Abstract
Background
World Health Organization expert groups recommended mortality trials of four repurposed antiviral drugs — remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a — in patients hospitalized with coronavirus disease 2019 (Covid-19).
Methods
We randomly assigned inpatients with Covid-19 equally between one of the trial drug regimens that was locally available and open control (up to five options, four active and the local standard of care). The intention-to-treat primary analyses examined in-hospital mortality in the four pairwise comparisons of each trial drug and its control (drug available but patient assigned to the same care without that drug). Rate ratios for death were calculated with stratification according to age and status regarding mechanical ventilation at trial entry.
Results
At 405 hospitals in 30 countries, 11,330 adults underwent randomization; 2750 were assigned to receive remdesivir, 954 to hydroxychloroquine, 1411 to lopinavir (without interferon), 2063 to interferon (including 651 to interferon plus lopinavir), and 4088 to no trial drug. Adherence was 94 to 96% midway through treatment, with 2 to 6% crossover. In total, 1253 deaths were reported (median day of death, day 8; interquartile range, 4 to 14). The Kaplan–Meier 28-day mortality was 11.8% (39.0% if the patient was already receiving ventilation at randomization and 9.5% otherwise). Death occurred in 301 of 2743 patients receiving remdesivir and in 303 of 2708 receiving its control (rate ratio, 0.95; 95% confidence interval [CI], 0.81 to 1.11; P=0.50), in 104 of 947 patients receiving hydroxychloroquine and in 84 of 906 receiving its control (rate ratio, 1.19; 95% CI, 0.89 to 1.59; P=0.23), in 148 of 1399 patients receiving lopinavir and in 146 of 1372 receiving its control (rate ratio, 1.00; 95% CI, 0.79 to 1.25; P=0.97), and in 243 of 2050 patients receiving interferon and in 216 of 2050 receiving its control (rate ratio, 1.16; 95% CI, 0.96 to 1.39; P=0.11). No drug definitely reduced mortality, overall or in any subgroup, or reduced initiation of ventilation or hospitalization duration.
Conclusions
These remdesivir, hydroxychloroquine, lopinavir, and interferon regimens had little or no effect on hospitalized patients with Covid-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay. (Funded by the World Health Organization; ISRCTN Registry number, ISRCTN83971151; ClinicalTrials.gov number, NCT04315948.)
In February 2020, a World Health Organization (WHO) research forum on coronavirus disease 2019 (Covid-19) recommended evaluation of treatments in large, randomized trials,1 and other WHO expert groups identified four repurposed antiviral drugs that might have at least a moderate effect on mortality: remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a.2 In March 2020, the WHO began a large, simple, international, open-label, randomized trial involving hospital inpatients to evaluate the effects of these four drugs on in-hospital mortality. The trial was adaptive; unpromising drugs could be dropped and others added. Hydroxychloroquine, lopinavir, and interferon were eventually dropped from the trial, but others, such as monoclonal antibodies, will be added. We report interim results for the original four drugs.
Methods
Trial Design
The protocol, which was published previously3 and is available with the full text of this article at NEJM.org, was designed to involve hundreds of hospitals in dozens of countries. Trial procedures were minimal but rigorous, with data entry through a cloud-based Good Clinical Practice–compliant clinical data management system that recorded demographic characteristics, respiratory support, coexisting illnesses, and local availability of trial drugs before generating the treatment assignment. Written informed consent was provided by patients, or if they were unable to do so, by their legal representatives.3 Consent forms were retained by signatories and encrypted for records. The enrollment of patients who provided consent took just a few minutes. Eligible patients were 18 years of age or older, were hospitalized with a diagnosis of Covid-19, were not known to have received any trial drug, were not expected to be transferred elsewhere within 72 hours, and, in the physician’s view, had no contraindication to any trial drug.
The same cloud-based system was used to report any suspected unexpected serious adverse reaction. It was also used to record death in the hospital or discharge alive (with documentation of respiratory support in the hospital, trial-drug timing, use of nontrial drugs, and probable cause of death). National and global monitors raised or resolved queries (or both) and checked progress and completeness.
Treatment Regimens
The trial drugs were remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a (given with lopinavir until July 4). The hydroxychloroquine, lopinavir, and interferon regimens were discontinued for futility on, respectively, June 19, July 4, and October 16, 2020. Participants were randomly assigned in equal proportions to receive no trial drug or one of the trial drug regimens that was locally available (up to five options; all patients were to receive the local standard of care). In this open-label trial, no placebos were used.
The controls for a drug were patients assigned to the standard of care at a time and place in which that drug was locally available (except that when interferon was being given only with lopinavir, its controls were patients given only lopinavir). Assignment to the standard of care at a hospital in which more than one trial drug was available would put that patient into the control group for each of those drugs. Hence, there was partial overlap among the four control groups. Each comparison between a trial drug and its control, however, was evenly randomized (in a 1:1 ratio) and unbiased, because both groups were affected equally by differences between countries or hospitals and by time trends in patient characteristics or the standard of care.
Daily doses were those already used for other diseases, but to maximize any efficacy without undue cardiac risk, the hydroxychloroquine dose was based on that for amoebic liver abscess rather than the lower dose for malaria.4 (Hydroxychloroquine slightly prolongs the QT interval, and an unduly high dose or rapid administration might cause arrhythmias or hypotension.) Treatments stopped at discharge.
The regimen for remdesivir (intravenous) was 200 mg on day 0 and 100 mg on days 1 through 9. The regimen for hydroxychloroquine (oral) was four tablets at hour 0, four tablets at hour 6, and, starting at hour 12, two tablets twice daily for 10 days. Each tablet contained 200 mg of hydroxychloroquine sulfate (155 mg of hydroxychloroquine base per tablet; a little-used alternative involved 155 mg of chloroquine base per tablet). The regimen for lopinavir (oral) was two tablets twice daily for 14 days. Each tablet contained 200 mg of lopinavir (plus 50 mg of ritonavir, to slow hepatic lopinavir clearance). Other formulations were not provided, so patients who were receiving mechanical ventilation received no trial lopinavir while they were unable to swallow. The regimen for interferon (mainly subcutaneous) was three doses over a period of 6 days (the day of randomization and days 3 and 6) of 44 μg of subcutaneous interferon beta-1a; where intravenous interferon was available, patients receiving high-flow oxygen, ventilation, or extracorporeal membrane oxygenation (ECMO) were instead to be given 10 μg intravenously daily for 6 days.
Outcomes
The protocol-specified primary objective was to assess effects on in-hospital mortality (i.e., death during the original hospitalization; follow-up ceased at discharge), regardless of whether death occurred before or after day 28. The only protocol-specified secondary outcomes were the initiation of mechanical ventilation and hospitalization duration. Although no placebos were used, appropriate analyses of these secondary outcomes can still be informative. Add-on studies that were led from Canada, France, India, and Norway recorded other outcomes (not reported here).
Oversight and Funding
The trial was registered at the ISRCTN Registry and ClinicalTrials.gov, with the core protocol approved by the WHO ethics review committee and local protocols approved by national ethics committees and regulatory authorities. Trial conduct was in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The only exclusions from the intention-to-treat analyses were the few patients with no, or uncertain, consent to follow-up. All other randomly assigned patients were included. The WHO was the global cosponsor and governments the national cosponsors, with trial governance by the executive group of the international steering committee. External statistical analyses for the independent data and safety monitoring committee were unseen by the executive group or the WHO, with two exceptions. After outside evidence of the futility of hydroxychloroquine and lopinavir became available, the executive group requested unblinded analyses of the findings just for these two drugs. In addition, after deciding in a blinded fashion to report all interim results, the executive group revised this manuscript, which has been drafted only by the WHO trial team and external statisticians. Remdesivir was donated by Gilead Sciences, hydroxychloroquine by Mylan, lopinavir by AbbVie, Cipla, and Mylan, and interferon beta-1a by Merck (subcutaneous) and Faron Pharmaceuticals (intravenous).
Sample Size
The protocol stated, “The larger the number entered the more accurate the results will be, but numbers entered will depend on how the epidemic develops. … it may be possible to enter several thousand hospitalised patients with relatively mild disease and a few thousand with severe disease, but realistic, appropriate sample sizes could not be estimated at the start of the trial.” The executive group, whose members were unaware of the findings, made the decision to release the interim results.
Statistical Analysis
The intention-to-treat analyses related outcome to assigned treatment. The primary analyses were of in-hospital mortality among all randomly assigned patients (each drug vs. its control). The only protocol-specified subgroup analyses involved patients who already had severe disease at entry and those who did not. Severity was not protocol-defined, but separate analyses are provided regarding those receiving some supplemental oxygen or none and for those already receiving ventilation at entry or not. Rate ratios for death (or, equivalently, hazard ratios) and P values are from log-rank analyses stratified according to six strata of age and ventilation status at entry. Graphs of mortality according to time are from unstratified Kaplan–Meier methods, with denominators chosen to yield in-hospital mortality. (For example, if 99 of 100 patients were discharged alive before the last one died, the in-hospital mortality would be 1% and at the time of that death the probability of not having died in the hospital was multiplied by 99/100; this denominator included those already discharged.)
The risk on day N was calculated by first excluding patients with an outcome not reported or an entry fewer than N days before data-set closure (or transferred elsewhere before day N); then, the number of in-hospital deaths on day N was divided by the total number of patients in the hospital on day N or discharged alive before day N. This denominator (or “risk set”), which includes those discharged before day N, was also used to calculate the contribution of day N to log-rank analysis and Cox analysis of in-hospital mortality. Denominators for the few deaths on day 0, but not on later days, included patients with no follow-up reported (because if any patient died on the day of randomization, this would probably have been reported).
If the stratified log-rank observed minus expected number of deaths is O−E with variance V, the loge rate ratio is calculated as (O−E)/V with variance 1/V and a normal distribution. If event times are accurate and b is the log hazard ratio and L(b) the Cox log-likelihood, the first and second derivatives of L(b) at b=0 are (O−E) and –V.5 Forest plots (with 95% confidence intervals only for overall trial results; otherwise, with 99% confidence intervals to allow for subgroup multiplicity) and chi-square statistics (sum of [O−E]2/V, without any P value) help interpret any heterogeneity of rate ratios between subgroups. All rate ratios describe proportional risk reductions; absolute risk reductions would also depend on background risks. Analyses were performed with the use of SAS software, version 9.4, and R software, version 4.02.
Meta-analyses of the major trial results are based on the inverse-variance–weighted average of b=loge rate ratio from each stratum of each trial, with the use of odds ratios when hazard ratios or rate ratios for death were unavailable. (This weighted average is derived from the sums of [O−E] and of V over strata.5) In general, the more deaths in a stratum the larger V is and, correspondingly, the smaller is the variance of the loge rate ratio, so the more weight that stratum gets. The variance that is attributed to the result in each stratum and to the overall weighted average reflects only the play of chance at randomization. Homogeneity of different rate ratios is not needed for such a weighted average to be informative.
Results
Patient Characteristics and Adherence
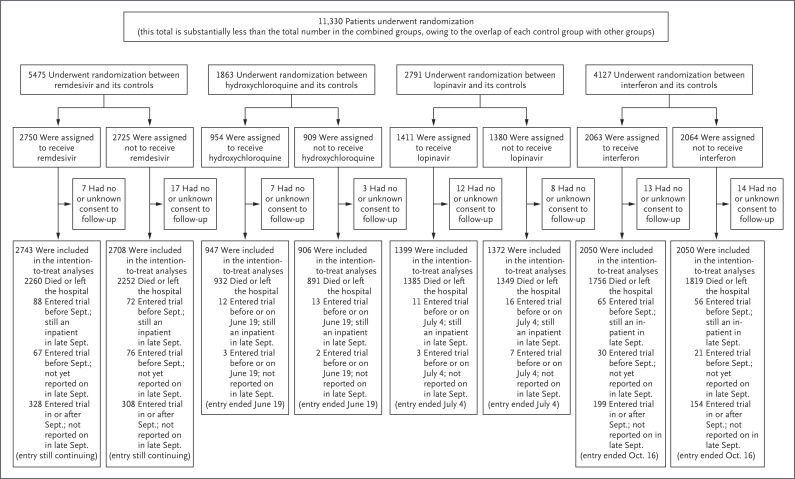
From March 22 to October 4, 2020, a total of 11,330 patients were entered in the trial from 405 hospitals in 30 countries in all six WHO regions. Of these patients, 64 (0.6%) had no, or uncertain, consent to follow-up, which left 11,266 in the intention-to-treat analyses. A total of 2750 patients were assigned to receive remdesivir, 954 to hydroxychloroquine, 1411 to lopinavir (without interferon), 2063 to interferon (including 651 to interferon plus lopinavir), and 4088 to no trial drug (Figure 1); reporting is 97% complete for those who were entered more than 1 month earlier and 99.7% complete for those who were entered more than 3 months earlier. All 3 patients for whom the diagnosis of Covid-19 was later ruled out were included in the analyses and survived. Table 1 shows patient characteristics: 9120 (81%) were younger than 70 years of age, 6985 (62%) were male, 2768 (25%) had diabetes, 916 (8%) were already receiving ventilation, and 7002 (62%) underwent randomization on days 0 or 1. For each drug, patient characteristics were well balanced by the unstratified 1:1 randomization between it and its control. Deaths were at a median of day 8 (interquartile range, 4 to 14), and discharges were at a median of day 8 (interquartile range, 5 to 12).
Figure 1. Information to October 4, 2020, on Trial Entry, Follow-up, and Intention-to-Treat Analyses.
After it was determined which treatments were locally available, random assignment (with equal probability) was between the local standard of care and the available treatments. After the exclusion of 64 of 11,330 patients (0.6%) who had provided either no or uncertain consent regarding follow-up, 11,266 remained in the intention-to-treat analyses. Each pairwise intention-to-treat analysis was between a particular trial drug and its control (i.e., patients who could have been assigned to a particular trial drug but were concurrently assigned to the same care without it). There is partial overlap of each control group with other groups.
Table 1. Entry Characteristics According to Random Assignment, and Adherence to That Assignment.*.
| Variable | Any Intention-to-Treat Analysis (N=11,266) | Remdesivir vs. Its Control |
Hydroxychloroquine vs. Its Control |
Lopinavir vs. Its Control |
Interferon vs. Its Control† |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entered Trial |
Died in Hospital‡ | 28-Day Mortality§ |
Active (N=2743) |
Control (N=2708) |
Active (N=947) |
Control (N=906) |
Active (N=1399) |
Control (N=1372) |
Active (N=2050) |
Control (N=2050) |
|
| no. (%) | no. | % | no. of patients | ||||||||
| Entry characteristics | |||||||||||
| Age | |||||||||||
| <50 yr | 3995 (35) | 237 | 6.2 | 961 | 952 | 335 | 317 | 511 | 501 | 720 | 697 |
| 50–69 yr | 5125 (45) | 618 | 12.8 | 1282 | 1287 | 410 | 396 | 597 | 596 | 934 | 973 |
| ≥70 yr | 2146 (19) | 398 | 20.4 | 500 | 469 | 202 | 193 | 291 | 275 | 396 | 380 |
| Respiratory support | |||||||||||
| No supplemental oxygen at entry | 3204 (28) | 78 | 2.5 | 661 | 664 | 345 | 341 | 528 | 539 | 482 | 490 |
| Supplemental oxygen at entry | 7146 (63) | 844 | 12.8 | 1828 | 1811 | 517 | 483 | 759 | 719 | 1429 | 1430 |
| Already receiving ventilation | 916 (8) | 331 | 39.0 | 254 | 233 | 85 | 82 | 112 | 114 | 139 | 130 |
| Lesions in both lungs | |||||||||||
| No | 1266 (11) | 49 | 3.7 | 287 | 259 | 154 | 170 | 235 | 256 | 162 | 155 |
| Yes | 8832 (78) | 1043 | 12.7 | 2175 | 2153 | 656 | 618 | 985 | 945 | 1723 | 1718 |
| Not imaged at entry | 1168 (10) | 161 | 14.9 | 281 | 296 | 137 | 118 | 179 | 171 | 165 | 177 |
| Previous days in the hospital | |||||||||||
| 0 | 3289 (29) | 319 | 9.8 | 724 | 712 | 296 | 281 | 423 | 403 | 678 | 677 |
| 1 | 3713 (33) | 384 | 10.8 | 917 | 938 | 317 | 312 | 442 | 445 | 681 | 662 |
| ≥2 | 4264 (38) | 550 | 14.6 | 1102 | 1058 | 334 | 313 | 534 | 524 | 691 | 711 |
| Geographic region | |||||||||||
| Europe and Canada¶ | 2488 (22) | 188 | 7.8 | 715 | 698 | 286 | 267 | 349 | 350 | 254 | 244 |
| Latin America‖ | 1941 (17) | 400 | 22.7 | 470 | 514 | 97 | 96 | 145 | 148 | 474 | 478 |
| Asia and Africa** | 6837 (61) | 665 | 10.3 | 1558 | 1496 | 564 | 543 | 905 | 874 | 1322 | 1328 |
| Other characteristics | |||||||||||
| Male sex | 6985 (62) | 852 | 13.0 | 1706 | 1725 | 574 | 535 | 851 | 802 | 1303 | 1278 |
| Current smoker | 830 (7) | 93 | 11.8 | 178 | 161 | 92 | 82 | 141 | 124 | 136 | 138 |
| Coexisting conditions | |||||||||||
| Diabetes | 2768 (25) | 379 | 14.7 | 707 | 666 | 199 | 205 | 341 | 324 | 489 | 537 |
| Heart disease | 2337 (21) | 319 | 14.7 | 571 | 567 | 193 | 194 | 289 | 290 | 427 | 456 |
| Chronic lung disease | 635 (6) | 102 | 17.2 | 151 | 145 | 62 | 66 | 95 | 87 | 114 | 109 |
| Asthma | 529 (5) | 56 | 11.5 | 139 | 139 | 41 | 46 | 65 | 56 | 75 | 97 |
| Chronic liver disease | 135 (1) | 21 | 17.2 | 36 | 41 | 15 | 14 | 15 | 23 | 11 | 22 |
| Adherence to assigned treatment | |||||||||||
| Percent taking trial drug midway through scheduled duration††‡‡ | 96 | 2 | 95 | 6 | 94 | 2 | 94 | 2 | |||
| Percent ever reported as discharged who were still in the hospital at various times†† | |||||||||||
| On day 7 | 69 | 59 | 64 | 54 | 68 | 59 | 55 | 51 | |||
| On day 14 | 22 | 19 | 23 | 20 | 31 | 22 | 19 | 18 | |||
| On day 21 | 9 | 8 | 11 | 10 | 12 | 11 | 8 | 7 | |||
A total of 64 patients who did not provide clear informed consent regarding follow-up were excluded. Comparisons are of each trial drug with concurrent assignment to the same treatment without it. Because the control groups overlap, the total number (11,266) is less than the sum of the numbers in the pairwise comparisons. The few patients (always <0.4%) with a particular characteristic not yet known were merged with the largest category of that characteristic: 33 were merged with male sex, 40 were merged with an age of 50 to 69, and 45 were merged with previous days in the hospital of 2 or more.
Interferon randomization was interferon plus lopinavir as compared with lopinavir until July 4, 2020, then it was interferon as compared with the local standard of care.
Shown are any in-hospital deaths, regardless of whether they occurred before or after day 28 (total, 1253 deaths).
Shown is the Kaplan–Meier 28-day risk of in-hospital death, expressed as a percentage (overall value, 11.8%). Percentages may not total 100 because of rounding.
Countries in Europe were Albania, Austria, Belgium, Finland, France, Ireland, Italy, Lithuania, Luxembourg, North Macedonia, Norway, Spain, and Switzerland.
Countries included Argentina, Brazil, Colombia, Honduras, and Peru.
Countries included Egypt, India, Indonesia, Iran, Kuwait, Lebanon, Malaysia, Pakistan, the Philippines, Saudi Arabia, and South Africa.
Percentage of patients (rather than number of patients) is shown for this variable.
Adherence was calculated only among patients who died or were discharged alive and was defined as the percentage of patients who were taking the trial drug midway through its scheduled duration (or midway through the time from entry to death or discharge, if this was shorter).
There were 1253 in-hospital deaths (the primary outcome, including those before and after day 28). The Kaplan–Meier risk of in-hospital death to day 28 was 11.8%; a few in-hospital deaths occurred later. This risk depended on several factors, particularly age (20.4% if ≥70 years and 6.2% if <50 years) and ventilation status (39.0% if the patient was already receiving ventilation and 9.5% otherwise).
Table 1 also shows adherence. For remdesivir, the scheduled treatment duration was 10 days (or to death or discharge). Of those assigned to remdesivir, 98% began treatment. Midway through this period, 96% of the patients were still taking it (as compared with only 2% of those in the relevant group). Similarly, for other drugs adherence midway was 94% to 95%, and crossover was 2 to 6%. Trial treatments ceased on schedule (if the patient was still in the hospital). Absolute differences (active vs. control) in the use of glucocorticoids (i.e., corticosteroids) and other nontrial drugs were 0.2 to 3.5 percentage points (Table S2 in the Supplementary Appendix, available at NEJM.org).
Primary Outcome
For each pairwise comparison of a drug and its control, Figure 2 and Figures S1 through S5 show the results of unstratified Kaplan–Meier analyses of in-hospital mortality (with numbers of patients who underwent randomization, in-hospital deaths each week and after day 28, and weekly denominators), along with rate ratios for death stratified according to age and ventilation status; Figure 3 shows the stratified rate ratios according to age and according to ventilation status. No trial drug had any definite effect on mortality, either overall (each P>0.10) or in any subgroup defined according to age, ventilation status at entry, other entry characteristics, geographic region, or glucocorticoid use (Figs. S6 through S9).
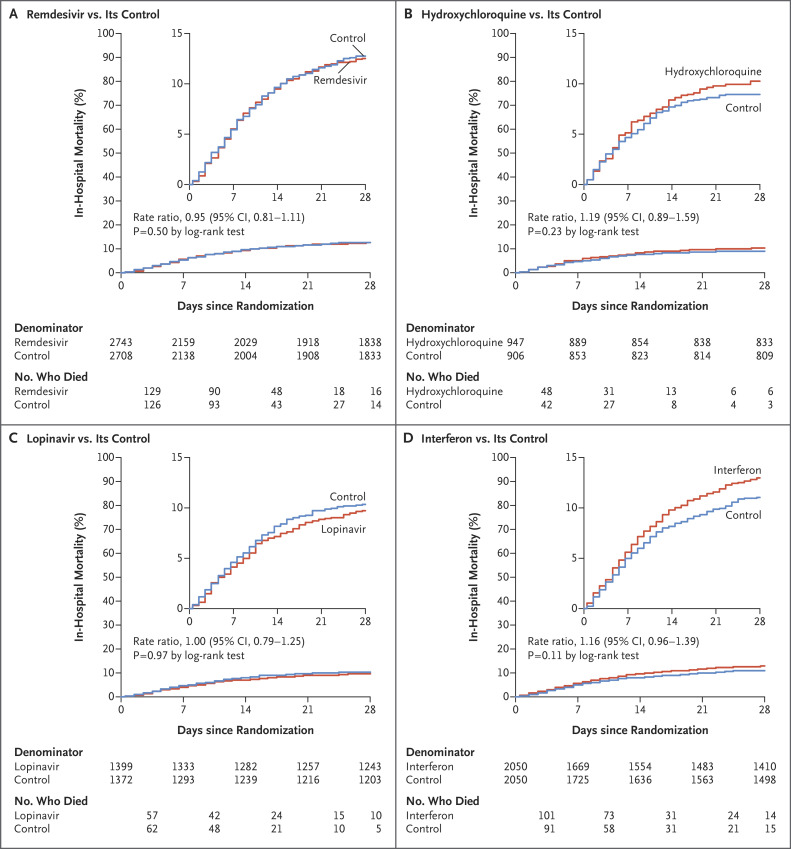
Figure 2. Effects of Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon on In-Hospital Mortality.
Shown are Kaplan–Meier graphs of in-hospital mortality at any time (the primary outcome), comparing each treatment with its control without standardization for any initial patient characteristics. Insets show the same data on an expanded y axis. The rate ratios for death were standardized for age and for ventilation status at entry. Denominators for the few events on day 0, but not thereafter, include patients with no follow-up. Numbers of deaths are by week, and then deaths after day 28. CI denotes confidence interval.
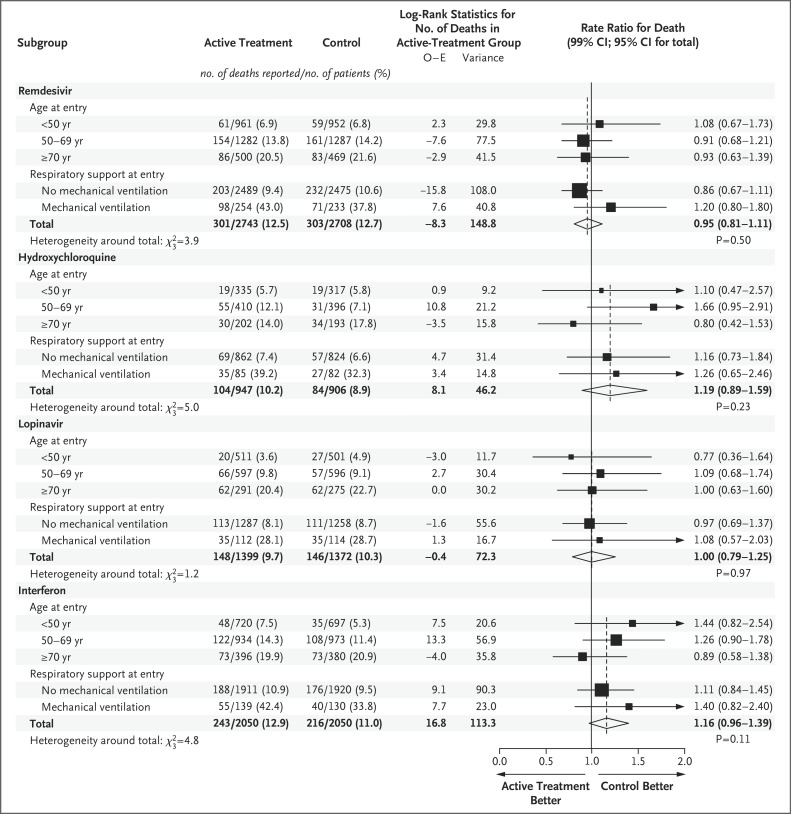
Figure 3. Rate Ratios for In-Hospital Death, Subdivided by Age and Respiratory Support at Trial Entry.
Analyses in subgroups of age are stratified according to respiratory status at trial entry and vice versa, so each total is stratified for both factors. The percentages show Kaplan–Meier 28-day mortality. O−E denotes the observed minus expected number of deaths in patients assigned to active treatment. Diamonds show 95% confidence intervals for treatment effects. Squares and horizontal lines show treatment effects in particular subgroups and their 99% confidence intervals, with an arrow if the upper 99% confidence limit is outside the range shown. The area of each square is proportional to the variance of O−E in the subgroup it describes..
Death occurred in 301 of 2743 patients receiving remdesivir and in 303 of 2708 receiving its control (rate ratio, 0.95; 95% confidence interval [CI], 0.81 to 1.11; P=0.50), in 104 of 947 patients receiving hydroxychloroquine and in 84 of 906 receiving its control (rate ratio, 1.19; 95% CI, 0.89 to 1.59; P=0.23), in 148 of 1399 patients receiving lopinavir and in 146 of 1372 receiving its control (rate ratio, 1.00; 95% CI, 0.79 to 1.25; P=0.97), and in 243 of 2050 patients receiving interferon and in 216 of 2050 receiving its control (rate ratio, 1.16; 95% CI, 0.96 to 1.39; P=0.11). Unstratified comparisons yielded similarly null findings (Figure 2), as did analyses that excluded patients receiving glucocorticoids and multivariable sensitivity analyses that estimated trial drug effects simultaneously (Table S3). If mechanical ventilation prevented oral administration of lopinavir or other trial drugs, then this could have reduced any effects on mortality of assignment to those drugs, but prespecified analyses of mortality among patients not already receiving ventilation at entry also indicated no definite protective effect of any trial drug (Figure 3).
Secondary Outcomes
The prespecified secondary outcomes were ventilation and time to discharge. No trial drug reduced the initiation of ventilation among patients not already receiving ventilation. Ventilation was initiated after randomization in 295 patients receiving remdesivir and in 284 receiving its control, in 75 patients receiving hydroxychloroquine and in 66 receiving its control, in 126 patients receiving lopinavir and in 121 receiving its control, and in 209 patients receiving interferon and in 210 receiving its control (Table S1). Figure S10 shows the results for the combined outcome of in-hospital death or ventilation initiation.
In this open-label trial, patients who would be considered fit for discharge might be kept in the hospital somewhat longer just because they were being given a trial drug, but information on time to recovery can be obtained by comparing the effects of different drugs on time to discharge. Each of the three trial treatments that were scheduled to last more than 7 days increased the percentage of patients remaining in the hospital at day 7 (Table 1). If one of these three drugs had appreciably accelerated recovery, then the sizes of these effects should have differed, but they did not. Figures S11 through S16 plot time to discharge for all patients, those receiving supplemental oxygen, those not receiving supplemental oxygen, those receiving ventilation, those not receiving ventilation, and those receiving any respiratory support. Each drug delayed discharge by approximately 1 to 3 days while it was being given. Directly randomized comparisons of one trial drug with another (Fig. S17) likewise showed no appreciable differences in discharge rates while both drug regimens continued or after both had ended.
The supplementary analyses (Tables S2 and S3) tabulate co-medication (only small absolute differences were found between each trial drug and its control) and provide a multivariable Cox regression fitting all four treatment effects simultaneously (rate ratios for death were similar to those in Figure 3). The analyses also (in Figs. S1 through S9) subdivide 28-day mortality graphs according to ventilation status at entry and give subgroup analyses of rate ratios for death according to other characteristics and according to glucocorticoid use (with no noteworthy subgroup-specific or geographic variation).
All active treatment ended within 14 days, and the numbers of deaths during this 14-day period with any cardiac cause mentioned on the electronic death record were seven with remdesivir and eight with its control, four with hydroxychloroquine and two with its control, six with lopinavir and three with its control, and six with interferon and eight with its control (Fig. S18). Many deaths from Covid-19 involve multiorgan failure, but no death in a patient assigned to a trial drug was attributed specifically by the doctor reporting the death to renal or hepatic disease.
Meta-Analyses
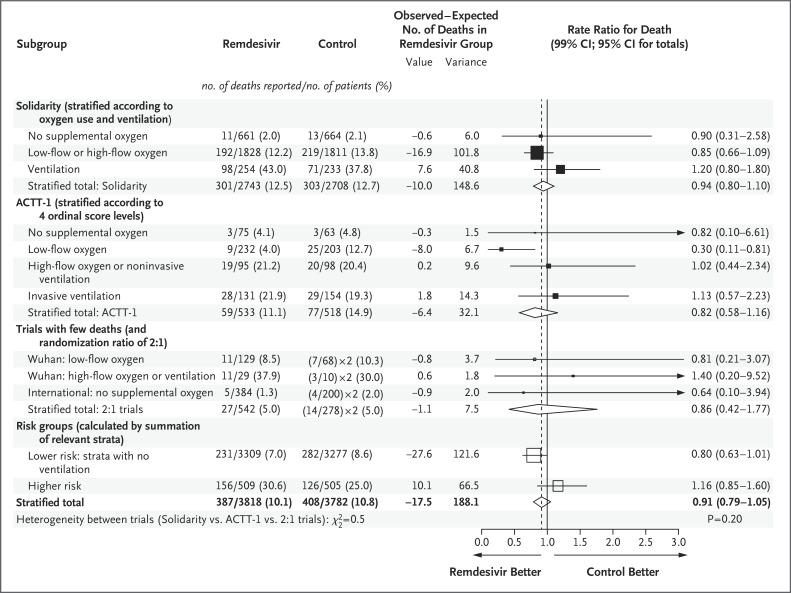
There are four trials that have compared remdesivir with control: the Solidarity trial (604 deaths in 5451 randomly assigned patients), the Adaptive Covid-19 Treatment Trial (ACTT-1) (136 deaths in 1062 patients; mortality was a secondary outcome), and two smaller trials (41 deaths).6-9 Figure 4 shows the mortality results from each trial, stratified according to initial respiratory support. Within each trial, summation of the observed minus expected numbers of deaths with remdesivir in each stratum led to the stratified rate ratio for death in that trial. Summation of these trial-specific observed-minus-expected subtotals then led to an appropriately weighted average of the results from all trials, which yielded a rate ratio for death (remdesivir vs. control) of 0.91 (95% CI, 0.79 to 1.05).5 Figures S19 and S20 show the mortality results in the trials of hydroxychloroquine (rate ratio, 1.09; 95% CI, 0.98 to 1.21) and of lopinavir (rate ratio, 1.01; 95% CI, 0.91 to 1.13).
Figure 4. Meta-Analysis of Mortality in Trials of Random Assignment of Remdesivir or Its Control to Hospitalized Patients with Covid-19.
Percentages show Kaplan–Meier 28-day mortality. Values for observed minus expected number of deaths (O−E) are log-rank O−E for the Solidarity trial, O−E from 2-by-2 tables for the Wuhan7 and international8 trials, and w.loge hazard ratio for each stratum in the Adaptive Covid-19 Treatment Trial (ACTT-1)6 (with the weight w being the inverse of the variance of the loge hazard ratio, which was calculated from the confidence interval of the hazard ratio). Rate ratios were calculated by taking the loge rate ratio to be (O−E)/V with a Normal distribution and variance 1/V. Subtotals or totals of (O−E) and of V yield inverse-variance–weighted averages of the loge rate ratios. For balance, controls in the 2:1 trials were counted twice in the control totals and subtotals. Diamonds show 95% confidence intervals for treatment effects. Squares and horizontal lines show treatment effects in particular subgroups and their 99% confidence intervals, with an arrow if the upper 99% confidence limit is outside the range shown. The area of each square is proportional to the variance of O−E in the subgroup it describes.
Discussion
The main outcomes of mortality, initiation of ventilation, and hospitalization duration were not definitely reduced by any trial drug, either overall or in any particular subgroup. The findings for mortality and for initiation of ventilation cannot have been appreciably biased by the open-label design without placebos, or by variation in local care or patient characteristics, and were little affected when homogeneity was increased by stratification according to geographic region, age, or use of ventilation at entry. No trial drug reduced the initiation of mechanical ventilation. The similarity of this null effect for all four drugs is further evidence that none has any material effect on major disease progression, a conclusion supported by analyses of the combined outcome of death or ventilation initiation.
Although assignment to any of the active trial treatments in this open-label trial somewhat delayed discharge from the hospital, this could have been because some recovered patients otherwise fit for discharge were kept in the hospital merely to continue their trial treatment. In all patients and in those not receiving ventilation, assignment to each active trial drug increased the time to discharge by approximately 1 to 3 days while treatment continued. Because no treatment had much effect on death or progression to ventilation, the similarity of these four moderate delays of discharge suggests that none of the four treatments had a pharmacologic effect that substantially reduced time to recovery (i.e., fitness for discharge). In particular, it suggests at most only a small effect of remdesivir on time to recovery, a conclusion supported by the directly randomized comparisons between remdesivir and the other three trial drugs.
ACTT-1, which examined remdesivir, was placebo-controlled,6 which avoids any bias in time to discharge. In that trial, however, the proportion of lower-risk patients (i.e., those not already receiving high-flow oxygen or ventilation) happened to be appreciably greater in the remdesivir group than in the placebo group. This chance imbalance might account for some of the differences in time to recovery between ACTT-1 and the Solidarity trial.
The chief aim of the Solidarity trial was to help determine whether any of four repurposed antivirals could at least moderately affect in-hospital mortality. Its results should be considered in the context of the evidence on mortality from all trials, but for remdesivir and for interferon it provides more than three fourths of that evidence (Figure 4). Stratification of the findings according to initial respiratory support again facilitates allowance for the remdesivir group in ACTT-1 having, by chance, started with a greater proportion of low-risk patients and a smaller proportion of high-risk patients than the placebo group. The stratified rate ratios for death in the Solidarity trial and ACTT-1 are compatible with each other, and either singly or together they are compatible with there being little or no effect of remdesivir on mortality.
With an appropriately weighted average of the stratified results from each of the four trials,5 the rate ratio for death with remdesivir as compared with control was 0.91 (95% CI, 0.79 to 1.05). Interpretation of this should chiefly reflect not the P value (P=0.20) or point estimate (rate ratio, 0.91) but the confidence interval (0.79 to 1.05), which shows the range of rate ratios for death that are compatible with the weighted average of the findings from all trials. This does not support the suggestion that remdesivir can prevent a substantial fraction of all deaths. The confidence interval is compatible with prevention of a small fraction of all deaths, but it is also compatible with prevention of no deaths.
Statistical uncertainties are magnified if attention is restricted to particular subgroups or time periods.10 If remdesivir has no effect on mortality, then chance could well produce somewhat favorable findings in a subgroup of the results for all trials or striking findings in a selected subgroup of a particular trial (as in the unplanned subgroup of ACTT-1 in which the rate ratio for death was 0.30) (Figure 4). Although both the Solidarity trial and ACTT-1 envisaged separate analyses involving lower-risk and higher-risk patients, they did not define how this subdivision would apply to mortality analyses. The ACTT-1 protocol prespecified separate analyses of time to recovery among those with mild-to-moderate disease not receiving supplemental oxygen, as did the recent Food and Drug Administration reanalyses,11 which categorized anyone receiving even low-flow supplemental oxygen as having severe disease. This subdivision, however, leaves few deaths in the no-supplemental-oxygen category (death in 3 of 75 patients with remdesivir and in 3 of 63 with placebo in ACTT-1, in 11 of 661 patients with remdesivir and in 13 of 664 with its control in the Solidarity trial, and in 5 of 384 patients with remdesivir and in 4 of 200 with the standard of care in an international trial with a 2:1 randomization ratio8).
To augment these small numbers of deaths, the subtotals in Figure 4 include low-flow oxygen with no supplemental oxygen, which yields a large lower-risk subgroup and a small higher-risk subgroup. With this nonprespecified subgrouping, there appears to be an absolute reduction of approximately 1 to 2 percentage points in mortality among lower-risk inpatients and an absolute increase of approximately 5 to 6 percentage points among higher-risk inpatients. These absolute differences in the meta-analysis of all four trials are similar to the absolute differences seen when the Solidarity trial is subdivided according to ventilation status at entry. Neither subgroup should, however, be considered in isolation from the other or from the confidence interval for overall mortality.
For hydroxychloroquine and lopinavir, the Solidarity trial showed no definite effect on mortality in any subgroup. The only other substantial trial is the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12,13 which for these two drugs was larger than the Solidarity trial and also showed no benefit. Combination of both trials reinforces these null findings (Figs. S19 and S20).
For hydroxychloroquine, the joint rate ratio for death (combining the Solidarity and RECOVERY trials) was 1.10 (95% CI, 0.98 to 1.23), with no apparent benefit whether the patient was receiving ventilation or not. This confidence interval rules out any material benefit from this hydroxychloroquine regimen in hospitalized patients with Covid-19. It is compatible with some adverse effect but is not good evidence for any adverse effect and is not a safety signal. Despite concerns that the loading dose could be temporarily cardiotoxic,4 in neither trial was there any excess mortality during the first few days, and cardiac deaths were too few to be reliably informative. A recent meta-analysis identified 15 small, randomized trials with nonzero mortality14; combining all 17 hydroxychloroquine trials yields a rate ratio of 1.09 (95% CI, 0.98 to 1.21), which still rules out any material benefit.
For lopinavir, which was always administered with ritonavir, the joint rate ratio for death (combining the Solidarity and RECOVERY trials and the only informative smaller trial15) was 1.01 (95% CI, 0.91 to 1.13). Although lopinavir tablets could not be swallowed by patients receiving ventilation, there was no apparent benefit in analyses that involved only those not already receiving ventilation at entry. This confidence interval suggests no material effect on mortality and rules out a 10% proportional reduction. An add-on study within the Solidarity trial, Discovery, recorded many clinical variables and identified an unexpected increase in the creatinine level (perhaps because blood lopinavir levels are higher than in patients with human immunodeficiency virus infection receiving similar doses16,17), but the Solidarity and RECOVERY trials recorded no specifically renal or hepatic deaths with lopinavir.
For interferon beta-1a, no other large trials exist. With 4000 patients, the rate ratio for death in the Solidarity trial was 1.16 (95% CI, 0.96 to 1.39), or 1.12 (95% CI, 0.83 to 1.51) without lopinavir co-administration; these findings suggest no mortality reduction. Subcutaneous and intravenous interferon have different pharmacokinetic characteristics,18,19 and glucocorticoids could affect interferon signaling,20,21 but the clinical relevance of both issues is unclear. Most interferon was administered subcutaneously, because intravenous interferon was used only in patients receiving high-flow oxygen or ventilation, and distribution of it began only in late May, just before strong evidence emerged of glucocorticoid efficacy in such patients.22,23 Hence, few patients received intravenous interferon without a glucocorticoid. Approximately half the patients who were assigned to interferon (and half their controls) received glucocorticoids, but the rate ratio for death with interferon as compared with its control seemed unaffected by glucocorticoid use. Randomization to interferon was discontinued on October 16, but other trials continue. A report that nebulized interferon beta-1a might be effective involved only approximately 100 patients with Covid-19 (ClinicalTrials.gov number, NCT04385095), but the ongoing placebo-controlled ACTT-3 of subcutaneous interferon beta-1a aims to involve 1000 patients (NCT04492475), with examination of time to recovery.
For each of these four repurposed nonspecific antivirals, several thousand patients have now undergone randomization in various trials. The unpromising overall findings from the regimens tested suffice to refute early hopes, based on smaller or nonrandomized studies, that any of these regimens will substantially reduce inpatient mortality, the initiation of mechanical ventilation, or hospitalization duration. Narrower confidence intervals would be helpful (particularly for remdesivir), but the main need is for better treatments. The Solidarity trial has been recruiting approximately 2000 patients per month, and efficient factorial designs may allow it to assess further treatments, such as immune modulators or anti–SARS-Cov-2 monoclonal antibodies.
Acknowledgments
We thank the thousands of patients and their families who participated in this trial and the hundreds of medical staff who randomly assigned and cared for them. The Ministries of Health of participating member states and national institutions provided critical support in trial implementation. Derk Arts of Castor EDC donated and managed Castor’s cloud-based clinical data capture and management system, with blinding to trial findings. Anonymized data handling or analysis was performed at the Universities of Bern, Bristol, and Oxford. Nicholas J. White and colleagues provided unpublished data on the pharmacokinetic characteristics of hydroxychloroquine to help the WHO select the regimen, the members of the Discovery data and safety monitoring committee shared clinical variables, the investigators of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial shared log-rank statistics, the investigators of the Adaptive Covid-19 Treatment Trial (ACTT-1) shared subgroup hazard ratios, and Bin Cao shared details of the Wuhan trial. Collaborators, committee members, data analysts, and data management systems charged no costs.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The members of the writing and steering committees are as follows: Hongchao Pan, Ph.D., Richard Peto, F.R.S., Ana-Maria Henao-Restrepo, M.D., Marie-Pierre Preziosi, Ph.D., Vasee Sathiyamoorthy, Ph.D., Quarraisha Abdool Karim, Ph.D., Marissa M. Alejandria, M.D., César Hernández García, Ph.D., Marie-Paule Kieny, Ph.D., Reza Malekzadeh, M.D., Srinivas Murthy, M.D., K. Srinath Reddy, M.D., Mirta Roses Periago, M.D., Pierre Abi Hanna, M.D., Florence Ader, Ph.D., Abdullah M. Al-Bader, Ph.D., Almonther Alhasawi, M.D., Emma Allum, M.Math., Athari Alotaibi, M.Sc., Carlos A. Alvarez-Moreno, Ph.D., Sheila Appadoo, M.P.H., Abdullah Asiri, M.B., B.S., Pål Aukrust, Ph.D., Andreas Barratt-Due, Ph.D., Samir Bellani, B.Sc., Mattia Branca, Ph.D., Heike B.C. Cappel-Porter, M.Math., Nery Cerrato, M.D., Ting S. Chow, M.D., Najada Como, Ph.D., Joe Eustace, B.Ch., M.H.S., Patricia J. García, Ph.D., Sheela Godbole, M.B., B.S., Eduardo Gotuzzo, M.D., Laimonas Griskevicius, Ph.D., Rasha Hamra, Pharm.D., Mariam Hassan, M.B., B.S., Mohamed Hassany, M.D., David Hutton, B.Sc., Irmansyah Irmansyah, M.D., Ligita Jancoriene, Ph.D., Jana Kirwan, M.A., Suresh Kumar, M.B., B.S., Peter Lennon, B.B.S., Gustavo Lopardo, M.D., Patrick Lydon, M.Sc., Nicola Magrini, M.D., Teresa Maguire, Ph.D., Suzana Manevska, M.D., Oriol Manuel, M.D., Sibylle McGinty, Ph.D., Marco T. Medina, M.D., María L. Mesa Rubio, M.D., Maria C. Miranda-Montoya, M.D., Jeremy Nel, M.B., Ch.B., Estevao P. Nunes, Ph.D., Markus Perola, Ph.D., Antonio Portolés, Ph.D., Menaldi R. Rasmin, M.D., Aun Raza, M.D., Helen Rees, M.R.C.G.P., Paula P.S. Reges, M.D., Chris A. Rogers, Ph.D., Kolawole Salami, M.D., Marina I. Salvadori, M.D., Narvina Sinani, Pharm.D., Jonathan A.C. Sterne, Ph.D., Milena Stevanovikj, Ph.D., Evelina Tacconelli, Ph.D., Kari A.O. Tikkinen, Ph.D., Sven Trelle, M.D., Hala Zaid, Ph.D., John-Arne Røttingen, Ph.D., and Soumya Swaminathan, M.D.
Manuscript preparation, revision, and submission were controlled by the World Health Organization (WHO) trial team and writing committee. Any views expressed are those of the writing committee, not necessarily of the WHO. No funder or donor unduly influenced analyses, manuscript preparation, or submission; their comments merely clarified methods, not changing analyses or conclusions. Donors of trial drugs were shown the main results for their drug in the last week of September.
This article was published on December 2, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the World Health Organization. Other grants are listed in the Supplementary Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org..
References
- 1.World Health Organization. A coordinated global research roadmap: 2019 novel coronavirus. March 2020. (https://www.who.int/blueprint/priority-diseases/key-action/Coronavirus_Roadmap_V9.pdf?ua=1).
- 2.World Health Organization. R&D blueprint and Covid-19 (https://www.who.int/teams/blueprint/covid-19).
- 3.World Health Organization. An international randomised trial of additional treatments for Covid-19 in hospitalised patients who are all receiving the local standard of care (https://www.who.int/publications/m/item/an-international-randomised-trial-of-additional-treatments-for-covid-19-in-hospitalised-patients-who-are-all-receiving-the-local-standard-of-care).
- 4.White NJ, Watson JA, Hoglund RM, Chan XHS, Cheah PY, Tarning J. Covid-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med 2020;17(9):e1003252-e1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-1717. [DOI] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe Covid-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate Covid-19: a randomized clinical trial. JAMA 2020;324:1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020;383:1827-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer 2011;104:1057-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA Center for Drug Evaluation and Research. FDA’s approval of Veklury (remdesivir) for the treatment of Covid-19: summary review (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf).
- 12.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;383:2030-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with Covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020;396:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in Covid-19: an international collaborative meta-analysis of randomized trials. October 22, 2020. (https://www.medrxiv.org/content/10.1101/2020.09.16.20194571v2). preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venisse N, Peytavin G, Bouchet S, et al. Concerns about pharmacokinetic (PK) and pharmacokinetic-pharmacodynamic (PK-PD) studies in the new therapeutic area of Covid-19 infection. Antiviral Res 2020;181:104866-104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregoire M, Le Turnier P, Gaborit BJ, et al. Lopinavir pharmacokinetics in Covid-19 patients. J Antimicrob Chemother 2020;75:2702-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchwalder PA, Buclin T, Trinchard I, Munafo A, Biollaz J. Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res 2000;20:857-866. [DOI] [PubMed] [Google Scholar]
- 19.Jalkanen J, Hollmén M, Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Crit Care 2020;24:335-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalkanen J, Pettilä V, Huttunen T, Hollmén M, Jalkanen S. Glucocorticoids inhibit type I IFN beta signaling and the upregulation of CD73 in human lung. Intensive Care Med 2020;46:1937-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flammer JR, Dobrovolna J, Kennedy MA, et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol 2010;30:4564-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Corticosteroids for Covid-19: living guidance, 2 September 2020 (https://apps.who.int/iris/handle/10665/334125).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.