon A, Tyrakis PA, Macias D, et al. An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32(5):669–683.e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res.2010;86(2):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, Pilon-Thomas S, Guvenis A, Gillies RJ. Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology. 2018;154(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses energy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72(11):2746–2756. [DOI] [PubMed] [Google Scholar]
- 30. Kareva I, Hahnfeldt P. The emerging “Hallmarks” of metabolic reprogramming and immune evasion: distinct or linked? Cancer Res. 2013;73(9):2737–2742. [DOI] [PubMed] [Google Scholar]
- 31. Bohn T, Rapp S, Luther N, et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 2018;19(12):1319–1329. [DOI] [PubMed] [Google Scholar]
- 32. Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ilhan-Mutlu A, Osswald M, Liao Y, et al. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. 2016;15(4):702–710. [DOI] [PubMed] [Google Scholar]
- 34. Kodack DP, Chung E, Yamashita H, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci U S A. 2012;109(45):E3119–E3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. [DOI] [PubMed] [Google Scholar]
- 36. Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–1874. [DOI] [PubMed] [Google Scholar]
- 38. Wang HC, Chan LP, Cho SF. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front Oncol. 2019;9:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oelkrug C, Ramage JM. Enhancement of T cell recruitment and infiltration into tumours. Clin Exp Immunol. 2014;178(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cogdill AP, Andrews MC, Wargo JA. Hallmarks of response to immune checkpoint blockade. Br J Cancer. 2017;117(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu X, Giobbie-Hurder A, Liao X, et al. Angiopoietin-2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol Res. 2017;5(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park J-S, Kim I-K, Han S, et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30(6):953–967. [DOI] [PubMed] [Google Scholar]
- 44. Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4(7):e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–625. [DOI] [PubMed] [Google Scholar]
- 47. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. [DOI] [PubMed] [Google Scholar]
- 48. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. [DOI] [PubMed] [Google Scholar]
- 50. Gavalas NG, Tsiatas M, Tsitsilonis O, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107(11):1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–4166. [PubMed] [Google Scholar]
- 52. Oussa NA, Dahmani A, Gomis M, et al. VEGF requires the receptor NRP-1 to inhibit lipopolysaccharide-dependent dendritic cell maturation. J Immunol. 2016;197(10):3927–3935. [DOI] [PubMed] [Google Scholar]
- 53. Oyama T, Ran S, Ishida T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160(3):1224–1232. [PubMed] [Google Scholar]
- 54. Kishuku M, Nishioka Y, Abe S, et al. Expression of soluble vascular endothelial growth factor receptor-1 in human monocyte-derived mature dendritic cells contributes to their antiangiogenic property. J Immunol. 2009;183(12):8176–8185. [DOI] [PubMed] [Google Scholar]
- 55. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. [DOI] [PubMed] [Google Scholar]
- 56. Maenhout SK, Thielemans K, Aerts JL. Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology. 2014;3(10):e956579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wada J, Suzuki H, Fuchino R, et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009;29(3):881–888. [PubMed] [Google Scholar]
- 58. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 59. Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–137. [DOI] [PubMed] [Google Scholar]
- 60. Augustin HG, Young Koh G, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 61. Scholz A, Lang V, Henschler R, et al. Angiopoietin-2 promotes myeloid cell infiltration in a beta(2)-integrin-dependent manner. Blood. 2011;118(18):5050–5059. [DOI] [PubMed] [Google Scholar]
- 62. Coffelt SB, Chen YY, Muthana M, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186(7):4183–4190. [DOI] [PubMed] [Google Scholar]
- 63. De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. [DOI] [PubMed] [Google Scholar]
- 64. Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178(11):7405–7411. [DOI] [PubMed] [Google Scholar]
- 65. Kloepper J, Riedemann L, Amoozgar Z, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016;113(16):4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmittnaegel M, Rigamonti N, Kadioglu E, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9(385):eaak9670. [DOI] [PubMed] [Google Scholar]
- 67. Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113(16):4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rahbari NN, Kedrin D, Incio J, et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med. 2016;8(360):360ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bessho H, Wong B, Huang D, et al. Effect of Ang-2-VEGF-A bispecific antibody in renal cell carcinoma. Cancer Invest. 2015;33(8):378–386. [DOI] [PubMed] [Google Scholar]
- 70. Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16(7):447–462. [DOI] [PubMed] [Google Scholar]
- 72. Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha–dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109(41):E2784–E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. De Silva DM, Roy A, Kato T, et al. Targeting the hepatocyte growth factor/Met pathway in cancer. Biochem Soc Trans. 2017;45(4):855–870. [DOI] [PubMed] [Google Scholar]
- 74. Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70(24):10090–10100. [DOI] [PubMed] [Google Scholar]
- 75. Ilangumaran S, Villalobos-Hernandez A, Bobbala D, Ramanathan S. The hepatocyte growth factor (HGF)–MET receptor tyrosine kinase signaling pathway: diverse roles in modulating immune cell functions. Cytokine. 2016;82:125–139. [DOI] [PubMed] [Google Scholar]
- 76. Owusu BY, Galemmo R, Janetka J, Klampfer L. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers. 2017;9(4):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hartmann S, Bhola NE, Grandis JR. HGF/Met signaling in head and neck cancer: impact on the tumor microenvironment. Clin Cancer Res. 2016;22(16):4005–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Okunishi K, Dohi M, Nakagome K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175(7):4745–4753. [DOI] [PubMed] [Google Scholar]
- 79. Benkhoucha M, Santiago-Raber M-L, Schneiter G, et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2010;107(14):6424–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20(6):660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Agrawal S, Ganguly S, Hajian P, Cao J-N, Agrawal A. PDGF upregulates CLEC-2 to induce T regulatory cells. Oncotarget. 2015;6(30):28621–28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16(2):159–178. [DOI] [PubMed] [Google Scholar]
- 85. Presta M, Chiodelli P, Giacomini A, Rusnati M, Ronca R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol Ther. 2017;179:171–187. [DOI] [PubMed] [Google Scholar]
- 86. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. [DOI] [PubMed] [Google Scholar]
- 87. Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. [DOI] [PubMed] [Google Scholar]
- 88. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Du Four S, Maenhout SK, Niclou SP, Thielemans K, Neyns B, Aerts JL. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res. 2016;6(11):2514–2531. [PMC free article] [PubMed] [Google Scholar]
- 92. Horikawa N, Abiko K, Matsumura N, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. 2017;23(2):587–599. [DOI] [PubMed] [Google Scholar]
- 93. Liu X-D, Hoang A, Zhou L, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3(9):1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lorgis V, Maura G, Coppa G, et al. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol. 2012;107(2):351–358. [DOI] [PubMed] [Google Scholar]
- 95. Levin VA, Mendelssohn ND, Chan J, et al. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol. 2015;122(1):145–150. [DOI] [PubMed] [Google Scholar]
- 96. Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014;8(3):696–706. [DOI] [PubMed] [Google Scholar]
- 97. Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. [DOI] [PubMed] [Google Scholar]
- 98. Falcon BL, Hashizume H, Koumoutsakos P, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175(5):2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Reardon DA, Lassman AB, Schiff D, et al. Phase 2 and biomarker study of trebananib, an angiopoietin-blocking peptibody, with and without bevacizumab for patients with recurrent glioblastoma. Cancer. 2018;124(7):1438–1448. [DOI] [PubMed] [Google Scholar]
- 100. Manzur M, Hamzah J, Ganss R. Modulation of G protein signaling normalizes tumor vessels. Cancer Res. 2009;69(2):396–399. [DOI] [PubMed] [Google Scholar]
- 101. Ganss R. Keeping the balance right: regulator of g protein signaling 5 in vascular physiology and pathology. Prog Mol Biol Transl Sci. 2015;133:93–121. [DOI] [PubMed] [Google Scholar]
- 102. Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–414. [DOI] [PubMed] [Google Scholar]
- 103. Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998;4(4):408–414. [DOI] [PubMed] [Google Scholar]
- 104. Talmadge JE, Tribble HR, Pennington RW, Phillips H, Wiltrout RH. Immunomodulatory and immunotherapeutic properties of recombinant γ-interferon and recombinant tumor necrosis factor in mice. Cancer Res. 1987;47(10):2563–2570. [PubMed] [Google Scholar]
- 105. Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A. 2012;109(20):7841–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Deng J, Liu X, Rong L, et al. IFNgamma-responsiveness of endothelial cells leads to efficient angiostasis in tumours involving down-regulation of Dll4. J Pathol. 2014;233(2):170–182. [DOI] [PubMed] [Google Scholar]
- 107. Kammertoens T, Friese C, Arina A, et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017;545(7652):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. He B, Jabouille A, Steri V, et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J Pathol. 2018;245(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Treps L. EnLIGHTenment of tumor vessel normalization and immunotherapy in glioblastoma. J Pathol. 2018;246(1):3–6. [DOI] [PubMed] [Google Scholar]
- 110. Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–617. [DOI] [PubMed] [Google Scholar]
- 111. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zheng X, Fang Z, Liu X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest. 2018;128(5):2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. García-Figueiras R, Goh VJ, Padhani AR, et al. CT Perfusion in oncologic imaging: a useful tool? AJR Am J Roentgenol. 2013;200(1):8–19. [DOI] [PubMed] [Google Scholar]
- 114. Wang M, Li B, Sun H, et al. Correlation study between dual source CT perfusion imaging and the microvascular composition of solitary pulmonary nodules. Lung Cancer. 2019;130:115–120. [DOI] [PubMed] [Google Scholar]
- 115. Yao J, Yang Z-G, Chen H-J, Chen T-W, Huang J. Gastric adenocarcinoma: can perfusion CT help to noninvasively evaluate tumor angiogenesis? Abdom Imaging. 2011;36(1):15–21. [DOI] [PubMed] [Google Scholar]
- 116. Heist RS, Duda DG, Sahani DV, et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc Natl Acad Sci U S A. 2015;112(5):1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schmitz S, Rommel D, Michoux N, et al. Dynamic contrast-enhanced computed tomography to assess early activity of cetuximab in squamous cell carcinoma of the head and neck. Radiol Oncol. 2015;49(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cao N, Cao M, Chin-Sinex H, Mendonca M, Ko SC, Stantz KM. Monitoring the effects of anti-angiogenesis on the radiation sensitivity of pancreatic cancer xenografts using dynamic contrast-enhanced computed tomography. Int J Radiat Oncol Biol Phys. 2014;88(2):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cao M, Liang Y, Shen C, Miller KD, Stantz KM. Developing DCE-CT to quantify intra-tumor heterogeneity in breast tumors with differing angiogenic phenotype. IEEE Trans Med Imaging. 2009;28(6):861–871. [DOI] [PubMed] [Google Scholar]
- 120. Yang J, Liao C, Liu Y, et al. MR imaging biomarkers evaluating vascular normalization window after anti-vessel treatment. Oncotarget. 2018;9(15):11964–11976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hectors SJ, Jacobs I, Lok J, et al. Improved evaluation of antivascular cancer therapy using constrained tracer-kinetic modeling for multiagent dynamic contrast-enhanced MRI. Cancer Res. 2018;78(6):1561–1570. [DOI] [PubMed] [Google Scholar]
- 122. Liang J, Cheng Q, Huang J, et al. Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis. 2019;22(3):457–470. [DOI] [PubMed] [Google Scholar]
- 123. Wu C, Pineda F, Hormuth II DA, Karczmar GS, Yankeelov TE. Quantitative analysis of vascular properties derived from ultrafast DCE-MRI to discriminate malignant and benign breast tumors. Magn Reson Med. 2019;81(3):2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am. 2011;22(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shi C, Liu D, Xiao Z, et al. Monitoring tumor response to antivascular therapy using non-contrast intravoxel incoherent motion diffusion-weighted MRI. Cancer Res. 2017;77(13):3491–3501. [DOI] [PubMed] [Google Scholar]
- 126. Song X-L, Wang L, Ren H, Wei R, Ren J-L, Niu J. Intravoxel incoherent motion imaging in differentiation borderline from malignant ovarian epithelial tumors: correlation with histological cell proliferation and vessel characteristics. J Magn Reson Imaging. 2020;51(3):928–935. [DOI] [PubMed] [Google Scholar]
- 127. Lee H-J, Rha SY, Chung YE, et al. Tumor perfusion-related parameter of diffusion-weighted magnetic resonance imaging: correlation with histological microvessel density. Magn Reson Med. 2014;71(4):1554–1558. [DOI] [PubMed] [Google Scholar]
- 128. Klau M, Mayer P, Bergmann F, et al. Correlation of histological vessel characteristics and diffusion-weighted imaging intravoxel incoherent motion–derived parameters in pancreatic ductal adenocarcinomas and pancreatic neuroendocrine tumors. Invest Radiol. 2015;50(11):792–797. [DOI] [PubMed] [Google Scholar]
- 129. Pan J-H, Zhu S, Huang J, et al. Monitoring the process of endostar-induced tumor vascular normalization by non-contrast intravoxel incoherent motion diffusion-weighted MRI. Front Oncol. 2018;8:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hoyt K, Umphrey H, Lockhart M, Robbin M, Forero-Torres A. Ultrasound imaging of breast tumor perfusion and neovascular morphology. Ultrasound Med Biol. 2015;41(9):2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lassau N, Coiffier B, Kind M, et al. Selection of an early biomarker for vascular normalization using dynamic contrast-enhanced ultrasonography to predict outcomes of metastatic patients treated with bevacizumab. Ann Oncol. 2016;27(10):1922–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen DR, Lin C, Wang YF. Window of opportunity: a new insight into sequential bevacizumab and paclitaxel in two cases of metastatic triple-negative breast cancer. Exp Ther Med. 2015;10(3):885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kristian A, Revheim ME, Qu H, et al. Dynamic 18F-FDG-PET for monitoring treatment effect following anti-angiogenic therapy in triple-negative breast cancer xenografts. Acta Oncol. 2013;52(7):1566–1572. [DOI] [PubMed] [Google Scholar]
- 134. Vangestel C, Van de Wiele C, Van Damme N, et al. (99)mTc-(CO)(3) His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab. J Nucl Med. 2011;52(11):1786–1794. [DOI] [PubMed] [Google Scholar]
- 135. Li W, Quan YY, Li Y, Lu L, Cui M. Monitoring of tumor vascular normalization: the key points from basic research to clinical application. Cancer Manag Res. 2018;10:4163–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bauerschlag DO, Hilpert F, Meier W, et al. Evaluation of potentially predictive markers for anti-angiogenic therapy with sunitinib in recurrent ovarian cancer patients. Transl Oncol. 2013;6(3):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wada H, Nagano H, Yamamoto H, et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int. 2006;26(4):414–423. [DOI] [PubMed] [Google Scholar]
- 138. Hacker UT, Escalona-Espinosa L, Consalvo N, et al. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial. Br J Cancer. 2016;114(8):855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schulz P, Fischer C, Detjen KM, et al. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25(10):3325–3335. [DOI] [PubMed] [Google Scholar]
- 140. Goede V, Coutelle O, Neuneier J, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;103(9):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2(7):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wu X, Giobbie-Hurder A, Liao X, et al. VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer Immunol Res. 2016;4(10):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 145. Grimm M-O, Leucht K, Grünwald V, Foller S. New first line treatment options of clear cell renal cell cancer patients with PD-1 or PD-L1 immune-checkpoint inhibitor-based combination therapies. J Clin Med. 2020;9(2):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25(2):515–523. [DOI] [PubMed] [Google Scholar]
- 147. Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(8):1109–1123. [DOI] [PubMed] [Google Scholar]
- 148. Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol. 2018;52(Pt 2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]