Abstract
Our access to a unique material of postmortem brains obtained from decades of data collection enabled a stereological analysis of the neuron numbers and correlation of results with individual premorbid intelligence quotient (IQ) data. In our sample of 50 brains from men, we find that IQ does not correlate with the number of brain cells in the human neocortex and was only weakly correlated to brain weight. Our stereological examination extended to measures of several other parameters that might be of relevance to intelligence, including numbers of cerebral glial cells (astrocytes, oligodendrocytes, and microglia) and the volume of key areas in the gray and white matter and of the cerebral ventricles, also showing near-zero nonsignificant correlations to IQ.
Keywords: Børge Priens Prøve (BPP), cell numbers, human brain, intelligence quotient, stereology
Introduction
The definition and measurement of human intelligence has been one of the most significant developments in the history of psychological science, with major theoretical and practical implications. Intelligence, defined as a general cognitive ability, is often measured as an intelligence quotient (IQ). This index is well established and highly reproducible; it remains largely stable throughout adult life and is relatively independent of the method used to measure it (Mackintosh 2011). Scores in two of the most internationally used intelligence tests, the Wechsler’s Adult Intelligence Scale (WAIS) and Raven’s Progressive Matrices (RPM), typically have a correlation coefficient of ~0.7 or higher, despite being assessed in almost entirely different settings (McLeod and Rubin 1962). From childhood onwards, IQ has a close link to academic performance and the individual’s future professional attainment, whereas negative social factors such as drug and alcohol abuse, and criminality in general are associated with a relatively low IQ (Mackintosh 2011). There is strong evidence that differences in intelligence are not only functional, but also have an organic basis. The presence of a well-established and substantial genetic contribution to intelligence (Deary et al. 2012, Bouchard 2014) has led to numerous imaging studies correlating intelligence and the anatomy of the brain. Accordingly, previous volumetric studies have reported a specific link between intelligence and the total volume of the brain’s gray matter, while others have reported correlations with the volume of the cerebral cortex (Luders et al. 2009) and other areas of the brain (Ganjavi et al. 2011).
Magnetic resonance (MR) imaging enables the acquisition of brain volumetric data in individuals with present documentation of IQ and other traits. To our knowledge, only one in vitro study has tested for such correlations in a comparatively large number of individuals (Witelson et al. 2006). That study compared postmortem brain volumes with premorbid IQ test scores (WAIS) obtained from 100 non-neurological patients, reporting a small positive correlation between IQ and brain volume. Gender, handedness, and specific scores in WAIS subtests all modulated this finding. However, Witelson et al. (2006) considered only gross brain weight and did not examine the microscopic details of the brain. Thus, it remains unknown whether intelligence correlates with the number of brain cells.
It has not previously been possible to test for correlations of IQ with numbers of specific cell populations because this requires the application of stereology in brains from individuals for whom there is documentation of premorbid IQ. Stereology is a discipline that is based on mathematically and statistically proven methods, that when applied properly, lead to unbiased results. The present material is, to our knowledge, unique, and is the product of a decades long brain collection program. We aimed to test the hypothesis whether intelligence correlates with the number of neurons in the cerebral cortex and its subregions. In addition, we measured several other parameters that might be of relevance to intelligence, including cerebral glial cell (astrocytes, oligodendrocytes, and microglia) numbers and the volumes of key areas in the gray and white matter, and of the cerebral ventricles.
Materials and Methods
Patients
Fifty-two brains from individuals for whom there was documentation of premorbid IQ, were available for the study. Of these, we had to exclude two brains for technical reasons, leaving us with brains from 50 Danish males aged 20–52 years at death, which was due to various non-neurological diseases (Table 1).
Table 1.
Demographic and autopsy-related data
| Age at death | Body height (cm) | Body weight (kg) | Occupation | Cause of death | Brain weight (g) | IQ |
|---|---|---|---|---|---|---|
| 20 | 178 | 67 | — | Ruptured heart | 1500 | 94 |
| 21 | 185 | — | Factory worker | Puncture of the heart | 1400 | 89 |
| 21 | 183 | 77 | Unemployed | Acute myocardial infarction | 1485 | 95 |
| 28 | 180 | 76 | Unemployed | Knife wound (homicide) | 1400 | 90 |
| 33 | 176 | — | Operator | Cancer of the rectum | 1570 | 107 |
| 34 | 181 | 79 | Special worker | Bleeding (homicide) | 1230 | 83 |
| 35 | 176 | 86 | — | Acute myocardial infarction | 1390 | 81 |
| 36 | 178 | 86 | School teacher | Sepsis | 1550 | 120 |
| 37 | 182 | 76 | — | Acute myocardial infarction | 1620 | 96 |
| 37 | 187 | 76 | Innkeeper | Drowning | 1446 | 73 |
| 38 | 180 | 96 | Navigator | Acute myocardial infarction | 1390 | 110 |
| 39 | 176 | 55 | Electrician | Cardiomyopathy | 1558 | 96 |
| 39 | 183 | 80 | Fire inspector | Acute myocardial infarction | 1550 | 109 |
| 39 | 179 | 85 | Entrepreneur | Liver failure | 1460 | 97 |
| 39 | 181 | 72 | Laborer | Lung cancer | 1210 | 108 |
| 39 | 187 | 96 | — | Acute myocardial infarction | — | 91 |
| 39 | 177 | — | Engineer | Acute myocardial infarction | 1860 | 113 |
| 40 | 179 | 85 | Refinery worker | Acute myocardial infarction | 1620 | 92 |
| 40 | 167 | 75 | Caretaker | Acute myocardial infarction | 1710 | 80 |
| 40 | 173 | 76 | Brewer | Acute myocardial infarction | — | 115 |
| 41 | 174 | 81 | — | Cardiomyopathy | 1432 | 106 |
| 41 | 186 | 79 | — | Acute pancreatitis | 1600 | 92 |
| 41 | 173 | 78 | Welder | Terminal uremia | 1240 | 107 |
| 41 | 190 | 87 | Insurance agent | Lung cancer | 1340 | 96 |
| 42 | 186 | 107 | — | Aorta aneurysm | 1500 | 116 |
| 42 | 167 | 61 | Fisherman | Lung cancer | 1460 | 78 |
| 43 | 170 | 80 | — | Acute myocardial infarction | 1150 | 82 |
| 43 | 176 | 89 | Laborer | Acute myocardial infarction | 1680 | 72 |
| 43 | 175 | 82 | Haulier, plumber | Acute myocardial infarction | 1560 | 89 |
| 43 | 172 | 89 | Electrician | Acute myocardial infarction | 1350 | 96 |
| 44 | 181 | 93 | — | Pulmonary embolism | 1610 | 93 |
| 44 | 172 | 81 | Early retirement | Drowning | 1500 | 86 |
| 44 | 179 | 89 | Medical doctor | Acute myocardial infarction | 1570 | 127 |
| 44 | 185 | 88 | Chimney sweeper | Pulmonary embolism | — | 99 |
| 45 | 186 | 82 | Operator | Liver failure | 1538 | 75 |
| 44 | — | — | — | Acute myocardial infarction | 1420 | 125 |
| 45 | 175 | 68 | Operator | Acute lung edema | 1350 | 106 |
| 46 | 186 | 66 | Fitter | Pulmonary heart disease | 1460 | 96 |
| 46 | 170 | 79 | Baker | Hypernephroma | 1520 | 104 |
| 46 | 174 | — | Mechanic | Hypernephroma | 1400 | 79 |
| 46 | 174 | 67 | Disability pension | Liver failure | 1500 | 107 |
| 47 | 176 | 81 | Slaughterhouse worker | Acute myocardial infarction | 1270 | 79 |
| 47 | 174 | 78 | Concrete worker | Acute myocardial infarction | 1630 | 88 |
| 47 | 172 | 88 | Locksmith | Bleeding stomach ulcer | 1519 | 94 |
| 47 | 180 | 77 | Driver | Acute myocardial infarction | 1579 | 95 |
| 48 | 176 | 46 | — | Lung edema | 1340 | 86 |
| 49 | 172 | 78 | Laborer | Acute myocardial infarction | 1400 | 62 |
| 49 | 175 | 74 | Early retirement | Suicide with medicinea | 1360 | 102 |
| 50 | 182 | 78 | Carpenter | Aorta aneurysm | 1760 | 109 |
| 52 | 171 | 77 | Farmer, CEO | Acute myocardial infarction | 1340 | 106 |
| Mean (range) | 174 [167–190] | 71 [46–107] | 1475 [1150–1860] | 96 [62–127] |
Note: aDied without any previously known diseases.
The brains had been collected between 1985 and 1991, at a time where there were no donation programs in Denmark and where the vast majority of people died at the hospital. The collection and treatment of brains were all conducted in strict accordance with Danish laws regarding autopsied human tissue at that time, thus providing a unique consecutive collection of brains of Danish men. The Danish Data Protection Agency (j. nr: 2012-58-0004) has approved the Brain Bank. Some of the studied subjects have been used in previous studies from our laboratory. We have reused tissue blocks and sections from 24 of the brains and obtained 26 new brains solely for this project. All the cell counts were performed de novo in the 50 brains and by the same stereologist. We included four cases with a history of alcohol dependence and six cancer patients. This is justified by our previous work showing that chronic alcoholics do not lose neocortical neurons (Jensen and Pakkenberg 1993), and by the absence of cases dying with coma, cachexia, or otherwise any prolonged agonal interval. Following data acquisition, it transpired that neocortical cell numbers of the four cases with alcohol dependence did not deviate from those of remainder of the sample (P = 0.13). Likewise, neocortical numbers in the cancer subgroup did not differ from that in the remainder of the sample (P = 0.36). None of the included cases had met exclusion criteria for drug abuse, psychiatric disturbances, diabetes, hypertension, or dementia.
Tissue Preparation
All the brains were stored in fixative (10% neutral buffered formalin, pH = 7.2) for at least 5 months before being studied. Right or left hemisphere was chosen systematically at random. The frontal-, temporal-, parietal-, and occipital lobes were delineated and painted with different colors of water-proof ink to distinguish the brain regions from each other (Pakkenberg and Gundersen 1997). The sampled hemispheres were embedded in agar before being cut into 4.25-mm thick slabs with a random starting point within a 4.25 mm interval. All the slabs were then photographed for estimating the total volumes, surface areas, and cortical thickness using point counting, test-lines, and the Cavalieri estimator (Gundersen and Jensen 1987). Then, 2-mm thick columns (i.e., rods) were sampled systematically at random from every third slab. About 8–12 rods were subsampled from each cortical lobe before being dehydrated in a gradient ethanol series and randomly rotated around the vertical axis (Fig. 1 and corresponding text in Pakkenberg and Gundersen 1997). The seemingly low number of tissue samples required for sampling in one brain is calculated from the principles of systematic, uniform, and random sampling, which allows the investigator to obtain any desired precision. The rods were embedded in Historesin (2-hydroxyethyl methacrylate, Kulzer, Germany) followed by cutting of a central 40-μm thick section that was stained using a modified Giemsa solution, and then used for cell counting. During the preparation of the rods intended for cell counting, extra rods were collected to measure shrinkage before and after processing. No net shrinkage was detected.
Figure 1.
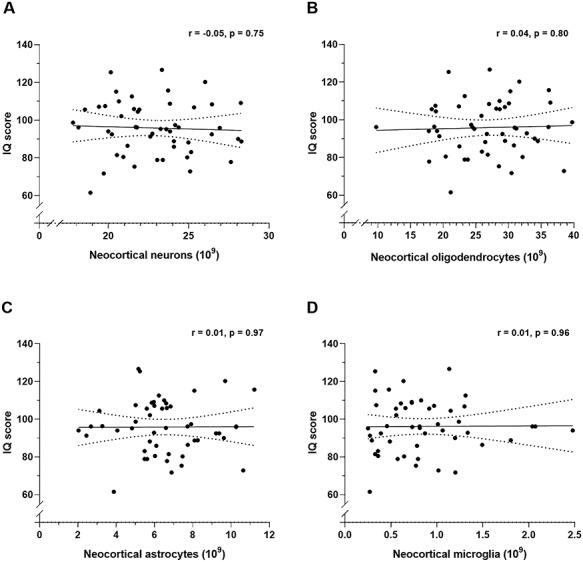
Pearson’s correlation coefficients (r) between IQ score and estimates of total number of neocortical neurons (A,  ), oligodendrocytes (B,
), oligodendrocytes (B,  ), astrocytes (C,
), astrocytes (C,  ), and microglia (D,
), and microglia (D,  ). The correlations are shown with 95% confidence intervals (dotted lines).
). The correlations are shown with 95% confidence intervals (dotted lines).
Estimation of Cell Numbers
To estimate the total cell numbers (Gundersen 1986), we used optical disectors, which are 3D probes consisting of an unbiased counting frame (Gundersen 1977) that can be moved in the z-direction down through the tissue section. We used an Olympus BX50 microscope equipped with a motorized x–y stage (Märzhäuser, Germany) and a microcator for z-analysis (Heidenhain, Germany). The tissue images were captured with a high-resolution color video camera (Basler, Germany), and projected onto a computer screen, where optical disectors were applied using NewCAST software (Visiopharm, Hørsholm, Denmark). In the present study, the area of the unbiased counting frames was set to 5000 μm2 in the frontal-, parietal-, and temporal lobes, and 2500 μm2 in the occipital lobe. Based on z-distribution analysis, the average section thickness was 40 μm, resulting in a disector height of 15 μm, a 5-μm guard zone at the top of the sections and a 20-μm guard zone of at the bottom of the section. The step-length between sampling sites was set to 1000 μm.
The different cell types were identified by accepted morphological criteria, with neurons having a large nucleus, single dark stained nucleolus and a visible cytoplasm. Oligodendrocytes were small and rounded, with no visible cytoplasm, astrocytes were larger than oligodendrocytes and with a pale nucleus and a granulated appearance, and microglia were small, comma-shaped cells (Garcia-Cabezas et al. 2016). Since the cell counts were performed using Giemsa-stained sections, our results are based on cell morphology alone. However, astroglia-, oligodendroglia-, microglia-, and neuron-specific immunohistochemistry has ongoingly corroborated our cell-identification criteria (Salvesen et al. 2017). The cell counts were performed using a ×60 oil-immersion objective (numerical aperture = 1.42, Olympus, Denmark) at a final on-screen magnification of ×1380. A uniform distribution of the cells within the disector height was confirmed by analyzing the z-distribution of particles.
To obtain the numerical density (NV) of each cell type in one hemisphere (unilaterally), we used the following equation (Gundersen and Jensen 1987): NV = ∑Q−/(Vdis × ∑P), where ∑Q− is the total number of cells counted, Vdis is the volume of the disector, and ∑P is the total number of disectors sampled in the region of interest. Then, the total number of cells is estimated by multiplying NV with the Cavalieri volume (Vref = T x (a/p) × ∑P, where T is the thickness of the slab, a/p is the area per point on the counting grid, and Pi is the number of points hitting the tissue within the region of interest). Finally, the total number of each cell type was estimated by multiplying by two, to obtain bilateral numbers. These estimated results can have varying degrees of precision as determined by the investigator (indicated by the coefficient of error (CE)). In general, “optimal” precision is achieved when the CE value is less than half of the observed biological variance (CV), as OCV2 = ICV2 + CE2, where OCV is the observed CV and ICV is the inherent CV (Boyce et al. 2010). According to this formula, we are able to adjust the CE to suit the CV by adjusting the amount of sampling. Since the biological variance of the total number of neurons between examined brains in this study was ~12%, we aimed at a precision of about half the biological variance (in this study ~4%).
IQ Scores
The IQ scores for the 50 men, who were 18–20 years of age at examination, were derived from an intelligence test used for evaluating potential recruits into the armed forces, the Børge Priens Prøve (BPP), which is a test correlating very highly with scores from the internationally used WAIS and Raven’s IQ tests (Mortensen et al. 1989, Teasdale 2009). The BPP test, unchanged in content between 1957 and 2010, has been taken by ~90% of all Danish males at age 18+, and individual scores can be obtained retrospectively from computerized registers (Christensen et al. 2015). We retrieved the scores from the Danish Conscription Database, which has authorization from the Danish Data Registration Agency to release such data to be used in studies of intelligence and health (jr.nr: 2014-41-2911). The BPP has four subtests, totaling 78 items. The total score for the BPP has very satisfactory psychometric properties (Nielsen et al. 2019). The 50 men in the sample were born between 1937 and 1962, such that their BPP testing occurred during a period with markedly improved BPP performance (Teasdale and Owen 1987). Therefore, we transformed the raw BPP scores to IQs, adjusting for the generational difference (the so-called “Flynn effect,” Trahan et al. 2014). This was achieved as follows. Using the Danish extensive population norms for the range of birth years, we computed IQ scores normed for the corresponding years, for example, the IQ for a man tested in 1970 was scaled from BPP score in relation to the mean and standard deviation for the ~25 000 men who had been tested in that year. Across our sample, the mean IQ was somewhat lower than the population average, but with very close to the expected variation (mean = 96, SD = 14), and therefore adequately represents the general population. Although there is little variation in the age at which IQ was measured, the ages at death ranged between 20 and 52 years. However, IQ varies little across the age range (Mackintosh 2011) and, indeed, the correlation coefficient for IQ as a function of age of death in our sample did not indicate any relationship (r = 0.01, data not shown).
Statistics
With n = 50, we had a power of 80% to detect a correlation between IQ and total neocortical neuron numbers of 0.34. Correlations were analyzed using Pearson’s Product Moment formula and 95% confidence limits for these were calculated using Bootstrap procedures. The statistical analyses were performed using SPSS (vers. 26) and the significance was set at P < 0.05 (two-tailed). Graphical presentation was completed using GraphPad Prism (vers. 8). As can be seen in Table 2, correlations do not deviate significantly from zero and the confidence limits deviate widely around that value.
Table 2.
Power and confidence limits
| Power | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | n | Delta | Alpha | |||||||||
| 0.4 | 50 | 2.8 | 80% | |||||||||
| Confidence limits | Neocortex | WM | CG | |||||||||
| Neurons | Astro | Oligo | Micro | Volume | Surface | Thickness | Volume | Volume | ||||
| Pearson correlation IQ with | −0.05 | 0.01 | 0.04 | 0.01 | −0.04 | −0.12 | 0.11 | −0.08 | −0.001 | |||
| Bootstrap, 95% | Lower | −0.33 | −0.27 | −0.23 | −0.34 | −0.32 | −0.37 | −0.17 | −0.39 | −0.25 | ||
| confidence interval | Upper | 0.24 | 0.27 | 0.28 | 0.17 | 0.22 | 0.15 | 0.38 | 0.22 | 0.28 | ||
Results
In our sample of 50 male brains, IQ scores did not correlate significantly with the total number of neurons (Fig. 1A), oligodendrocytes (Fig. 1B), astrocytes (Fig. 1C) or microglia (Fig. 1D) in the neocortex, nor with the cortical volume (Fig. 2A), surface area (Fig. 2B) and thickness (Fig. 2C). This also applied to estimates of the four separate lobes (frontal-, temporal-, parietal-, and occipital cortices; see Supplementary Material). Neither did IQ score correlate significantly with the volumes of white matter (Fig. 2D), central gray matter (Fig. 2E) or lateral ventricles (Fig. 2F), nor with the brain weight (Fig. 3A), or body height (Fig. 3B). All of these correlation coefficients were less than 0.2. Finally, the total number of neurons correlated negatively with age of death (Fig. 3C), but not brain weight (Fig. 3D).
Figure 2.
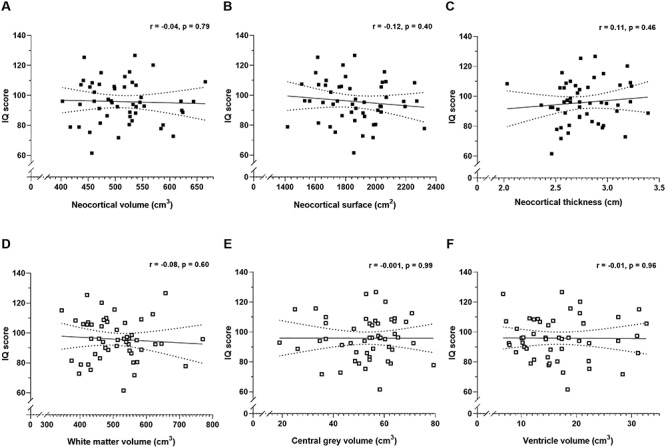
Pearson’s correlation coefficients (r) between IQ score and estimates of total neocortical volume (A, ■), surface area (B, ■), and mean thickness (C, ■). Also shown are the correlations between IQ score and the volume of white matter, (D, □), central gray (E, □), and cerebral ventricles (F, □). The correlations are shown with 95% confidence intervals (dotted lines).
Figure 3.
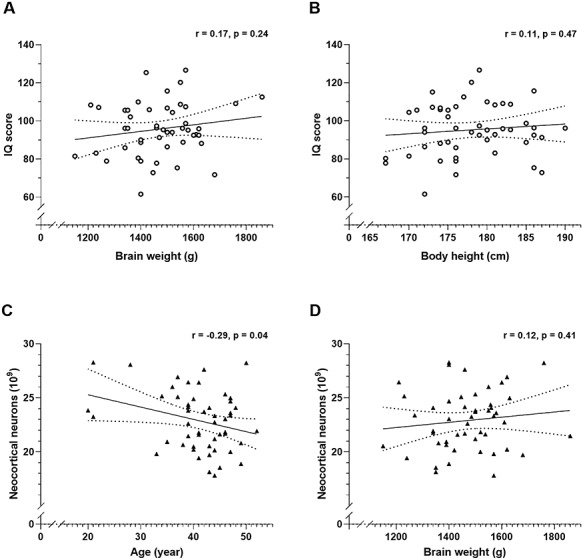
Pearson’s correlation coefficients (r) between IQ score and brain weight (A, ○), and body height (B, ○) and correlations between total number of neocortical neurons and age (C, ▲), and brain weight (D, ▲). The correlations are shown with 95% confidence intervals (dotted lines).
Discussion
Large brains contain not only more neurons, but also more glia cells, more subcortical gray matter and a larger white matter fiber network compared with small brains (Marner et al. 2003), but none of these parameters correlated significantly with IQ in this study using a sample size of 50 male brains.
The repeated demonstration of a marked genetic contribution to intelligence (Bouchard 2014) implies some neural basis to variance in intelligence, but the present negative results in our sample of 50 male brains are not consistent with any important association with neocortical neuron numbers but rather could have relation to other factors such as network properties, synapse numbers or other structural components. Speculatively, the lack of significant correlations in this study might help to explain why the rather large difference in neocortical neuron number between men and women (16% higher in men, Pakkenberg and Gundersen 1997) does not match with the minor gender difference in IQ (Halpern and LaMay 2000) and that highly demented female Alzheimer’s disease patients have normal neocortical neuron numbers (Regeur et al. 1994, Pelvig et al. 2003). One limitation of the present study is the small sample size, and our negative results must be interpreted with caution. Further, cell quantification was performed using Giemsa-stained sections, and our results are therefore based on cell morphology alone. However, neuron-, oligodendroglia-, astroglia-, and microglia-specific immunohistochemistry verified our cell identification criteria.
As stated above, various reports correlating IQ-scores to estimates of brain size such as brain weight, head circumference, computed tomography- (CT) and MR imaging (MRI)-based brain volume estimates, have shown results with correlations ranging from 0 to 0.6 (Gignac and Bates 2017). The apparent link between brain volumes and IQ-scores may relate to factors such as cell density and/or neuronal circuit complexity, myelin thickness and dendritic arborization (Jung and Haier 2007). However, the great preponderance of studies on this topic is based on CT and MR imaging, which is uninformative about cell populations. In contrast to many IQ studies, our stereological data did not find that IQ correlates with macroscopic (brain weight, volumes, cortical thickness, and surface area) estimates. However, while MRI-based volumetric quantification offers high-resolution brain images of living participants, the results from CT or MRI studies cannot always be directly compared with results from physical sections. For example, Furlong et al. (2013) made a direct comparison between postmortem 3 T MRI and stereology on physical tissue sections from 16 cerebral hemispheres to estimate the volume of the cortex and subcortex, area of the pial surface and gray–white matter boundary, and thickness of the cerebral cortex. The results showed poor agreement between MRI and stereology, especially for pial surface (MRI = 1165 cm2, stereology = 2134 cm2, P < 0.05), thickness of the cerebral cortex (MRI = 3.7 mm, stereology = 2.3 mm, P < 0.001) as well as volume of the cerebral cortex (MRI = 530 cm3, stereology = 454 cm3, P < 0.001) and subcortex (MRI = 432 cm3, stereology = 520 cm3, P < 0.001). However, the two methods gave very similar results for whole brain volume (MRI = 962 cm3, stereology = 974 cm3, P = 0.5). The authors concluded that the major cause for the differences was due to the resolution of the MR images which was not sufficient to always allow reliable delineation of the cerebral sulci. Further, it should be recognized that the present lack of significant correlation between IQ and brain volumes may reflect the number of brains available for this analysis, which consequently affects the statistical power. Thus, with a sample size of n = 50, an 80% probability of finding a two-tailed significance would only be obtained, if the correlation was 0.4. This is a value above most reported studies finding positive brain volume-IQ correlations.
Our results found only minor relationships between IQ and neuroanatomical measures obtained from stereological analysis of physical sections. It may be, however, that dynamic functional measures, such as position emission tomography (PET) or functional MRI, have greater promise as correlates of intelligence. These methods measure neural metabolism and activity or functional connectivity between brain regions, which maybe therefore have a stronger functional correlation to intelligence as a property of living brains. For example, one PET study has examined the regional glucose metabolic rate in a small group of participants performing the Raven’s Advanced Progressive Matrices (RAPM) (Jung and Haier 2007). That study showed an inverse correlation between high RAPM-scores and low regional glucose metabolic rate, which suggest the occurrence of increased “neuronal efficacy” in high-performing subjects. However, follow-up studies have produced conflicting results showing both increased and decreased brain metabolism in subjects with high RAPM-scores (Neubauer and Fink 2009, Basten et al. 2015).
In summary, this is the first study to estimate and correlate the total number of neocortical cells with IQ. In our unique collection of 50 consecutive collected male brains, we found no correlation between cell numbers and IQ. We speculate that this lack of correlation could be due to other factors being more important for IQ such as the neuronal circuit complexity, synapse numbers, or dendritic arborization.
Notes
We thank Professor Merete Osler M.D. Dr Med. Sci., Department of Epidemiology, University of Copenhagen, who provided us with the BPP data. Conflict of Interest: None declared.
Funding
The Bispebjerg Research grant.
Supplementary Material
References
- Basten U, Hilger K, Fiebach CJ. 2015. Where smart brains are different: a quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Dermatol Int. 51:10–27. [Google Scholar]
- Bouchard T. 2014. Genes, evolution and intelligence. Behav Genet. 44:549–577. [DOI] [PubMed] [Google Scholar]
- Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJ. 2010. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 38:1011–1025. [DOI] [PubMed] [Google Scholar]
- Christensen GT, Molbo D, Angquist LH, et al.. 2015. Cohort profile: the Danish conscription database (DCD): a cohort of 728 160 men born from 1939 through 1959. Int J Epidemiol. 44:432–440. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Yang J, Davies G, Harris SE, Tenesa A, Liewald D, Luciano M, Lopez LM, Gow AJ, Corley J, et al.. 2012. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 482:212–215. [DOI] [PubMed] [Google Scholar]
- Furlong C, Garcia-Fiñana M, Puddephat M, Anderson A, Fabricius K, Eriksen N, Pakkenberg B, Roberts N. 2013. Application of stereological methods to estimate post-mortem brain surface area using 3T MRI. Magn Reson Imaging. 31:456–465. [DOI] [PubMed] [Google Scholar]
- Ganjavi H, Lewis JD, Bellec P, MacDonald PA, Deborah P, Waber DP, Evans AC, Karama S. 2011. The brain development cooperative group. Negative associations between corpus callosum midsagittal area and IQ in a representative sample of healthy children and adolescents. PlosOne. 6:e19698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, John YJ, Barbas H, Zikopoulus B. 2016. Distinction of neurons, glia and endothelial cells in the cereb cortex: an algorithm based on cytological features. Front Neuroanat. 10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignac GE, Bates TC. 2017. Brain volume and intelligence: the moderating role of intelligence measurement quality. Dermatol Int. 64:18–29. [Google Scholar]
- Gundersen HJG. 1977. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 111:219–223. [Google Scholar]
- Gundersen HJ. 1986. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 143:3–45. [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. 1987. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 147:229–263. [DOI] [PubMed] [Google Scholar]
- Halpern DF, LaMay ML. 2000. The smarter sex: a critical review of sex differences in intelligence. Educ Psychol Rev. 12:229–246. [Google Scholar]
- Jensen GB, Pakkenberg B. 1993. Do alcoholics drink their neurons away? Lancet. 342:1201–1204. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. 2007. The Parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 30:135–154. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Toga AW. 2009. Neuroanatomical correlates of intelligence. Dermatol Int. 37:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. 2011. IQ and human intelligence. New York: Oxford University Press Inc. [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. 2003. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 462:144–152. [DOI] [PubMed] [Google Scholar]
- McLeod HN, Rubin J. 1962. Correlation between raven progressive matrices and WAIS. J Consult Psychol. 26:190–191. [Google Scholar]
- Mortensen EL, Reinisch JM, Teasdale TW. 1989. Intelligence as measured by the WEIS and a military draft board group test. Scand J Psychol. 30:315–318. [Google Scholar]
- Neubauer AC, Fink A. 2009. Intelligence and neural efficiency. Neurosci Biobehav Rev. 33:1004–1023. [DOI] [PubMed] [Google Scholar]
- Nielsen T, Kreiner S, Teasdale TW. 2019. Assessment of cognitive ability at conscription for the Danish army: is a single total score sufficient? Scand J Psychol. 107:e161–e169. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. 1997. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 384:312–320. [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeur L, Pakkenberg B. 2003. Neocortical glial cell numbers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 16:212–219. [DOI] [PubMed] [Google Scholar]
- Regeur L, Jensen GB, Pakkenberg H, Evans SM, Pakkenberg B. 1994. No global neocortical nerve cell loss in brains from patients with senile dementia of Alzheimer’s type. Neurobiol Aging. 15:347–352. [DOI] [PubMed] [Google Scholar]
- Salvesen L, Winge K, Brudek T, Agander TK, Løkkegaard A, Pakkenberg B. 2017. Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb Cortex. 27:400–410. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Rapp MA, Huys QJ, Beck A, Wüstenberg T, Deserno L, Buchholz HG, Kalbitzer J, Buchert R, Bauer M, et al.. 2013. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 34:1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale TW. 2009. The Danish draft board’s intelligence test, Børge Prien’s Prøve: psychometric properties and research applications through 50 years. Scand J Psychol. 50:633–638. [DOI] [PubMed] [Google Scholar]
- Teasdale TW, Owen DR. 1987. National secular trends in intelligence and education: a twenty-year cross-sectional study. Nature. 325:119–121. [Google Scholar]
- Trahan LH, Stuebing KK, Fletcher JM, Hiscock M. 2014. The Flynn effect: a meta-analysis. Psychol Bull. 140:1332–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. 2006. Intelligence and brain size in 100 post-mortem brains: sex, lateralization and age factor. Brain. 129:386–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


