Abstract
Acquiring a foreign language is challenging for many adults. Yet certain individuals choose to acquire sometimes dozens of languages and often just for fun. Is there something special about the minds and brains of such polyglots? Using robust individual-level markers of language activity, measured with fMRI, we compared native language processing in polyglots versus matched controls. Polyglots (n = 17, including nine “hyper-polyglots” with proficiency in 10–55 languages) used fewer neural resources to process language: Their activations were smaller in both magnitude and extent. This difference was spatially and functionally selective: The groups were similar in their activation of two other brain networks—the multiple demand network and the default mode network. We hypothesize that the activation reduction in the language network is experientially driven, such that the acquisition and use of multiple languages makes language processing generally more efficient. However, genetic and longitudinal studies will be critical to distinguish this hypothesis from the one whereby polyglots’ brains already differ at birth or early in development. This initial characterization of polyglots’ language network opens the door to future investigations of the cognitive and neural architecture of individuals who gain mastery of multiple languages, including changes in this architecture with linguistic experiences.
Keywords: fMRI, polyglots, multilingualism, neural plasticity, expertise
Introduction
In the presence of linguistic input, a typically developing child effortlessly acquires a language or multiple languages. Sometime during late childhood/early adolescence, after the so-called critical period, acquiring new languages becomes substantially more difficult (e.g., Lenneberg 1967; Johnson and Newport 1989; Hyltenstam and Abrahamsson 2003; Birdsong 2005; DeKeyser and Larson-Hall 2005; Pinker 2009; Hartshorne et al. 2018). Yet, many individuals learn new languages in their adult years, with some of them doing so of their own volition, suggesting they find the process enjoyable (e.g., Erard 2012). “Polyglots” are a subset of these individuals, who obtain proficiency in multiple languages, sometimes dozens (“hyper-polyglots”; Hudson 2016). Definitions of polyglotism vary in the literature, but most reserve this term for individuals who have acquired at least some of the languages after the critical period (cf., multilinguals, who grow up in environments where multiple languages are spoken, like Belgium, Singapore, India, Morocco, or Mali) (e.g., Hyltenstam 2016; Krzeminska 2016).
Extraordinary cases of polyglotism have been reported (e.g., Krashen and Kiss 1996; Baker and Jones 1998; Erard 2012), and modern-day polyglots continue to attract enormous attention from the general public, as evidenced by millions of views that videos of and about polyglots receive (e.g., THNKR 2013; Doner 2014; Machova 2018) and by the high readership of popular articles about polyglots (e.g., Leland 2012; Thurman 2018). However, scientific investigations of polyglotism are almost nonexistent (e.g., Erard 2005, 2012; Reiterer et al. 2009; Biedron and Pawlak 2016; Hyltenstam 2016). At least two broad questions can be asked about this population. First, we can ask whether polyglots exhibit any innate predispositions—manifesting genetically, neurally, and/or cognitively—to language learning. And second, we can ask whether learning multiple languages leads to changes in one’s cognition and neural architecture with respect to the language system or more generally. Both questions have wide-ranging implications for our understanding of the human mind and brain, including issues of innateness, the relationship between language and other domains, the limits of human cognition, and brain plasticity. Here we begin to tackle these questions by comparing the brains of polyglots and non-polyglot individuals using functional brain imaging.
To the best of our knowledge, the only prior study that has examined brains of polyglots was a postmortem examination of the brain of Emil Krebs (E.K.), a German polyglot, who studied 120 languages and allegedly mastered over 60. Amunts et al. (2004) examined the microanatomy of Broca’s area (Brodmann areas 44 and 45) in E.K. compared with 11 male control brains and observed reliable cytoarchitectonic differences, with no differences observed in a control, visual area (Brodmann Area 18). However, two factors make the observed differences difficult to interpret. First, the microarchitectural features examined have not been linked to functional brain responses or behavior, which is not surprising given that this level of structural detail is not accessible to current imaging methodologies for living brains. And second, the postmortem nature of the study precludes matching the polyglot and control participants on cognitive abilities, like general IQ. The latter is especially important given that Broca’s area houses both language-selective and domain-general areas that have been linked to fluid intelligence (e.g., Fedorenko, Duncan, et al. 2012; Fedorenko and Blank 2020; Woolgar et al. 2018).
Potentially relevant evidence comes from research on bilingualism. Brains of individuals who know more than one language have been argued to differ from those of monolinguals, both functionally (e.g., Kovelman et al. 2008; Li et al. 2014; Schafer and Constable 2009; Park et al. 2012; Román et al. 2015) and structurally (e.g., Mechelli et al. 2004; cf., Keller et al. 2001 and Greve et al. 2013 for evidence that even extreme functional differences—left vs. right language lateralization—are not accompanied by large/any differences in anatomy). For example, according to the “bilingual neural signature” hypothesis (Kovelman et al. 2008, 2008), early exposure to two languages is associated with a particular pattern of neural activity. Specifically, bilinguals exhibit stronger and more extensive activation within classic language areas during the processing of either language compared with monolinguals processing the same language (e.g., Kovelman et al. 2008; Park et al. 2012; Jasinska and Petitto 2013; Román et al. 2015; Coderre et al. 2016; cf., Parker-Jones et al. 2012 and Palomar-Garcia et al. 2015, who argue that this pattern is limited to language production tasks). These stronger responses could reflect more effortful language processing due to interference from the second language, concurrent activation of both languages, or richer/deeper processing of linguistic input resulting from generally increased linguistic awareness due to experience with multiple, sometimes diverse, languages.
More controversially, effects of bilingualism have been argued to extend beyond linguistic processing to affect domain-general executive control and theory of mind, or mentalizing, abilities. For example, stronger activity in executive control brain areas has been reported in bilinguals, compared with monolinguals, during some linguistic tasks (e.g., Rodriguez-Fornells et al. 2002; Hernandez 2009). Others have further suggested that the frequent need to manage crosslinguistic conflict in bilinguals, including switching between languages during production, leads to superior performance on some nonlinguistic executive function tasks (for reviews, see Bialystok 2009; Bialystok et al. 2009; Hilchey and Klein 2011; Kroll and Bialystok 2013) and more efficient use of brain areas that support domain-general executive control (e.g., Abutalebi et al. 2012; for reviews, see Adesope et al. 2010; Pliatsikas and Luk 2016; Grundy et al. 2017). However, the bilingual advantage hypothesis has not received robust and consistent support across populations, paradigms, and research groups (e.g., Paap and Greenberg 2013; Duñabeitia et al. 2014; Paap et al. 2015; von Bastian et al. 2016; Donnelly et al. 2019; Nichols et al. 2020). The evidence is also mixed in the theory of mind domain, with some reporting a bilingual advantage (e.g., Goetz 2003; Kovács 2009; Rubio-Fernández and Glucksberg 2012), but others failing to detect differences between bilinguals and monolinguals (e.g., Kyuchukov and de Villiers 2009; Ryskin et al. 2014; Cox et al. 2016; Dahlgren et al. 2017). These mixed findings may result from the high heterogeneity that characterizes the bilingual population (Bialystok 2009; Leivada et al. 2020; Pilatsikas 2020). For example, Blanco-Elorrieta and colleagues recently argued that the bilingual advantage may only occur in bilinguals who switch languages frequently due to external constraints, for example, because they reside in dense code-switching communities or commonly engage in simultaneous translation (Blanco-Elorrieta and Pylkkänen 2018).
In summary, the need to manage two or more languages within one mind/brain appears to lead to some changes in the language network, and possibly also in the domain-general executive network and/or the theory of mind network, although the evidence for the effects on nonlinguistic abilities is equivocal. Based on these findings, we might expect to find similar, or even more pronounced, changes in the relevant brain networks in polyglots.
In the current study, we used functional MRI to probe the brains of 17 polyglots (languages spoken: 5–55), 9 of whom qualified as “hyper-polyglots” by Erard’s (2012) definition, having some knowledge of 10 or more languages. The polyglots were compared with pairwise-matched (on age, sex, handedness, and IQ) monolinguals, as well as a larger control population (n = 217; for evidence that large samples are needed to discover genuine group differences see, e.g., Ramus et al. 2018; Brysbaert 2019; Assem et al. 2020). We examined activity during native language processing (English) in the fronto-temporal language network, which selectively supports high-level language comprehension (e.g., Fedorenko et al. 2011; Monti et al. 2012; Fedorenko and Blank 2020), including both lexico-semantic and combinatorial syntactic and semantic processing (e.g., Keller et al. 2001; Fedorenko et al. 2010, 2020; Fedorenko, Nieto-Castañon, et al. 2012; Bautista and Wilson 2016).
We asked two research questions. First, we asked whether the language network differs between polyglots and controls, focusing on neural markers that we have previously established to be stable within individuals over time, like the strength and extent of activation, and lateralization (Mahowald and Fedorenko 2016). A priori, one can make two opposite predictions. On the one hand, polyglots, like bilinguals (e.g., Kovelman et al. 2008; Park et al. 2012; Jasinska and Petitto 2013; Román et al. 2015; Coderre et al. 2016), might exhibit stronger and more extensive activations during linguistic processing, compared with control participants. This pattern would align with prior findings of more extensive activations in experts in other domains (e.g., Maguire et al. 1997; Schneider et al. 2002; Olesen et al. 2004; Russel et al. 2010) and would suggest that polyglotism and bilingualism manifest similarly in the brain. Alternatively, polyglots might exhibit weaker and less extensive activations, reflecting greater processing efficiency, in line with prior work on activation reduction as a function of practice with a task (e.g., Poldrack et al. 1998; Fletcher et al. 1999; Gauthier et al. 1999; Schneider et al. 2002; Maguire et al. 2003; McCandliss et al. 2003; Calvo-Merino et al. 2004; Kelly and Garavan 2005; Landau and d’Esposito 2006; Bavelier et al. 2012; Bernardi et al. 2013; Protzner et al. 2016), including linguistic tasks (e.g., Reichle et al. 2000; Xue et al. 2006; Prat et al. 2007; Xue and Poldrack 2007; Prat and Just 2010; Grogan et al. 2012; Glezer et al. 2015; Hervais-Adelman et al. 2018). Further, aptitude for nonnative language learning has been linked with functional responses in the right homologs of the language areas (e.g., Kepinska et al. 2017a; Qi et al. 2019; see Qi and Legault 2020 for a review), leading to a possible prediction of differences in the activity of the right hemisphere language areas. Finally, reduced lateralization of language processing has been reported in several language disorders (e.g., Sommer et al. 2001; Kleinhans et al. 2008; Bishop 2013). If polyglotism is characterized by an aptitude for language learning, perhaps it would be associated with increased lateralization (e.g., Gotts et al. 2013; Mellet et al. 2014; cf., Novoa et al. 1988; Amunts et al. 2004).
And second, we asked whether the between-population differences are restricted to the language network or extend to other networks that support high-level cognition. In particular, we examined activity in the fronto-parietal domain-general multiple demand (MD) network (e.g., Duncan 2010, 2013), which has been linked to executive control and fluid intelligence, and the fronto-parietal default mode network (DMN) (e.g., Buckner et al. 2008; Buckner and DiNicola 2019), implicated in social cognition, recollection and prospection, and semantic processing. As discussed above, some of these high-level abilities have been argued to be affected by knowing more than one language. Further, non-native language learning ability has been associated with functional responses and functional correlation patterns in both the executive control network (e.g., Kepinska et al. 2017a, 2017b) and the DMN (e.g., Kepinska et al. 2017b). Together, the three networks cover substantial portions of the frontal, temporal, and parietal association cortex. Whether superior executive or theory of mind abilities should be associated with stronger and more extensive versus weaker and less extensive activity remains debated (see, e.g., Assem et al. 2020 for a discussion of this issue in the domain of executive abilities). As a result, we leave the predictions of possible polyglot versus non-polyglot differences open as to the direction of the effects.
Materials and Methods
Participants
Polyglots
Polyglots were defined as individuals who (a) have some level of proficiency in at least five languages (their native language and four other languages) and (b) have advanced proficiency in at least one language other than their native language. Because this population has not been studied extensively in the past, these criteria are necessarily somewhat arbitrary. They are based on the fact that most individuals living in a predominantly monolingual society, like the USA, typically study just one foreign language in school and/or college. So, having some proficiency in four foreign languages is sufficiently unusual. Participants assessed their own proficiency in listening, speaking, reading, and writing in each language they have some familiarity with, on a scale from 0 = no knowledge to 5 = native proficiency. A total score of 16 or higher for a language was used as an indicator of advanced proficiency. Seventeen polyglots were recruited from the Boston community (Mage = 30.5 years [standard deviation {SD} = 8.6]; 9 males; 16 right-handed [as determined by the Edinburgh handedness inventory; Oldfield 1971]; all native speakers of English; MKBIT non-verbal = 124 [SD = 8]). The median number of languages spoken with some level of proficiency was 7 (Mlanguages = 13.9, range: 5–55 languages; see Table 1 for detailed linguistic background). Nine of the polyglots qualified as “hyper-polyglots,” having some knowledge of 10 or more languages (Erard 2012). The mean self-rated proficiency for L1 (native language) was 20.0 (SD = 0), as expected, for L2, 17.7 (SD = 1.7, range: 16–20), for L3, 15.84 (SD = 2.52, range: 11–20), for L4, 12.5 (SD = 3.9, range: 6–20), and for L5, 9.9 (SD = 3.7, range: 4–16). Thus, in addition to having native-like proficiency in their L2, most of these individuals had quite high proficiency in their L3 and L4 and some in their L5. All polyglots were born in the USA. Eleven polyglots were raised in monolingual households, while six grew up in bilingual families. Approximately 64% of nonnative languages spoken by polyglots (219 languages across the 17 polyglots) were learned by them on their own using various self-learning tools (e.g., language learning software, textbooks, audio programs; see Hyltenstam (2018) for a discussion of “learner autonomy” in polyglots); ~ 26% were acquired through language classes; and the remaining ~ 10% were learned through immersion (3% in childhood through exposure to languages spoken by parents and family members; 7% in adulthood through travel to foreign countries).
Table 1.
Demographic and linguistic background information for the polyglots
| ID | Sex, age at testing, IQ, handedness | N Lang. | Languages spoken |
|---|---|---|---|
| Poly01 | M, 20, 130, R | 26 | Englisha (20), Frenchc (17), Arabicc (14), Persianc (14), Germanc (13), Siniticc (12), Semitic (12), Romance (11), Slavic (8), Turkic (8), Bantu (8), Romance (8), Indo-Iranian (8), Japonic (7), Indo-Iranian (7), Germanic (5), Hellenicc (4),Chadic (4), Germanic (4), Niger-Congo (4), Niger-Congo (4), Algic (4), Malayo-Polynesian (4), Romancec (4), Germanic (4), Semiticc (3) |
| Poly02 | M, 29, 130, R | 16 | Englisha (20), Germanc (18), Spanishc (16), Mandarinc,b (14), Hungarianc (12),Germanic (8), Sinitic (8), Slavic (6), Semitic (6), Semitic (5), Japonic (5), Germanic (5), Moseten-Chonan (4), Mongolic (4), Koreanic (3), Turkic (3) |
| Poly03 | F, 29, 132, R | 9 | Englisha (20), Japanesea,b,c (19), Frenchc (17), Korean (15), Mandarinc (10), Romance (10), Semitic (8), Sign-basedc (4), Semiticc (4) |
| Poly04 | F, 19, 125, R | 5 | Englisha (20), Spanisha (20), Italianc (19), Frenchc (15), Mandarinc (13) |
| Poly05 | F, 28, 109, R | 5 | Englisha (20), Japanesec (20), Spanishc (20), Portuguesea,b (20), Frenchc (12) |
| Poly06 | M, 43, 132, R | 12 | Englisha (20), Mandarinc (19), Russianc (12), Japanesec (10), German (10), Romancec (10), Romance (9), Austroasiatic (8), Semitic (6), Moseten-Chonan (4), Turkic (4), Turkic (4) |
| Poly07 | F, 30, 132, L | 5 | Englisha (20), Japanesec (16), Spanishc (11), Portuguese (6), Mandarin (4) |
| Poly10 | M, 32, 105, R | 7 | Englisha (20), Germanc,b (20), Arabicc,b (18), Italianc (16), Hebrewc (13), Indo-Iranian (8),Romance (6) |
| Poly11 | M, 29, 130, R | 55 | Englisha (20), Mandarinc,b (19), Koreanc (16), Japanesec,b (13), Vietnameseb (13), Romancec (10), Romancec (10), Semiticc (9), Slavicc (9), Sinitic (9), Dravidian (8), Germanicc (8), Romance (8), Sinitic (7), Semitic (7), Indo-Iranian (7), Germanic (7), Esperanto (6), Uralic (6), Hellenic (6), Semitic (6), Sinitic (6), Semiticc (6), Indo-Iranian (6), Semitic (6), Semitic (6), Austroasiatic (5), Kra-Dai (5), Germanic (5), Germanic (5), Celtic (5), Mongolic (5), Germanic (5), Indo-Iranian (5), Semitic (5), Turkic (5), Malayo-Polynesian (5), Indo-Iranian (4), Slavic (4), Romance (4), Quechuan (4), Indo-Iranian (4), Niger-Congo (4), Niger-Congo (4), Niger-Congo (4), Niger-Congo (4), Sino-Tibetan (4), Celtic (4), Sinitic (4), Sino-Tibetan (4), Uralic (4), Dene-Yeniseian (4), Germanic (4), Lojban (4), Indo-Iranian (4) |
| Poly13 | F, 22, 130, R | 7 | Englisha (20), Portuguesea (16), Frenchc (16), Japanesec (12), Spanishc (11), Romance (8), Slavic (4) |
| Poly14 | M, 28, 120, R | 6 | Englisha (20), Icelandicc (16), Spanishc (15), Frenchc (14), German (8), Sinitic (8) |
| Poly15 | F, 27, 120, R | 5 | Englisha (20), Germanc (16), Russiana (12), Frenchc (7), Hebrew (5) |
| Poly16 | M, 27, 120, R | 5 | Englisha (20), Mandarinc (17), Spanishc (13), French (9), Koreanc (6) |
| Poly17 | F, 24, 120, R | 5 | Englisha (20), Portuguesec (16), Spanishc (15), Arabicc (8), Swahili (4) |
| Poly18 | F, 51, 120, R | 26 | Englisha (20), Spanishc (16), Germanc,b (16), Frenchc (16), Italian (12), Romance (11), Semitic (11), Hellenic (9), Slavic (8), Semitic (7), Indo-Iranian (7), Slavic (7), Germanic (6), Semitic (6), Germanic (6), Germanic (5), Japonic (5), Sinitic (5), Germanic (5), Koreanic (5), Celtic (5), Indo-Iranian (5), Niger-Congo (4),Baltic (4), Slavic (4), Turkic (4) |
| Poly19 | M, 43, 130, R | 13 | Englisha (20), Spanishc (16), Bulgarianb (16), French (8), Romanian (6), Slavic (6), Sinitic a (5), Germanic (5), Germanic (5), Turkic (4), Uralicc,b (4), Sinitic (4), Japonicc (4) |
| Poly20 | M, 39, 130, R | 29 | Englisha (20), Mandarinc,b (19), Indonesianc,b (16), Passamaquoddy-Maliseet (16), Penobscot (16), Algonkian (16), Celticc (16), Malayo-Polynesian (16), Algonkian(14), Hmongic (14), Romancec (13), Romancec (13), Celtic (13), Celticc (12), Semiticc,b (12), Celtic (12), Afro-Asiatic (12), Vasconic (10), Iroquoian (10), Germanic (9), Slavic (8), Slavic (8), Uralic (8), Albanian (8), Sino-Tibetan (8), Muskogean (8), Mongolicc (8), Sign languagec (8), Quechuan (6) |
Self-reported language proficiency scores are provided in brackets next to each language (the maximal score of 20 corresponds to native proficiency). To protect the participants’ identities, we provide only the language family of the relevant language for L6 and any additional languages. Symbols next to each language indicate the ways in which that language was learned.
Note: No symbol: self-training.
aParents/immersion as a child.
bImmersion as an adult.
cFormal training.
Matched Monolingual Controls
Polyglot participants were pairwise-matched with monolinguals on age (M polyglot: 30.5 [SD = 8.6] vs. M non-polyglot: 31.6 [SD = 10.1]; t(32) = 0.33, n.s.), sex (9 males in each group), handedness (as determined by the Edinburgh handedness inventory [Oldfield 1971]; 1 left-handed individual in each group both of whom had a left-lateralized language network), and nonverbal IQ, as measured by KBIT (Kaufman and Kaufman 2004; M polyglot: 124 [SD = 8] vs. M non-polyglot: 119.6 [SD = 7.2]; t(32) = 1.74, n.s.). The mean number of languages with any level of proficiency for the non-polyglot controls was 1.4 (SD = 0.5, range: 1–2). All non-polyglot controls with some knowledge of a second language identified as novice L2 speakers and thus qualify as monolinguals.
A Larger Group of Controls
To examine the key neural measures relative to a larger distribution from the population, we further included data from a relatively large (n = 217) set of non-polyglot participants—nonoverlapping with the set of 17 pairwise-matched monolinguals described above—from the Fedorenko lab’s database, each of whom had completed a language localizer experiment (Fedorenko et al. 2010) as part of different studies (Mage = 23.8 years [SD = 6.1]; 71 males; 202 right-handed, 9 left-handed, and 6 ambidextrous [as determined by the Edinburgh handedness inventory (Oldfield 1971) or self-report]; MKBIT = 119.5 [SD = 11.3]; all native speakers of English; mean number of languages spoken with any level of proficiency = 2.9 [SD = 1.3, range: 1–9]). This group consisted of 172 monolinguals, 43 bilinguals (self-rated proficiency in 2 languages 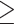 16 out of 20; second languages spoken: Arabic, Armenian, French, German, Korean, Mandarin, Polish, Portuguese, Spanish, and Swahili), and 2 trilinguals (self-rated proficiency in 3 languages
16 out of 20; second languages spoken: Arabic, Armenian, French, German, Korean, Mandarin, Polish, Portuguese, Spanish, and Swahili), and 2 trilinguals (self-rated proficiency in 3 languages 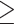 16 out of 20; English–Mandarin–Cantonese and English–Spanish–Portuguese). In this group, individuals who indicated some familiarity with 4 or more languages did not have advanced proficiency in any of their nonnative languages and thus do not qualify as polyglots.
16 out of 20; English–Mandarin–Cantonese and English–Spanish–Portuguese). In this group, individuals who indicated some familiarity with 4 or more languages did not have advanced proficiency in any of their nonnative languages and thus do not qualify as polyglots.
All participants gave informed consent in accordance with the requirements of the Committee on the Use of Humans as Experimental Subjects at MIT and were paid for their participation.
Experimental Design, Materials, and Procedure
All participants completed a language localizer task (Fedorenko et al. 2010). Furthermore, 16 of the 17 polyglots and pairwise-matched controls and 151 of the 217 participants in the larger control group (including 120 monolinguals, 30 bilinguals, and 1 trilingual) additionally completed a localizer for the MD network (Duncan 2010, 2013), which can also be used to define the regions of the DMN (e.g., Mineroff et al. 2018). Some participants completed one or two additional tasks for unrelated studies. The entire scanning session lasted ~2 h.
Language Localizer
The polyglots and the pairwise-matched monolinguals passively read English sentences and lists of pronounceable nonwords in a blocked design. The Sentences > Nonwords contrast targets brain regions that support high-level language comprehension, including lexico-semantic and combinatorial processes (e.g., Keller et al. 2001; Fedorenko et al. 2010, 2020; Fedorenko, Nieto-Castañon, et al. 2012; Bautista and Wilson 2016) and has been shown to be robust to changes in materials, task, timing parameters, and other aspects of the procedure (Fedorenko et al. 2010; Fedorenko 2014; Mahowald and Fedorenko 2016; Scott et al. 2017). Each trial started with 100-ms pretrial fixation, followed by a 12-word-long sentence or a list of 12 nonwords presented on the screen one word/nonword at a time at the rate of 450 ms per word/nonword. Then, a line drawing of a hand pressing a button appeared for 400 ms, and participants were instructed to press a button whenever they saw this icon, and finally a blank screen was shown for 100 ms, for a total trial duration of 6 s. The simple button-pressing task was included to help participants stay awake and focused. Each block consisted of three trials and lasted 18 s. Each run consisted of 16 experimental blocks (8 per condition) and 5 fixation blocks (14 s each), for a total duration of 358 s (5 min 58 s). Each participant performed two runs, with condition order counterbalanced across runs. One hundred sixty-five of the 217 participants in the larger control group performed the same version of the localizer task. The remaining 52 performed versions that differed slightly in the design and procedure (Table 2) that have been shown to not affect the activations (e.g., Mahowald and Fedorenko 2016).
Table 2.
Information on which subsets of participants in the sample of 217 non-polyglots performed which version of the language localizer
| Number of participants | Language localizer version | Conditions | Materials | Trials per block | Blocks per run/per condition per run |
|---|---|---|---|---|---|
| n = 165 | SNloc_ips179 | Sentences, Nonwords | 12-word-/nonword-long sequences | 3 | 16/8 |
| n = 29 | SNloc_ips189 | Sentence, Nonwords | 12-word-/nonword-long sequences | 3 | 16/8 |
| n = 19 | SWNloc_ips198 | Sentences, Wordlists, Nonwords | 12-word-/nonword-long sequences | 3 | 18/6 |
| n = 3 | SWJN_v1_ips252 | Sentences, Wordlists, Jabberwocky, Nonwords | 12-word-/nonword-long sequences | 5 | 16/4 |
| n = 1 | SWJN_v2_ips232 | Sentences, Wordlists, Jabberwocky, Nonwords | 8-word-/nonword-long sequences | 5 | 16/4 |
Information on the procedure and timing for the SNloc_ips179 version (used for the majority of the participants, as well as the polyglots and pairwise-matched monolinguals) is provided in the Methods section. Information on the procedure and timing for the other versions can be found in Mahowald and Fedorenko (2016), Table 2.
Multiple Demand and Default Mode Network Localizer
Participants performed a spatial working memory task, where they had to keep track of four (easy condition) or eight (hard condition) locations in a 3 x 4 grid (Blank et al. 2014). In the easy condition, the locations were presented one at a time, and in the hard condition, they were presented two at a time. In both conditions, subjects performed a two-alternative forced-choice task at the end of each trial to indicate the set of locations that they just saw. The Hard > Easy contrast targets the brain regions of the MD network, a bilateral fronto-parietal network, which supports executive control and fluid intelligence (Duncan 2010, 2013; Fedorenko et al. 2013). The reverse, Easy > Hard, contrast can be used to identify the brain regions of the DMN, a bilateral network, which has been implicated in social cognition, recollection and prospection, and semantic processing (Buckner et al. 2008; Buckner and DiNicola 2019), because a well-established functional signature of this network is deactivation to demanding tasks, with greater deactivation to more demanding conditions. Indeed, DMN regions defined with the Easy > Hard contrast from the spatial working memory task show exactly this profile (Mineroff et al. 2018), and contrasts between fixation and the Easy or Hard condition yield similar areas (unpublished data from the Fedorenko lab).
Each trial lasted 8 s (see Fedorenko et al. 2011, for the timing details). Each block consisted of four trials and lasted 32 s. Each run consisted of 12 experimental blocks (6 per condition) and 4 fixation blocks (16 s in duration each), for a total duration of 448 s (7 min 28 s). Each participant completed two runs, with condition order counterbalanced across runs.
fMRI Data Acquisition, Preprocessing, and Modeling
Structural and functional data were collected on the whole-body 3 Tesla Siemens Trio scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 179 sagittal slices with 1 mm isotropic voxels (TR = 2530 ms, TE = 3.48 ms). Functional, blood oxygenation level–dependent (BOLD) data were acquired using an EPI sequence (with a 90° flip angle and using GRAPPA with an acceleration factor of 2), with the following acquisition parameters: 31 4-mm-thick near-axial slices, acquired in an interleaved order with a 10% distance factor; 2.1 x 2.1 mm in-plane resolution; field of view of 200 mm in the phase encoding anterior to posterior (A > P) direction; matrix size of 96 x 96 voxels; TR of 2000 ms; and TE of 30 ms. Prospective acquisition correction (Thesen et al. 2000) was used to adjust the positions of the gradients based on the participant’s motion one TR back. The first 10 s of each run were excluded to allow for steady-state magnetization.
fMRI data were preprocessed and analyzed (for first-level data modeling) with SPM5 and custom MATLAB scripts. (We used the older version of SPM here because the data for some of the control participants were collected and analyzed many years ago, and we wanted to have all the data analyzed through the same pipeline, for better comparability; see Fig. SI-4 in Diachek et al. 2020, for illustration that neural responses obtained through an SPM5 vs. SPM12 pipeline are similar.) Each subject’s data were motion-corrected (realigned to the mean image of the first functional run using 2nd-degree b-spline interpolation) and then normalized into a common brain space (the Montreal Neurological Institute [MNI] template) (estimated for the mean image using trilinear interpolation) and resampled into 2-mm isotropic voxels. The data were then smoothed with a 4-mm FWHM Gaussian filter and high-pass filtered (at 200 s). For each localizer task, a standard mass univariate analysis was performed whereby a general linear model (GLM) estimated the effect size of each condition in each experimental run. These effects were each modeled with a boxcar function (representing entire blocks) convolved with the canonical hemodynamic response function. The model also included first-order temporal derivatives of these effects, as well as nuisance regressors representing entire experimental runs and offline-estimated motion parameters. All the individual activation maps are available at OSF: https://osf.io/td7am/.
Language, Multiple Demand, and Default Mode Network fROI Definition and Estimation of the Neural Features of Interest
For each participant, functional regions of interest (fROIs) were defined using the Group-constrained Subject-Specific (GSS) approach (Fedorenko et al. 2010), whereby a set of parcels or “search spaces” (i.e., brain areas within which most individuals in prior studies showed activity for the localizer contrast; Fig. 1) is intersected with each individual participant’s activation map for the same contrast.
Figure 3.
Functional response properties of the RH language, MD, and DMN networks in polyglots versus non-polyglots. The Sentences > Nonwords (Language), Hard > Easy (MD), and Easy > Hard (DMN) effect sizes and region volumes are shown as box-and-whisker plots for polyglots and matched non-polyglots (red and blue dots, respectively) and as a histogram for a larger sample of non-polyglots (n = 151). No group differences were found in the RH language network or in either of the control networks.
Figure 1.
Functional response properties of the LH language network in polyglots versus non-polyglots. Left: The Sentences > Nonwords effect sizes and region volumes are shown as box-and-whisker plots for polyglots and matched non-polyglots (red and blue dots, respectively) and as a histogram for a larger sample of non-polyglots (n = 217). Right: The effect sizes and region volumes for the three groups (polyglots, matched non-polyglots, larger set of non-polyglots) are shown as bar plots for the six language regions separately. Group differences in effect sizes and region volumes were present in the LH language network.
To define the language fROIs, we used six parcels derived from a group-level representation of data for the Sentences > Nonwords contrast in 220 participants. These parcels included three regions in the left frontal cortex: two located in the inferior frontal gyrus (LIFG and LIFGorb) and one located in the middle frontal gyrus (LMFG) and three regions in the left temporal and parietal cortices spanning the entire extent of the lateral temporal lobe and extending into the angular gyrus (LAntTemp, LPostTemp, and LAngG). Additionally, we examined activations in the right hemisphere homologs of the language regions. To define the fROIs in the right hemisphere, the left hemisphere parcels were mirror-projected onto the RH to create six homologous masks. By design, the parcels cover large swaths of cortex in order to be able to accommodate interindividual variability. Hence the mirrored versions are likely to encompass RH language regions despite possible hemispheric asymmetries in the precise locations of activations (Mahowald and Fedorenko 2016). (Note that these parcels and the parcels for the other two networks were defined/selected independently of the current study and used across many prior published studies; the use of the same parcels and localizer contrasts across studies, and research groups, allows for straightforward comparisons of results, ensuring robust, replicable, and cumulative science [e.g., Poldrack et al. 2017; Fedorenko and Blank 2020]).
To define the MD fROIs, we used 18 anatomical parcels in the frontal and parietal cortex of the two hemispheres (Tzourio-Mazoyer et al. 2002). These regions included the bilateral opercular IFG (L/R IFGop), MFG (L/R MFG), orbital MFG (L/R MFGorb), insular cortex (L/R Insula), precentral gyrus (L/R PrecG), supplementary and presupplementary motor areas (L/R SMA), inferior parietal cortex (L/R ParInf), superior parietal cortex (L/R ParSup), and anterior cingulate cortex (L/R ACC). (These anatomical parcels are highly overlapping with a set of functional parcels derived from a group-level representation of data for the Hard > Easy spatial working memory contrast in 197 participants. We chose to use the anatomical parcels here for consistency with prior studies [Fedorenko et al. 2013; Blank et al. 2014].)
To define the DMN fROIs, we used eight anatomical parcels in the frontal and parietal cortices of the two hemispheres. These regions included the posterior cingulate (L/R PostCing), frontal medial orbital cortex (L/R FrontMedOrb), frontal medial superior cortex (L/R FrontMedSup), and the precuneus (L/R Precuneus). In addition, we included two parcels—in the left and right temporo-parietal junction (L/R TPJ)—derived from a group-level representation of data for the False Belief > False Photograph contrast in 462 participants (Dufour et al. 2013). (These parcels are highly overlapping with a set of functional parcels derived from a group-level representation of data for the Easy > Hard spatial working memory contrast in 197 participants.)
We examined three features of the activations for our four networks of interest (left hemisphere (LH) language, right hemisphere (RH) language, MD, and DMN): (i) effect sizes, (ii) extent of activation (region volumes), and (iii) lateralization based on the extent of activation. All three measures have been shown to be reliable within individuals over time (e.g., Mahowald and Fedorenko 2016; Assem et al. 2020). The first two measures have further been shown to be strongly correlated (e.g., Mahowald and Fedorenko 2016); as a result, whatever differences emerge with respect to effect sizes are expected to also manifest for the extent-of-activation measures.
To compute effect sizes, individual fROIs were defined by selecting—within each parcel—the top 10% of most localizer-responsive voxels based on the t-values for the relevant contrast (Sentences > Nonwords for the language network localizer, Hard > Easy spatial working memory for the MD network localizer, and Easy > Hard spatial working memory for the DMN localizer). To maintain independence between the data used to define the fROIs versus to characterize their responses (Kriegeskorte et al. 2009), we used an across-run cross-validation procedure, where (i) the first run was used to define the fROIs and the second run to estimate the responses (in percent BOLD signal change); (ii) the second run was used to define the fROIs and the first run to estimate the responses; and finally, (iii) the estimates were averaged across the two left-out runs to derive a single value per participant per fROI.
To compute region volumes, we counted the number of voxels that showed a significant effect (at the P < 0.001 whole-brain uncorrected threshold) for the relevant localizer contrast within each parcel.
Finally, to estimate the degree of lateralization (for the language network only), we subtracted the number of activated voxels for the Sentences > Nonwords contrast (at the P < 0.001 whole-brain uncorrected threshold) across all the right hemisphere language parcels from the number of Sentences > Nonwords voxels in all the left hemisphere parcels and divided the resulting value by the total number of Sentences > Nonwords voxels across hemispheres. The resulting lateralization values range from 1 (exclusively left hemisphere activations) to −1 (exclusively right hemisphere activations).
All the extracted functional measures are available at OSF: https://osf.io/td7am/.
Statistical Analyses
To test whether high-level language-processing regions differ in their functional properties between polyglots and non-polyglots, we used GLMs and Bayesian linear regressions with Group (polyglots vs. non-polyglots) as a predictor of the Sentences > Nonwords effect sizes (in the LH and RH separately), region volumes (in the LH and RH separately), and lateralization. Bayes factor (BF10) statistics were calculated using the JASP software package (JASP Team 2019). We did not correct the results for the use of three neural measures. First, as noted above, effect sizes and region volumes are strongly correlated (e.g., Mahowald and Fedorenko 2016); as a result, we treated the region volume analyses as complementary to the effect size analyses, expecting them to mirror each other (which they did, at least at the network level). And second, the lateralization measure, albeit largely independent from the effect size/region volume measures, was used to evaluate a distinct hypothesis.
For each of the three measures, the GLM and Bayesian linear regression analyses described above were conducted across the language network. For the effect size and region volume measures, we further examined each of the 6 fROIs separately (correcting for the number of fROIs within each network), to test for potential differences among the regions. To examine the effects across the network, effect sizes were averaged across the regions within each network. Region volume measures were summed across the regions within each network and normalized by dividing the number of activated voxels by the total number of voxels in the network (i.e., 6794 voxels total for each of the LH and RH language networks).
To circumvent the issue of a relatively small sample of polyglots, we also assessed the probability that the polyglots (or the matched non-polyglots) were drawn from the same distribution as a relatively large population (n = 217) of non-polyglot individuals, for each neural measure. This was done via permutation tests, by randomly sampling (10 000 times) 17 data points from the large set of non-polyglots and comparing the observations from the polyglots (or the matched controls) to the distribution of these random samples. These analyses complement the critical analyses performed with the carefully pairwise-matched monolingual controls. (In a supplementary analysis aimed at comparing polyglots and bilinguals, we further divided the n = 217 sample into a set of 172 monolinguals and a set of 43 bilinguals (the remaining 2 of the 217 participants were trilingual, as noted above, and were excluded from this analysis). Using the set of monolinguals as the normative distribution, we then randomly sampled (10 000 times) 17 data points and 43 data points from this distribution and compared the observations from the polyglots and from the bilinguals to the distributions of these random samples.)
Next, to test whether nonlanguage brain networks differ in their functional properties between polyglots and non-polyglots, we used GLMs and Bayesian linear regressions with Group (polyglots vs. non-polyglots) as a predictor of (a) the Hard > Easy effect sizes and region volumes for the bilateral MD network, which supports executive functions (Duncan 2010), and (b) the Easy > Hard effect sizes and region volumes for the bilateral DMN network, which has been implicated in social cognition, recollection and prospection, and semantic processing (Buckner et al. 2008; Buckner and DiNicola 2019). All the analyses were parallel to those carried out on the LH and RH language networks above. For region volume normalization, the following values were used: 41 012 voxels total for the MD network and 15 070 voxels total for the DMN network. To compare the polyglots to the larger sample of non-polyglots, the same permutation tests were used as those described above.
To test whether the patterns of polyglot versus non-polyglot differences differed between the LH language network and the other networks examined, the key measures (effect sizes and region volumes for each relevant contrast) served as dependent variables in three linear mixed-effects models (performed using the lme4 package in R; Bates et al. 2014) which included a fixed effect for a Network (LH language vs. control, where control was the RH language network, the MD network, or the DMN) x Group (polyglots vs. non-polyglots) interaction and random intercepts for participants. Significance values were obtained using the likelihood ratio tests.
Results
The LH Language Network is Smaller and Less Active in Polyglots
The polyglots’ LH language network showed lower activation and was smaller in its extent compared with both the matched controls (t(32) = 3.67, P < 0.001, d = 1.36, BF10s = 70; t(32) = 4.04, P < 0.001, d = 1.57, BF10s = 294; Fig. 1; see Fig. 2 for sample individual language activation maps in polyglots vs. controls) and the larger sample of non-polyglots (Ps < 0.001; Fig. 1). The observed group differences were also reliable in most individual fROIs (Fig. 1, right panel): the polyglots showed weaker responses than the controls in the LAntTemp, LPostTemp, LIFG, and LMFG fROIs (ts(32) > 2.34, Ps < 0.03, ds > 0.86, FDR-corrected for the number of fROIs here and below; although note that in the Bayesian analyses, moderate or strong evidence for group differences in activation of the LH language network was found only for LAntTemp, LPostTemp, and LMFG fROIs, BF10s > 3.26), and smaller region volumes in the LAntTemp, LPostTemp, LMFG, and LIFGorb fROIs (ts(32) > 2.62, Ps < 0.02, ds > 0.88, BF10s > 3.62). Further, the polyglots (but neither the matched monolinguals nor the bilinguals) showed reliably weaker responses and smaller regions relative to the larger set of monolinguals (n = 172) (Ps < 0.03; SI Fig. 1, available at OSF: https://osf.io/td7am/). In fact, the bilinguals showed numerically stronger responses relative to the monolinguals, in line with the “bilingual neural signature” hypothesis (Kovelman et al. 2008); no similar trend was observed for the region volumes.
Figure 2.
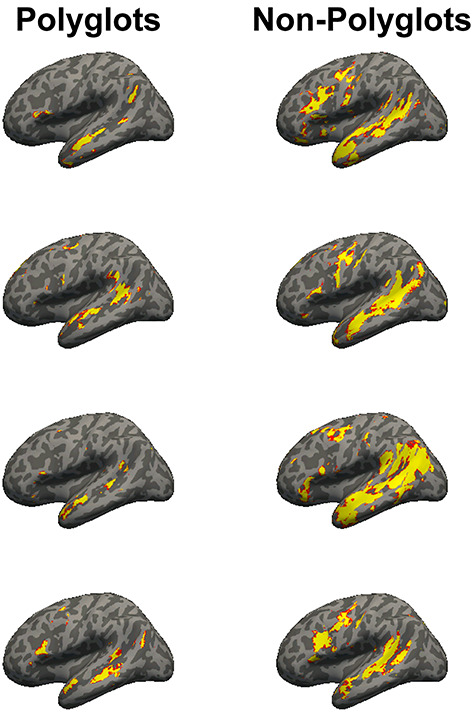
Whole-brain maps of language activity (at the whole-brain threshold of P < 0.001, uncorrected) in four polyglot: matched-control pairs (see SI Fig. 2, available at OSF: https://osf.io/td7am/, for the maps of the remaining pairs). Polyglots exhibited weaker and less extensive activity. (Note that the maps are binary: the red color at the edges of the activations simply reflects lower certainty in projecting volume-based activations onto a surface; all the analyses were performed in the volume, and surfaces are only used for visualization.)
Reduced Neural Activity in Polyglots is Restricted to the LH Language Network
We found no evidence that the strength or extent of activation in the RH language network differed between the polyglots and the matched controls (ts(32) < 1, Ps > 0.52, ds < 0.22, BF10s < 0.38; Fig. 3) or the larger sample of non-polyglots (Ps > 0.26). Similarly, we found no evidence of group differences in two control domain-general networks (Fig. 3): MD network (polyglots vs. matched controls: ts(30) < 0.20, Ps > 0.80, ds < 0.08, BF10s < 0.34; polyglots vs. a larger sample: Ps < 0.13) and DMN (polyglots vs. matched controls: ts(30) < 0.69, Ps > 0.49, ds < 0.26, BF10s < 0.40; polyglots vs. a larger sample: Ps < 0.25). Further, the effects observed in the LH language network differed reliably from those observed in each of the other three networks (RH language, MD, DMN), as evidenced by reliable Network x Group interactions (χ2s(1) > 4.11, Ps < 0.04).
In the presence of similar strength and extent of activation in the RH language network between the polyglots and controls, the weaker LH language activations led to a significant group difference in the degree of language lateralization, with the polyglots exhibiting less lateralized responses (t(32) = 2.51, P = 0.02, d = 0.85, BF10 = 3.34). This result was corroborated by the permutation test that found less lateralized responses in the polyglots compared with the larger sample of non-polyglots (P = 0.02).
Discussion
Much past research has focused on developmental and acquired impairments that affect the acquisition and/or processing of language (e.g., Bloom and Lahey 1978; Goodglass 1993; Gorno-Tempini et al. 2011; Schwartz 2017). Understanding how a cognitive system may malfunction is a powerful approach that has yielded core insights into the architecture of the human mind (e.g., Caramazza and Coltheart 2006). However, a complementary, and potentially equally powerful, approach is to probe the minds and brains of individuals who exhibit superior performance in the relevant domain as a result of aptitude/talent or extensive experience (e.g., Obler and Fein 1988; Winner 1996; Gladwell 2008; Russell et al. 2009; Barrett et al. 2013).
Linguistic aptitude/talent or expertise can manifest in many ways—in line with the multi-componential nature of language comprehension and production—from an exceptionally large vocabulary (e.g., avid readers), to fast and eloquent speech (e.g., orators), to the ability to quickly come up with rhymes (e.g., rappers) or find the precise word or phrase to express an idea (e.g., journalists or novelists), to the extraordinary spelling ability (e.g., Spelling Bee champions), to the ability to hear subtle distinctions in foreign speech or imitate foreign words. In the current study, we focused on knowledge of multiple foreign languages. Any of these abilities could have an innate predisposition, develop as a result of extensive experience and practice, or—perhaps most likely—result from the combination of the two. Understanding whether and how the minds and brains of linguistically gifted individuals or linguistic experts, including polyglots, differ from those of typical language users can inform critical issues in cognitive science and neuroscience, including innateness, the relationship between language and other domains, the limits of human cognition, and brain plasticity.
This work is the first to characterize the functional properties of the language network in the brains of polyglots—individuals with some knowledge of five or more languages. To illuminate the neural architecture of the language system of polyglots, we conducted a cross-sectional fMRI study (see Poldrack 2000, on the benefits and limitations of this approach) where we compared neural responses in the language network—and two control brain networks—of 17 polyglots (range of languages spoken: 5–55) with those of 17 carefully matched monolingual controls as well as a larger set of non-polyglots (n = 217). Four results emerged clearly, as elaborated below.
First, the polyglots appear to have a smaller language network. Compared with non-polyglot controls, polyglots recruited less extensive cortical areas within the fronto-temporal language network of the left hemisphere (reflected in smaller region volumes) and activated these areas to a lesser degree (reflected in smaller response magnitudes). These differences in the properties of the language network are in line with prior reports of functional changes in the brain in response to knowledge and skill acquisition (e.g., Poldrack et al. 1998; Fletcher et al. 1999; Gauthier et al. 1999; Schneider et al. 2002; McCandliss et al. 2003; Calvo-Merino et al. 2004; Kelly and Garavan 2005; Landau and d’Esposito 2006; Bavelier et al. 2012; Bernardi et al. 2013; Protzner et al. 2016), including in the domain of language (Reichle et al. 2000; Xue et al. 2006; Prat et al. 2007; Xue and Poldrack 2007; Prat and Just 2010; Grogan et al. 2012; Glezer et al. 2015). At the same time, our finding of decreased activity within the language network in polyglots stands in sharp contrast to the pattern of increased activity reported previously in bilinguals (e.g., Kovelman et al. 2008; Park et al. 2012; Jasinska and Petitto 2013; Román et al. 2015; Coderre et al. 2016) and observed as a numerical trend here (SI Fig. 1). This difference suggests that more efficient use of neural resources for language processing does not simply stem from knowledge of more than one language, but rather requires (a) experience with a larger number of languages, and/or (b) acquiring some of these languages after the critical period, and/or (c) special aptitude for language learning. Hence, polyglots form a special group of language users whose neurocognitive mechanisms for language processing differ from those of bilinguals.
Neural differences between any group of experts and controls are notoriously difficult to interpret, however, because cross-sectional designs fail to determine whether the observed differences result from the extensive experience/training or whether instead they initially spur some individuals to seek training in the relevant domain (e.g., Yarrow et al. 2009; Zatorre et al. 2012). Thus, reduced language activity in polyglots might reflect extensive linguistic experience: Language representation and processing may become more efficient as a result of acquiring multiple languages. This would parallel activation reduction in other domains, like motor learning (e.g., Poldrack et al. 1998; Fletcher et al. 1999; Kelly and Garavan 2005; Bernardi et al. 2013). Kelly and Garavan (2005) refer to this experientially induced reduction of neural activity as a “processing efficiency change.” However, it is also possible that individuals who eventually become polyglots represent and process language more efficiently from the start, even as they acquire their first (native) language. Without establishing a genetic basis for polyglotism (e.g., Graham and Fisher 2013), combined with longitudinal investigations of individuals as they acquire new languages (Osterhout et al. 2006), we cannot conclusively determine the causal direction of the observed group difference. However, exploring interindividual variability within the polyglot population may provide some clues. An increasing number of studies are examining effects of individual differences in language use on cognition and brain architecture (e.g., Abutalebi and Green 2016; Grundy et al. 2017; Pliatsikas 2020; Leivada et al. 2020), including in multilingual individuals (Rothman et al. 2019). As larger samples of polyglots accumulate, we can begin to probe the effects of the number of languages spoken and/or patterns of language use on cognitive and neural outcomes.
Second, the difference between the polyglots and controls was restricted to the language network in the dominant (left) hemisphere. Activations in the right hemisphere homologs of the language regions were similar between the two groups. The role of the right hemisphere language network in linguistic/cognitive processing is widely debated (e.g., Gazzaniga and Hillyard 1971; Jung-Beeman 2005; Lindell 2006; Vigneau et al. 2011). Our results do not inform this debate directly. However, the fact that the observed group differences were restricted to the left hemisphere adds to the evidence that the LH and RH language regions constitute complementary but distinct networks (e.g., Gotts et al. 2013; Chai et al. 2016) that can be differentially affected by linguistic experience, or possess distinct early, possibly genetically driven, biases.
Third, the polyglots exhibited a reduced degree of left lateralization for language compared with non-polyglots. Interestingly, reduced lateralization of linguistic function has been previously reported in numerous developmental disorders, including those characterized by language impairments, such as autism, specific language impairment, dyslexia, and schizophrenia (e.g., Herbert et al. 2002; Hale et al. 2005; Yuan et al. 2006; Wehner et al. 2007; De Guibert et al. 2011; Oertel-Knöchel and Linden 2011). Reduced language lateralization has also been argued to index lower linguistic ability in neurotypical individuals (e.g., Bishop 2013; Mellet et al. 2014). Our observation of reduced language lateralization in individuals with (at least one form of) superior linguistic abilities appears to contradict this idea. However, the underlying causes of lateralization reduction in these different populations are distinct. In linguistic disorders, reduced lateralization is due to the greater engagement of the right hemisphere (e.g., Takeuchi et al. 2004; Kleinhans et al. 2008; Tesink et al. 2009; Anderson et al. 2010; Jouravlev et al. 2020), whereas in the polyglots, it results from reduced activity in the left hemisphere. Thus, reduced lateralization can apparently characterize both ends of the linguistic performance/ability spectrum: linguistic impairments (in the presence of increased RH activity) and superior linguistic abilities/extensive linguistic expertise (in the presence of reduced LH activity).
Finally, we found no evidence that polyglotism has a widespread effect across the brain. The strength and extent of activation were similar between the polyglots and controls in two domain-general brain networks linked to high-level cognition, including some aspects of language/meaning/communication—the MD network, which supports executive functions (Duncan 2010), and the DMN, which supports internally directed cognition (Buckner et al. 2008; Buckner and DiNicola 2019). This result argues against ubiquitous between-group differences in information processing and is in line with prior work that has suggested that the language network is functionally distinct from other high-level large-scale networks (e.g., Fedorenko et al. 2011; Monti et al. 2012; Blank et al. 2014; Fedorenko and Varley 2016; Mineroff et al. 2018; Fedorenko and Blank 2020). One limitation of the current study is that we did not use a theory of mind localizer (e.g., Saxe and Kanwisher 2003) to identify the brain regions that support social cognition/mentalizing. Although the DMN has sometimes been discussed as encompassing the social cognition network or at least overlapping with it (e.g., Mars et al. 2012; Spreng and Andrews-Hanna 2015), recent work has clearly established that the social cognition network is robustly separable from—albeit closely juxtaposed to—the network that supports episodic cognition and is strongly linked to the hippocampus (Braga and Buckner 2017; DiNicola et al. 2020). As a result, the question of whether polyglotism may affect the neural network that supports social reasoning deserves further investigation.
To conclude, compared with matched monolingual controls, as well as a larger population of non-polyglot participants, the polyglots have smaller language regions that respond less strongly during native language processing. This difference is restricted to the left hemisphere language network and may reflect more efficient processing in polyglots (Kelly and Garavan 2005; Poldrack et al. 1998; Fletcher et al. 1999). However, uncovering the nature of this difference—innate/early-emerging versus driven by the experience of acquiring multiple languages—will require longitudinal studies and studies that probe the genetic basis of polyglotism.
Funding
E.F. was supported by National Institutes of Health awards R00-HD057522, R01-DC016607, and R01-DC016950.
Notes
We thank (i) EvLab members for help with fMRI data collection and helpful comments; (ii) Ted Gibson, Nancy Kanwisher, Simon Fisher, Michael Erard, Esti Blanco-Elorrieta, Rachel Ryskin, and Saima Malik Moraleda for helpful discussions and/or comments on the manuscript; (iii) Hannah Small and Evgeniia Diachek for help with the figures and the OSF page; (iv) all our polyglots and other participants; (v) Judith Thurman, Simon Calder, Patrick Cox, Yvonne Stapp, and Christian Saunders for fun and helpful conversations about this and related work; and (vi) Marc Brysbaert and three anonymous reviewers for constructive comments that have helped improve the manuscript. The authors would also like to acknowledge the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT and the support team (Steven Shannon and Atsushi Takahashi). Conflict of Interest: The authors declare no competing financial interests.
Contributor Information
Olessia Jouravlev, Brain & Cognitive Sciences Department, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Cognitive Science, Carleton University, Ottawa, ON K1S5B6, Canada.
Zachary Mineroff, Brain & Cognitive Sciences Department, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Idan A Blank, Brain & Cognitive Sciences Department, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Psychology, University of California Los Angeles, Los Angeles, CA 90095, USA.
Evelina Fedorenko, Brain & Cognitive Sciences Department, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
References
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A. 2012. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex. 22(9):2076–2086. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green DW. 2016. Neuroimaging of language control in bilinguals: neural adaptation and reserve. Biling: Lang Cogn. 19(4):689–698. [Google Scholar]
- Adesope OO, Lavin T, Thompson T, Ungerleider C. 2010. A systematic review and meta-analysis of the cognitive correlates of bilingualism. Rev Educ Res. 80(2):207–245. [Google Scholar]
- Amunts K, Schleicher A, Zilles K. 2004. Outstanding language competence and cytoarchitecture in Broca’s speech region. Brain Lang. 89(2):346–353. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, Alexander AL, Bigler ED, Lainhart JE. 2010. Decreased left posterior insular activity during auditory language in autism. Am J Neuroradiol. 31(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Blank I, Mineroff Z, Ademoglu A, Fedorenko E. 2020. Multiple demand (MD) system’s activity predicts individual differences in working memory and fluid intelligence. Cortex. doi: 10.1016/j.cortex.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Jones SP. 1998. Encyclopedia of bilingualism and bilingual education multilingual matters. Bristol: Multilingual Matters.
- Barrett KC, Ashley R, Strait DL, Kraus N. 2013. Art and science: how musical training shapes the brain. Front Psychol. 4:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S.. 2014. lme4: linear mixed-effects models using eigen and S4.
- Bautista A, Wilson SM. 2016. Neural responses to grammatically and lexically degraded speech. Lang Cognit Neurosci. 31(4):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Achtman RL, Mani M, Föcker J. 2012. Neural bases of selective attention in action video game players. Vision Res. 61:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G, Ricciardi E, Sani L, Gaglianese A, Papasogli A, Ceccarelli R, Franzoni F, Galetta F, Santoro G, Goebel R et al. . 2013. How skill expertise shapes the brain functional architecture: an fMRI study of visuo-spatial and motor processing in professional racing-car and naïve drivers. PloS one. 8(10):e77764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedroń A, Pawlak M. 2016. New conceptualizations of linguistic giftedness. Lang Teach. 49(2):151–185. [Google Scholar]
- Bialystok E. 2009. Bilingualism: the good, the bad, and the indifferent. Biling: Lang Cogn. 12(1):3–11. [Google Scholar]
- Bialystok E, Craik FI, Green DW, Gollan TH. 2009. Bilingual minds. Psychol Sci Public Interest. 10(3):89–129. [DOI] [PubMed] [Google Scholar]
- Bishop DV. 2013. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 340(6138):1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsong D. 2005. Interpreting age effects in second language acquisition. Handbook of bilingualism: psycholinguistic approaches. Oxford: Oxford University Press, pp. 109–127.
- Blanco-Elorrieta E, Pylkkänen L. 2017. Bilingual language switching in the laboratory versus in the wild: the spatiotemporal dynamics of adaptive language control. J Neurosci. 37(37):9022–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, Pylkkänen L. 2018. Ecological validity in bilingualism research and the bilingual advantage. Trends Cognit Sci. 22(12):1117–1126. [DOI] [PubMed] [Google Scholar]
- Blank I, Kanwisher N, Fedorenko E. 2014. A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J Neurophysiol. 112(5):1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom L, Lahey M.. 1978. Language development and language disorders. New Jersey: John Wiley & Sons.
- Braga RM, Buckner RL. 2017. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 95(2):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M. 2019. How many participants do we have to include in properly powered experiments? A tutorial of power analysis with reference tables. J Cognit. 2(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network. Ann NY Acad Sci. 1124(1):1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM. 2019. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 20(10):593–608. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. 2004. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex. 15(8):1243–1249. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Coltheart M. 2006. Cognitive neuropsychology twenty years on. Cognit Neuropsych. 23(1):3–12. [DOI] [PubMed] [Google Scholar]
- Chai LR, Mattar MG, Blank IA, Fedorenko E, Bassett DS. 2016. Functional network dynamics of the language system. Cereb Cortex. 26(11):4148–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre EL, Smith JF, Van Heuven WJ, Horwitz B. 2016. The functional overlap of executive control and language processing in bilinguals. Biling: Lang Cognit. 19(3):471–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Bak TH, Allerhand M, Redmond P, Starr JM, Deary IJ, MacPherson SE. 2016. Bilingualism, social cognition and executive functions: a tale of chickens and eggs. Neuropsychol. 91:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren S, Almén H, Dahlgren Sandberg A. 2017. Theory of mind and executive functions in young bilingual children. J Gen Psych. 178(5):303–307. [DOI] [PubMed] [Google Scholar]
- De Guibert C, Maumet C, Jannin P, Ferré JC, Tréguier C, Barillot C, Le Rumeur E, Allaire C, Biraben A. 2011. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain. 134(10):3044–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser R, Larson-Hall J. 2005. What does the critical period really mean? Handbook of bilingualism: psycholinguistic approaches. Oxford: Oxford University Press, 88:108. [Google Scholar]
- Diachek E, Blank I, Siegelman M, Fedorenko E. 2020. The domain-general multiple demand (MD) network does not support core aspects of language comprehension: a large-scale fMRI investigation. J Neurosci. 40(23):4536–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicola LM, Braga RM, Buckner RL. 2020. Parallel distributed networks dissociate episodic and social functions within the individual. J Neurophys. 123(3):1144–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly S, Brooks PJ, Homer BD. 2019. Is there a bilingual advantage on interference-control tasks? A multiverse meta-analysis of global reaction time and interference cost. Psychon Bull Rev. 26(4):1122–1147. [DOI] [PubMed] [Google Scholar]
- Doner T. 2014. Breaking the language barrier. TEDxTeen Talk. [Video File].Retrieved from https://youtu.be/xNmf-G81Irs. [Google Scholar]
- Dufour N, Redcay E, Young L, Mavros PL, Moran JM, Triantafyllou C, Gabrieli JDE, Saxe R. 2013. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PloS One. 8(9):e75468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñabeitia JA, Hernández JA, Antón E, Macizo P, Estévez A, Fuentes LJ, Carreiras M. 2014. The inhibitory advantage in bilingual children revisited. Exp Psychol. 61:234–251. [DOI] [PubMed] [Google Scholar]
- Duncan J. 2010. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cognit Sci. 14(4):172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J. 2013. The structure of cognition: attentional episodes in mind and brain. Neuron. 80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard M. 2005. The gift of the gab. New Scientist. 8:40–43. [Google Scholar]
- Erard M. 2012. Babel no more: the search for the world's most extraordinary language learners. New York: Simon and Schuster. [Google Scholar]
- Fedorenko E. 2014. The role of domain-general cognitive control in language comprehension. Front Psychol. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. 2011. Functional specificity for high-level linguistic processing in the human brain. PNAS. 108(39):16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Blank IA. 2020. Broca’s area is not a natural kind. Trends Cognit Sci. 24(4):270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. 2012. Language-selective and domain-general regions lie side by side within Broca’s area. Curr Biol. 22(21):2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Nieto-Castañon A, Kanwisher N. 2012. Lexical and syntactic representations in the brain: an fMRI investigation with multi-voxel pattern analyses. Neuropsych. 50(4):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. 2013. Broad domain generality in focal regions of frontal and parietal cortex. PNAS. 110(41):16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh PJ, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. 2010. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol. 104(2):1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Mineroff Z, Siegelman M, Blank I. 2020. Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition. 203:104348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Varley R. 2016. Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Ann NY Acad Sci. 1369(1):132–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P, Büchel C, Josephs O, Friston K, Dolan R. 1999. Learning-related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cereb Cortex. 9(2):168–178. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. 1999. Activation of the middle fusiform face area increases with expertise in recognizing novel objects. Nat Neurosci. 2(6):568. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Hillyard SA. 1971. Language and speech capacity of the right hemisphere. Neuropsychol. 9(3):273–280. [DOI] [PubMed] [Google Scholar]
- Gladwell M. 2008. Outliers: the story of success. Boston: Little, Brown and Company. [Google Scholar]
- Glezer LS, Kim J, Rule J, Jiang X, Riesenhuber M. 2015. Adding words to the brain's visual dictionary: novel word learning selectively sharpens orthographic representations in the VWFA. J Neurosci. 35(12):4965–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz PJ. 2003. The effects of bilingualism on theory of mind development. Biling: Lang Cognit. 6(1):1–15. [Google Scholar]
- Goodglass H. 1993. Understanding aphasia. Cambridge, MA: Academic Press. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SEEA, Ogar JM, Rohrer JD, Black S, Boeve BF. 2011. Classification of primary progressive aphasia and its variants. Neurol. 76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A. 2013. Two distinct forms of functional lateralization in the human brain. PNAS. 110(36):E3435–E3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SA, Fisher SE. 2013. Decoding the genetics of speech and language. Curr Opin Neurobiol. 23(1):43–51. [DOI] [PubMed] [Google Scholar]
- Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, Brysbaert M. 2013. A surface-based analysis of language lateralization and cortical asymmetry. J Cog Neurosci. 25(9):1477–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan A, Jones ŌP, Ali N, Crinion J, Orabona S, Mechias ML, Ramsden S, Green DW, Price CJ. 2012. Structural correlates for lexical efficiency and number of languages in non-native speakers of English. Neuropsychol. 50(7):1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy JG, Anderson JA, Bialystok E. 2017. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 1396(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Zaidel E, McGough JJ, Phillips JM, McCracken JT. 2006. Atypical brain laterality in adults with ADHD during dichotic listening for emotional intonation and words. Neuropsych. 44(6):896–904. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK, Tenenbaum JB, Pinker S. 2018. A critical period for second language acquisition: evidence from 2/3 million English speakers. Cognit. 177:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J et al. . 2002. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 52(5):588–596. [DOI] [PubMed] [Google Scholar]
- Hernandez AE. 2009. Language switching in the bilingual brain: what’s next? Brain Lang. 109(2–3):133–140. [DOI] [PubMed] [Google Scholar]
- Hervais-Adelman A, Egorova N, Golestani N. 2018. Beyond bilingualism: multilingual experience correlates with caudate volume. Brain Struct Funct. 223(7):3495–3502. [DOI] [PubMed] [Google Scholar]
- Hilchey MD, Klein RM. 2011. Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psych Bul Rev. 18(4):625–658. [DOI] [PubMed] [Google Scholar]
- Hudson R. 2016. The limits of multilingualism. Retrieved fromhttp://dickhudson.com/hyperpolyglots.
- Hyltenstam K, Abrahamsson N. 2003. Maturational constraints in SLA. The handbook of second language acquisition, New Jersey: John Wiley & Sons, pp. 538–588.
- Hyltenstam K. 2016. Advanced proficiency and exceptional ability in second languages. Vol 51 Walter de Gruyter GmbH & Co KG. [Google Scholar]
- Hyltenstam K. 2018. Polyglotism: a synergy of abilities and predisposition. High-level language proficiency in second language and multilingual contexts, Cambridge: Cambridge University Press, pp. 170–196.
- Jasinska KK, Petitto LA. 2013. How age of bilingual exposure can change the neural systems for language in the developing brain: a functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev Cognit Neurosci. 6:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. 1989. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognit Psych. 21(1):60–99. [DOI] [PubMed] [Google Scholar]
- JASP Team 2019. JASP (Version 0.10.1) [Computer software].
- Jouravlev O, Kell A, Mineroff Z, Haskins AJ, Kanwisher N, Fedorenko E. 2020. Reduced language lateralization is a robust marker of the broader autism phenotype. https://www.biorxiv.org/content/10.1101/2020.02.10.942698v1. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. 2005. Bilateral brain processes for comprehending natural language. Trends Cognit Sci. 9(11):512–518. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 2004. Kaufman brief intelligence test. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Keller TA, Carpenter PA, Just MA. 2001. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 11(3):223–237. [DOI] [PubMed] [Google Scholar]
- Kelly AC, Garavan H. 2005. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 15(8):1089–1102. [DOI] [PubMed] [Google Scholar]
- Kepinska O, Rover M, Caspers J, Schiller NO. 2017a. On neural correlates of individual differences in novel grammar learning: an fMRI study. Neuropsychol. 98:156–168. [DOI] [PubMed] [Google Scholar]
- Kepinska O, Rover M, Caspers J, Schiller NO. 2017b. Whole-brain functional connectivity during acquisition of novel grammar: distinct functional networks depend on language learning abilities. Behav Brain Res. 320:333–346. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Müller RA, Cohen DN, Courchesne E. 2008. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 1221:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács ÁM. 2009. Early bilingualism enhances mechanisms of false-belief reasoning. Dev Sci. 12(1):48–54. [DOI] [PubMed] [Google Scholar]
- Kovelman I, Baker SA, Petitto LA. 2008. Bilingual and monolingual brains compared: a functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualism. J Cognit Neurosci. 20(1):153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I, Shalinsky MH, Berens MS, Petitto LA. 2008. Shining new light on the brain's “bilingual signature”: a functional near infrared spectroscopy investigation of semantic processing. Neuroimage. 39(3):1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashen S, Kiss N. 1996. Notes on a polyglot: Kato Lomb. System. 24(2):207–210. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 12(5):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Bialystok E. 2013. Understanding the consequences of bilingualism for language processing and cognition. J Cog Psych. 25(5):497–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeminska M. 2016. The cult of the polyglot. Retrieved fromhttps://latg.org/2016/07/19/the-cult-polyglot#.XQoudy0ZM8Y.
- Kyuchukov H, De Villiers J. 2009. Theory of mind and evidentiality in Romani-Bulgarian bilingual children. Psych Lang Commun. 13(2):21–34. [Google Scholar]
- Landau SM, d’Esposito M. 2006. Sequence learning in pianists and nonpianists: an fMRI study of motor expertise. Cognit Affect Behav Neurosci. 6(3):246–259. [DOI] [PubMed] [Google Scholar]
- Leivada E, Westergaard M, Duñabeitia JA, Rothman J. 2020. On the phantom-like appearance of bilingualism effects on neurocognition: (How) should we proceed? Biling: Lang Cognit. 1–14. doi: 10.1017/S1366728920000358. [DOI]
- Leland J. 2012. Adventures of a Teenage Polyglot. The New York Times; March 9 Retrieved fromhttps://www.nytimes.com/2012/03/11/nyregion/a-teenage-master-of-languages-finds-online-fellowship.html. [Google Scholar]
- Lenneberg EH. 1967. The biological foundations of language. Hosp Pract. 2(12):59–67. [Google Scholar]
- Li P, Legault J, Litcofsky KA. 2014. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex. 58:301–324. [DOI] [PubMed] [Google Scholar]
- Lindell AK. 2006. In your right mind: right hemisphere contributions to language processing and production. Neuropsych Rev. 16(3):131–148. [DOI] [PubMed] [Google Scholar]
- Machova L. 2018. The secrets of learning a new language. TED talk. [Video file] Retrieved from https://www.ted.com/talks/lydia_machova_the_secrets_of_learning_a_new_language. [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. 1997. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 17(18):7103–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N. 2003. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 13(2):250–259. [DOI] [PubMed] [Google Scholar]
- Mahowald K, Fedorenko E. 2016. Reliable individual-level neural markers of high-level language processing: a necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage. 139:74–93. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. 2012. On the relationship between the “default mode network” and the “social brain”. Front Human Neurosci. 6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. 2003. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cognit Sci. 7(7):293–299. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. 2004. Structural plasticity in the bilingual brain. Nature. 431(7010):757–757. [DOI] [PubMed] [Google Scholar]
- Mellet E, Zago L, Jobard G, Crivello F, Petit L, Joliot M, Mazoyer B, Tzourio-Mazoyer N. 2014. Weak language lateralization affects both verbal and spatial skills: an fMRI study in 297 subjects. Neuropsychol. 65:56–62. [DOI] [PubMed] [Google Scholar]
- Mineroff Z, Blank IA, Mahowald K, Fedorenko E. 2018. A robust dissociation among the language, multiple demand, and default mode networks: evidence from inter-region correlations in effect size. Neuropsychol. 119:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, Osherson DN. 2012. Thought beyond language: neural dissociation of algebra and natural language. Psych Sci. 23(8):914–922. [DOI] [PubMed] [Google Scholar]
- Nichols ES, Wild CJ, Stojanoski B, Battista ME, Owen AM. 2020. Bilingualism affords no general cognitive advantages: a population study of executive function in 11,000 people. Psych Sci. 0956797620903113. [DOI] [PubMed] [Google Scholar]
- Novoa L, Fein D, Obler LK. 1988. Talent in foreign languages: a case study. The exceptional brain: neuropsychology of talent and special abilities, New York: Guilford Press, pp. 294–302.
- Obler LK, Fein DE. 1988. The exceptional brain: neuropsychology of talent and special abilities. New York: Guilford Press. [Google Scholar]
- Oertel-Knöchel V, Linden DEJ. 2011. Cerebral asymmetry in schizophrenia. Neuroscientist. 17(5):456–467. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. 2004. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 7(1):75. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychol. 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Osterhout L, McLaughlin J, Pitkänen I, Frenck-Mestre C, Molinaro N. 2006. Novice learners, longitudinal designs, and event-related potentials: a means for exploring the neurocognition of second language processing. Lang Learn. 56(s1):199–230. [Google Scholar]
- Paap KR, Greenberg ZI. 2013. There is no coherent evidence for a bilingual advantage in executive processing. Cognit Psych. 66(2):232–258. [DOI] [PubMed] [Google Scholar]
- Paap KR, Johnson HA, Sawi O. 2015. Bilingual advantages in executive functioning either do not exist or are restricted to very specific and undetermined circumstances. Cortex. 69:265–278. [DOI] [PubMed] [Google Scholar]
- Palomar-García MÁ, Bueichekú E, Ávila C, Sanjuán A, Strijkers K, Ventura-Campos N, Costa A. 2015. Do bilinguals show neural differences with monolinguals when processing their native language? Brain Lang. 142:36–44. [DOI] [PubMed] [Google Scholar]
- Park HR, Badzakova-Trajkov G, Waldie KE. 2012. Language lateralisation in late proficient bilinguals: a lexical decision fMRI study. Neuropsych. 50(5):688–695. [DOI] [PubMed] [Google Scholar]
- Parker Jones Ō, Green DW, Grogan A, Pliatsikas C, Filippopolitis K, Ali N, Lee HL, Ramsden S, Gazarian K, Prejawa S et al. . 2012. Where, when and why brain activation differs for bilinguals and monolinguals during picture naming and reading aloud. Cereb Cortex. 22(4):892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas C. 2020. Understanding structural plasticity in the bilingual brain: the dynamic restructuring model. Biling: Lang Cognit. 23(2):459–471. [Google Scholar]
- Pliatsikas C, Luk G. 2016. Executive control in bilinguals: a concise review on fMRI studies. Biling: Lang Cognit. 19(4):699–705. [Google Scholar]
- Pinker S. 2009. Language learnability and language development, with new commentary by the author. Vol 7 Cambridge, Massachusetts: Harvard University Press. [Google Scholar]
- Poldrack RA. 2000. Imaging brain plasticity: conceptual and methodological issues—a theoretical review. Neuroimage. 12(1):1–13. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, Yarkoni T. 2017. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 18(2):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JD. 1998. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb Cortex. 8(1):1–10. [DOI] [PubMed] [Google Scholar]
- Prat CS, Just MA. 2010. Exploring the neural dynamics underpinning individual differences in sentence comprehension. Cereb Cortex. 21(8):1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat CS, Keller TA, Just MA. 2007. Individual differences in sentence comprehension: a functional magnetic resonance imaging investigation of syntactic and lexical processing demands. J Cognit Neurosci. 19(12):1950–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner AB, Hargreaves IS, Campbell JA, Myers-Stewart K, Hees S, Goodyear BG, Sargious P, Pexman PM. 2016. This is your brain on scrabble: neural correlates of visual word recognition in competitive scrabble players as measured during task and resting-state. Cortex. 75:204–219. [DOI] [PubMed] [Google Scholar]
- Qi Z, Han M, Wang Y, Angeles C, Liu Q, Garel K, Ee SC, Whitfield-Gabrieli S, Gabrieli JDE, Perrachione TK. 2019. Speech processing and plasticity in the right hemisphere predict variation in adult foreign language learning. NeuroImage. 192:76–87. [DOI] [PubMed] [Google Scholar]
- Qi Z, Legault J. 2020. Neural hemispheric organization in successful adult language learning: is left always right? Psych Learn Motivation. 72:119. [Google Scholar]
- Ramus F, Altarelli I, Jednorog K, Zhao J, Di Covella LS. 2018. Neuroanatomy of developmental dyslexia: pitfalls and promise. Neurosci Biobehav Rev. 84:434–452. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Carpenter PA, Just MA. 2000. The neural bases of strategy and skill in sentence–picture verification. Cognit Psych. 40(4):261–295. [DOI] [PubMed] [Google Scholar]
- Reiterer S, Pereda E, Bhattacharya J. 2009. Measuring second language proficiency with EEG synchronization: how functional cortical networks and hemispheric involvement differ as a function of proficiency level in second language speakers. Second Lang Res. 25(1):77–106. [Google Scholar]
- Rodriguez-Fornells A, Rotte M, Heinze HJ, Nösselt T, Münte TF. 2002. Brain potential and functional MRI evidence for how to handle two languages with one brain. Nature. 415(6875):1026–1029. [DOI] [PubMed] [Google Scholar]
- Román P, González J, Ventura-Campos N, Rodríguez-Pujadas A, Sanjuán A, Ávila C. 2015. Neural differences between monolinguals and early bilinguals in their native language during comprehension. Brain Lang. 150:80–89. [DOI] [PubMed] [Google Scholar]
- Rothman J, Alonso JG, Puig-Mayenco E. 2019. Third language acquisition and linguistic transfer (Vol. 163). Cambridge: Cambridge University Press. [Google Scholar]
- Rubio-Fernández P, Glucksberg S. 2012. Reasoning about other people's beliefs: bilinguals have an advantage. J Exp Psych: LMC. 38(1):211. [DOI] [PubMed] [Google Scholar]
- Russell R, Duchaine B, Nakayama K. 2009. Super-recognizers: people with extraordinary face recognition ability. Psychon Bull Rev. 16(2):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R, Yue X, Nakayama K, Tootell RB. 2010. Neural differences between developmental prosopagnosics and super-recognizers. J Vision. 10(7):582–582. [Google Scholar]
- Ryskin RA, Brown-Schmidt S, Canseco-Gonzalez E, Yiu L, Nguyen E. 2014. Visuospatial perspective-taking in conversation and the role of bilingual experience. J Memory Lang. 74:46–76. [Google Scholar]
- Saxe R, Kanwisher N. 2003. People thinking about thinking people: the role of the temporo-parietal junction in ``theory of mind''. Neuroimage. 19(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Constable RT. 2009. Variation in language networks in monolingual and bilingual English speakers: consequences for language mapping for surgical preplanning. J Clin Exp Neuropsych. 31(8):945–954. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A. 2002. Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci. 5(7):688. [DOI] [PubMed] [Google Scholar]
- Schwartz RG. 2017. Handbook of child language disorders. New York: Psychology Press. [Google Scholar]
- Scott TL, Gallée J, Fedorenko E. 2017. A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cognit Neurosci. 8(3):167–176. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Ramsey NF, Kahn RS. 2001. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 52(1):57–67. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Andrews-Hanna JR. 2015. The default network and social cognition. Brain mapping: an encyclopedic reference. 1316:165–169. [Google Scholar]
- Takeuchi M, Harada M, Matsuzaki K, Nishitani H, Mori K. 2004. Difference of signal change by a language task on autistic patients using functional MRI. J Med Invest. 51(1–2):59–62. [DOI] [PubMed] [Google Scholar]
- Tesink CM, Buitelaar JK, Petersson KM, Van der Gaag RJ, Kan CC, Tendolkar I, Hagoort P. 2009. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 132(7):1941–1952. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. 2000. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 44(3):457–465. [DOI] [PubMed] [Google Scholar]
- THNKR 2013. Teen speaks over 20 languages [Video File]. Retrieved fromhttps://www.youtube.com/watch?v=Km9-DiFaxpU.
- Thurman J. 2018. The mystery of people who speak dozens of languages. September 3. The New Yorker; Retrieved fromhttps://www.newyorker.com/magazine/2018/09/03/the-mystery-of-people-who-speak-dozens-of-languages. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15(1):273–289. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Jobard G, Petit L, Crivello F, Emmanuel Mellet, Zago Laure, Mazoyer B, Tzourio-Mazoyer N. 2011. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing?: insights from a meta-analysis. Neuroimage. 54(1): 577–593. [DOI] [PubMed] [Google Scholar]
- von Bastian CC, Souza AS, Gade M. 2016. No evidence for bilingual cognitive advantages: A test of four hypotheses. J Exp Psych Gen. 145(2): 246. [DOI] [PubMed] [Google Scholar]
- Wehner DT, Ahlfors SP, Mody M. 2007. Effects of phonological contrast on auditory word discrimination in children with and without reading disability: a magnetoencephalography (MEG) study. Neuropsych. 45(14):3251–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner E. 1996. Gifted children. Vol 1 New York: Basic Books. [Google Scholar]
- Woolgar A, Duncan J, Manes F, Fedorenko E. 2018. Fluid intelligence is supported by the multiple-demand system not the language system. Nat Hum Behav. 2(3):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. 2006. Language experience shapes fusiform activation when processing a logographic artificial language: an fMRI training study. Neuroimage. 31(3):1315–1326. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. 2007. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. J Cogn Neurosci. 19(10):1643–1655. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Brown P, Krakauer JW. 2009. Inside the brain of an elite athlete: the neural processes that support high achievement in sports. Nat Rev Neurosci. 10(8):585. [DOI] [PubMed] [Google Scholar]
- Yuan W, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, Strawsburg RH, Holland SK. 2006. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 47(3):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. 2012. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 15(4):528. [DOI] [PMC free article] [PubMed] [Google Scholar]


