Summary
Early lymphocyte recovery following autologous stem cell transplant for Non-Hodgkin’s Lymphoma has been reported to be associated with improved outcome. The significance of early lymphocyte recovery following a stem cell transplant in NHL subtype -Diffuse Large B cell Lymphoma in the rituximab era remains unclear. Patients who underwent an ASCT at our institution for diffuse large B cell Lymphoma during the time period 1998–2008 (n=115) were included in the study. Patient characteristics were well-balanced in both rituximab naïve and rituximab exposed groups. Overall survival of the entire cohort was comparable with previous studies. Prior rituximab therapy did not affect lymphocyte recovery on day 14 (p=0.95) or day 28 (p=0.27). Lymphocyte recovery on day 14 and day 28 and prior rituximab had no impact on transplant outcome on long term follow-up despite early benefit. Other factors such as age, stage at presentation, number of salvage regimens, mobilization procedure, conditioning regimen, pre-transplant radiation therapy, and pre-transplant disease status (CR vs. PR in chemotherapy-sensitive disease) had no impact on survival. In conclusion, our data showed that the survival benefit with early lymphocyte recovery and prior rituximab seen in previous reports may be lost with longer follow up. Prior rituximab therapy does not appear to influence the lymphocyte count at day 14, 28 following ASCT. Our findings suggest that future trials should consider manipulating the immune system as a post- transplant intervention to improve long term outcome.
Keywords: Diffuse large B-cell lymphoma, rituximab, autologous stem cell transplant, early lymphocyte recovery, Absolute lymphocyte count
Introduction:
Relapsed diffuse large B cell lymphoma (DLBCL) is often curable with high dose therapy followed by autologous stem cell transplantation (ASCT). The Parma trial published in the prerituximab era is the only prospective randomized trial that established ASCT as the standard of care for eligible patients with relapsed refractory large B cell lymphoma (Philip, et al 1995). Several factors have been recognized as potential prognostic factors on transplant outcome. In non-Hodgkin’s lymphoma, chemotherapy sensitive disease, rituximab use either at initial diagnosis or at disease relapse, higher stem cell dose infusion, relapse greater than 12 months from the time of initial diagnosis have all been associated with improved outcome in patients undergoing ASCT (Nademanee, et al 2000, Yoon, et al 2009). Rituximab appears to improve the clinical outcome among patients undergoing high dose therapy (Fenske, et al 2009). Recently it has been suggested that patients who received ASCT for NHL, early recovery of the lymphocyte count (ELR), defined typically as an absolute lymphocyte count (ALC) at approximately two to four weeks following therapy has been associated with superior progression-free and overall survival in patients with NHL (Gordan, et al 2003, Porrata, et al 2001).
Recovery of certain lymphocyte subsets suggests anti-tumor effects of self immune system. Specifically, early recovery of natural killer cell subtype is of clinical significance and may be of prognostic value (Porrata, et al 2008).
Previous studies that have identified ELR as an independent prognostic factor in predicting outcome grouped several NHL histologies and did not specifically look at DLBCL. Long term impact of rituximab, which is known to deplete B lymphocytes by binding to the CD20 domain, on early lymphocyte recovery and transplant outcome is lacking.
To address these major short comings, we evaluated patients with only diffuse large B cell histology who were either rituximab naïve or exposed to rituximab during their course of treatment followed by ASCT. Here, we study the effects of ELR on patient outcome.
Patients & Methods
Sample size and data collection
One hundred fifteen consecutive patients who underwent ASCT for relapsed DLBCL at a single institution between January 1998 and December 2008 were included in our systematic analysis. In order to perform clinically meaningful analysis of eight different variables on outcome we determined that a sample size of at least eighty patients with complete data set was required for this study. This study was approved by the Vanderbilt University Institutional Review Board and was in accordance with the United States Federal Regulations and Declaration of Helsinki. Patient’s basic demographic data (age, gender), disease-related information (stage, grade, histology, age at diagnosis, stage at diagnosis, and pre-transplant therapy including presence or absence of rituximab), stem cell mobilization, conditioning regimens, engraftment, relapse, and survival information were collected from the institution’s electronic medical record. The primary end point of this study was to determine if ALC at day 14 following an ASCT in patients with DLBCL can predict survival in the rituximab era.
Diffuse large B cell lymphoma and histologies
Pathology that was reviewed and confirmed by hematopathologists at Vanderbilt University as DLBCL was included in the study. Patients with a diagnosis of T cell rich large B cell, histiocytic rich large B cell and follicular grade 3 with diffuse B cell like features were also included. Patients with transformed lymphoma were excluded from the study.
Absolute lymphocyte count
Absolute lymphocyte count (109/l) at days 14± 1day and day 28 ± 1 day following autologous stem cell infusion were reported. Based on prior reports, it was determined that absolute lymphocyte counts of 500 cells/μL at day 14 or 1000 cells/μL at day 28 were accepted cutoff values and could be dichotomized at this level of significance.
Rituximab exposure
Patients who had no exposure to rituximab either at the time of diagnosis or at relapse were termed rituximab naïve and patients who have had any exposure during the course of therapy and have received at least two doses of 375mg/mt2 of rituximab prior to ASCT were defined as being exposed to rituximab.
Stem cell mobilization and conditioning regimens
Stem cells were collected either via peripheral blood or bone marrow harvest. Patients who were inadequately mobilized from peripheral blood underwent bone marrow harvest. Stem cells were mobilized with cyclophosphamide alone, G-CSF alone or with cyclophosphamide and G-CSF combination. Conditioning regimens included: CBV (cyclophosphamide 7200 mg/m2, etoposide 2000 mg/m2, and BCNU 400 mg/ m2), cyclophosphamide 60mg/m2and total body irradiation (TBI) 12Gy, BEAM (BCNU 300 mg/m2, etoposide 100 mg/m2, ARA-C 100 mg/m2, and melphalan 140mg/m2) or BEAC (BCNU 300 mg/m2, etoposide 100 mg/m2, ARA-C 100 mg/m2, and cyclophosphamide 35mg/kg).
Response criteria
Response criteria were based on guidelines from the International workshop on NHL (Cheson, et al 1999). Complete remission (CR) was defined as complete radiological regression of all prior measurable disease or bone marrow involvement. Partial response was defined as a reduction of 75% or greater reduction in the sum of the products of the longest and perpendicular diameter of measurable lesions. Based on these criteria, all data was individually verified regarding the best response status just prior to ASCT after administration of at least 2–4 cycles of chemotherapy.
Statistical analysis
Data was analyzed using SPSS software (v.17). Overall survival was reported by using Kaplan-Meir analysis. The difference between survival curves was tested for statistical significance using log-rank test. ALC at two time intervals (Day 14 and Day 28) were assessed as a continuous variable and dichotomized as either 500 cells/μL at day 14 or ≥ 1000 cells/μL at day 28 as previously described or as ≥ median lymphocyte count at day 14 (660 cells/μL) or day 28 (1020 cells/μL). Univariate analysis was performed on several other disease related factors that were determined a priori. The factors analyzed include, remission status at the time of transplant, stem cell dose, number of chemotherapy regimens and prior rituximab exposure. Chi-square tests were used to determine the relationship between all categorical variables. Cox-proportional hazard model was use for multivariable analysis. For association between continuous variables and categories Mann-Whitney U rank sum tests were used. All p-values reported were two-sided and statistical significance was declared at p<0.05. Cases with missing values were deleted from final analysis.
Results
Patient characteristics
Baseline characteristics are reported in Table 1. A total of one hundred thirteen patients were included in the final analysis. Two patients were excluded due to lack of complete data set. There were no significant differences among patients who were exposed to rituximab or those who were rituximab naïve regarding age, sex, stage, pre-transplant remission status, number of pre-transplant regimens or conditioning regimens. Patients in the rituximab exposed group did have a statistically significant number of patients with a diagnosis of DLBCL – with follicular grade 3 type. Sixty nine (78.4%) patients continued to be in CR at day 100 post- ASCT evaluation. Nineteen (21.6%) patients had persistent diseases requiring further therapy.
Table 1.
Baseline characteristics of patients who were exposed to rituximab and those who were Rituximab naïve.
| Patient Characteristics | Rituximab n=69 | Rituximab Naïve n=41 |
|---|---|---|
| Sex | ||
| Male | 43 | 27 |
| Female | 26 | 14 |
| Age at diagnosis | ||
| Median | 52 years | 46 years |
| Range | 24–69 | 24–64 |
| Stage at Diagnosis (%) | ||
| (I,II) | 36% | 29% |
| (III/IV) | 64% | 71% |
| Histology (%) | ||
| DLBCL | 81% | 98% |
| DLBCL- T Cell rich B cell | 9% | 2% |
| DLBCL- follicular grade 3 | 10% | 0% (p=0.01) |
| # Pre-transplant salvage regimens | ||
| Mean | 1.4 | 1.3 |
| Range | 1–5 | 1–4 |
| Pretransplant disease status (%) | ||
| Complete Remission | 56% | 32% (p=0.07) |
| Partial Response | 35% | 41% |
| Conditioning Regimen (%) | ||
| CBV | 95% | 76% |
| Cy/TBI | 1% | 18% |
| BEAC or BEAM | 4% | 6% |
DLBCL: Diffuse Large B cell lymphoma, CBV: cyclophosphamide, BCNU and etoposide, Cy/TBI: Cytoxan/Total body radiation, BEAC: BCNU, etoposide, ARA-C, cyclophosphamide, BEAM: BCNU, etoposide, ARA-C and melphalan
Overall survival of patients following autologous stem cell transplant
The median survival of patients following ASCT for DLBCL was 38.7 months (range 3–186 months) (Figure 1A). At the time analysis 69 (60.5%) patients were alive after median follow-up of 22.3 months (range: 3.2–184.3 months). 18 (15.6%) patients were alive beyond 5 years post-ASCT. Prior rituximab showed a initial survival advantage, however on long term follow-up, benefit was lost due to late relapses (p=0.12) (Figure 1B).
Figure 1a:
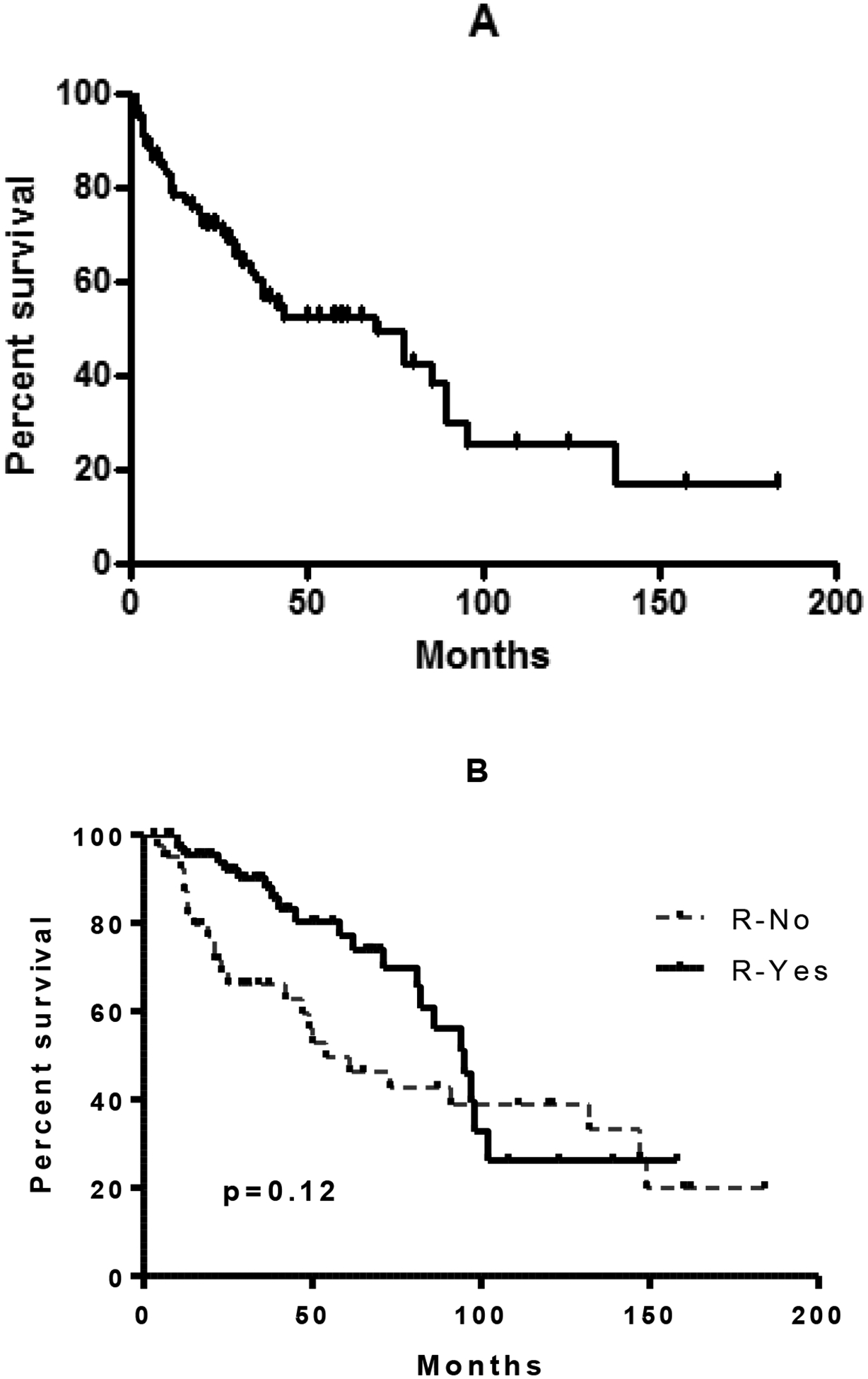
Overall survival of 110 patients with DLBCL after ASCT. Figure 1b: Survival of all patients who were rituximab naïve and rituximab exposed.
Univariate and multivariate analysis
In a univariate analysis from the time of ASCT, it appears that prior rituximab therapy significantly improved the overall outcome of patients with a hazard ratio (HR) of 0.5 (95% CI: 0.27–0.95) and p=0.03. In the multivariate analysis considering factors such as age, stage and ALC14, prior rituximab therapy has no impact on patient outcome HR=0.71 (95% CI: 0.33–1.47) Table 2.
Table 2:
Univariate analysis of survival
| 2.92 | 0.38 to 22.67 | 0.58 | |
| 3.32 | 0.44 to 25.27 | ||
| 2.94 | 0.38 to 22.52 | ||
| 0.53 | 0.23 to 1.21 | 0.11 | |
| 0.6 | 0.32 to 1.13 | 0.11 | |
| Pre- SCT disease status (CR vs. PR) | 0.95 | 0.47 to 1.9 | 0.06 |
| 1.53 | 0.21 to 11.19 | 0.19 | |
| 0.93 | 0.32 to 2.68 | 0.89 | |
| 1.29 | 0.53 to 3.18 | 0.31 | |
| 0.92 | 0.43 to 1.94 | 0.18 |
Pre-transplant disease status and outcome
Forty (56%) patients were in CR and 25 (35%) in PR in patients with prior rituximab group compared with13 (32%) and 17 (41%) in CR and PR respectively among rituximab naïve cohort. The difference in rates of CR did not reach statistical significance (p=0.07). Pre-transplant disease status (CR vs. PR) did not have a significant impact on survival (Figure 2A). The number of salvage regimens prior to ASCT also did not significantly impact the absolute lymphocyte count or survival (Figure 2B).
Figure 2a and 2b:
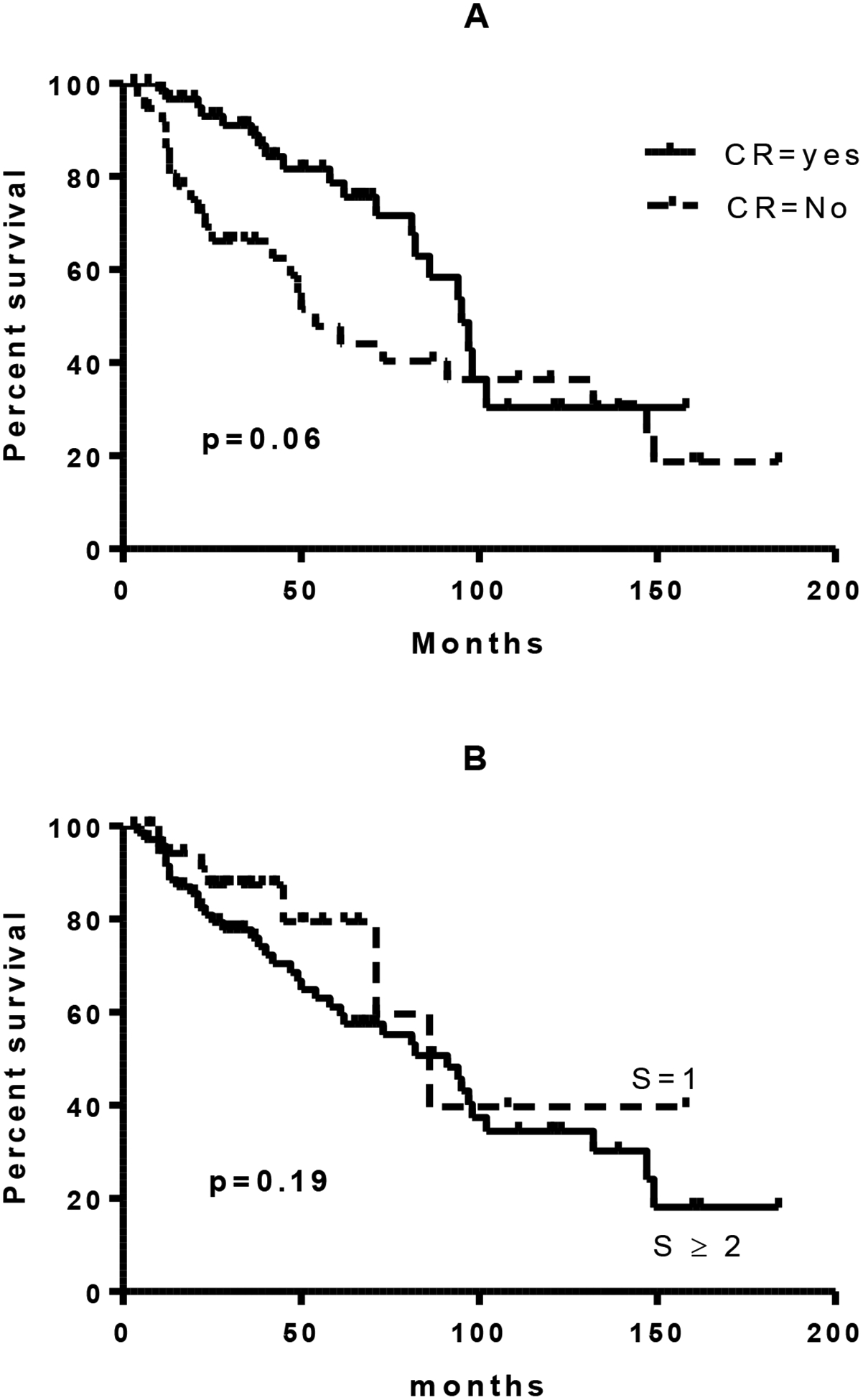
Survival of patients as a function of pre-transplant disease status or number of pre-transplant salvage regimens
Lymphocyte recovery and outcome
Absolute lymphocyte count at day +14 (ALC-14) and at day +28 (ALC-28) after ASCT were available on 90/113 patients and 85/113 patients, respectively. There was no significant difference in either ALC-14 or ALC-28 in patients who were rituximab exposed or naïve (Figure 3A, 3B.). Neither ALC-14 nor ALC-28 was associated with a significant difference in transplant outcome, irrespective of rituximab status (Figure 4.). Both ALC-14 and ALC-28 were analyzed as continuous and categorical variables without any significant difference in outcome. Analyzing ALC neither above or below 500 cells/μL nor above or below the median ALC resulted in any significant difference in outcome (data not shown). Patients with an absolute lymphocyte count of 1000cells/μL at day 28 had an initial improved survival compared with patients with less than 1000cells/μL at day 28 (p=0.03). This survival benefit was however lost with long term follow up.
Figure 3:
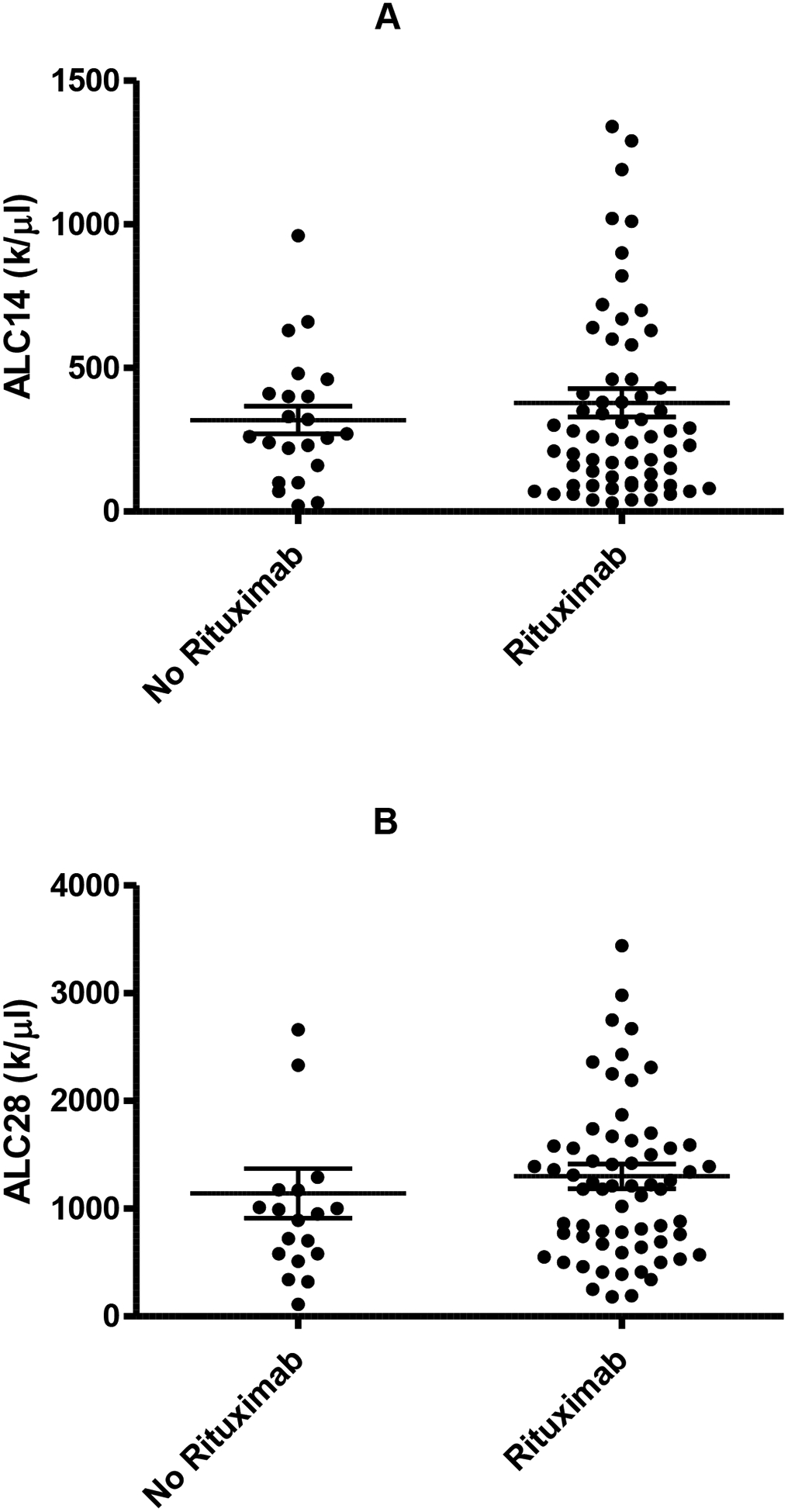
Effect of rituximab on absolute lymphocyte count post ASCT on day +14 (A; no rituximab n=22; rituximab n=65) and day +28 (B; no rituximab n=19; rituximab n=65).
Figure 4:
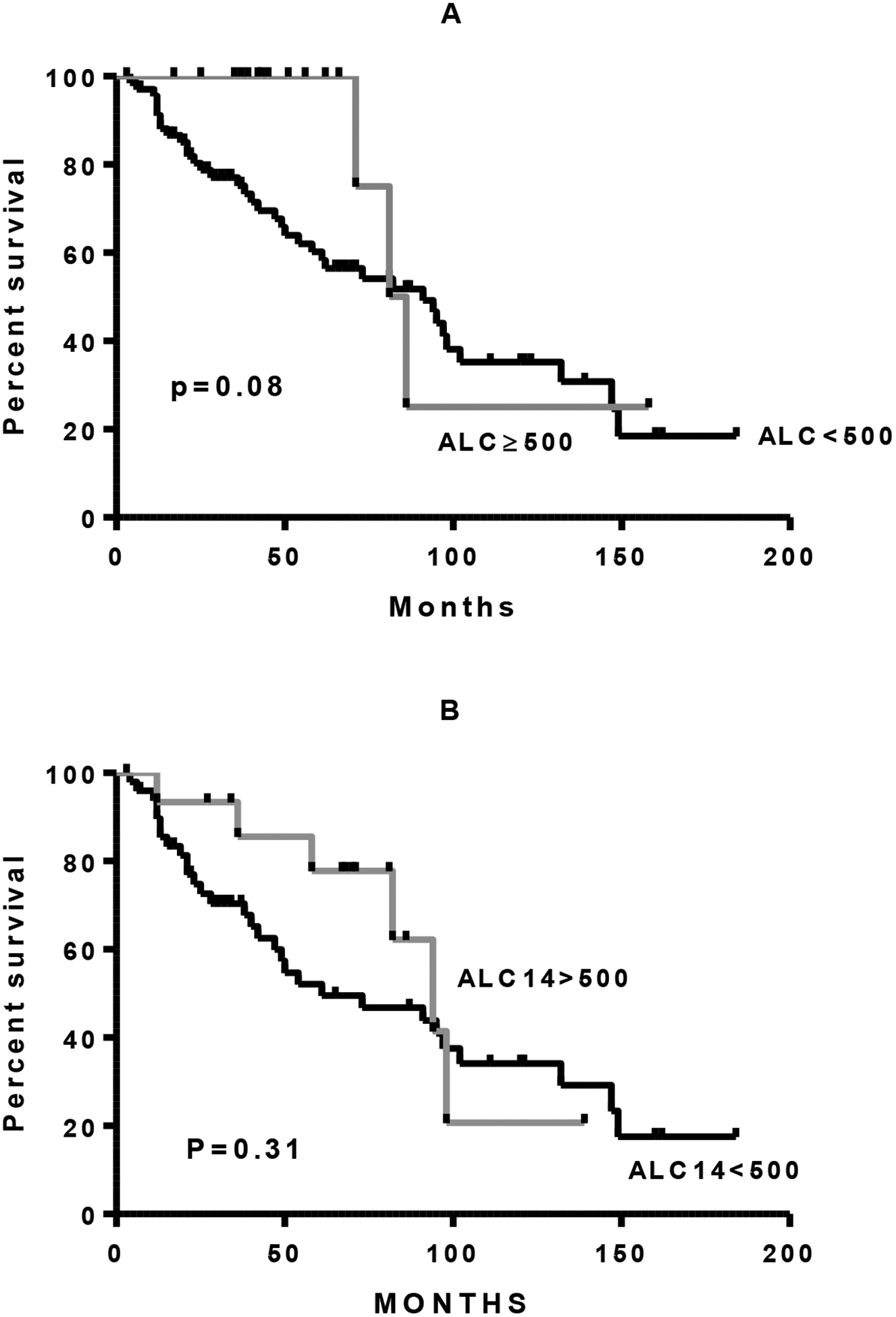
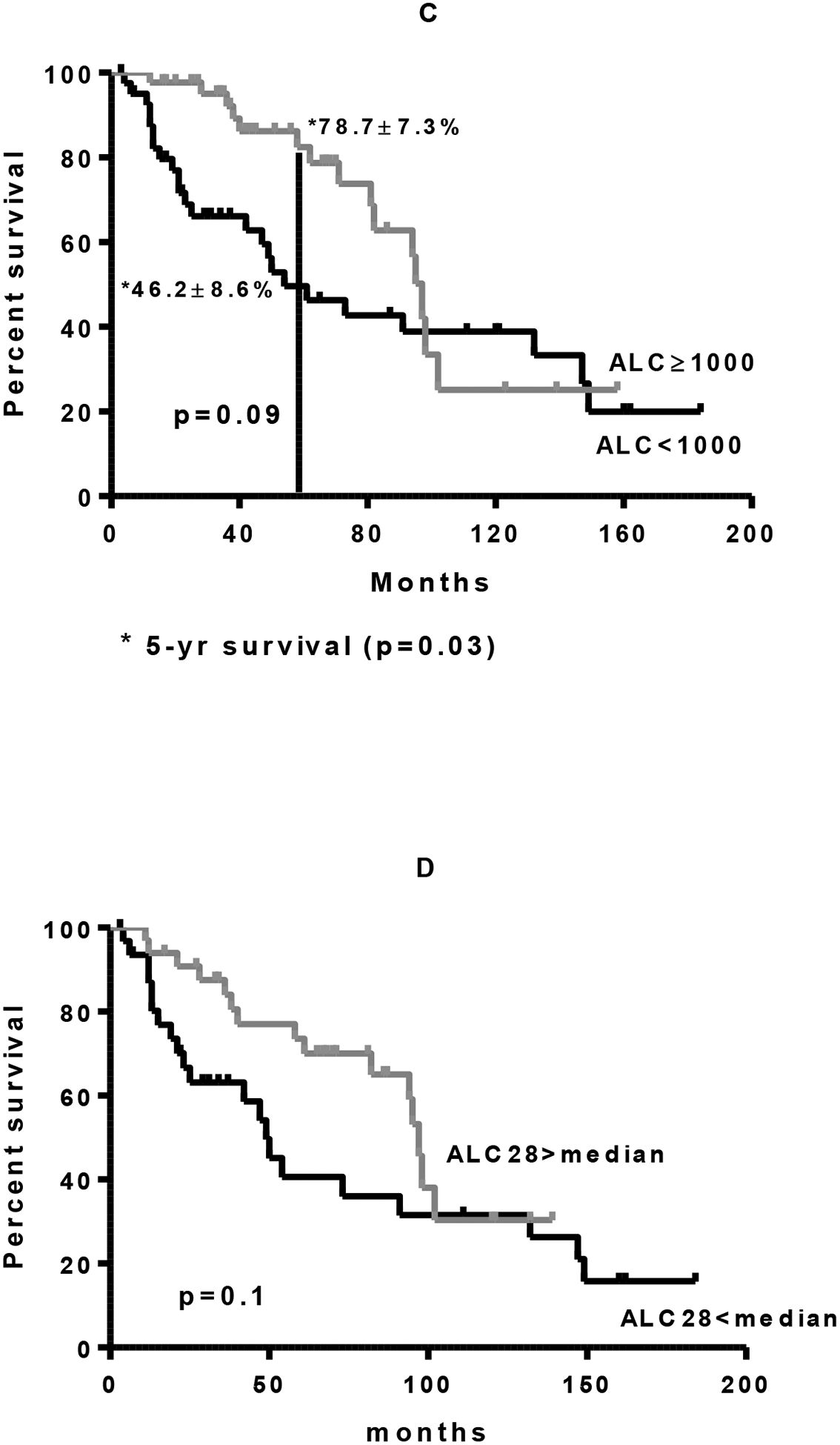
Overall survival with DLBCL after ASCT as a function of absolute lymphocyte count (ALC) of less than 500 cells/μL versus greater than or equal to 500 cells/μL. A. Day +14 entire cohort. B. Day +14 only patients who received rituximab. C. Day +28 entire cohort. D. Day +28 only patients who received rituximab.
Discussion
Our results demonstrate that the early survival advantage seen post- ASCT in patients with an early lymphocyte recovery is lost with long-term follow-up. The advantages of prior rituximab exposure were also lost in the long term. Our data also showed prior rituximab had no impact on early lymphocyte recovery.
The current standard of care for eligible patients with relapsed DLBCL is high dose therapy followed by an ASCT. Previous data suggests that lymphocyte recovery greater than or equal to 500cells/μL at day +14 after ASCT is an independent prognostic factor and is associated with improved disease-free and overall survival in NHL as well as other hematological malignancies (Kim, et al 2006, Porrata, et al 2005, Porrata, et al 2008). These studies however grouped patients with several histologies. The number of peripheral blood autograft absolute lymphocytes infused appears to correlate with day 15 absolute lymphocyte count (Porrata, et al 2004)
The role of rituximab on lymphocyte recovery and its effects on other lymphocyte subsets such as T cells and NK cells are largely unknown. Rituximab is known to cause lymphopenia by the depleting the B cells. Rituximab does not appear to impact the T cells or NK cells. These effects may last for as long as six months for full recovery of lymphocytes (Ghielmini, et al 2004). In mantle cell lymphoma, the absolute lymphocyte count at day 15 (ALC-15) following an ASCT was associated with a significant improvement in clinical outcome. ALC-15 ≥ 500 cell/μl had an improved progression free and overall survival (Joao, et al 2006). The exact mechanism as to why early lymphocyte recovery would lead to improved survival in patients following ASCT is unclear. It has been hypothesized that early effective immune recovery may contribute to the graft versus tumor effect. Our results however do not draw similar conclusions in diffuse large B cell lymphoma. The quantitative early recovery of lymphocytes is effective in eradicating residual disease as demonstrated by the early survival advantage. This does not however translate to prolonged disease free interval suggesting the loss of ability of immune reconstitution cells to maintain the graft versus tumor effect. In a recently published study by Porrata et al, included lymphocyte subsets analysis in patients who were all treated with prior rituximab therapy, reported the NK cell subset was associated with superior PFS and OS among a heterogeneous group of NHL patients (Porrata, et al 2008).
Our study lacks data on lymphocyte subsets. We however report our analysis in a large homogenous population with the longest follow up of patients. Our results are in agreement with the published results by Tiwari et al., who demonstrated that the routine use of GM-CSF post transplant alters the prognostic significance of early lymphocyte recovery (Tiwari, et al 2007). In diffuse large B cell lymphoma, lymphopenia at the time of diagnosis or relapse defined as an ALC of <1.0×109/L was an independent prognostic marker for overall survival. This was an independent prognostic factor irrespective of the international prognostic index (Cox, et al 2008, Porrata, et al). Furthermore, the use of rituximab improves overall survival in patients with a low ALC (Oki, et al 2008). In the future; it would be interesting to see if specific lymphocyte subsets at the time of relapse can predict response to chemotherapy as immune reconstitution involves several components of the immune response including reconstitution of functional B cells and effective antigen presenting cells, reappearance of cytotoxic T lymphocytes and NK cells that aid in eradication of minimal residual disease (Guillaume, et al 1998).
In conclusion, this study demonstrates that rituximab does not have an impact on absolute lymphocyte count at day 14 or 28 after ASCT for DLBCL. The survival benefit with early lymphocyte recovery and prior rituximab exposure may be lost with longer follow up. Future studies should focus on lymphocyte subsets in the rituximab era. Our study is limited by the inherent weakness related to its retrospective nature. There is a need for prospective studies with post- transplant treatment interventions to prevent late relapses even in patients with favorable group with prior rituximab exposure and rapid early lymphocyte recovery after ASCT.
Table 3 :
Multivariate analysis of survival:
| Factor | HR | 95% CI | p-value |
|---|---|---|---|
| Age | 1.01 | 0.977 to 1.044 | 0.56 |
| Stage (I vs. others) | 1.49 | 0.700 to 3.186 | 0.30 |
| Pre-SCT Rituximab | 0.70 | 0.331 to 1.470 | 0.34 |
| ALC 14 (> median 660×109/l) | 1.00 | 0.999 to 1.001 | 0.73 |
Acknowledgements
The authors would like to acknowledge Carole Hunt for her contribution towards this manuscript.
Grant support: Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH, and 5K-12 CA090625-09
References
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R & Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol, 17, 1244. [DOI] [PubMed] [Google Scholar]
- Cox MC, Nofroni I, Ruco L, Amodeo R, Ferrari A, La Verde G, Cardelli P, Montefusco E, Conte E, Monarca B & Aloe-Spiriti MA (2008) Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leuk Lymphoma, 49, 1745–1751. [DOI] [PubMed] [Google Scholar]
- Fenske TS, Hari PN, Carreras J, Zhang MJ, Kamble RT, Bolwell BJ, Cairo MS, Champlin RE, Chen YB, Freytes CO, Gale RP, Hale GA, Ilhan O, Khoury HJ, Lister J, Maharaj D, Marks DI, Munker R, Pecora AL, Rowlings PA, Shea TC, Stiff P, Wiernik PH, Winter JN, Rizzo JD, van Besien K, Lazarus HM & Vose JM (2009) Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant, 15, 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, Hess U, Zucca E, Stupp R, Kovacsovics T, Helg C, Lohri A, Bargetzi M, Vorobiof D & Cerny T (2004) Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood, 103, 4416–4423. [DOI] [PubMed] [Google Scholar]
- Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA & Moreb JS (2003) Correlation of early lymphocyte recovery and progression-free survival after autologous stem-cell transplant in patients with Hodgkin’s and non-Hodgkin’s Lymphoma. Bone Marrow Transplant, 31, 1009–1013. [DOI] [PubMed] [Google Scholar]
- Guillaume T, Rubinstein DB & Symann M (1998) Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood, 92, 1471–1490. [PubMed] [Google Scholar]
- Joao C, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA & Markovic SN (2006) Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant, 37, 865–871. [DOI] [PubMed] [Google Scholar]
- Kim H, Sohn HJ, Kim S, Lee JS, Kim WK & Suh C (2006) Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma. Bone Marrow Transplant, 37, 1037–1042. [DOI] [PubMed] [Google Scholar]
- Nademanee A, Molina A, Dagis A, Snyder DS, O’Donnell MR, Parker P, Stein A, Smith E, Planas I, Kashyap A, Spielberger R, Fung H, Krishnan A, Bhatia R, Wong KK, Somlo G, Margolin K, Chow W, Sniecinski I, Vora N, Slovak M, Niland JC & Forman SJ (2000) Autologous stem-cell transplantation for poor-risk and relapsed intermediate- and high-grade non-Hodgkin’s lymphoma. Clin Lymphoma, 1, 46–54. [DOI] [PubMed] [Google Scholar]
- Oki Y, Yamamoto K, Kato H, Kuwatsuka Y, Taji H, Kagami Y & Morishima Y (2008) Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients’ survival benefit from rituximab. Eur J Haematol, 81, 448–453. [DOI] [PubMed] [Google Scholar]
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL & et al. (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med, 333, 1540–1545. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, Gastineau DA, Dispenzieri A, Ansell SM, Micallef IN, Geyer SM & Markovic SN (2001) Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood, 98, 579–585. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Gertz MA, Litzow MR, Lacy MQ, Dispenzieri A, Inwards DJ, Ansell SM, Micallef IN, Gastineau DA, Elliott M, Hogan WJ, Hayman SR, Tefferi A & Markovic SN (2005) Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis. Clin Cancer Res, 11, 1210–1218. [PubMed] [Google Scholar]
- Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Litzow MR, Winters JL & Markovic SN (2008) Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant, 14, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Hogan WJ & Markovic SN New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant, 16, 376–383. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Litzow MR, Inwards DJ, Gastineau DA, Moore SB, Pineda AA, Bundy KL, Padley DJ, Persky D, Ansell SM, Micallef IN & Markovic SN (2004) Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant, 33, 291–298. [DOI] [PubMed] [Google Scholar]
- Tiwari D, Gao F, Hidalgo J, Adkins DR, Vij R, DiPersio JF & Khoury HJ (2007) Prognostic significance of early lymphocyte recovery after post-autografting administration of GM-CSF in non-Hodgkin’s lymphoma. Bone Marrow Transplant, 40, 671–675. [DOI] [PubMed] [Google Scholar]
- Yoon DH, Sohn BS, Jang G, Kim EK, Kang BW, Kim C, Kim JE, Kim S, Lee DH, Lee JS, Park SJ, Park CJ, Huh J & Suh C (2009) Higher infused CD34+ hematopoietic stem cell dose correlates with earlier lymphocyte recovery and better clinical outcome after autologous stem cell transplantation in non-Hodgkin’s lymphoma. Transfusion, 49, 1890–1900. [DOI] [PubMed] [Google Scholar]


