Abstract
Subjective emotional experience that is congruent with a given situation (i.e., target emotions) is critical for human survival (e.g., feeling disgusted in response to contaminated food motivates withdrawal behaviors). Neurodegenerative diseases including frontotemporal dementia and Alzheimer’s disease affect brain regions critical for cognitive and emotional functioning, resulting in increased experience of emotions incongruent with the situation (i.e., non-target emotions, such as feeling happy when seeing someone grieving). We examined neuroanatomical correlates of subjective experience of non-target emotions in 147 patients with neurodegenerative diseases and 26 healthy individuals. Participants watched three films intended to elicit particular target emotions and rated their experience of negative and positive target and non-target emotions after watching each film. We found that smaller volume in left hemisphere regions (e.g., caudate, putamen, and dorsal anterior insula) was associated with greater experience of negative non-target emotions. Follow-up analyses confirmed that these effects were left-lateralized. No correlates emerged for positive non-target emotions. These findings suggest that volume loss in left-hemisphere regions produces a more diffuse, incongruent experience of non-target emotions. These findings provide a potential neuroanatomical basis for understanding how subjective emotional experience is constructed in the brain and how this can be disrupted in neurodegenerative disease.
Keywords: voxel-based morphometry, frontotemporal dementia, subjective feeling, affect
Introduction
Imagine someone encountering a plate of contaminated food. Without much conscious thought, this person would likely display a disgust facial expression, be flooded with subjective feelings of revulsion, and act to discard the food immediately. This scenario and the associated feeling of revulsion might lead this person to recall other times when eating spoiled food led to becoming ill. To warn close others, the person might communicate the feeling of disgust and other details about the scenario to help others avoid the same food in the future.
Now consider a different case. A person has a neurodegenerative disease that produces difficulties in experiencing emotions that are congruent with a given situation. The person might experience enjoyment in response to the same contaminated food and approach the food rather than withdrawing. The person may even share this “positive experience” with companions, making companions feel confused or emotionally distressed. This person’s companions may even become physically ill if they do not know that the food is contaminated and proceed to eat it.
Historically, emotion researchers have predominantly focused on studying the subjective experience of “target emotions”—the emotions that are typical or congruent with a given situation and associated with adaptive behaviors (e.g., experiencing disgust in response to contaminated food is associated with withdrawal and expulsion behaviors). The experience of “non-target emotions”, such as experiencing amusement or sadness in response to contaminated food, is an important aspect of emotional functioning that has largely been unstudied. In an effort to address this gap, in two recent studies, we found that (a) patients with Alzheimer’s disease (AD) and frontotemporal dementia (FTD) reported experiencing more non-target emotions compared with patients with other neurodegenerative diseases and healthy controls (HC) (Chen et al. 2017a) and (b) more negative non-target emotions reported by patients were associated with worse caregiver mental health (Chen et al. 2017b). The present study used a lesion approach to examine associations between gray and white matter volumes and increased subjective experience of non-target emotions in patients with AD, FTD, and other neurodegenerative diseases.
Subjective Experience of Target Emotions
Subjective experience of emotions is evolutionarily critical. In the example above, feelings of disgust in response to contaminated food can help individuals engage in appropriate coping behaviors and inform conspecifics of preferences and likely future actions (Keltner and Haidt 1999; Levenson 1999). Affective researchers have long been interested in studying how individuals come to experience an emotion subjectively. In peripheralist views, the experience of emotions is derived from interoceptive information from the somatic (e.g., facial expressions of emotions) and visceral responses (e.g., heart rate changes) that are produced by emotions (James 1884; Levenson 2014). Based on these views, Craig (2009) suggested that the anterior insula plays a critical role by integrating interoceptive information with homeostatic, environmental, hedonic, motivational, social, and cognitive information (that stems from other regions of the brain, for example, amygdala, temporal cortex, nucleus accumbens, anterior cingulate cortex, and orbitofrontal cortex) to form a subjective experience of emotion. In the constructionist views, both interoceptive information and contextual information from the external environment contribute to subjective emotional experience. In the constructionist neural model of emotions, generation of subjective emotional experience involves (a) a brain circuit that represents pleasant or unpleasant affect (including brain regions such as the anterior insula, amygdala, medial orbitofrontal cortex, and subgenual cingulate), and (b) a secondary brain circuit (e.g., dorsal medial prefrontal cortex and posterior cingulate cortex) that conceptualizes these pleasant and unpleasant affective presentation (Barrett 2017; Satpute et al. 2015) to form meaningful, subjective emotional experience.
Increased Subjective Experience of Non-target Emotions in AD and FTD
While previous research on subjective experience of emotions has predominantly focused on target emotions, non-target emotions have been largely overlooked. Experiencing non-target emotions in situations that normally elicit strong target emotions may reflect alterations in the way the brain processes emotions. From a functional perspective, experiencing non-target emotions can interfere with effective coping, emotional learning, and social communication (Keltner and Haidt 1999; Levenson 1999), such as making the person approach the contaminated food and share it with companions in the above-mentioned case. We have previously found that, compared with HC and patients with other neurodegenerative diseases, patients with AD experienced more positive non-target emotions, and patients with FTD experienced more positive and negative non-target emotions. Increased experience of non-target emotions may result from two sources: (a) alterations in bodily reactions (e.g., patients exhibited facial expressions and autonomic nervous system responses associated with non-target emotions), (b) alterations in the evaluation and interpretation (or conceptualization) of the bodily reactions and other information that accompanies the emotional stimuli (e.g., the patients did not exhibit responses associated with non-target emotions; rather, they misinterpreted bodily reactions and other internal/external information). In our previous study, patients with FTD and AD did not differ from comparison groups in facial expression of non-target emotions (Chen et al. 2017a). This supports the second explanation, which emphasizes problems in evaluation and interpretation of bodily reactions and/or external cues. Problems in evaluation and interpretation of bodily reactions and/or external cues may be explained by either peripheralist or constructionist views of emotion, although these views may predict different parts of the brain being involved in this deficit (e.g., anterior insula versus medial prefrontal cortex, respectively).
The Present Study
Because FTD and AD affect large-scale brain networks (Seeley et al. 2009), the neuroanatomical mechanisms underlying increased experience of non-target emotions remain unclear. In the present study, we conducted whole brain voxel-based morphometry (VBM) analyses to examine areas of volume loss associated with increased experience of non-target emotions in patients and HC from our previous study (Chen et al. 2017a) who had valid, artifact-free MRI scans. Based on our previous findings that the experience of non-target emotions might result from errors in evaluation and interpretation processes (Chen et al. 2017a), we hypothesized that greater experience of non-target emotions would be associated with smaller volume in brain regions that subserve these functions, which could include the anterior insula (according to the peripheralist neural model; Craig 2009) and dorsal medial prefrontal cortex and posterior cingulate cortex (according to the constructionist neural mode; Satpute et al. 2015) or all of these regions.
Materials and Methods
Participants
In the previous study (Chen et al. 2017a), we examined a sample of 226 participants including 189 patients with neurodegenerative diseases and 37 neurologically HC. The present study included 173 participants from the same participant sample who had valid, artifact-free MRI scans, including 147 patients with neurodegenerative diseases and 26 HC. Patients were recruited through the Memory and Aging Center at the University of California, San Francisco (UCSF) where they received diagnoses based on consensus criteria (McKhann et al. 1984; Litvan et al. 1996; Strong et al. 2009; Gorno-Tempini et al. 2011; Rascovsky et al. 2011; Armstrong et al. 2013). HC were recruited from the community and did not have a history of neurological, psychiatric, or cognitive disorders. The final sample consisted of patients with three subtypes of FTD: behavioral variant FTD (bvFTD; n = 38), semantic variant primary progressive aphasia (svPPA; n = 25), and nonfluent variant primary progressive aphasia (nfvPPA; n = 15); patients with AD (n = 38), progressive supranuclear palsy (PSP; n = 14), and corticobasal syndrome (CBS; n = 17). Details concerning specific patterns of neurodegeneration typically associated with these different diagnoses have been published elsewhere (Seeley et al. 2009; Brown et al. 2017). Patients who participated in this study were at mild to moderate stages of the diseases and sufficiently healthy to come to the Berkeley Psychophysiology Laboratory at the University of California, Berkeley (UCB) to complete the laboratory session. Table 1 shows participants’ sociodemographic and functional characteristics.
Table 1.
Sociodemographic and functional characteristics of patients and HC, MEAN (SEM)
| Whole research sample | Diagnostic groups | |||||||
|---|---|---|---|---|---|---|---|---|
| bvFTD | svPPA | nfvPPA | AD | PSP | CBS | HC | ||
| N | 173 | 38 | 25 | 15 | 38 | 14 | 17 | 26 |
| Sex | 94 M, 79 F | 26 M, 12 F | 14 M, 11 F | 8 M, 7 F | 20 M, 18 F | 7 M, 7F | 9 M, 8 F | 10 M, 16 F |
| Age | 34–85; 64.28 (0.62) | 61.37 (1.37)† | 64.16 (1.07) | 69.2 (2.1) | 62.16 (1.52) | 66.71 (1.53) | 66.12 (1.40) | 66.38 (1.63) |
| CDR Total | 0–3; 0.71 (0.04) | 1.2 (0.11)*** | 0.7 (0.09)*** | 0.57 (0.13)*** | 0.79 (0.04)*** | 0.75 (0.09)*** | 0.59 (0.11)*** | 0 (0.00) |
| CDR Box | 0–13; 3.80 (0.23) | 6.32 (0.51)*** | 3.9 (0.53)*** | 2.2 (0.60)* | 4.14 (0.31)*** | 5.21 (0.68)*** | 3.5 (0.60)*** | 0 (0.00) |
| Semantic Knowledge | 1–16; 13.68 (0.25) | 13.91 (0.48)† | 9.46 (0.89)*** | 14.86 (0.36) | 13.76 (0.39)* | 15.42 (0.19) | 14.19 (0.68) | 15.65 (0.14) |
| Film recognition | 0.33–1; 0.97 (0.01) | 0.96 (0.02) | 0.96 (0.02) | 1.00 (0.00) | 0.96 (0.02) | 1.00 (0.00) | 0.98 (0.02) | 0.97 (0.02) |
Note: bvFTD = behavioral variant frontotemporal dementia. svPPA = semantic variant primary progressive aphasia. nfvPPA = nonfluent variant primary progressive aphasia. PSP = progressive supranuclear palsy. CBS = corticobasal syndrome. AD = Alzheimer’s disease. HC = healthy controls. F = Female. M = Male. CDR-Total = Dementia Rating Scale total score. CDR-Box = Dementia Rating Scale sum of boxes. Annotations indicate significant or trending effects as compared with the HC group. †P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001.
Experimental Design
We included patients with various neurodegenerative diseases as well as HC to increase the neuroanatomical and behavioral heterogeneity, which is useful for mapping relationships between altered experience of positive and negative non-target emotions and regional neurodegeneration (Verstaen et al. 2016). During a “film watching task” in the laboratory session at UCB, participants watched three films and after each film reported their experience of positive and negative non-target emotions (as well as subjective experience of target emotions). Video recordings of facial expressions were rated by trained coders using the Emotional Expressive Behavior coding system (EEB; Gross and Levenson 1993) to identify target and non-target emotions. Prior to their UCB visit, participants were evaluated at UCSF, where their MRI scans and neurological and neuropsychological assessment measures (e.g., severity of dementia) were collected. For full details, see Procedure and Emotion Measure sections below.
As noted earlier, differences among diagnostic groups in subjective experience as well as facial expressions of non-target emotions were examined in a previous study using a larger sample of patients (Chen et al. 2017a). In the current study, we first conducted preliminary analyses using a subset of this larger sample (i.e., those with valid MRI scans available) to determine if the findings from the previous study concerning differences in non-target emotions and emotional facial expressions among various diagnostic groups were still observed. Importantly, in the previous study, we had focused on FTD and AD syndromes compared with motor syndromes and HC. Thus, patients with a diagnosis of bvFTD, svPPA, or nfvPPA were all grouped together in the FTD group; patients with a diagnosis of CBS or PSP were grouped together in the motor syndromes group. In the preliminary analyses of the present study, we took a different approach by examining the data from each diagnosis separately rather than from larger groups. Thus, analyses were performed to compare bvFTD, svPPA, nfvPPA, AD, PSP, and CBS with the HC group. This approach allows us to understand whether patients with different diagnoses experienced non-target emotions to similar or different degrees.
We performed whole-brain VBM analyses to test our hypothesis that the experience of positive and negative non-target emotions was associated with smaller combined gray and white matter volume in brain regions thought to be involved in the generation of subjective emotional experience. The main VBM analyses included a set of covariates to account for differences in head size, dementia severity, diagnosis (Braak and Braak 1995; Seeley et al. 2009), and scanner type (Abdulkadir et al. 2011). To ensure that our VBM findings were robust (i.e., not solely confounded by differences in semantic knowledge; see Functional measures section below for details), we performed additional VBM analyses in which we included covariate(s) that were significantly correlated with greater experience of both positive and negative non-target emotions (see preliminary analyses section for complete details).
Procedure
All participants (including patients and HC) first visited UCSF, where they underwent detailed clinical interviews, neurological examination, functional assessment (e.g., dementia severity), neuropsychological evaluation (e.g., semantic knowledge), and structural MRI. Within 4 months for patients and 12 months for HC of this UCSF visit, participants visited UCB for a comprehensive day-long assessment of emotional functioning (Levenson 2007). Informed consent was obtained upon arrival at both sites. All procedures were approved by the UCSF and UCB Institutional Review Boards.
The present study focused on a film-watching task that consisted of three trials. In each trial, participants relaxed for 60 seconds, watched a film clip selected to induce a specific target emotion, and then rated their experience of the target emotion and nine non-target emotions when they were watching the film clip (see Emotion measures section below for details). Participants’ facial and upper torso behaviors were recorded by a partially hidden camera. The three trials in the film-viewing task occurred in a fixed order (Fig. 1).
Figure 1.
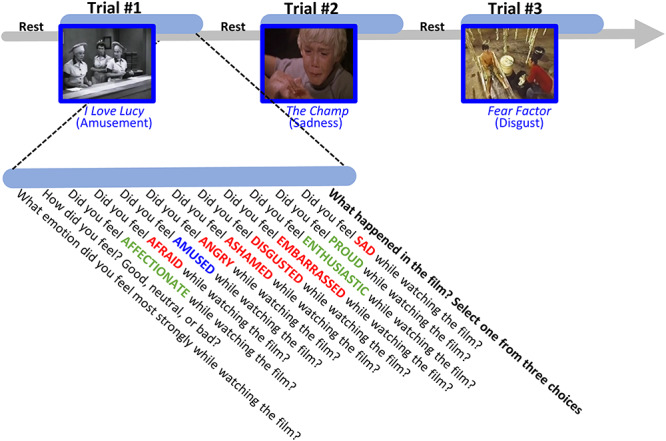
Task procedure. The film watching task consisted of three trials. In each trial, participants watched a short film clip which was selected to induce a specific type of emotion (i.e., target emotion of either amusement, sadness, or disgust). Immediately following the film clip, participants rated how much they experienced the target emotions (in blue) and nine positive (in green) and negative (in red) non-target emotions in a fixed order while watching the film. Next, participants were asked to identify what happened in the film by choosing from three multiple choice options.
In the first trial, participants watched a scene from the TV sitcom I Love Lucy, which depicts two female workers trying to wrap chocolates and keep up with the rapid pace of a conveyor belt as they stuff chocolate candy into their mouths. In the second trial, they watched a scene from the movie The Champ, which depicts a boy crying after his father dies after a boxing match. In the third trial, they watched a scene from the TV show Fear Factor, which depicts a young man sucking fluids out of cow intestines, spitting the fluids into a cup, and then drinking the fluid. These three film clips were selected to induce the target emotions of amusement, sadness, and disgust, respectively. The validity of these film clips for inducing these target emotions has been demonstrated in previous studies (Gross and Levenson 1995; Eckart et al. 2012; Shiota and Levenson 2012; Sturm et al. 2015).
To ensure participants attended to and understood the film clips, we asked them to identify what happened in the film by choosing from three multiple-choice options. Responses were coded as correct (1) or incorrect (0).
Emotion Measures
Subjective experience of non-target emotions. (In the current and previous studies (Chen et al., 2017a, 2017b), the experience of non-target emotions was defined as any emotions that were not intended to be induced by the film clips. However, we have observed that the experience of certain non-target emotions could be common. For example, HC often reported feeling affection (in addition to sadness, the target emotion) in response to watching a boy crying after his father’s death. To ensure that findings of our study reflected changes associated with neurodegenerative diseases, we performed additional VBM analyses which focused on non-target emotions that HC did not endorse (i.e., mean response of HC was not significantly different from zero based on nonparametric one-sample Wilcoxon tests; therefore, affection and enthusiasm for the amusement film, affection for the sadness film, and angry for the disgust film were excluded from the analyses). Findings from these analyses are presented in the Supplemental Fig. S1. These analyses revealed neuroanatomical correlates that were very similar to those reported in the main text. After each film clip, participants reported the degree (0 = not at all; 1 = a little; 2 = a lot) to which they felt affectionate, afraid, amused, angry, ashamed, disgusted, embarrassed, enthusiastic, proud, and sad while watching the film. These 10 emotions were presented in the same order (i.e., alphabetically) for all trials. The target emotions in the three trials were amusement (first trial), sadness (second trial), and disgust (third trial). Non-target emotions were defined as the nine other emotions (besides the target emotion) in each trial. Using the same analytic approach used in the previous study (Chen et al. 2017a), we first aggregated positive and negative non-target emotions for each trial to capture valence changes in the subjective experience of non-target emotions (Chen et al. 2017a). For example, in the third trial where the target emotion was disgust, the average of positive non-target emotions included affection, enthusiasm, and pride, and the average of negative non-target emotions included anger, fear, shame, sadness, and embarrassment. Figure 2A shows the specific emotions included in the positive and negative non-target emotion measures for the three task trials. Aggregating emotions resulted in two emotion categories (i.e., positive and negative non-target emotions), which helped us (a) focus on mapping alterations to positive and negative emotions (rather than examining specific discrete emotions); (b) increase data heterogeneity (because each specific non-target emotion was rated on a 3-point scale); (c) control Type I error by reducing the number of statistical tests. For each participant, we then computed the average ratings of the positive and negative non-target emotions across the three trials of the task. Subjective experience of non-target emotions was not examined for individual films because of our interest in studying the experience of non-target emotions as a more general characteristic, rather than something specific to a particular emotional stimulus. In addition, preliminary analyses did not reveal significant interactions between film trials and diagnostic groups (Fs < 1.82, Ps > 0.05). Similarly, we also computed the average ratings of the target emotions across the three trials, see Figure 3A.
Figure 2.
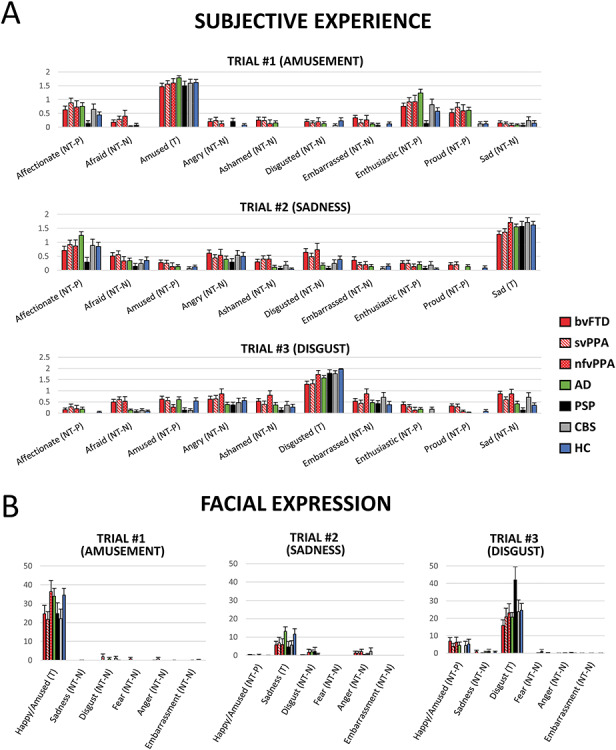
(A) Subjective experience of ten emotions by trial and by diagnostic group. (B) Facial expressions of six emotions by trial and by diagnostic group. T = target emotions; NT-P = positive non-target emotions; NT-N = negative non-target emotions. bvFTD = behavioral variant frontotemporal dementia. svPPA = semantic variant primary progressive aphasia. nfvPPA = nonfluent variant primary progressive aphasia. PSP = progressive supranuclear palsy. CBS = corticobasal syndrome. AD = Alzheimer’s disease. HC = healthy controls.
Figure 3.
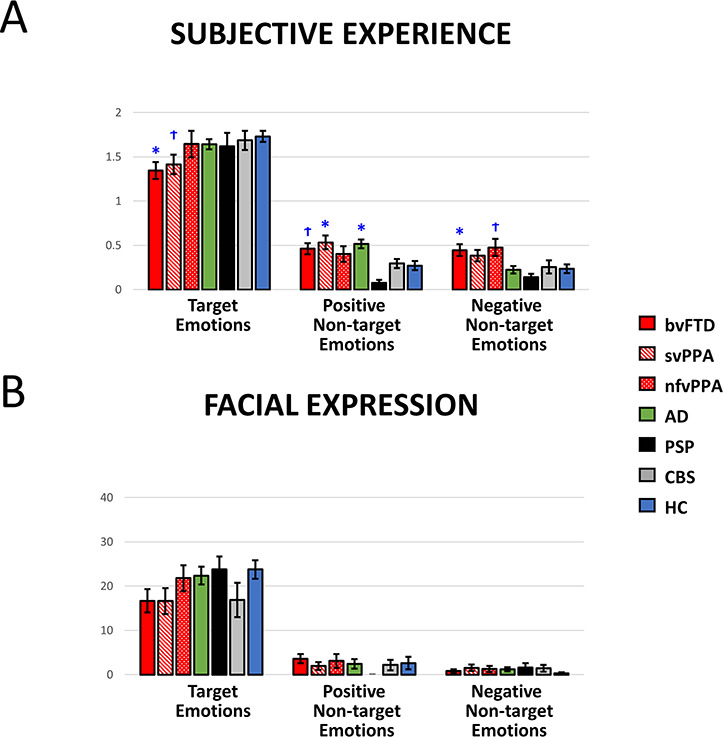
Averaged (A) subjective experience and (B) facial expressions of target, positive and negative non-target emotions in the film watching task. M ± 1 SEM. T = Target emotion. NT-P = Positive non-target emotions. NT-N = Negative non-target emotions. bvFTD = behavioral variant frontotemporal dementia. svPPA = semantic variant primary progressive aphasia. nfvPPA = nonfluent variant primary progressive aphasia. PSP = progressive supranuclear palsy. CBS = corticobasal syndrome. AD = Alzheimer’s disease. HC = healthy controls. Annotations indicate significant or trending effects as compared with the HC group. †P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001.
Facial expressions of non-target emotions. Our previous study using a larger sample size (N = 226) found no diagnostic group differences in facial expressions of non-target emotions (Chen et al. 2017a). To determine if the previous findings remained with the smaller sample used in the present study (N = 173; note that in this study, we also used a different approach by focusing on separate diseases rather than larger groups), facial expressions of positive and negative non-target emotions were also computed. Facial expressions of happiness/amusement, sadness, disgust, anger, fear, and embarrassment during a pre-selected 30-second “hot spot” (i.e., the most emotionally powerful segment) of each film clip were coded second-by-second on a 4-point scale (0 = no expression, 1 = slight, 2 = moderate, 3 = strong expression) by trained research assistants using the Expressive Emotional Behavior Coding System (EEB; Gross and Levenson 1993). Total scores over the 30-second “hot spot” were computed for each emotion (for complete details concerning the behavioral coding see Olney et al. 2011; Eckart et al. 2012; Sturm et al. 2015; Verstaen et al. 2016).
Facial expressions of the target emotions for the three trials were defined as amusement, sadness, and disgust, respectively. Facial expressions of non-target emotions for the three trials were defined as the expression of emotions that were not targeted in the trial. Figure 2B shows a list of target and non-target facial expressions of emotions for each trial. Consistent with the subjective experience data, facial expressions of non-target emotions were aggregated across the three trials based on valence (i.e., positive non-target and negative non-target). For comparison, we also computed averaged facial expressions of target emotions for each trial, see Figure 3B. EEB only codes for one positive emotion: amusement. Thus, for the first trial (amusement film) where facial expression of amusement was the target facial expression, there was no positive non-target emotion measure.
Functional Measures
Dementia severity. Dementia severity was included as a covariate because it is typically positively correlated with the severity of neurodegeneration (Braak and Braak 1995). At UCSF, clinicians assessed the severity of dementia with the Clinical Dementia Rating Scale (CDR), which consists of six domains of impairments: (a) memory, (b) orientation, (c) judgment and problem-solving, (d) community affairs, (e) home and hobbies, and (f) personal care (Morris 1993). Two CDR scores were obtained: (i) total score, which ranges from 0 to 3 (i.e., CDR-Total; 0 = normal, 0.5 = very mild dementia; 1 = mild dementia, 2 = moderate dementia, 3 = severe dementia) and (ii) sum of the boxes (i.e., CDR-Box), which ranges from 0 to 18 (Table 1; for both scores, higher values indicate greater dementia severity). In data analyses, we used CDR-Box as a covariate instead of CDR-Total because the former provided greater sensitivity (i.e., larger range) to the degree of patient impairment.
Semantic knowledge. Semantic knowledge was included as a potential covariate in the additional VBM analyses to ensure that our main VBM findings did not simply reflect patient language impairment that would impair their ability to understand the semantic meaning of the emotion terms (Patterson et al. 2007). Semantic knowledge was assessed during the UCSF neuropsychological evaluation using a modified version of the Peabody Picture Vocabulary test (Dunn and Dunn 1981; Kramer et al. 2003), which included 16 items. In response to the clinician verbalizing a word, the participant matches the word to a picture representing a verb, adjective, animate object, or inanimate object from 4 picture choices (either by pointing or verbalizing the number of the picture). Scores range from 0 to 16, with lower scores indicating greater impairments in semantic knowledge. Note that PPVT scores were not available for 13 participants.
Memory (recognition) of the film content. Patients’ correct recognition of the content of the film clips was included as a potential covariate in the additional VBM analyses to ensure that our main VBM findings did not simply reflect patient impairment in memory functioning. As mentioned, participants were asked to identify what happened in the film by choosing from three multiple choice options. Responses were coded as correct or incorrect. Percentage of correct responses over all three trials was computed for each participant. Scores range from 0 to 1, with higher scores indicating being able to attend, understand, and remember the general content of the emotional film clips.
Neuroimaging
Structural neuroimaging acquisition. 122 MRIs (70.5%) were acquired on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil located at the UCSF Neuroscience Imaging Center using volumetric magnetization prepared rapid gradient echo (MPRAGE) (160 sagittal slices; slice thickness, 1.0 mm; FOV, 256 × 230 mm; matrix, 256 × 230; voxel size, 1.0 × 1.0 × 1.0 mm; TR, 2300 ms; TE, 2.98 ms; flip angle, 9°). 37 MRIs (21.4%) were acquired on a 4 T Bruker MedSpec system at the San Francisco Veterans Administration Hospital with an 8-channel head coil controlled by a Siemens Trio console, using an MPRAGE sequence (192 sagittal slices; slice thickness, 1 mm; FOV, 256 × 224 mm; matrix, 256 × 224; voxel size, 1.0 × 1.0 × 1.0 mm; TR, 2840 ms; TE, 3 ms; flip angle, 7°). 14 MRIs (8.1%) were acquired on a 1.5 T Siemens Magnetom VISION system (Siemens, Iselin, NJ) at the San Francisco Veterans Administration Hospital, equipped with a standard quadrature head coil, using a MPRAGE sequence (164 coronal slices; slice thickness, 1.5 mm; field of view [FOV], 256 × 256 mm; matrix, 256 × 256; voxel size, 1.0 × 1.5 × 1.0 mm; repetition time [TR], 10 ms; echo time [TE], 4 ms; flip angle, 15°). There were no diagnostic differences (six patient diagnoses and HCs) in the proportion of MRI scans acquired through 1.5 T (X2 (6, 173) = 4.07, P = 0.67) or 4T scanners (X2 (6, 173) = 7.41, P = 0.28). All MRIs were visually inspected for scan quality.
Preprocessing. We utilized statistical parametric mapping version 12 default parameters (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) for preprocessing with the light clean-up procedure in the morphological filtering step. We then corrected structural T1 images for bias field and segmented images into gray matter, white matter, and cerebrospinal fluid, and spatially normalized into Montreal Neurological Institute (MNI) space (Ashburner and Friston 2005). We used default tissue probability priors (voxel size, 2.0 × 2.0 × 2.0 mm) of the International Consortium for Brain Mapping. Segmented images were then visually inspected for adequate gray matter and white matter segmentation. Smoothing was then performed on these images with an 8 mm full-width at half-maximum Gaussian kernel.
Statistical Analyses
Preliminary analyses. We first characterized participants by performing descriptive statistics for the whole research sample as well as the seven diagnostic groups (bvFTD, svPPA, nfvPPA, AD, PSP, CBS, and HC). We then performed one-way ANOVA and chi-square tests to examine differences in characteristics between diagnostic groups. To determine whether findings in our earlier paper (i.e., FTD and AD patients reported experiencing greater non-target emotions but did not exhibit greater facial expressions of these emotions, as compared with HC; Chen et al. 2017a) were also found in the current paper using the smaller sample of participants with valid MRI scans, we performed four separate one-way ANOVAs for subjective experience and facial behaviors of positive and negative non-target emotions. Significant group effects were followed by post hoc comparisons in which each patient group was compared against the HC group (2-sided) with multiple comparisons corrected with the Dunnett t method.
In addition, we performed bivariate Pearson’s correlations to examine the associations between subjective experience of positive and negative non-target emotions and a set of variables that could confound our main VBM findings. Variables significantly correlated with both positive and negative non-target emotional experience were included as covariates in the additional VBM analyses.
Neuroanatomical correlates. First, we characterized the areas of neurodegeneration by examining structural differences in combined gray and white matter maps between each patient group and HC (analyses adjusted for patient age, sex, and total intracranial volume; as will be discussed in detail below, analyses also adjusted for the type of scanner used for data acquisition). Second, we conducted whole-brain VBM analyses to examine the relationship between positive or negative non-target emotional experience and combined structural gray and white matter maps, which provide a useful approach to correlate atrophy with behavior in patients with neurodegenerative disease (Wilson et al. 2010; Sturm et al. 2015). To account for variation in disease progression, dementia severity (CDR-Box) was included as a covariate. To account for individual differences in head size, total intracranial volume (the sum of volume for gray matter, white matter, and cerebrospinal fluid) was included as a covariate. Analyses also included six diagnostic covariates (dummy coded 1 for the patient diagnosis of interest or 0 for the remaining groups) to ensure that our findings did not simply reflect diagnostic differences. Although neuroimaging analyses that include images collected across different types of scanners have robust effects and are unlikely to cause artifacts at strict statistical thresholds (Abdulkadir et al. 2011), we included two additional covariates (dummy coded 1 for the scanner of interest or 0 for the remaining scanners) as covariates to account for different scanner types used for data collection.
We examined statistical maps and report findings at PFWE < 0.05. Minimum cluster size reported was 350mm3. We ran five thousand permutation analyses to derive a study-specific error distribution (Hayasaka and Nichols 2004) using vlsm2 (Bates et al. 2003). Permutation analysis is a resampling approach for significance testing through which a test statistic is compared with the null distribution derived from the present study’s data set and is an accurate representation of Type 1 error at P < 0.05 across the entire mask. The combined peak and extent thresholds were used to determine the one-tailed T-threshold for multiple comparisons correction at PFWE < 0.05. This approach has been used in similar research in this patient population (Sturm et al. 2013, 2018a; Yokoyama et al. 2015). Images were overlaid with MRIcron on an MNI average brain based on the gray and white matter templates used for preprocessing.
Following the main VBM analyses, we performed additional VBM analyses in which we included semantic knowledge as an additional covariate. In preliminary data analyses (see below for details), we found that participants with poorer performance in semantic knowledge reported more positive and negative non-target emotions. Therefore, the additional VBM analyses helped rule out the possibility that our main VBM findings were simply confounded by disease-related changes in semantic knowledge.
Results
Preliminary Analyses
Participant characteristics. Table 1 includes demographic characteristics of the present study’s 173 participants. Descriptive statistics revealed that the majority of participants were right-handed, male, and the mean age was 64.28. Regarding film recognition, the average percentage for correct recognition was 0.97, suggesting that most participants were able to understand and then recognize the contents of the film clips. We then performed ANOVAs and chi-square to test differences between diagnostic groups. As expected, ANOVAs revealed significant group effects for dementia severity and semantic knowledge (Fs > 18.05, Ps < 0.001). Compared with HC, all patient groups had significantly greater dementia severity (in both CDR Total and CDR Box), and the svPPA and AD groups performed significantly worse on the semantic knowledge test (Ps < 0.05). We also found a significant age effect between groups, F(4, 137) = 3.49, P < 0.01); post hoc comparisons revealed a trending effect that the bvFTD group was younger then the HC (P = 0.059).
Covariates in the additional VBM. Next, we performed bivariate Pearson correlations between subjective experience of positive and negative non-target emotions and a set of variables that could potentially influence our main VBM findings. As shown in Table 2, we found that worse semantic knowledge was associated with greater experience of both positive and negative non-target emotions. Thus, this variable was included in the additional VBM analyses as a covariate. We also found that older age and worse film recognition were associated with greater experience of positive non-target emotions. However, these effects were not found for the experience of negative non-target emotions. Therefore, we did not include age or film recognition as covariates in the additional VBM analyses.
Table 2.
Bivariate Pearson’s correlations between subjective experience of positive and negative non-target emotions and potential covariates
| Subjective experience of non-target emotions | |||
|---|---|---|---|
| Positive | Negative | ||
| R | R | Note | |
| Sex | 0.03 | 0.06 | |
| Age | −0.16* | 0.05 | |
| CDR Box | 0.26*** | 0.23** | Included in main and additional VBM as a covariate |
| Semantic knowledge | −0.45*** | −0.31*** | Included in additional VBM as a covariate |
| Film recognition | −0.25*** | −0.09 | |
| Subjective experience: | |||
| Target emotions | 0.03 | 0.11 | |
| Facial expression: | |||
| Target emotions | −0.07 | −0.09 | |
| Positive non-target emotions | 0.02 | −0.12 | |
| Negative non-target emotions | −0.01 | −0.01 | |
Note: VBM = voxel-based morphometry analyses. *P < 0.05; **P < 0.01; ***P < 0.001.
Diagnostic differences in subjective experience of non-target emotions. To determine if the diagnostic differences found in the previous study (Chen et al. 2017a) were observed in the present study with a smaller sample size—particularly, here we focused on the specific diagnostic groups (e.g., bvFTD, svPPA, and nfvPPA) versus HC, rather than larger groups (e.g., FTD) versus controls—we first performed an ANOVA and found a significant group effect for positive non-target emotions, F(6, 166) = 5.36, P < 0.001. Post hoc comparisons indicated that compared with the HC group, patients with svPPA (P = 0.018) and AD (P = 0.014) reported greater positive non-target emotions. An ANOVA revealed a significant group effect for negative non-target emotions, F(6, 166) = 3.63, P = 0.002. Post hoc comparisons revealed that patients with bvFTD reported greater negative non-target emotions than HC (P = 0.046). These results are presented in Figure 3A.
Diagnostic differences in facial expressions of non-target emotions. The previous study did not find diagnostic differences for facial expressions of non-target emotions (Chen et al. 2017a). The same pattern of findings was revealed when examining this relationship in the smaller sample used in the present study, as ANOVA results did not reveal group effects for facial expressions of positive non-target emotions, F(6, 163) = 0.72, P = 0.63, or negative non-target emotions, F(6, 164) = 0.62, P = 0.72. These results are presented in Figure 3B.
Distribution of Neurodegeneration
Each patient group had the expected pattern of white and gray matter volume loss (Fig. 4). Compared with HC, the AD group had smaller volume in the hippocampi, precuneus, and posterior temporal regions; the bvFTD group had smaller volume in medial frontal, cingulate, and insula regions; the svPPA group had smaller volume in left anterior temporal and insula regions; the nfvPPA group had smaller volume in left dorsal frontal and insula regions; the CBS group had smaller volume in supplementary motor area and medial frontal regions and the PSP group had smaller volume in the dorsal midbrain (Seeley et al. 2009; Brown et al. 2017).
Figure 4.
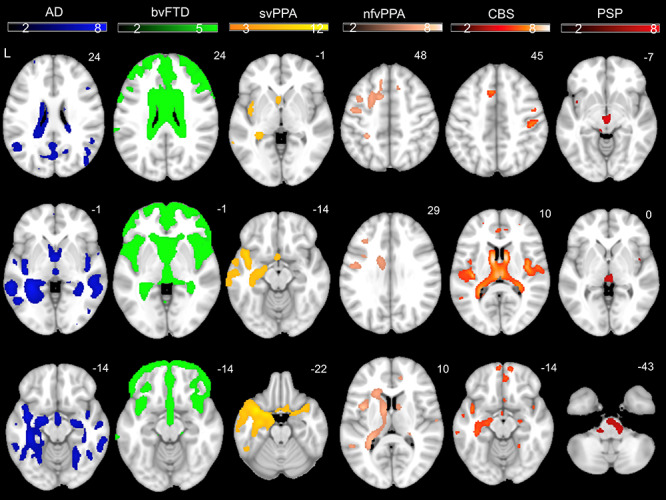
Color bar represents T-scores for regions with smaller volume in patient groups compared with HC after adjusting for age, sex, scanner type, and total intracranial volume (PFWE < 0.05). Results are overlaid on an MNI template brain.
Neuroanatomical Correlates of Subjective Experience of Non-target Emotions
Main VBM analyses. We performed whole brain VBM analyses (while adjusting for six patient diagnoses, dementia severity, scanner type, and total intracranial volume) and found that greater experience of negative non-target emotions was associated with smaller volume in a large cluster, which included the left putamen and extended to the left caudate, pallidum, thalamus, anterior cingulate cortex, and dorsal anterior insula (PFWE < 0.05). No clusters emerged for positive non-target emotions or target emotions. See Table 3 for T-scores and significance levels for all associated regions; Figure 5 top for the statistical maps.
Table 3.
Neural correlates of subjective experience of negative non-target emotions. Smaller volume in left hemisphere regions was associated with greater subjective experience of negative non-target emotions in response to three film clips when adjusting for diagnosis (dummy coded variables for six patient groups), semantic knowledge, disease severity, scanner field strength, and total intracranial volume. MNI coordinates (x, y, z) given for maximum T-score for the cluster. Results reported at PFWE < 0.05. Minimum cluster size reported is 350 mm3
| Anatomical region | Cluster volume (mm3) | MNI coordinates | Max T-score | Corrected P | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
Main analyses:
Negative non-target emotions | ||||||
| Left putamen | 17033* | −15 | 16 | −4 | 4.50 | 0.0138 |
| Left caudate | a | |||||
| Left pallidum | a | |||||
| Left thalamus | a | |||||
| Left anterior cingulate cortex | a | |||||
| Left dorsal anterior insula | a | |||||
|
Additional analyses:
Negative non-target emotions, adjusting for semantic knowledge | ||||||
| Left caudate | 7638* | −12 | 18 | −8 | 3.87 | 0.0250 |
| Left putamen | a | |||||
| Left dorsal anterior insula | a | |||||
|
Exploratory analyses:
Negative non-target emotions (laterality index), adjusting for semantic knowledge | ||||||
| Left parahippocampal gyrus | 608** | −18 | −12 | −32 | 4.39 | 0.0692 |
| Left caudate | 543** | −6 | 3 | 10 | 4.28 | 0.0802 |
*Results significant at PFWE < 0.05., **Results significant at PFWE < 0.09, aSignifies that these regions were included in the cluster above but did not meet criteria on their own.
Figure 5.
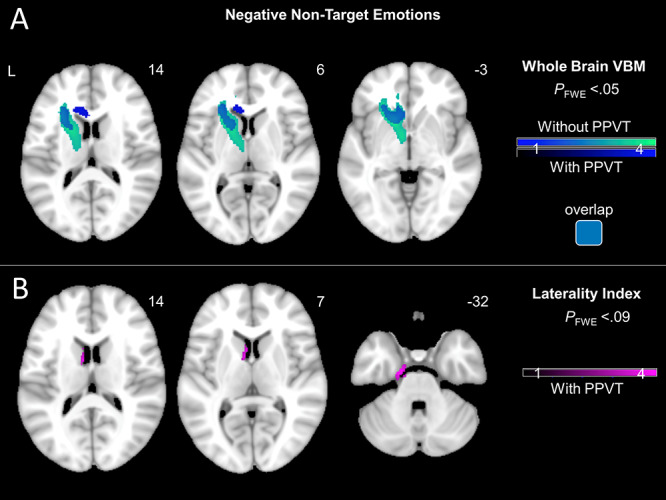
(A) T-score maps of brain areas for which smaller volume was associated with greater subjective experience of negative non-target emotions after adjusting for diagnosis (six dummy variables accounting for patient groups), disease severity (CDR-Box), scanner type, and total intracranial volume. Results shown with and without adjusting for semantic knowledge (PPVT). Whole brain VBM analyses revealed that greater experience of negative non-target emotions was associated with smaller volume in left hemisphere regions, including caudate, putamen, and dorsal anterior insula (PFWE < 0.05). (B) T-score maps of brain areas for which left-worse-than-right atrophy was associated with greater subjective experience of negative non-target emotions after adjusting for diagnosis (six dummy variables accounting for patient groups), disease severity (CDR-Box), scanner type, total intracranial volume, and semantic knowledge (PPVT). No clusters survived FWE correction. At a less stringent threshold, greater experience of negative non-target emotions was associated with left-worse-than-right atrophy in the caudate and parahippocampal gyrus (PFWE < 0.09).
Additional VBM analyses: Adjusting for semantic knowledge. We performed additional whole brain VBM analyses (using the same covariates as above) while also adjusting for semantic knowledge. We found that greater experience of negative non-target emotions was associated with smaller volume in a cluster that included the left caudate and extended to the left putamen, and dorsal anterior insula (PFWE < 0.05). No clusters emerged for positive non-target emotions or target emotions. See Table 3 for T-scores and significance levels for all associated regions; Figure 5 top for the statistical maps.
Exploratory analyses: Testing lateralization of the effects. Although our findings were localized to the left hemisphere, these results do not necessarily imply that these effects are truly unilateral. Analogous regions in the right hemisphere may also be biologically relevant but might not have reached statistical significance. Thus, we ran additional post hoc VBM analyses (with the same covariates as above, including semantic knowledge) using lateralization indices for a more direct comparison of left and right hemisphere structures. Lateralization indices quantify the differential atrophy between left and right hemispheres of the brain, which is a useful measure in neurodegenerative disease samples to determine the critical role of structures in one hemisphere compared with the other (Guo et al. 2016; Sturm et al. 2018a; Takeda et al. 2019). We computed lateralization indices using combined gray and white matter preprocessed structural images. The average intensity in each voxel in the left hemisphere (VL) and in the right hemisphere (VR) of the brain was entered into the following equation: (VL—VR)/(VL + VR), following previous methods (Guo et al. 2016; Sturm et al. 2018b; Takeda et al. 2019). Lower scores indicated worse left-than-right atrophy. No clusters emerged at a stringent threshold (PFWE < 0.05). At a less stringent threshold (PFWE < 0.09), we found that left-worse-than-right atrophy in the caudate and parahippocampal gyrus was associated with greater experience of negative non-target emotions. See Table 3 for T-scores and significance levels for all associated regions; Figure 5 bottom for the statistical maps.
Overlap of VBM findings with distribution of neurodegeneration. To further examine the associations between the above VBM findings and disease-related brain volume loss, we visualized together maps of structural differences between controls and our patient groups (Fig. 4) and maps of neural correlates for negative non-target emotions (including whole brain analyses [Fig. 5A with PPVT accounted for] and laterality analyses [Fig. 5B]). As shown in Figure 6, results from the whole brain VBM analysis overlap with patient structural differences in the left caudate and insula, whereas results from the laterality index analysis overlap with patient structural differences in the left caudate.
Figure 6.
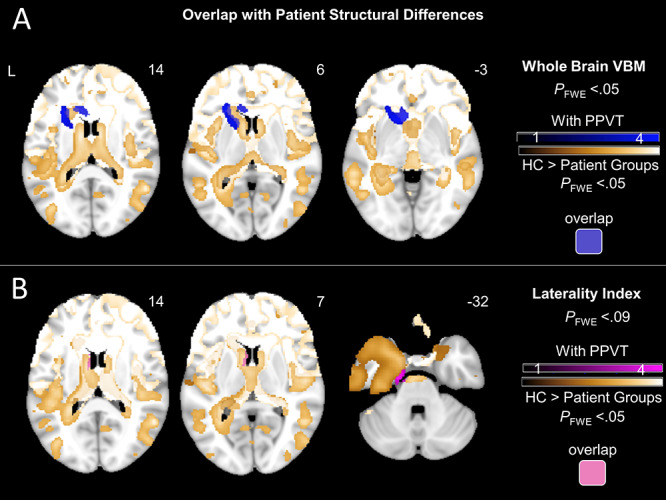
All patient maps have been added as separate gold overlays to visualize areas of overlap with neural correlates of negative non-target emotions more easily. (A) T-score maps of brain areas for which smaller volume was associated with greater subjective experience of negative non-target emotions after adjusting for diagnosis (six dummy variables accounting for patient groups), disease severity (CDR-Box), scanner type, total intracranial volume, and semantic knowledge (PPVT) overlaid with T-score maps for patient structural differences relative to controls. Results from whole brain VBM overlap with patient structural differences in the left caudate and insula. (B) T-score maps of brain areas for which left-worse-than-right atrophy was associated with greater subjective experience of negative non-target emotions after adjusting for diagnosis (six dummy variables accounting for patient groups), disease severity (CDR-Box), scanner type, total intracranial volume, and semantic knowledge (PPVT) overlaid with T-score maps for patient structural differences relative to controls. Results from laterality index overlap with patient structural differences in the left caudate. Results are overlaid on an MNI template brain.
Discussion
The present study found that smaller volumes in several left-hemisphere regions (i.e., caudate, putamen, pallidum, thalamus, dorsal anterior insula, and anterior cingulate cortex) were associated with greater subjective experience of negative non-target emotions. These findings were robust in the main VBM analyses, which adjusted for six diagnoses, dementia severity, total intracranial volume, and scanner field strength. In additional VBM analyses, which adjusted for semantic knowledge, several of these findings (i.e., left caudate, putamen, dorsal anterior insula) remained stable. Results from exploratory laterality analyses confirm these “left-side” effects, finding “left-worse-than-right” atrophy in the caudate and parahippocampal gyrus was associated with greater subjective experience of negative non-target emotions. However, for subjective experience of positive non-target emotions, no correlates emerged. Visualizing areas of overlap suggest that most regions in the additional VBM and laterality analyses (all with PPVT adjusted) overlapped with regions of patient volume loss.
Neuroanatomical Correlates of Negative Non-target Emotions
Left dorsal anterior insula and dorsal striatum. Greater experience of negative non-target emotions was associated with smaller volume in the left anterior insula. According to peripheralist’s views, the anterior insula integrates signals from various brain regions (e.g., posterior insula, striatum, ACC; Craig 2009) and “interprets” (a) interoceptive information, including proprioception (derived from the action of the somatic nervous system; e.g., facial expressions), as well as visceral perception (derived from the action of the autonomic nervous system; e.g., cardiac activity) that can occur when we experience emotions, and (b) sociocontextual information associated with emotional stimuli (Craig 2009). Volume loss in this region may result in uninterpreted, partially interpreted, or inaccurately interpreted internal and external signals. Our findings here, together with previous findings that participants in different diagnostic groups differed in subjective experience, but not facial expressions of non-target emotions (Chen et al. 2017a), support the notion that increased experience of non-target emotions emerges from altered interpretation of information, rather than altered emotional responding.
Greater subjective experience of negative non-target emotions was also associated with smaller volume in the dorsal striatum, including the caudate and putamen. The dorsal striatum has strong functional connections to the anterior insula (Postuma and Dagher 2005; Robinson et al. 2012; Ghaziri et al. 2018). The involvement of dorsal striatum in motor planning has long been recognized (Albin et al. 1989; Grillner et al. 2005). More recently, emerging evidence suggests that the dorsal striatum is also involved in higher-order cognition and social and affective processing, such as representing the subjective values of actions (e.g., positive versus negative values are associated with extending the arm forward to grab a slice of cake versus touching a piece of moldy bread; Balleine et al. 2007; Carretié et al. 2009; Badgaiyan 2010). In our study, volume loss in the dorsal striatum may be associated with impairment in the patients’ ability to encode affective meaning of motor actions (e.g., the association between nose-wrinkling and the emotion “disgust”), which in turn disrupts the interpretation/appraisal process during the search for the most-likely cause of the bodily changes (i.e., facial expressions of target emotions and other corresponding physiological activations; Schachter and Singer 1962). Moreover, these regions remained stable after adjusting for semantic knowledge, suggesting they may be particularly important for integrating internal and external cues that require minimal semantic processing.
Other left hemisphere regions . We also found associations between greater subjective experience of negative non-target emotions and smaller volumes in several other left-hemisphere regions, including the anterior cingulate cortex, thalamus, and parahippocampal gyrus. Notably, these regions have strong structural and functional connections to the anterior insula (Seeley et al. 2007; Craig 2009; Medford and Critchley 2010) and are thought to be involved in relaying sensory information from the body and external environment (i.e., thalamus; Damasio and Carvalho 2013; Sturm et al. 2018a) and evaluating this information in the social context (i.e., anterior cingulate and parahippocampal cortices; Drevets et al. 2008; Etkin et al. 2011; Ochsner et al. 2012; Aminoff et al. 2013; Rudebeck et al. 2014; Satpute et al. 2015; Rigney et al. 2017). Therefore, volume loss in these regions may further disrupt the interpretation/appraisal processes by providing the anterior insula with biased information about sensory inputs and their sociocontextual meanings.
While certain neural correlates for non-target negative emotions (e.g., left caudate and insula) overlapped with patient structural volume differences compared with controls, other neural correlates (e.g., putamen) did not (Fig. 6). This lack of one-to-one overlap may be due to our whole brain VBM approach, which does not limit the scope of findings to only regions with significant volume loss in patients compared with controls. Therefore, patient structural differences in specific brain areas related to non-target emotions (e.g., putamen) may not have met the statistical threshold (PFWE < 0.05) but could still have volume loss. In addition, it is possible that volume loss in these regions was not directly related to the neurodegenerative disease process but instead reflected typical changes in aging (Wang et al. 2019). Nevertheless, these alternative causes of brain changes do not change our main conclusion that smaller volume in left-lateralized brain structures correlates with greater experience of negative non-target emotions (in a sample of patients with neurodegenerative diseases and age-matched controls).
Interim summary . Altogether, these findings support our hypothesis that the experience of non-target emotions in neurodegenerative diseases results from errors in evaluation and interpretation processes due to neurodegeneration in brain regions that support these functions. The involvement of thalamus and anterior insula supports peripheralist (James 1884; Craig 2009; Levenson 2014) views that body signals, interpretation of these signals, and integration with other internal and external information are critical for the generation of subjective experience of emotions. On the other hand, the involvement of the dorsal striatum, anterior cingulate cortex, and parahippocampal cortex supports the constructionist (Satpute et al. 2015) and peripheralist views that the evaluation of the stimuli as well as contextual and external information also plays a key role in the generation of subjective emotional experience. It should be noted that although effects for some regions were relatively less robust (i.e., became statistically insignificant when the analyses adjusted for semantic knowledge, or only emerged in the laterality analyses) as compared with effects for other regions, these neural findings altogether suggest the importance of evaluation and interpretation in the generation of a more focused and congruent subjective experience of emotions.
Interestingly, several other brain regions often posited to play an important role in generating subjective emotional experience (e.g., posterior insula, amygdala, and dorsal medial prefrontal cortex) did not emerge in our analyses. Differences between the prior literature and our present findings may reflect differences in emotional stimuli used across studies. Our study focused on emotional experience in response to films selected to induce amusement, sadness, and disgust, whereas other studies have selected stimuli to induce other target emotions such as fear (e.g., Feinstein et al. 2011). This may help explain why brain regions critical for detecting threat (e.g., amygdala) were not revealed in our study.
Neuroanatomical Correlates of Positive Non-target Emotions
We did not find neural correlates for subjective experience of positive non-target emotions. In preliminary analyses, however, we found that the experience of positive non-target emotions appeared to be more strongly correlated with semantic knowledge (r = −0.45), as compared with the experience of negative non-target emotions (r = −0.31). We also found that greater experience of positive non-target emotions was associated with older age and lower film recognition accuracy, which was not the case for the experience of negative non-target emotions. These findings point to the possibility that, in neurodegenerative diseases, increased experience of positive versus negative non-target emotions may involve different psychological and neurobiological mechanisms. It is possible that increased positive non-target emotional experience may be more influenced by combined effects of older age, worse memory functioning, and impairment in semantic knowledge and processing (i.e., differentiating the meaning of amused, affection, or enthusiasm) than negative non-target emotional experience. We have noticed that positive emotions assessed in our study have more complicated semantic meanings and overall lower word frequency than the negative emotions we assessed (e.g., mean word frequency scores for positive emotion words examined in this study were 1162.5; for negative emotion words were 3575; SubtlexUS 2017). In addition, at the group level, impairment in semantic knowledge (see Table 1) and self-reported experience of positive non-target emotions (see Fig. 3A) were both greater for svPPA and AD patients as compared with HC.
Lateralization to the Left Hemisphere
Interestingly, we found that left hemisphere neurodegeneration was related to increased experience of negative non-target emotions. Laterality analyses confirmed these effects by showing that left-worse-than-right atrophy in the caudate was associated with greater experience of negative non-target emotions. Emotion researchers have also been interested in the lateralization of the brain for emotional processing. There is evidence that some aspects of emotions are lateralized. For example, in behavioral and autonomic nervous system responding, the left hemisphere has been found to be important for the generation of approach behaviors and parasympathetic nervous system responses, whereas the right hemisphere has been found to be specialized for the generation of withdrawal behaviors and sympathetic nervous system responses (Davidson et al. 1990; Berkman and Lieberman 2010; Guo et al. 2016). Our findings in which volume loss in the left but not right hemisphere regions was associated with increased experience of negative non-target emotions are consistent with previous models that describe the left hemisphere being specialized for the interpretation/reasoning of the internal and external worlds (Gazzaniga and LeDoux 1978; Gazzaniga 2005). Together, results from the present study suggest that whereas the generation of positive and negative emotional responses (e.g., approach versus withdraw behaviors, sympathetic versus parasympathetic nervous system activation) is lateralized to different hemispheres (Davidson et al. 1990; Guo et al. 2016), the generation of a more focused and congruent subjective experience of emotions may be lateralized to the left hemisphere, presumably due to its important role in evaluating and interpreting internal (e.g., interoceptive) and external (contextual) information associated with the emotional stimuli.
Implications
Our results have implications for emotion theory and affective neuroscience research. First, we found that volume loss in specific brain regions was related to alterations in the subjective experience of emotions but not to facial expressions of the same emotions. These findings are consistent with peripheralist (Levenson 2014) and some contemporary neural models of emotions (Adolphs 2017) that view subjective experience and other aspects of emotional responding (e.g., facial expressions) as being subserved by different neural circuitry. Second, our findings that increased subjective experience of non-target emotions was associated with neurodegeneration in left hemisphere regions are consistent with peripheralist and constructionist views that the generation of subjective experience of emotions requires the individuals to “make sense” of the situation (either internal situation such as bodily change, or external situation such as the social context, or both) and these processes are implemented by a large-scale brain networks rather than a single brain region (Craig 2009; Satpute et al. 2015). Finally, findings from this and other studies (Chen et al. 2017b; Johnson et al. 2017) highlight the importance of evaluating both target and non-target emotions in emotion research. For example, fMRI studies of neural activity associated with emotion elicitation might benefit from considering activation related to the full range of emotions that participants are experiencing (i.e., both those targeted by the elicitation procedures and those that are not).
Our findings also have implications for understanding clinical features of neurodegenerative diseases. When communicating with clinicians and family members, patients with neurodegenerative diseases may have difficulty providing a focused and accurate description of their internal emotional experiences. The present study provided neuroanatomical (i.e., volume loss in left-hemisphere regions) and psychological (i.e., deficits in evaluating and interpreting internal and external information) explanations for this difficulty, which furthers our understanding of the nature of emotional changes that occur in these diseases. In addition, we have previously found that family members caring for patients who report more negative non-target emotions had worse mental health (Chen et al. 2017b). Thus, findings of the current study indicate patient-based biomarkers (Hua et al. 2019) that may predict family members at heightened risk for developing mental health problems during caregiving.
Strengths and Limitations
The present study had a number of strengths, including (a) focusing on non-target emotions, a largely unstudied emotional symptom in patients with neurodegenerative diseases that have negative consequences for social interactions and relationships (Chen et al. 2017b); (b) using a relatively large sample of patients with a range of different neurodegenerative diseases and HC, thus maximizing neuroanatomical and behavioral heterogeneity and increasing statistical power; (c) using a variety of emotion-eliciting film clips, which increases the generalizability of findings; and (d) adjusting for semantic knowledge in additional analyses, which rule out the possibility that our findings simply reflect patient impairment in semantic functioning.
The study also had several limitations. First, we only focused on two aspects of emotional responding (subjective experience and facial expressions) and did not consider other important aspects that can also contribute to subjective experience of emotions (e.g., autonomic nervous system and activity in nonfacial muscles; Levenson 2014). Second, patients viewed the emotional film clips and rated their subjective emotional experience in fixed rather than randomized orders. Third, we only elicited one positive (amusement) and two negative (sadness and disgust) emotions and only obtained self-report of four positive and six negative non-target emotions. Examining other emotions would lead to a greater confidence in conclusions concerning a broader range of non-target emotions. Fourth, although functional decline typically precedes structural volume loss in neurodegenerative diseases (Zhou et al. 2010), structural volume loss by itself cannot determine changes in neuronal function. Future studies could examine functional neuroimaging data to identify more precise mechanisms of neuronal functional decline related to emotional responding.
Conclusions
This is the first study to examine neuroanatomical correlates of subjective experience of non-target emotions. Our results implicated a left-lateralized effect. Smaller volume in distributed left hemisphere regions thought to be critical for the evaluation and interpretation of the internal and external environment was associated with greater subjective experience of negative non-target emotions. These effects could not be explained by patient impairment in semantic knowledge. These findings advance our knowledge of how neurodegenerative diseases can negatively impact patients’ emotional functioning and may shed light on how subjective experience of emotions “is formed” in healthy brains (i.e., what is necessary to form more targeted, less diffused subjective experience of emotions).
Funding
This research was supported by the National Institute on Aging (RO1AG041762 and P01AG019724 to R.W.L; K99 AG059947 to K.-H.C.) and by the National Institute of Mental Health (T32 MH020006 fellowship to A.Y.H.).
Supplementary Material
Contributor Information
Kuan-Hua Chen, University of California, Berkeley, CA, USA.
Alice Y Hua, University of California, Berkeley, CA, USA.
Sandy J Lwi, University of California, Berkeley, CA, USA.
Claudia M Haase, Northwestern University, Evanston, IL, USA.
Howard J Rosen, University of California, San Francisco, CA, USA.
Bruce L Miller, University of California, San Francisco, CA, USA.
Robert W Levenson, University of California, Berkeley, CA, USA.
Notes
Correspondence should be addressed to Robert W. Levenson, 2121 Berkeley Way, Room 3302, University of California, Berkeley, Berkeley, CA 94720-1650. E-mail: boblev@berkeley.edu. We thank Scott Newton and Kia Nesmith for their assistance with subject recruitment, Deepak Paul for his help with data management and technical support, and all of our research assistants for their assistance with data collection and the coding of facial expressions of emotion. We also thank all patients and caregivers who donated their time so generously to participate in this research.Conflict of Interest: The authors report no conflict of interest.
References
- Abdulkadir A, Mortamet B, Vemuri P, Jack CR Jr, Krueger G, Klöppel S. 2011. Effects of hardware heterogeneity on the performance of svm Alzheimer's disease classifier. Neuroimage. 58:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. 2017. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc Cogn Affect Neurosci. 12:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12:366–375. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. 2013. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 17:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M et al. . 2013. Criteria for the diagnosis of corticobasal degeneration. Neurology. 80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD. 2010. Dopamine is released in the striatum during human emotional processing. Neuroreport. 21:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. 2007. The role of the dorsal striatum in reward and decision-making. J Neurosci. 27:8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. 2003. Voxel-based lesion–symptom mapping. Nat Neurosci. 6:448. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. 2010. Approaching the bad and avoiding the good: lateral prefrontal cortical asymmetry distinguishes between action and valence. J Cogn Neurosci. 22:1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. 1995. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 16:271–278. [DOI] [PubMed] [Google Scholar]
- Brown JA, Hua AY, Trujillo A, Attygalle S, Binney RJ, Spina S, Lee SE, Kramer JH, Miller BL, Rosen HJ et al. . 2017. Advancing functional dysconnectivity and atrophy in progressive supranuclear palsy. Neuroimage Clin. 16:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Ríos M, Gándara BS, Tapia M, Albert J, López-Martín S, Álvarez-Linera J. 2009. The striatum beyond reward: caudate responds intensely to unpleasant pictures. Neuroscience. 164:1615–1622. [DOI] [PubMed] [Google Scholar]
- Chen K-H, Lwi SJ, Hua AY, Haase CM, Miller BL, Levenson RW. 2017a. Increased subjective experience of non-target emotions in patients with frontotemporal dementia and Alzheimer’s disease. Curr Opin Behav Sci. 15:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-H, Wells JL, Otero M, Lwi SJ, Haase CM, Levenson RW. 2017b. Greater experience of negative non-target emotions in patients with neurodegenerative diseases is related to lower emotional well-being in caregivers. Dement Geriatr Cogn Disord. 44:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB. 2013. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 14:143–152. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. 1990. Approach-withdrawal and cerebral asymmetry : emotional expression and brain physiology I. J Pers Soc Psychol. 58:330–341. [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. 1981. Manual for the peabody picture vocabulary test-revised. Circle Pines (MN): American Guidance Service. [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, Levenson RW. 2012. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia. 50:786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. 2011. The human amygdala and the induction and experience of fear. Curr Biol. 21:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS. 2005. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 6:653–659. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, LeDoux J. 1978. The integrated mind. New York (NY): Plenum. [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Boucher O, Houde J-C, Descoteaux M, Obaid S, Gilbert G, Rouleau I, Nguyen DK. 2018. Subcortical structural connectivity of insular subregions. Sci Rep. 8:8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa S, Ogar J, Rohrer J et al. . 2011. Classification of primary progressive aphasia and its variants. Neurology. 76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. 2005. Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends Neurosci. 28:364–370. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. 1993. Emotional suppression: physiology, self-report, and expressive behavior. J Pers Soc Psychol. 64:970–986. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. 1995. Emotion elicitation using films. Cogn Emot. 9:87–108. [Google Scholar]
- Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, Crawford R, Stables L, Kramer JH, Rankin K et al. . 2016. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc Natl Acad Sci U S A. 113:E2430–E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. 2004. Combining voxel intensity and cluster extent with permutation test framework. Neuroimage. 23:54–63. [DOI] [PubMed] [Google Scholar]
- Hua AY, Wells JL, Haase CM, Chen KH, Rosen HJ, Miller BL, Levenson RW. 2019. Evaluating patient brain and behavior pathways to caregiver health in neurodegenerative diseases. Dement Geriatr Cogn Disord. 47:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. 1884. What is an emotion. Mind. 9:188–205. [Google Scholar]
- Johnson SL, Haase CM, Beermann U, Sanchez AH, Tharp JA, Lwi SJ, Casey JJ, Nguyen NK. 2017. Positive urgency and emotional reactivity: evidence for altered responding to positive stimuli. Emotion. 17:442–449. [DOI] [PubMed] [Google Scholar]
- Keltner D, Haidt J. 1999. Social functions of emotions at four levels of analysis. Cogn Emot. 13:505–521. [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. 2003. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 16:211–218. [DOI] [PubMed] [Google Scholar]
- Levenson RW. 1999. The intrapersonal functions of emotion. Cogn Emot. 13:481–504. [Google Scholar]
- Levenson RW. 2007. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment emotion elicitation with neurological patients. New York (NY): Oxford University Press, pp. 158–168. [Google Scholar]
- Levenson RW. 2014. The autonomic nervous system and emotion. Emot Rev. 6:100–112. [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH et al. . 1996. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 47:1–9. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 34:939–944. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. 2010. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. 1993. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney NT, Goodkind MS, Lomen-Hoerth C, Whalen PK, Williamson CA, Holley DE, Verstaen A, Brown LM, Miller BL, Kornak J et al. . 2011. Behaviour, physiology and experience of pathological laughing and crying in amyotrophic lateral sclerosis. Brain. 134:3458–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 8:976–987. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. 2005. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Swieten JC, Seelaar H, Dopper EGP, Onyike CU et al. . 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney AE, Koski JE, Beer JS. 2017. The functional role of ventral anterior cingulate cortex in social evaluation: disentangling valence from subjectively rewarding opportunities. Soc Cogn Affect Neurosci. 13:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA et al. . 2012. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 60:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, Murray EA. 2014. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci U S A. 111:5391–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Wilson-Mendenhall CD, Kleckner IR, Barrett LF. 2015. Emotional experience In: Toga AW, editor. Brain mapping: An encyclopedic reference. Waltham (MA): Elsevier, pp. 65–72. [Google Scholar]
- Schachter S, Singer J. 1962. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 69:379–399. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. 2009. Neurodegenerative diseases target large-scale human brain networks. Neuron. 62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. 2012. Turn down the volume or change the channel? Emotional effects of detached versus positive reappraisal. J Pers Soc Psychol. 103:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN et al. . 2009. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 10:131–146. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Brown JA, Hua AY, Lwi SJ, Zhou J, Kurth F, Eickhoff SB, Rosen HJ, Kramer JH, Miller BL et al. . 2018a. Network architecture underlying basal autonomic outflow: evidence from frontotemporal dementia. J Neurosci. 38:8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Sible IJ, Datta S, Hua AY, Perry DC, Kramer JH, Miller BL, Seeley WW, Rosen HJ. 2018b. Resting parasympathetic dysfunction predicts prosocial helping deficits in behavioral variant frontotemporal dementia. Cortex. 109:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Eckart JA, Zakrzewski J, Rosen HJ, Miller BL, Seeley WW, Levenson RW. 2015. Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex. 64:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP. 2013. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci U S A. 110:9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SubtlexUS: American word frequencies 2017. [accessed 19 May 2017]. http://subtlexus.lexique.org/.
- Takeda A, Sturm VE, Rankin KP, Ketelle R, Miller BL, Perry DC. 2019. Relationship turmoil and emotional empathy in frontotemporal dementia. Alzheimer Dis Assoc Disord. 33(3):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstaen A, Eckart JA, Muhtadie L, Otero MC, Sturm VE, Haase CM, Miller BL, Levenson RW. 2016. Insular atrophy and diminished disgust reactivity. Emotion. 16:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Q, Luo J, Hu M, Zuo C. 2019. Effects of age and sex on subcortical volumes. Front Aging Neurosci. 11:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. 2010. Connected speech production in three variants of primary progressive aphasia. Brain. 133:2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, Bonham LW, Sturm VE, Adhimoolam B, Karydas A, Coppola G, Miller BL, Rankin KP. 2015. The 5-HTTLPR variant in the serotonin transporter gene modifies degeneration of brain regions important for emotion in behavioral variant frontotemporal dementia. Neuroimage Clin. 9:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


