The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has reminded us of the critical role of an effective host immune response and the devastating effect of immune dysregulation. This year marks 10 years since the first description of a cytokine storm that developed after chimeric antigen receptor (CAR) T-cell therapy1 and 27 years since the term was first used in the literature to describe the engraftment syndrome of acute graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation.2 The term “cytokine release syndrome” was coined to describe a similar syndrome after infusion of muromonab-CD3 (OKT3).3 Cytokine storm and cytokine release syndrome are life-threatening systemic inflammatory syndromes involving elevated levels of circulating cytokines and immune-cell hyperactivation that can be triggered by various therapies, pathogens, cancers, autoimmune conditions, and monogenic disorders.
From a historical perspective, cytokine storm was previously referred to as an influenza-like syndrome that occurred after systemic infections such as sepsis and after immunotherapies such as Coley’s toxins.4 Yersinia pestis infection (i.e., the plague) has led to major pandemics (e.g., the Black Death) and triggers alveolar macrophages to produce excessive amounts of cytokines, resulting in cytokine storm.5 An exaggerated immune response was suspected to contribute to the lethality of the 1918–1919 influenza pandemic. In fact, a reconstructed H1N1 virus isolated from the 1918 pandemic, as compared with common reference strains of the virus that causes influenza A, triggered marked pulmonary inflammation in mice.6 Recognition that the immune response to the pathogen, but not the pathogen itself, can contribute to multiorgan dysfunction and that similar cytokine storm syndromes could occur with no obvious infection led to the investigation of immunomodulators and cytokine-directed therapies. One of the earliest targeted therapies for abrogation of a cytokine storm was the anti–interleukin-6 receptor monoclonal antibody tocilizumab, which was developed for the treatment of idiopathic multicentric Castleman’s disease in the 1990s. A host of other disorders have been described as causes of cytokine storm and targeted with immune-directed therapies, such as sepsis, primary and secondary hemophagocytic lymphohistiocytosis (HLH), autoinflammatory disorders, and coronavirus disease 2019 (Covid-19).
No single definition of cytokine storm or the cytokine release syndrome is widely accepted, and there is disagreement about how these disorders differ from an appropriate inflammatory response. The National Cancer Institute’s definition, based on the Common Terminology Criteria for Adverse Events (CTCAE), is too broad, since the criteria for an inflammatory syndrome can also apply to other physiological states, and the definition of the American Society for Transplantation and Cellular Therapy is based on criteria that focus too specifically on iatrogenic causes of cytokine storm alone.7 Although cytokine storm is easy to identify in disorders with elevated cytokine levels in the absence of pathogens, the line between a normal and a dysregulated response to a severe infection is blurry, especially considering that certain cytokines may be both helpful in controlling an infection and harmful to the host. The interdependence of these inflammatory mediators further complicates the distinction between a normal and a dysregulated response.
It is important for the clinician to recognize cytokine storm because it has prognostic and therapeutic implications. In this review, we propose a unifying definition of cytokine storm; discuss the pathophysiological features, clinical presentation, and management of the syndrome; and provide an overview of iatrogenic, pathogen-induced, neoplasia-induced, and monogenic causes. Our goal is to provide physicians with a conceptual framework, a unifying definition, and essential staging, assessment, and therapeutic tools to manage cytokine storm.
Clinical Features and Laboratory Abnormalities
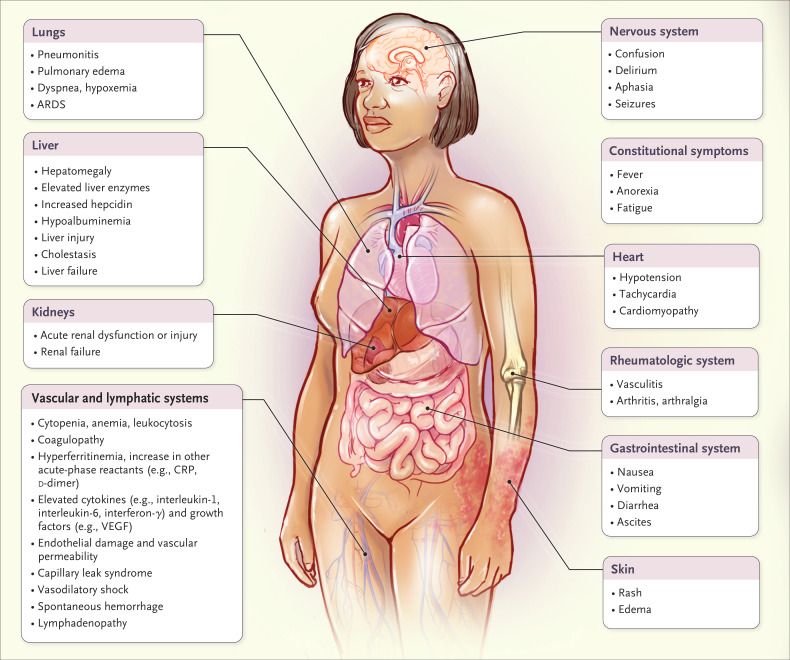
Cytokine storm is an umbrella term encompassing several disorders of immune dysregulation characterized by constitutional symptoms, systemic inflammation, and multiorgan dysfunction that can lead to multiorgan failure if inadequately treated (Figure 1). The onset and duration of cytokine storm vary, depending on the cause and treatments administered.7 Although the initial drivers may differ, late-stage clinical manifestations of cytokine storm converge and often overlap. Nearly all patients with cytokine storm are febrile, and the fever may be high grade in severe cases.8 In addition, patients may have fatigue, anorexia, headache, rash, diarrhea, arthralgia, myalgia, and neuropsychiatric findings. These symptoms may be due directly to cytokine-induced tissue damage or acute-phase physiological changes or may result from immune-cell–mediated responses. Cases can progress rapidly to disseminated intravascular coagulation with either vascular occlusion or catastrophic hemorrhages, dyspnea, hypoxemia, hypotension, hemostatic imbalance, vasodilatory shock, and death. Many patients have respiratory symptoms, including cough and tachypnea, that can progress to acute respiratory distress syndrome (ARDS), with hypoxemia that may require mechanical ventilation. The combination of hyperinflammation, coagulopathy, and low platelet counts places patients with cytokine storm at high risk for spontaneous hemorrhage.
Figure 1. Clinical Presentation of Cytokine Storm.
A wide range of clinical and laboratory abnormalities can be observed in cytokine storm. However, all cases involve elevated circulating cytokine levels, acute systemic inflammatory symptoms, and secondary organ dysfunction (often renal, hepatic, or pulmonary). ARDS denotes acute respiratory distress syndrome, CRP C-reactive protein, and VEGF vascular endothelial growth factor.
In severe cases of cytokine storm, renal failure, acute liver injury or cholestasis, and a stress-related or takotsubo-like cardiomyopathy can also develop.9 The combination of renal dysfunction, endothelial-cell death, and acute-phase hypoalbuminemia can lead to capillary leak syndrome and anasarca — changes that are similar to those observed in patients with cancer who are treated with high-dose interleukin-2.10 Neurologic toxicity associated with T-cell immunotherapy is referred to as immune effector cell–associated neurotoxicity syndrome or cytokine release syndrome–associated encephalopathy.7 The neurologic toxic effects are often delayed, developing several days after the onset of the cytokine storm.
The laboratory findings in cytokine storm are variable and influenced by the underlying cause. Nonspecific markers of inflammation such as C-reactive protein (CRP) are universally elevated and correlate with severity.11 Many patients have hypertriglyceridemia and various blood-count abnormalities, such as leukocytosis, leukopenia, anemia, thrombocytopenia, and elevated ferritin and d-dimer levels. Changes in circulating cell counts are most likely due to a complex interplay among cytokine-induced changes in production and mobilization of cells from the bone marrow, immune-mediated destruction, and chemokine-induced migration. Prominent elevations in serum inflammatory cytokine levels, such as interferon-γ (or CXCL9 and CXCL10, chemokines induced by interferon-γ), interleukin-6, interleukin-10, and soluble interleukin-2 receptor alpha, a marker of T-cell activation, are usually present. Highly elevated serum interleukin-6 levels are found in CAR T-cell therapy–induced cytokine storm and several other cytokine storm disorders.8
The approach to evaluating a patient with cytokine storm should accomplish the following three main goals: identifying the underlying disorder (and ruling out disorders that may mimic cytokine storm), establishing severity, and determining the clinical trajectory. A complete workup for infection, as well as laboratory assessment of kidney and liver function, should be performed in all suspected cases of cytokine storm. Measurements of inflammatory acute-phase biomarkers, such as CRP and ferritin, and blood counts should be obtained, since they correlate with disease activity. Arterial blood-gas measurement should be performed if the respiratory evaluation warrants it. Cytokine profiles may be helpful in determining the trend from baseline values, although these findings are typically not available soon enough to include as part of the immediate workup or to guide treatment decisions.
Establishing the disorder underlying the cytokine storm can be challenging. Cytokine storm is not a diagnosis of exclusion, and it can encompass many disorders. For example, patients may have both sepsis and cytokine storm. However, it is important to distinguish between cytokine storm due to an iatrogenic cause such as CAR T-cell therapy and cytokine storm due to systemic infection, since immunosuppressive treatments could be detrimental if used in patients with septicemia. Unfortunately, it is difficult to distinguish cytokine storm due to sepsis from cytokine storm due to CAR T-cell therapy on the basis of clinical features alone. Levels of serum cytokines — most prominently, interferon-γ — are often more elevated in patients with cytokine storm due to CAR T-cell therapy than in patients with sepsis-induced cytokine storm, who often have higher levels of circulating interleukin-1β, procalcitonin, and markers of endothelial damage.12 Thus, combinations of assays to rule out infection and measure serum cytokines can help to identify the cause of the cytokine storm. However, CAR T-cell therapy and other noninfectious causes can also occur with infections, and infections can develop during the course of therapy, so continued monitoring for infections is warranted. Disorders that should be ruled out in considering cytokine storm include anaphylaxis and physiological responses to microbial infections.
The grading systems used to predict and assess the severity of cytokine storm differ according to the cause. Serum biomarkers, including glycoprotein 130 (gp130), interferon-γ, and interleukin-1–receptor antagonist (IL1RA), can be used to predict the severity of cytokine storm induced by CAR T-cell therapy,13 with a separate grading scale used to assess the current severity.7 HScore and MS score are used for classifying HLH-associated cytokine storm, and HLH-2004 guides treatment. For the grading of cytokine storm due to other causes, the immune systems disorders section of CTCAE is used (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf).
Pathophysiological Features of Cytokine Storm
Inflammation involves a set of biologic mechanisms that evolved in multicellular organisms to contain invasive pathogens and resolve injuries by activating innate and adaptive immune responses. The immune system is expected to recognize foreign invaders, respond proportionally to the pathogen burden, and then return to homeostasis. This response requires a balance between sufficient cytokine production to eliminate the pathogen and avoidance of a hyperinflammatory response in which an overabundance of cytokines causes clinically significant collateral damage. Cytokines play a key role in coordinating antimicrobial effector cells and providing regulatory signals that direct, amplify, and resolve the immune response. Cytokines have short half-lives, which normally prevents them from having effects outside lymphoid tissue and sites of inflammation. Although typically considered to be pathologic, sustained production of cytokines that leads to elevated circulating levels may be necessary to appropriately control some disseminated infections. At increased levels, cytokines can have systemic effects and cause collateral damage to vital organ systems.
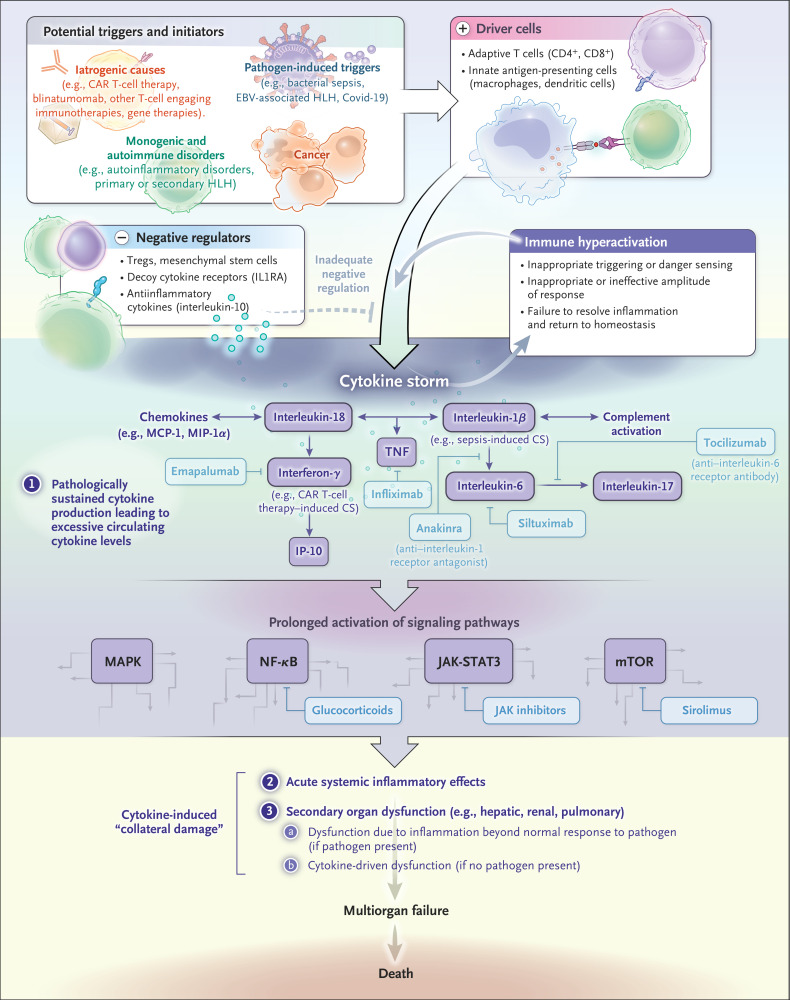
Immune hyperactivation in cytokine storm can occur as a result of inappropriate triggering or danger sensing, with a response initiated in the absence of a pathogen (e.g., in genetic disorders involving inappropriate inflammasome activation or idiopathic multicentric Castleman’s disease); an inappropriate or ineffective amplitude of response, involving excessive effector immune-cell activation (e.g., in cytokine storm due to CAR T-cell therapy), an overwhelming pathogen burden (e.g., in sepsis), or uncontrolled infections and prolonged immune activation (e.g., in HLH associated with Epstein–Barr virus [EBV]); or failure to resolve the immune response and return to homeostasis (e.g., in primary HLH) (Figure 2). In each of these states, there is a failure of negative feedback mechanisms that are meant to prevent hyperinflammation and the overproduction of inflammatory cytokines and soluble mediators. The excessive cytokine production leads to hyperinflammation and multiorgan failure. Regulatory cell types, decoy receptors for proinflammatory cytokines such as IL1RA, and antiinflammatory cytokines such as interleukin-10 are important for antagonizing inflammatory-cell populations and preventing immune hyperactivity.
Figure 2. Pathophysiological Features of Cytokine Storm.
Cytokine storm can occur as a result of inappropriate recognition (e.g., in hypersensitivity) or ineffective recognition with immune evasion (e.g., in Epstein–Barr virus [EBV]–associated hemophagocytic lymphohistiocytosis [HLH]), an inappropriate response with an exaggerated effector response and cytokine production (e.g., in chimeric antigen receptor [CAR] T-cell therapy) or an ineffective response due to immune evasion (e.g., in sepsis), or failure to terminate homeostasis or return to homeostasis (e.g., in HLH). Examples of drugs that can inhibit signaling pathways are shown in boxes. Covid-19 denotes coronavirus disease 2019, CS cytokine storm, IL1RA interleukin-1–receptor antagonist, IP-10 interferon-inducible protein 10, JAK-STAT3 Janus kinase–signal transducer and activator of transcription 3, MAPK mitogen-activated protein kinase, MCP-1 monocyte chemotactic protein 1, MIP-1α macrophage inflammatory protein 1α, mTOR mammalian target of rapamycin, NF-κB nuclear factor κB, TNF tumor necrosis factor, and Tregs regulatory T cells.
Given the lack of a unifying definition for cytokine storm14 and disagreement about the distinction between cytokine storm and a physiologic inflammatory response, we propose the following three criteria for identifying cytokine storm: elevated circulating cytokine levels, acute systemic inflammatory symptoms, and either secondary organ dysfunction (often renal, hepatic, or pulmonary) due to inflammation beyond that which could be attributed to a normal response to a pathogen (if a pathogen is present), or any cytokine-driven organ dysfunction (if no pathogen is present). Improvement in outcomes with cytokine neutralization or antiinflammatory agents further supports the pathologic role of excessive cytokines and the classification of a condition as a cytokine storm. However, lack of a treatment response does not necessarily rule out cytokine storm, because underlying conditions are likely to play a part, a different cytokine may be the disease driver, or the timing of treatment may have been poor.
In short, cytokine storm involves an immune response that causes collateral damage, which may be greater than the immediate benefit of the immune response. Thus, an exuberant inflammatory response to a large pathogen burden may be appropriate for controlling the infection if excessive secondary organ dysfunction does not occur, whereas similarly high levels of cytokines in cancer-associated HLH or idiopathic multicentric Castleman’s disease would be considered a pathologic state of cytokine storm because no pathogen requiring an immune response is involved and patients benefit from treatment with cytokine neutralization and other antiinflammatory agents. Circulating cytokine levels can be difficult to measure because cytokines have short half-lives, circulating levels may not accurately reflect local tissue levels, and measurements may not be easily obtained worldwide. We do not propose a specific threshold for elevations in cytokine levels above the normal range, and we do not recommend specific cytokine panels or list particular cytokines whose levels must be elevated, given the lack of available evidence. However, we believe that this is an important area for future research and could benefit from systematic assessment by a multidisciplinary consortium.
Cell Types Involved in Cytokine Storm
The cells of the innate immune system are the first line of defense against pathogens. Neutrophils, monocytes, and macrophages recognize pathogens, produce cytokines, and engulf pathogens and cells by phagocytosis. There are many other innate immune cells, such as dendritic cells, gamma–delta T cells, and natural killer (NK) cells.15 Innate immune cells use pattern-recognition receptors, which are not specific for any particular antigen, to recognize and respond to a wide variety of microbial invaders by producing cytokines that activate cells of the adaptive immune system.
Innate cells that are most often implicated in the pathogenesis of cytokine storm include neutrophils, macrophages, and NK cells. Neutrophils can produce neutrophil extracellular traps, a network of fibers that contribute to thrombi formation and amplify cytokine production during cytokine storm. Macrophages, which are tissue-resident cells that are often derived from circulating monocytes, do not divide; they have diverse functions, from the removal of senescent cells by engulfment, to tissue repair and immunoregulation, to antigen presentation. In many forms of cytokine storm, macrophages become activated and secrete excessive amounts of cytokines, ultimately causing severe tissue damage that can lead to organ failure. Hemophagocytic macrophages are often observed in bone marrow biopsy specimens from patients with cytokine storm. Interferon-γ can induce hemophagocytosis by macrophages, and this may contribute to the cytopenias commonly observed in patients with cytokine storm.16 The cytolytic function of NK cells is diminished in some forms of cytokine storm, which can lead to prolonged antigenic stimulation and difficulty resolving inflammation.17 Excess interleukin-6 may mediate the impairment in NK-cell function by lowering perforin and granzyme production.
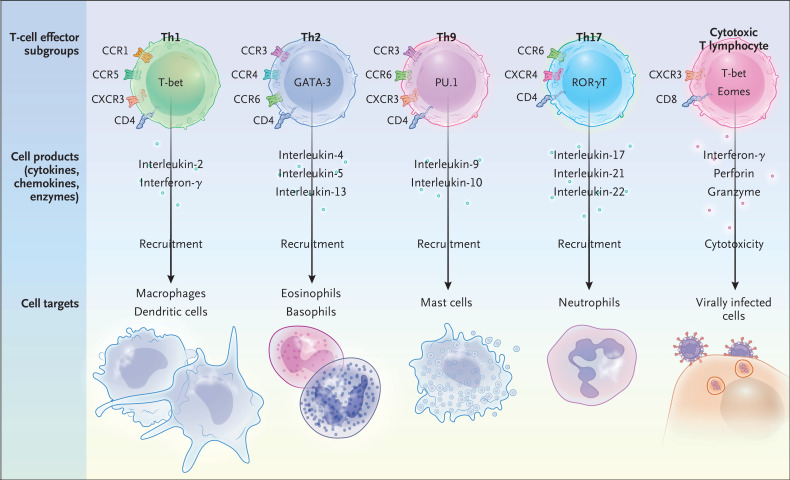
The adaptive immune system is composed of B cells and T cells. T cells differentiate into a number of subsets with distinct effector-cell functions potentially involved in cytokine storm (Figure 3). Type 1 helper T (Th1) cells and cytotoxic T lymphocytes (CTLs) are primarily responsible for the host defense against viral infections. Th1 cells regulate the recruitment of macrophages, whereas type 2 helper T (Th2) cells recruit eosinophils and basophils, type 9 helper T (Th9) cells recruit mast cells, and type 17 helper T (Th17) cells recruit neutrophils.18 An exaggerated Th1-type inflammatory response often occurs during cytokine storm. Th1 cells produce large quantities of interferon-γ, induce delayed hypersensitivity reactions, activate macrophages, and are essential for defense against intracellular pathogens.19 Iatrogenic causes of cytokine storm involving excessive T-cell activation, such as CAR T-cell and anti-CD28 antibody therapy, point to the ability of activated T cells to initiate cytokine storm. Impaired granule-mediated killing of infected cells or tumor cells by CTLs is a key aspect of some forms of cytokine storm.20 Data from mouse models of HLH and patients with cytokine storm indicate that the inability of CTLs to kill efficiently leads to prolonged activation of T cells, triggering a cascade of inflammatory tissue damage.21-23 Th17 cells have a major role in host defense, particularly antifungal protection, and abnormal Th17-cell function can lead to autoimmunity.24 An experimental model of macrophage activation syndrome (a form of secondary HLH) provides evidence that Th17 cells can be drivers of a cytokine storm that is independent of interferon-γ.25
Figure 3. T-Cell Effector Subgroups Involved in Cytokine Storm.
The master transcription factors (T-bet, GATA-3, PU.1, RORγT, and eomesodermin [eomes]), effector molecules, and cell targets are shown for the following T-cell subgroups: types 1, 2, 9, and 17 helper T (Th1, Th2, Th9, and Th17, respectively) cells and cytotoxic T lymphocytes.
B cells are not often associated with the pathogenesis of cytokine storm. However, the effectiveness of B-cell depletion in treating some cytokine storm disorders, such as human herpesvirus 8 (HHV-8)–associated multicentric Castleman’s disease, suggests that these cells are capable of initiating or propagating cytokine storm, particularly when virally infected.
Cytokines
As noted above, the recognition of cytokine storm as an entity is relatively recent. The advent of molecular cloning technologies led to the discovery of the panoply of cytokines and chemokines involved in cytokine storm (Table 1); the realization that diverse entities can cause cytokine storm (Table 2) also contributed to its recognition. The administration of recombinant cytokines (e.g., interleukin-1, interleukin-6, interleukin-12, interleukin-18, tumor necrosis factor [TNF], and interferon-γ) in animal models and for cancer treatment in humans induces severe toxic effects or lethality consistent with the central role of cytokines as mediators of hyperinflammation in cytokine storm.27-29 Conversely, reduction in symptoms and improvement in organ function with neutralization of specific cytokines with monoclonal antibodies also reveal that excessive levels of certain cytokines play a critical role in a number of cytokine storm disorders.
Table 1. Soluble Mediators in Cytokine Storm.*.
| Mediator | Main Cell Source | Type and Function |
|---|---|---|
| Cytokines and growth factors | ||
| Interleukin-1 | Macrophages, epithelial cells; pyroptotic cells | Proinflammatory alarmin cytokine; pyrogenic function, macrophage and Th17 cell activation |
| Interleukin-2 | T cells | Effector T-cell and regulatory T-cell growth factor |
| Interleukin-6 | Macrophages, T cells, endothelial cells | Proinflammatory cytokine; pyrogenic function, increased antibody production, induction of acute-phase reactants |
| Interleukin-9 | Th9 cells | Protection from helminth infections, activation of mast cells, association with type I interferon in Covid-1926 |
| Interleukin-10 | Regulatory T cells, Th9 cells | Antiinflammatory cytokine; inhibition of Th1 cells and cytokine release |
| Interleukin-12 | Dendritic cells, macrophages | Activation of the Th1 pathway; induction of interferon-γ from Th1 cells, CTLs, and NK cells; acting in synergy with interleukin-18 |
| Interleukin-17 | Th17 cells, NK cells, group 3 innate lymphoid cells | Promoting neutrophilic inflammation, protection from bacterial and fungal infections |
| Interleukin-18 | Monocytes, macrophages, dendritic cells | Proinflammatory alarmin cytokine; activation of Th1 pathway, acting in synergy with interleukin-12 |
| Interleukin-33 | Macrophages, dendritic cells, mast cells, epithelial cells | Proinflammatory alarmin cytokine; amplification of Th1 and Th2 cells, activation of NK cells, CTLs, and mast cells |
| Interferon-γ | Th1 cells, CTLs, group 1 innate lymphoid cells, and NK cells | Proinflammatory cytokine; activation of macrophages |
| Tumor necrosis factor | Macrophages, T cells, NK cells, mast cells | Increasing vascular permeability; pyrogenic function |
| GM-CSF | Th17 cells | Proinflammatory cytokine |
| VEGF | Macrophages | Angiogenesis |
| Chemokines | ||
| Interleukin-8 (CXCL8) | Macrophages, epithelial cells | Recruitment of neutrophils |
| MIG (CXCL9) | Monocytes, endothelial cells, keratinocytes | Interferon-inducible chemokine; recruitment of Th1 cells, NK cells, plasmacytoid dendritic cells |
| IP-10 (CXCL10) | Monocytes, endothelial cells, keratinocytes | Interferon-inducible chemokine; recruitment of macrophages, Th1 cells, NK cells |
| MCP-1 (CCL2) | Macrophages, dendritic cells, cardiac myocytes | Recruitment of Th2 cells, monocytes, dendritic cells, basophils |
| MIP-1α (CCL3) | Monocytes, neutrophils, dendritic cells, NK cells, mast cells | Recruitment of macrophages, Th1 cells, NK cells, eosinophils, dendritic cells; pyrogenic function |
| MIP-1β (CCL4) | Macrophages, neutrophils, endothelium | Recruitment of macrophages, Th1 cells, NK cells, dendritic cells |
| BLC (CXCL13) | B cells, follicular dendritic cells | Recruitment of B cells, CD4 T cells, dendritic cells† |
| Plasma proteins | ||
| CRP | Hepatocytes | Monomeric CRP increases interleukin-8 and MCP-1 secretion; interleukin-6 increases CRP expression |
| Complement | Hepatocytes, other cells | Complement activation contributes to tissue damage in cytokine storm; complement inhibition can reduce immunopathologic effects of cytokine storm |
| Ferritin | Ubiquitous | Primary site of iron storage in cells |
BLC denotes B-lymphocyte chemoattractant; Covid-19 coronavirus disease 2019; CRP C-reactive protein; CTLs cytotoxic T lymphocytes; CXCL C-X-C motif chemokine ligand; GM-CSF granulocyte–macrophage colony-stimulating factor; IP-10 interferon-inducible protein 10; MCP-1 monocyte chemoattractant protein 1; MIG monokine induced by interferon-γ; MIP-1α and MIP-1β macrophage inflammatory protein 1α and 1β, respectively; NK natural killer; Th1, Th2, Th9, and Th17 cells types 1, 2, 9, and 17 helper T cells, respectively; and VEGF vascular endothelial growth factor.
In idiopathic multicentric Castleman’s disease, the levels of CXCL13 are the most elevated of all the cytokines or chemokines.
Table 2. Clinical Causes of Cytokine Storm, Pathologic Drivers, and Therapeutic Approaches.*.
| Type of Cytokine Storm and Trigger | Cause | Pathologic Cellular or Cytokine Driver | Common Therapeutic Approaches |
|---|---|---|---|
| Iatrogenic | |||
| CAR T-cell therapy | Infusion of CAR T cells | Macrophages, CAR T cells, interleukin-6, interleukin-1β | Anti–interleukin-6 antibody, glucocorticoids |
| Blinatumomab | Infusion of CD19- and CD3-specific T-cell receptor–engaging antibody | Activated T cells, macrophages, interleukin-6 | Anti–interleukin-6 antibody, glucocorticoids |
| Pathogen-induced | |||
| Bacterial sepsis | Hematogenous bacterial infection | Heterogeneous and multifactorial drivers | Intravenous antibiotics |
| EBV-associated HLH | EBV infection in patient with genetic susceptibility | Interferon-γ, TNF, CD8+ T cells | B-cell–depleting therapy, glucocorticoids |
| HHV-8–associated MCD | HHV-8 infection in patient with HIV coinfection, genetic susceptibility, or both | Viral interleukin-6, interleukin-6 | B-cell–depleting therapy |
| Covid-19 | SARS-CoV-2 infection, potentially in a susceptible person | Unknown driver | Glucocorticoids |
| Monogenic and autoimmune | |||
| Primary HLH | Germline mutation in genes regulating granule-mediated cytotoxicity | CD8+ T cells, interferon-γ | T-cell inhibition or ablation, interferon-γ inhibitor, glucocorticoids |
| Secondary HLH, or MAS | Viral cause (EBV or CMV), autoimmune disorder (rheumatoid arthritis or adult-onset Still’s disease), or neoplastic disorder in patient with genetic susceptibility (lymphoma) | CD8+ T cells, interferon-γ, interleukin-1β, myeloid-cell autoinflammation | Treatment of the underlying cause, in addition to T-cell inhibition or ablation, interleukin-1β inhibitor, JAK1 and JAK2 inhibitors, glucocorticoids |
| Autoinflammatory disorders | Germline mutations in genes regulating the innate immune system and inflammasome activation | Innate cells, TNF, interleukin-1β | Anti-TNF antibody, anti–interleukin-1 antibody |
| Idiopathic MCD | Unknown cause | Interleukin-6, activated T cells, mTOR | Anti–interleukin-6 antibody, sirolimus, cyclosporine, cytotoxic chemotherapy, glucocorticoids |
CAR denotes chimeric antigen receptor, CMV cytomegalovirus, Covid-19 coronavirus disease 2019, EBV Epstein–Barr virus, HHV-8 human herpesvirus 8, HIV human immunodeficiency virus, HLH hemophagocytic lymphohistiocytosis, JAK1 Janus kinase 1, JAK2 Janus kinase 2, MAS macrophage activation syndrome, MCD multicentric Castleman’s disease, mTOR mammalian target of rapamycin, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
A complex, interconnected network of cell types, signaling pathways, and cytokines is involved in cytokine storm disorders. Interferon-γ, interleukin-1, interleukin-6, TNF, and interleukin-18 are key cytokines that often have elevated levels in cytokine storm and are thought to have central immunopathologic roles. The pattern of cytokine elevations varies on the basis of such factors as the microbiome, genetic features, and underlying disorders.30 The specific immune cells that secrete the various cytokines are not fully understood and most likely vary among cytokine storm disorders. Interferon-γ is primarily secreted by activated T cells and NK cells and is a potent activator of macrophages. Clinically, interferon-γ causes fever, chills, headache, dizziness, and fatigue.31 Emapalumab, a monoclonal antibody that binds interferon-γ, was recently approved for the treatment of cytokine storm in patients with primary HLH.32 This agent may also be useful in other cytokine storm disorders, such as macrophage activation syndrome or CAR T-cell–associated cytokine storm, although in the latter case, it may diminish antitumor effects.
Fever, a clinical hallmark of cytokine storm, can be elicited by interleukin-1, interleukin-6, or TNF through distinct mechanisms. Interleukin-1 is encoded by two genes (IL1A and IL1B), both of which bind to the same interleukin-1 receptor, activating a cascade of intracellular signaling pathways, including nuclear factor κB (NF-κB). The interleukin-1–receptor antagonist anakinra is effective as a single agent and in combination with other agents for the treatment of some forms of cytokine storm.33,34
Levels of interleukin-6, an important mediator of the acute inflammatory response and pathophysiological features of cytokine storm, are highly elevated across various underlying immunopathologic disorders35,36 and in mouse models of cytokine storm.37 Both tocilizumab, a monoclonal antibody directed at the interleukin-6 receptor (interleukin-6R), and siltuximab, which neutralizes interleukin-6 directly, have been shown to be effective in a number of cytokine storm disorders, including HLH, idiopathic multicentric Castleman’s disease, and CAR T-cell–induced cytokine storm.38
Interleukin-6 is one of the more complex cytokines, since it is produced by and acts on immune and nonimmune cells across multiple organ systems. It can signal through two main pathways, referred to as classic cis signaling and trans signaling.38 The membrane-bound interleukin-6R does not possess intracellular signaling domains but signals instead through interaction with membrane-bound gp130. In cis signaling, soluble interleukin-6 binds to membrane-bound interleukin-6R, forming an interleukin-6–interleukin-6R complex that binds to gp130, which then initiates signaling through its intracellular domain.
Downstream signal transduction is mediated by JAKs (Janus kinases) and STAT3 (signal transducer and activator of transcription 3), as well as by Akt–mTOR (mammalian target of rapamycin) and MAPK–ERK (mitogen-activated protein kinase–extracellular signal-regulated kinase) pathways. Membrane-bound gp130 is ubiquitously expressed, whereas expression of membrane-bound interleukin-6R is restricted largely to immune cells. Activation of cis signaling results in pleiotropic effects on the immune system, which can contribute to cytokine storm.38 In the presence of high circulating levels of interleukin-6, which can be present in cytokine storm, trans signaling occurs through the binding of interleukin-6 to the soluble form of interleukin-6R, forming a complex with a gp130 dimer on potentially all cell surfaces. The resultant interleukin-6–soluble interleukin-6R–gp130–JAK-STAT3 signaling is then activated in cells that do not express the membrane-bound interleukin-6R, such as endothelial cells. This results in systemic hyperinflammation involving secretion of monocyte chemoattractant protein 1 (MCP-1), interleukin-8, and additional interleukin-6, as well as increased vascular endothelial growth factor (VEGF) and reduced E-cadherin expression on endothelial cells, which contribute to vascular hyperpermeability, leakiness, hypotension, and pulmonary dysfunction.38
TNF is a potent, multifunctional, proinflammatory cytokine that belongs to the TNF–TNF receptor superfamily. In addition to inducing fever, augmenting systemic inflammation, and activating antimicrobial responses such as interleukin-6, TNF can induce cellular apoptosis and regulate immunity. TNF and other cytokines in the TNF–TNF receptor superfamily are potent inducers of NF-κB, leading to the expression of multiple proinflammatory genes. In mouse models of toxic shock, TNF is the cytokine driver of superantigen-driven cytokine storm.39 The effectiveness of anti-TNF therapies in certain autoinflammatory-driven cytokine storm conditions points to their potential role in the treatment of cytokine storm, but the limitations and dangers of anti-TNF therapies in patients with sepsis indicate that more work is needed.
Interleukin-18 is a member of the large interleukin-1 family40 that has recently been associated with cytokine storm disorders. Interleukin-18 and interleukin-1β are activated from precursors by inflammasomes. The inflammasome is a multimolecular cytosolic sensor that detects pathogenic microorganisms and sterile stressors and activates caspase-1 during the process of pyroptosis, which, in turn, causes the inactive precursor forms of interleukin-1β and interleukin-18 to become the active forms.41,42 Macrophages and dendritic cells are the primary sources of bioactive interleukin-18, which has many proinflammatory effects. Most important, it synergizes with interleukin-12 or interleukin-15 to stimulate secretion of interferon-γ from T cells and NK cells, and thus promotes Th1-type inflammatory responses. The interleukin-18 receptor is constitutively expressed on NK cells and induced on activation in most T cells. Interleukin-1β and interleukin-18 are also potent inducers of interleukin-6 secretion from macrophages.43
Patients with cytokine storm due to macrophage activation syndrome have high levels of interleukin-18 in serum,44 and interleukin-18 is a biomarker of severity that correlates with hyperferritinemia, elevated aminotransferase levels, and disease flare.45 The proinflammatory effects of interleukin-18 are normally kept in check by the interleukin-18–binding protein (IL18BP), which prevents the binding of interleukin-18 to its receptor.46 The ratio of free interleukin-18 to bound interleukin-18–IL18BP complexes in serum is an important indicator of the severity of the macrophage activation syndrome.44,47 Tadekinig alfa is a recombinant IL18BP currently under investigation as a treatment for hyperinflammation.
Chemokines are a class of cytokines that contribute to a variety of immune-cell functions, including leukocyte recruitment and trafficking. Dysregulated trafficking during inflammation may have a role in hyperinflammation. Numerous regulatory cytokines such as interleukin-10 and natural cytokine antagonists such as IL1RA serve as buffers to limit systemic off-target effects. Interleukin-10 inhibits the production of TNF, interleukin-1, interleukin-6, and interleukin-12 and down-regulates antigen presentation. Furthermore, in mice lacking interleukin-10, infection leads to cytokine storm.48 Though interleukin-10 and IL1RA are often elevated in cytokine storm, this finding most likely reflects a secondary, albeit insufficient, counterregulatory response to the proinflammatory cytokines. Anakinra is a therapeutic agent that mimics the endogenous immunoregulatory effects of IL1RA.
Plasma proteins such as complement proteins and other inflammatory mediators can contribute to the pathogenesis of cytokine storm. These soluble proteins recognize pathogens, amplify cellular responses, and provide feedback on cytokine signaling. In fact, cytokines can enhance the production of complement proteins, which in turn can enhance or inhibit cytokine production. Thus, complement can be highly effective in eliminating microbes but can also cause collateral damage if excessive. Hypocomplementemia, resulting from increased consumption by immune complexes, can be observed in cytokine storm.49 Complement inhibitors are under evaluation for the treatment of cytokine storm disorders.
Iatrogenic Cytokine Storm
Infusion of CAR T cells engineered to recognize and eliminate CD19+ lymphoma cells can induce cytokine storm, with supraphysiologic levels of interferon-γ and interleukin-6.50 The highly activated CAR T cells are clearly the initiators of the cytokine storm. Although some studies suggest that the driver cytokines are released by CAR T cells, resulting in a positive feedback loop of T-cell activation and inflammatory cytokine release,51 recent studies in mice suggest that the cytokines and factors mediating the severity of cytokine storm are produced not by the CAR T cells but by macrophages and can be reversed by interleukin-6 and interleukin-1 blockade.52-54 Tumor lysis most likely also contributes to the cytokine storm through the induction of pyroptosis in target cells.55 Since interleukin-6 blockade is highly effective at reversing symptoms and organ dysfunction in most patients, it is the likely cytokine driver of cytokine storm induced by CAR T-cell therapy. Glucocorticoids and interleukin-1 inhibition can also be effective in the treatment of this type of cytokine storm.
Cytokine storm can be observed with other T-cell–engaging immunotherapies as well, such as blinatumomab, a bispecific antibody that binds to CD19+ and CD3+ T cells.56 Like CAR T cells, activated T cells initiate the cytokine storm, and macrophage activation propagates blinatumomab-induced cytokine storm, which also responds to anti–interleukin-6 antibody therapy.36 The unfortunate consequences of another T-cell–activating treatment with the anti-CD28 superagonist TGN1412 show that rapid activation of large numbers of T cells can result in severe cytokine storm within minutes after infusion.57 However, cytokine storm does not develop in all patients treated with CAR T cells or blinatumomab, so additional factors, such as CAR structure and design,51 disease burden,58 and host genomic background,59 are likely to play a part. In a recent study of NK-cell CAR therapy, there were no reported cases of cytokine storm or even elevated interleukin-6 levels,60 possibly because of lower interleukin-6 production by NK cells than by T cells and different cross-talk with myeloid cells. Additional iatrogenic causes of cytokine storm include rituximab,35 gene therapies, immune checkpoint inhibitors, cardiac-bypass surgery,61 and allogeneic stem-cell transplantation, as well as bioterrorism agents such as staphylococcal enterotoxin B and Francisella tularensis.
Pathogen-Induced Cytokine Storm
Cytokine storm can also result from naturally occurring microbial infections. Though data on relative frequencies are limited, infections are most likely the most common trigger of cytokine storm. Distinguishing between appropriate cytokine production for controlling a widespread infection and excessive cytokine production is challenging. Disseminated bacterial infections causing sepsis induce the production of many cytokines that can lead to fever, cell death, coagulopathies, and multiorgan dysfunction. The collateral damage caused by the immune response as it attempts to clear the pathogen can be more deadly than the pathogen itself. Certain bacteria, including streptococcus species and Staphylococcus aureus, can produce superantigens that cross-link the major histocompatibility complex and T-cell receptors, leading to polyclonal activation of T cells, cytokine production, and toxic shock syndrome. Superantigens are the most powerful T-cell mitogens, and bacterial superantigen concentrations of less than 0.1 pg per milliliter are sufficient to stimulate T cells in an uncontrolled manner, resulting in fever, shock, and death.
In sepsis-associated cytokine storm, it is unclear which immune cell types and cytokines may be responsible for propagating the pathologic hyperinflammation. Antibiotics are the mainstay of treatment. The administration of monoclonal antibodies directed at specific cytokines and the use of apheresis or medical devices to remove cytokines from circulation have had generally disappointing results in clinical trials.62 Although the timing of treatment in these studies may have contributed to the lack of benefit, additional host or pathogen factors may be important, beyond the specifically elevated cytokine levels. For example, reanalysis of a negative trial of interleukin-1β blockade in patients with sepsis identified a subgroup of patients with elevated ferritin levels who seemed to benefit from the treatment.63
Disseminated viral infections can also induce profound cytokine storm. Patients with hyperinflammatory responses to microbes often have defects in pathogen detection, effector and regulatory mechanisms, or resolution of inflammation. For example, patients lacking functional perforin, which is critical for resolving infections and inflammation, have prolonged CD8+ T-cell production of interferon-γ and TNF, and HLH-associated cytokine storm develops in such patients when they are infected with EBV or cytomegalovirus.64 Experimental models suggest that cytokine storm occurs in these patients from defective perforin-mediated cytolysis that leads to prolonged engagement between lymphocytes and antigen-presenting cells and defective clearance of antigen-bearing dendritic cells, resulting in continuous activation and proliferation of T cells and macrophages, hemophagocytosis, and an autocrine loop of proinflammatory cytokines.21,65-67 Furthermore, retrospective analyses of data from persons who died from coagulopathies and hemophagocytosis during the H1N1 influenza pandemic of 2009 revealed germline mutations previously associated with HLH-associated cytokine storm.30 Thus, the pathogen initiates and T-cell activation propagates cytokine storm in patients with a genetic susceptibility. Cyclosporine and anti–interleukin-6 receptor monoclonal antibody therapy can be effective in some virus-driven forms of HLH-associated cytokine storm, indicating the critical role of T-cell activation and interleukin-6.
Another pathogen-induced form of cytokine storm is HHV-8–associated multicentric Castleman’s disease. In this disorder, uncontrolled infection with HHV-8 (also known as Kaposi’s sarcoma herpesvirus) leads to a cytokine storm driven primarily by excessive production of human interleukin-6 and viral interleukin-6 by HHV-8–infected plasmablasts.68 Patients with HHV-8–associated multicentric Castleman’s disease are immunocompromised as a result of human immunodeficiency virus infection or a genetic susceptibility, making it difficult to control the HHV-8 infection, which is a common, typically asymptomatic infection in the general population.69 A recent study showed that the effect of tocilizumab in patients with HHV-8–associated multicentric Castleman’s disease was minimal and short-lived, most likely because of viral interleukin-6 signaling that was independent of the neutralized interleukin-6 receptor.70 As with EBV-associated HLH,71 rituximab is highly effective in patients with HHV-8–associated multicentric Castleman’s disease, since B-cell depletion removes the primary reservoir for HHV-8.72 Many additional microbes can trigger cytokine storm, including other herpesviruses, such as herpes simplex virus, and other influenza viruses, such as H5N1.
Targeted treatment is more challenging in patients with viral infections than in patients with bacterial infections, since fewer antiviral agents are available. Intravenous immune globulin and convalescent plasma are sometimes used to help control the pathogen and provide beneficial immunomodulation. For some viral infections, treating patients with proinflammatory cytokines in the early stages of infection can help to control the virus before detrimental effects of the immune response occur.73
Monogenic or Autoimmune Cytokine Storm
In rare cases, a pathogen triggers cytokine storm in patients with monogenic disorders, and in other cases, cytokine storm has autoimmune, neoplastic, or idiopathic causes. In patients with primary HLH, various autosomal recessive monogenic abnormalities in granule-mediated cytotoxicity lead to cytokine storm. Common pathologic mutations include those occurring in PRF1, UNC13D, STXBP1, RAB27A, STX11, SH2D1A, XIAP, and NLRC4.23 In patients with secondary HLH, viral, autoimmune, or neoplastic disorders trigger cytokine storm, and such patients often have heterozygous polymorphisms in the same genes that are altered in primary HLH.65,74 Elevated levels of interferon-γ, TNF, interleukin-1, interleukin-4, interleukin-6, interleukin-8, interleukin-10, CXCL9, CXCL10, and interleukin-18 are frequently associated with HLH. Anti–interferon-γ antibody therapy with emapalumab has recently been approved for the treatment of primary HLH, as a bridge to allogeneic stem-cell transplantation, which is typically curative.
The beneficial effects of glucocorticoids, cyclosporine, anti–interleukin-1 antibody, JAK1 and JAK2 inhibitors, anti–interleukin-6 antibody, and cytotoxic chemotherapies in some patients with primary or secondary HLH suggest that pathways targeted by these agents are key to pathogenesis. Cyclophosphamide and etoposide, which are broadly cytotoxic but particularly effective at eliminating activated CD8+ T cells, are often effective in patients with primary HLH, secondary HLH (including macrophage activation syndrome), and corresponding models.75 Etoposide also targets macrophages, including those involved in regulating inflammation, which could be harmful. Generalized T-cell and B-cell ablation with alemtuzumab and T-cell ablation with antithymocyte globulin have been reported; ablation most likely works by depleting the pathogenic CD8+ T cells, among other cell types.76 Nonablative inhibition of T cells with cyclosporine can also be helpful.77
Autoinflammatory diseases are characterized by seemingly unprovoked inflammation and cytokine storm without signs of infection or autoimmunity. Affected patients have germline mutations in genes regulating the innate immune system and activation of the inflammasome. Several genetic disorders are associated with altered regulation of the innate immune system, including familial Mediterranean fever (MEFV), TNF receptor–associated periodic syndrome (TNFRSF1A), hyperimmunoglobulinemia D with periodic fever syndrome (MVK), familial cold autoinflammatory syndrome (NLRP3), the Muckle–Wells syndrome (NLRP3), neonatal-onset multisystem inflammatory disease (NLRP3), deficiency of ADA2 (CECR1), NLRC4 inflammasomopathies, X-linked lymphoproliferative type 2 disorder (XIAP), the Takenouchi–Kosaki syndrome (CDC42), and the Wiskott–Aldrich syndrome (CDC42). Although all patients with these disorders have periodic fevers, only a portion have cytokine storm. Given the primary genetic defects and the effective treatments that are available, innate cells are most likely the primary cell drivers involved, and TNF, interleukin-1, interleukin-18, or a combination of these cytokines probably drives pathogenesis. Patients with genetic immunodeficiency syndromes such as chronic granulomatous disease and STAT1 gain-of-function disease can, paradoxically, present with cytokine storm from overwhelming infections.78
Idiopathic multicentric Castleman’s disease is another cytokine storm disorder that is similar to HHV-8–associated multicentric Castleman’s disease, but the cause is unknown. Patients with the thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly (TAFRO) subtype tend to have the most severe cytokine storm.79 Although the cause is unknown, interleukin-6 is the driver of pathogenesis in a large portion of patients. As a result, tocilizumab, which targets the interleukin-6 receptor, and siltuximab, which targets interleukin-6 directly, were developed and approved by regulatory agencies in Japan (tocilizumab) and in the United States and dozens of other countries (siltuximab) for the treatment of idiopathic multicentric Castleman’s disease. Both siltuximab and tocilizumab have been shown to resolve disease flares and sustain remission in approximately one third to one half of patients.80 However, some patients with low circulating interleukin-6 levels have a response to interleukin-6 blockade, and some patients with high systemic interleukin-6 levels do not have a response. A seven-protein panel that can predict which patients with idiopathic multicentric Castleman’s disease are most likely to benefit from siltuximab was recently identified and validated (https://ashpublications.org/blood/article/132/Supplement%201/3716/265269/Serum-Proteomics-Reveals-Distinct-Subtypes?searchresult=1).
Patients with idiopathic multicentric Castleman’s disease who have progressive organ dysfunction and who do not have a response to anti–interleukin-6 therapy are often treated with combination cytotoxic chemotherapy to nonspecifically eliminate hyperinflammatory cells.81 Other elevated serum cytokines and cellular signaling pathways that could be considered for therapeutic targeting include CXCL13, CXCL10 (interferon-inducible protein 10 [IP-10]), VEGF-A,82 type I interferon,83 mTOR complex 1 (mTORC1),84 and JAK-STAT3. These findings have led to treatment with the mTORC1 inhibitor sirolimus in patients with idiopathic multicentric Castleman’s disease who do not have a response to anti–interleukin-6 therapy.85 Sirolimus therapy is being evaluated in an ongoing clinical trial involving patients with active disease who do not yet have fulminant cytokine storm (ClinicalTrials.gov number, NCT03933904).
Covid-19–Associated Cytokine Storm
Covid-19, which is caused by SARS-CoV-2, is characterized by heterogeneous symptoms ranging from mild fatigue to life-threatening pneumonia, cytokine storm, and multiorgan failure. Cytokine storm was also reported in patients with SARS and was associated with poor outcomes.86 Although the mechanisms of lung injury and multiorgan failure in Covid-19 are still under investigation,14 reports of hemophagocytosis and elevated cytokine levels — as well as beneficial effects of immunosuppressant agents — in affected patients, particularly those who are the most severely ill, suggest that cytokine storm may contribute to the pathogenesis of Covid-19.87,88
Serum cytokine levels that are elevated in patients with Covid-19–associated cytokine storm include interleukin-1β, interleukin-6, IP-10, TNF, interferon-γ, macrophage inflammatory protein (MIP) 1α and 1β, and VEGF.89,90 Higher interleukin-6 levels are strongly associated with shorter survival.91 The relative frequencies of circulating activated CD4+ and CD8+ T cells and plasmablasts are increased in Covid-19.92 In addition to the elevated systemic cytokine levels and activated immune cells, several clinical and laboratory abnormalities, such as elevated CRP and d-dimer levels, hypoalbuminemia, renal dysfunction, and effusions, are also observed in Covid-19, as they are in cytokine storm disorders. Laboratory test results reflecting hyperinflammation and tissue damage were found to predict worsening outcomes in Covid-19.93
Although immunologic dysregulation has been observed in severe cases of Covid-19,26 it is not known whether immune hyperactivity or a failure to resolve the inflammatory response because of ongoing viral replication or immune dysregulation underlies severe cases. The correlation between the nasopharyngeal viral load and cytokine levels (e.g., interferon-α, interferon-γ, and TNF), as well as a declining viral load in moderate but not severe cases, suggests that the immune response is positively associated with the viral burden.26 Alternatively, the discoveries of inborn errors of type I interferon immunity and autoantibodies against type I interferons in the most severe cases of Covid-19 suggest that an inadequate antiviral response may be contributory in some patients with Covid-19.94,95 Host immune responses and immune-related symptoms are extremely variable between asymptomatic patients (who have effective control of SARS-CoV-2) and patients with severe Covid-19 (who are unable to control the virus), which suggests that host immune dysregulation contributes to pathogenesis in some cases. Another hypothesized mechanism involves autoimmunity due to molecular mimicry between SARS-CoV-2 and a self-antigen. These mechanisms may be involved in subgroups of patients, such as children with postinfection multisystem inflammatory syndrome, a condition that seems to be ameliorated by immunomodulatory therapies such as intravenous immune globulin, glucocorticoids, and anti–interleukin-1 and anti–interleukin-6 therapies. Patients with multisystem inflammatory syndrome very clearly meet the definition of cytokine storm, since SARS-CoV-2 is no longer present; however, it is unclear whether the cytokine storm is a driver of Covid-19 or a secondary process. Furthermore, it is now clear that patients with SARS-CoV-2 infection can be asymptomatic or can have acute Covid-19 with heterogeneous severity, a chronic course of Covid-19, or multisystem inflammatory syndrome. A critical question concerns the factors that contribute to the severe cytokine storm–like phenotype observed in a small fraction of patients. Coexisting conditions such as hypertension, diabetes, and obesity are associated with more severe cases of Covid-19, possibly because of the preexisting chronic inflammatory state or a lower threshold for the development of organ dysfunction from the immune response.
Several important differences in therapeutic considerations should be noted between Covid-19–associated cytokine storm and many other cytokine storm disorders. First, cytokine storm triggered by infection with SARS-CoV-2 may require different therapies from those used for cytokine storm due to other causes. Cytokines may be both a key component of the cytokine storm and an essential factor in the antimicrobial response. Thus, blocking cytokine signaling may actually impair clearance of SARS-CoV-2, increase the risk of secondary infections, and lead to worse outcomes, as seen with influenza virus.96 Since interleukin-6 and other cytokines are potentially critical for both a healthy response to SARS-CoV-2 and a detrimental cytokine storm, it is particularly important that the right subgroups of patients with Covid-19 are selected for treatments at the right time. Despite positive anecdotal reports, two large, randomized, controlled trials of anti–interleukin-6 receptor antibody therapies did not show a survival benefit in hospitalized patients with Covid-19.97,98
Second, the primary site of infection and disease most likely contributes to differences in immune responses and mechanisms underlying the cytokine storm, which have implications for treatment. For example, selective elimination of the primary viral reservoir is beneficial in patients with HHV-8–associated multicentric Castleman’s disease but is not possible in patients with Covid-19.
Third, lymphopenia is not often observed in cytokine storm disorders, but it is a hallmark of severe Covid-19. It is currently unclear whether the lymphopenia observed in Covid-19 is due to tissue infiltration or destruction of lymphocytes.
Fourth, clotting issues can occur across cytokine storm disorders, but thromboembolic events appear to be more frequent in Covid-19–associated cytokine storm.99 Finally, although cytokine panels have not been measured simultaneously on the same platform across Covid-19–associated cytokine storm and other cytokine storm disorders, preliminary results suggest that circulating levels of several cytokines, such as interleukin-6, as well as other inflammatory markers, such as ferritin, are less severely elevated in Covid-19 than in some of the other cytokine storm disorders.26 Levels of inflammatory mediators in pulmonary tissue during infection with SARS-CoV-2 remain unknown.
Despite the many unknowns, a recent randomized, controlled trial showing that dexamethasone reduces mortality among the most severe cases of Covid-19, characterized by elevated CRP levels and supplemental oxygen requirements, and potentially worsens outcomes in milder cases suggests that excessive, late-stage inflammation contributes to mortality.88 A meta-analysis of seven randomized trials showed that 28-day all-cause mortality in critically ill patients with Covid-19 was lower among those who were treated with glucocorticoids than among those who received usual care or placebo.100 An observational study suggesting that patients with Covid-19 have a good response to glucocorticoids when the CRP level is high but a poor response when the level is low is consistent with these findings.101 Further support comes from positive anecdotal reports of targeted antagonists against interleukin-1, granulocyte–macrophage colony-stimulating factor, and JAK1 and JAK2 in patients with Covid-19.102-105 Likewise, the observation that proinflammatory agents such as inhaled interferon-β have a positive effect if given early in the disease course is consistent with a model in which immunostimulation that enhances antiviral activity is helpful early (and probably harmful late), whereas immunosuppression is helpful late and harmful early. As with dexamethasone, the timing of treatment and selection of subgroups of patients included in studies will most likely have an effect on outcomes.
Despite unknowns regarding the role of immune dysregulation and cytokine storm in Covid-19, hundreds of immunomodulatory drugs are currently under investigation.102 Many of these treatments have been used for other cytokine storm disorders. Canakinumab, an anti–interleukin-1β monoclonal antibody, and anakinra are both being studied for Covid-19–induced ARDS. Acalabrutinib, a selective inhibitor of Bruton tyrosine kinase that regulates B-cell and macrophage signaling and activation, may have promise for dampening the hyperinflammatory response in Covid-19.106 JAK1 and JAK2 inhibitors, which are approved for the treatment of a number of autoimmune and neoplastic conditions, have the potential to inhibit signaling downstream of type I interferon, interleukin-6 (and other gp130 family receptors), interferon-γ, and interleukin-2, among other cytokines.107 Much like anti–interleukin-6 antibody therapy, inhibition of Bruton tyrosine kinase and JAK could prove to be damaging or unhelpful if given too soon, when the immune response to SARS-CoV-2 is critical in controlling viral replication and clearance.
Therapeutics
The general treatment strategy for cytokine storm involves supportive care to maintain critical organ function, control of the underlying disease and elimination of triggers for abnormal immune system activation, and targeted immunomodulation or nonspecific immunosuppression to limit the collateral damage of the activated immune system. As noted throughout this review, a number of drugs are effective across multiple disorders under the cytokine storm umbrella and still more may be effective in multiple conditions that have not yet been studied.
Given the growing number of new therapeutics targeting various aspects of the immune system and our ability to probe the biologic mechanisms of disease, further research should focus on the identification of drugs that can be used across cytokine storm disorders and precision diagnostics for selecting the right drugs for the right patients, regardless of the underlying condition.108,109 A study involving patients with systemic juvenile idiopathic arthritis revealed subgroups of patients with cytokine profiles in which interleukin-6 and interleukin-18 predominated, pointing toward available therapeutic approaches.110 Likewise, biomarkers were recently shown to effectively predict which patients with adult-onset Still’s disease would have a response to anakinra or tocilizumab.111 The progress made in precision oncology suggests that similar efforts across cytokine storm disorders are warranted to identify specific therapeutic targets and signatures of response to certain drugs that cross disease boundaries. JAK signaling is an interesting target in cytokine storm, because multiple cytokine–receptor pairs can be targeted simultaneously, an approach that may be effective for multiple diseases driven by different cytokines. In addition, plasma exchange and plasma filtration columns for the adsorption of cytokines are both under evaluation for cytokine storm disorders.
It is important to consider several factors in managing cytokine storm. Neutralization of a particular cytokine whose level is elevated in the circulation with an existing agent (anti–interleukin-6, anti-TNF, anti–interferon-γ, or anti–interleukin-1β antibody) will not always be effective, and blocking a cytokine with a low or normal circulating level can be effective if it is a key component of the hyperinflammatory circuit or if its level is potentially elevated in tissue. In addition, the various therapies mentioned in this review have distinctive side-effect and risk profiles. All targeted agents have target-specific risks, and combination therapy has more potential risks than single-agent therapy. Furthermore, pathologic hyperinflammation itself is an immunodeficiency that can put patients at risk for infections, and immunosuppressive agents most likely increase the risk further. In this age of cytokine profiling and individualized medicine, patients must be monitored and given appropriate prophylaxis when treated empirically, and randomized, controlled trials should always be performed to assess efficacy and safety.
Advancing the research and treatment of cytokine storm will require pooling of samples for “omics” studies and collaboration among experts across conditions. The introduction of an International Classification of Diseases, 10th Revision, code for cytokine release syndrome in 2021 should facilitate electronic health record–based research into its natural history, pathogenesis, and treatments. Once sufficient scientific progress has been achieved toward biomarker-guided, individualized treatment of cytokine storm, reliable, quick, and accessible assays will be needed to measure soluble mediators of inflammation in plasma and tissues.
Summary
Mild, secondary organ dysfunction during an inflammatory response is evolutionarily acceptable if it allows the host to overcome the infection and survive. If the inflammatory response causes excessive organ dysfunction that puts host survival and reproductive fitness at risk (in the absence of ventilatory support and dialysis), then it is pathologic. Extensive regulatory mechanisms exist that modulate the immune response and prevent cytokine storm. Nevertheless, the disorder can still occur due to iatrogenic causes, pathogens, cancers, autoimmunity, and autoinflammatory mechanisms. Distinguishing between protective inflammatory responses and pathologic cytokine storm has important implications for treatment and is quite challenging. No unifying definition of cytokine storm exists, and there is much disagreement about what the definition should be and whether specific conditions such as Covid-19 should be included in the spectrum of cytokine storm disorders. We propose a unifying definition for cytokine storm that is based on the following criteria: elevated circulating cytokine levels, acute systemic inflammatory symptoms, and secondary organ dysfunction beyond that which could be attributed to a normal response to a pathogen, if a pathogen is present. Targeted therapeutic approaches to cytokine storm associated with idiopathic multicentric Castleman’s disease, HLH, or CAR T-cell therapy have turned deadly conditions into often reversible states. Given advances in “multi-omic” profiling and therapeutic modulation of the immune system, as well as concerted efforts to work across the cytokine storm umbrella, we expect to see continued improvements in outcomes.
Acknowledgments
We thank our colleagues Michael Jordan, Taku Kambayashi, Ivan Maillard, Sheila Pierson, Ruth-Anne Langan Pai, Dan Rader, Patricia Tsao, Frits van Rhee, Dermot Kelleher, Shanmuganthan Chandrakasan, Amber Cohen, Alexis Phillips, John Wherry, and Charles Dinarello for their critical review and feedback.
Disclosure Forms
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
References
- 1.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc 1993;25:1216-1217. [PubMed] [Google Scholar]
- 3.Chatenoud L, Ferran C, Bach JF. The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation. Curr Top Microbiol Immunol 1991;174:121-134. [DOI] [PubMed] [Google Scholar]
- 4.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci 1893;105:487-511. [PubMed] [Google Scholar]
- 5.Pechous RD, Sivaraman V, Price PA, Stasulli NM, Goldman WE. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog 2013;9(10):e1003679-e1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kash JC, Tumpey TM, Proll SC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 2006;443:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625-638. [DOI] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929-938. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother 2001;24:287-293. [DOI] [PubMed] [Google Scholar]
- 11.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diorio C, Shaw PA, Pequignot E, et al. Diagnostic biomarkers to differentiate sepsis from cytokine release syndrome in critically ill children. Blood Adv 2020;4:5174-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 2020;180:1152-1154. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013;38:792-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoller EE, Lykens JE, Terrell CE, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med 2011;208:1203-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez N, Virelizier J-L, Arenzana-Seisdedos F, Fischer A, Griscelli C. Impaired natural killer activity in lymphohistiocytosis syndrome. J Pediatr 1984;104:569-573. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F. Heterogeneity of human CD4+ T cells against microbes. Annu Rev Immunol 2016;34:317-334. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-173. [DOI] [PubMed] [Google Scholar]
- 20.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol 2019;10:119-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004;104:735-743. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011;118:5794-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulert GS, Cron RQ. The genetics of macrophage activation syndrome. Genes Immun 2020;21:169-181. [DOI] [PubMed] [Google Scholar]
- 24.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol 2009;27:485-517. [DOI] [PubMed] [Google Scholar]
- 25.Avau A, Mitera T, Put S, et al. Systemic juvenile idiopathic arthritis-like syndrome in mice following stimulation of the immune system with Freund’s complete adjuvant: regulation by interferon-γ. Arthritis Rheumatol 2014;66:1340-1351. [DOI] [PubMed] [Google Scholar]
- 26.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020;584:463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty GM, Lange JR, Langstein HN, Alexander HR, Buresh CM, Norton JA. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol 1992;149:1666-1670. [PubMed] [Google Scholar]
- 28.Cohen J. IL-12 deaths: explanation and a puzzle. Science 1995;270:908-908. [DOI] [PubMed] [Google Scholar]
- 29.Atkins MB. Interleukin-2: clinical applications. Semin Oncol 2002;29:Suppl 7:12-17. [DOI] [PubMed] [Google Scholar]
- 30.Schulert GS, Zhang M, Fall N, et al. Whole-exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J Infect Dis 2016;213:1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadhan-Raj S, Nathan CF, Sherwin SA, Oettgen HF, Krown SE. Phase I trial of recombinant interferon gamma by 1-hour i.v. infusion. Cancer Treat Rep 1986;70:609-614. [PubMed] [Google Scholar]
- 32.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med 2020;382:1811-1822. [DOI] [PubMed] [Google Scholar]
- 33.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 2020;72:326-334. [DOI] [PubMed] [Google Scholar]
- 34.Durand M, Troyanov Y, Laflamme P, Gregoire G. Macrophage activation syndrome treated with anakinra. J Rheumatol 2010;37:879-880. [DOI] [PubMed] [Google Scholar]
- 35.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999;94:2217-2224. [PubMed] [Google Scholar]
- 36.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013;121:5154-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Stegen SJ, Davies DM, Wilkie S, et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: identifying a window of therapeutic opportunity? J Immunol 2013;191:4589-4598. [DOI] [PubMed] [Google Scholar]
- 38.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity 2019;50:1007-1023. [DOI] [PubMed] [Google Scholar]
- 39.Faulkner L, Cooper A, Fantino C, Altmann DM, Sriskandan S. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J Immunol 2005;175:6870-6877. [DOI] [PubMed] [Google Scholar]
- 40.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 2013;39:1003-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237-241. [DOI] [PubMed] [Google Scholar]
- 42.Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 2019;26:99-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, Kullberg BJ, Verschueren I, Van Der Meer JW. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1beta. Eur J Immunol 2000;30:3057-3060. [DOI] [PubMed] [Google Scholar]
- 44.Mazodier K, Marin V, Novick D, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 2005;106:3483-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu M, Yokoyama T, Yamada K, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645-1653. [DOI] [PubMed] [Google Scholar]
- 46.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol 2013;4:289-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol 2013;25:439-448. [DOI] [PubMed] [Google Scholar]
- 48.Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 2011;121:2264-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelik M, Torok KS, Kietz DA, Hirsch R. Hypocomplementemia associated with macrophage activation syndrome in systemic juvenile idiopathic arthritis and adult onset Still’s disease: 3 cases. J Rheumatol 2011;38:396-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139-303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu XJ, Tang YM. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett 2014;343:172-178. [DOI] [PubMed] [Google Scholar]
- 52.Singh N, Hofmann TJ, Gershenson Z, et al. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy 2017;19:867-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24:739-748. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Fang Y, Chen X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol 2020;5(43):eaax7969-eaax7969. [DOI] [PubMed] [Google Scholar]
- 56.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57-66. [DOI] [PubMed] [Google Scholar]
- 57.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006;355:1018-1028. [DOI] [PubMed] [Google Scholar]
- 58.Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2016;2016:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahmer MK, Randolph A, Vitali S, Quasney MW. Genetic polymorphisms in sepsis. Pediatr Crit Care Med 2005;6:Suppl:S61-S73. [DOI] [PubMed] [Google Scholar]
- 60.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nebelsiek T, Beiras-Fernandez A, Kilger E, Möhnle P, Weis F. Routine use of corticosteroids to prevent inflammation response in cardiac surgery. Recent Pat Cardiovasc Drug Discov 2012;7:170-174. [DOI] [PubMed] [Google Scholar]
- 62.Fisher CJ Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA 1994;271:1836-1843. [PubMed] [Google Scholar]
- 63.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016;44:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood 2011;118:618-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, Bracaglia C, Prencipe G, et al. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J Immunol 2016;196:2492-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood 2013;121:5184-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pachlopnik Schmid J, Ho C-H, Chrétien F, et al. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med 2009;1:112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013;122:4189-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood 2020;135:1353-1364. [DOI] [PubMed] [Google Scholar]
- 70.Ramaswami R, Lurain K, Peer CJ, et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2020;135:2316-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chellapandian D, Das R, Zelley K, et al. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol 2013;162:376-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dalla Pria A, Pinato D, Roe J, Naresh K, Nelson M, Bower M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood 2017;129:2143-2147. [DOI] [PubMed] [Google Scholar]
- 73.Grajales-Reyes GE, Colonna M. Interferon responses in viral pneumonias. Science 2020;369:626-627. [DOI] [PubMed] [Google Scholar]
- 74.Kaufman KM, Linghu B, Szustakowski JD, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 2014;66:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol 2014;192:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer 2013;60:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mouy R, Stephan JL, Pillet P, Haddad E, Hubert P, Prieur AM. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases. J Pediatr 1996;129:750-754. [DOI] [PubMed] [Google Scholar]
- 78.Faitelson Y, Grunebaum E. Hemophagocytic lymphohistiocytosis and primary immune deficiency disorders. Clin Immunol 2014;155:118-125. [DOI] [PubMed] [Google Scholar]
- 79.Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 2016;91:220-226. [DOI] [PubMed] [Google Scholar]
- 80.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 2005;106:2627-2632. [DOI] [PubMed] [Google Scholar]
- 81.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood 2018;132:2115-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierson SK, Stonestrom AJ, Shilling D, et al. Plasma proteomics identifies a ‘chemokine storm’ in idiopathic multicentric Castleman disease. Am J Hematol 2018;93:902-912. [DOI] [PubMed] [Google Scholar]
- 83.Langan Pai R-A, Sada Japp A, Gonzalez M, et al. Type I IFN response associated with mTOR activation in the TAFRO subtype of idiopathic multicentric Castleman disease. JCI Insight 2020;5(9):e135031-e135031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arenas DJ, Floess K, Kobrin D, et al. Increased mTOR activation in idiopathic multicentric Castleman disease. Blood 2020;135:1673-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fajgenbaum DC, Langan R-A, Sada Japp A, et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J Clin Invest 2019;129:4451-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang K-J, Su I-J, Theron M, et al. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 2005;75:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473-474. [DOI] [PubMed] [Google Scholar]
- 88.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis 2020;95:332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020;369(6508):eabc8511-eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis 2020. September 25 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020. September 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bastard P, Rosen LB, Zhang Q, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020. September 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lauder SN, Jones E, Smart K, et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol 2013;43:2613-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2020. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020. October 21 DOI: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med 2020;15:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fajgenbaum DC, Khor JS, Gorzewski A, et al. Treatments administered to the first 9152 reported cases of COVID-19: a systematic review. Infect Dis Ther 2020;9:435-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol 2020;2(8):e465-e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest 2020. November 03 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2020. October 06 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol 2020;5(48):eabd0110-eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 2020;214:108393-108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol 2017;69:1135-1143. [DOI] [PubMed] [Google Scholar]
- 109.de Jesus AA, Hou Y, Brooks S, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest 2020;130:1669-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimizu M, Nakagishi Y, Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 2013;61:345-348. [DOI] [PubMed] [Google Scholar]
- 111.Vercruysse F, Barnetche T, Lazaro E, et al. Adult-onset Still’s disease biological treatment strategy may depend on the phenotypic dichotomy. Arthritis Res Ther 2019;21:53-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.