Abstract
Background:
Emergent linkages between musculoskeletal injury and the nervous system have increased interest to evaluate brain activity during functional movements associated with injury risk. Functional magnetic resonance imaging (fMRI) is a sophisticated modality that can be used to study brain activity during functional sensorimotor control tasks. However, technical limitations have precluded the precise quantification of lower-extremity joint kinematics during active brain scanning. The purpose of this study was to determine the validity of a new, MRI-compatible motion tracking system relative to a traditional multi-camera 3D motion capture system for measuring lower extremity joint kinematics.
Methods:
Fifteen subjects (9 females, 6 males) performed knee flexion-extension and leg press movements against guided resistance while laying supine. Motion tracking data were collected simultaneously using the MRI-compatible and traditional multi-camera 3D motion systems. Participants’ sagittal and frontal plane knee angles were calculated from data acquired by both multi-camera systems. Resultant range of angular movement in both measurement planes were compared between both systems. Instrument agreement was assessed using Bland-Altman plots and intraclass correlation coefficients (ICC).
Results:
The system demonstrated excellent validity in the sagittal plane (ICCs>0.99) and good to excellent validity in the frontal plane (0.84 < ICCs < 0.92). Mean differences between corresponding range of angular movement measurements ranged from 0.186 ° to 0.295 °.
Conclusions:
The present data indicate that this new, MRI-compatible system is valid for measuring lower extremity movements when compared to the gold standard 3D motion analysis system. As there is growing interest regarding the neural substrates of lower extremity movement, particularly in relation to injury and pathology, this system can now be integrated into neuroimaging paradigms to investigate movement biomechanics and its relation to brain activity.
Level of Evidence:
3
Keywords: Biomechanics, fMRI, lower extremity, motion analysis, movement system
BACKGROUND
Functional magnetic resonance imaging (fMRI) is one of the most commonly utilized methods for studying brain activity, particularly as a measurement tool to identify activity of brain regions important for motor control. Historically, fMRI was constrained to studying small scale upper extremity motor tasks (due to technological limitations and associated head motion artifact), but recent advancements have permitted fMRI to be used with lower extremity motor tasks, including leg flexion/extension movements,1-5 leg press exercises against resistance,1,6 stepping tasks,7,8 and cycling.9 Though these studies have provided foundational knowledge of brain mechanisms driving lower extremity motor control, associated movement deficits are not simultaneously quantified as traditional motion capture systems contain ferromagnetic materials unsafe for MRI (i.e., as they become kinetic projectiles created through magnetic forces) and produce data artifacts.
Typically, lower-extremity fMRI motor task paradigms rely on external cues to ‘standardize’ movements, (e.g., metronome, voice, or visual cues),1-5 and with a few exceptions,10,11 do not objectively quantify the subjects’ movements or verify task compliance via visual confirmation. Obtaining objective measures of performance—such as joint angle kinematics and range of motion—simultaneous with brain activation for within and between sessions in longitudinal trials is of particular concern to characterize intervention and pathology effects related to lower extremity movements (e.g., cerebrovascular accidents,12 anterior cruciate ligament reconstruction (ACLR),13 Parkinson's,14 Huntington's15). Compared to controls, differences in lower-extremity movement patterns associated with injury and/or pathology are well documented outside the constraints of the fMRI (i.e., in traditional motion capture laboratories)13,16-23 For example, patients who undergo ACLR—and even for those who are healthy but later go on to ACL injury—exhibit notably different lower extremity kinematics during loading tasks like the drop vertical jumps (DVJ) or leg press.13,16-18 Emergent evidence using fMRI further indicates that patients with ACLR also demonstrate increased visuomotor-related brain activity compared to non-injured controls during knee flexion and extension movements (theorized to occur because of patients’ enhanced visual reliance for movement).3 Though considerable pre-testing practice combined with a relatively simple movement (a single joint movement guided by a metronome-paced auditory cue) likely minimized any within or between group kinematic differences, patients and controls each completed these knee movements ∼72 times with the assumption that movement characteristics were equivalent within trial blocks and between the two groups. The well-documented movement differences known to be present between these groups (ACLR vs. healthy controls measured via traditional laboratories) suggests there may still have been some differential movement (e.g., subtle differences in total range of motion), which may have partially contributed to the reported differences in brain activation. Developing a system to precisely quantify movements during fMRI would supplement such paradigms by providing a critical covariate for the neuroimaging analyses. Further, if notable movement differences are observed even with standardization and pre-testing practice (possibly more likely during complex, multi joint fMRI paradigms), such a system could also provide online kinematic biofeedback to guide and/or correct movement in real-time – akin to systems successfully developed outside the constraints of MRI.24-28
Although ‘MRI-safe’ multi-camera motion capture systems have been used successfully,29-31 these systems occupy significant infrastructural resources within the constraints of an MRI room and require considerable calibration and setup. A single camera MRI compatible motion tracking system (High Field Moiré phase tracking by Metria Innovation Inc. [Milwaukee, WI]) has been successfully used for prospective head motion correction during fMRI. In brief, this system consists of an in-bore camera unit (with VGA resolution) tracking a single32-34 or double35 patterned and reflective marker, placed on the patient's head. A similar system, shown in Figure 1, was custom developed for quantifying lower extremity movement during fMRI capable of tracking a maximum of up to four markers.* A previous study tested the reliability of a similar retro-grate reflector (RGR) based motion capture system with a 1.3 megapixel sensor, by measuring orientations during a land and cut task with two markers aligned with the sagittal plane of the leg.36 Results of this study demonstrated that while tracking knee movement, the RGR system was within 0.2 ° (sagittal plane) and 0.5 ° (frontal plane) of a standard multicamera motion capture system. However, the camera hardware used in this previous study was limited to a single knee with the camera axis perpendicular to the sagittal plane of the body. Tracking bilateral knee movements in the fMRI would require the camera axis to be oriented perpendicular to the frontal plane of the body to track both thighs and shanks.
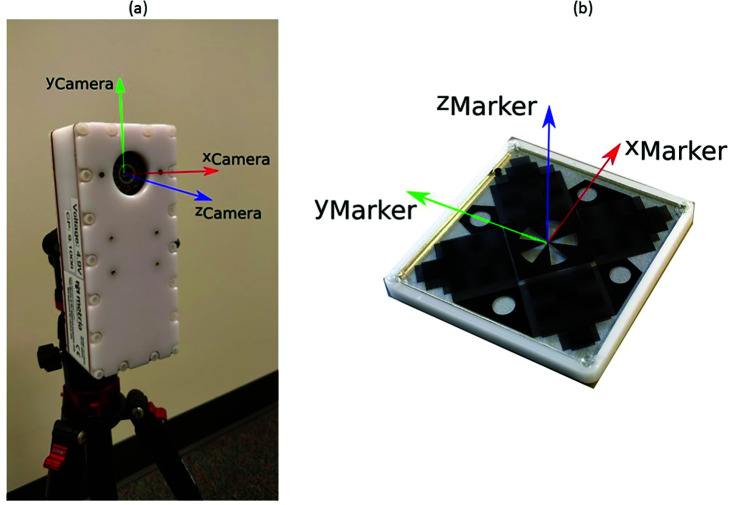
Figure 1.
(a) Camera unit with camera axis overlaid. (b) Marker for the MRI compatible motion capture system, overlaid with its axes.
The purpose of this study was to determine the validity of a new, MRI-compatible motion tracking system relative to a traditional multi-camera 3D motion capture system for measuring lower extremity joint kinematics. Three lower extremity motor tasks were utilized during active brain scanning (unilateral leg flexion/extension,2,3 unilateral leg press against resistance,1,6 bilateral leg press against resistance) to determine the validity of the MRI-compatible system for use with future fMRI research. Congruent with previous literature that positioned the camera perpendicular to the sagittal plane,36 the authors hypothesized that the MRI-compatible system would produce comparable and valid joint kinematics to those produced with a traditional motion capture system when the MRI-compatible camera was positioned facing the frontal plane.
METHODS
Participants
Fifteen subjects (9 females, 6 males; Mean age (years): 23.73, SD 4.91; Height (cm) 174.53 SD: 8.22; Weight (Kg) 75.06 SD: 13.15) from a convenience sample of staff and interns volunteered to participate in this study. Approval by the institutional review board of Cincinnati Children's Hospital Medical Center was received for the study protocol and participants provided informed consent before testing.
Equipment
Standard 3D motion capture data was collected at 240 Hz using a 44-camera motion tracking system (Motion Analysis Corp., Santa Rosa, CA). An MRI-compatible system (Figure 1a), custom developed by Metria Innovation Inc. (Milwaukee, WI) simultaneously captured movement data at 85 Hz. Standard optoelectronic spherical 9 mm reflective markers were used with the Motion Analysis system. The MRI-compatible system uses four markers (Figure 1b) which are 55mm x 55mm x 10mm in dimension. The system measures the position and orientation of the markers and by extension the leg segment they are attached to.
Data Collection
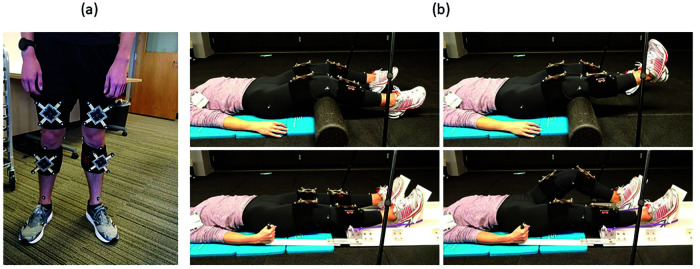
Measurements made with standard motion capture system were in the global (lab) coordinate system. Three standard optoelectronic spherical markers were also placed on each of the three perpendicular faces of the MRI-compatible camera module to approximate the orientation of the camera module's axes in the global coordinate system. These values were used to transform the measurements in the MRI-compatible camera coordinates to the global coordinates. All participants were fitted with standard spherical motion tracking markers on the lower extremity and four MRI-compatible markers. Standard spherical motion tracking markers were placed on the greater trochanter, mid lateral thigh, lateral and medial knee, tibial tubercle, mid shank and distal shank of both legs. The MRI-compatible markers were fitted in a cross-frame with four standard spherical motion tracking markers on the ends of the cross-arms, as shown in Figure 2a. Thus, each cross-frame referenced the MRI-compatible markers to the lab coordinate system.
Figure 2.
(a) Subject wearing the MRI compatible markers fitted in the cross frame and flanked by standard motion capture markers. (b) (Top) Right knee flexion-extension and (bottom) left unilateral leg press against resistance are shown for reference.
A static capture was performed with subjects in neutral standing position to establish segment coordinate system and transformation matrices for tracking markers on the cross frames attached to the respective leg segments. Following this, the subjects laid supine, with an angular pad supporting participants in knee flexion, and the MRI-compatible camera unit was positioned with the camera's z-axis perpendicular to the frontal plane (i.e. camera facing the frontal plane) of the subject and approximately three feet superior to their leg at maximum flexion. The subjects performed eight complete unilateral leg flexion-extension movement cycles on each leg (Figure 2b, top), individually. Next, the subjects were positioned into the leg press (Figure 2b, bottom), where they performed eight complete unilateral press-release movement cycles on each leg followed by eight complete bilateral press-release movement cycles. Participants complete all movements at their own pace. All data were processed in MATLAB (MathWorks, Natick, MA).
Data Analysis
Marker trajectories from the standard and MRI-compatible motion capture systems, and orientations from the MRI-compatible system were filtered using a low-pass fourth order Butterworth filter with a cutoff frequency of 12 Hz. The standard spherical markers on the cross-frame arms were used as tracking markers for the standard motion capture system. Trials were repeated if over three simultaneous frames were missing from the MRI-compatible motion capture system. Missing frames were spline interpolated. The static capture was used to define segment coordinate systems using the anatomical markers, and segment transformation matrices between the tracking markers and segment coordinate system. Transformation matrices were also defined between the coordinate system of each MRI-compatible marker and the respective segment coordinate system. The knee joint sagittal and frontal plane angles were calculated from the standard motion capture tracking data using joint coordinate system approach37 utilizing standard biomechanics procedures.38
To temporally align the data from the two systems, first, the angle (θ) between the vectors normal to the plane of the standard motion capture system marker group of the cross frames for each leg was calculated, then the angle (Φ) between the normal to the MRI-compatible markers were computed. θ and Φ were compared and served as reference to align the data temporally between the two motion capture systems. The data from standard motion capture system was interpolated at 85 Hz and was aligned with the MRI-compatible system's data for the remaining steps.
The MRI-compatible system reports the origin and pose of the markers in the camera coordinate system. To transform the data to the lab coordinate system, the estimated axes of the camera coordinate system were calculated in the lab coordinate system using the normal to the planes of the camera. The estimated x-axis and the xy-plane were used to calculate the orthonormal axes of the MRI-compatible camera in the lab coordinate system. The marker orientations were converted from camera coordinates to the lab coordinates. Using the transformations from the static capture, the orientation of the MRI-compatible markers were used to calculate the orientation of the segment coordinate system. Finally, the frontal and sagittal plane angles were calculated as in the previous steps.
Range of motion for each cycle was obtained by taking the difference of the maximum and minimum measures per cycle in the sagittal and frontal plane, where the cycle was defined by the sagittal plane angles. The mean of the range of motion across the 8 cycles was obtained. This measurement from both systems was compared and presence of any systematic bias between the two measurements was evaluated with Bland-Altman plots and outliers were determined with 95% limits of agreement (LoA) and 95% confidence interval (CI) limits.39,40 Intra-class correlation coefficients (ICC) using two-way, mixed effect absolute agreement single measurements were calculated along with the corresponding 95% CI limits.41 Excellent agreement was inferred at ICC above 0.90, good if between 0.75 and 0.90, moderate if between 0.50 and 0.75 and poor if below 0.50.42
Results
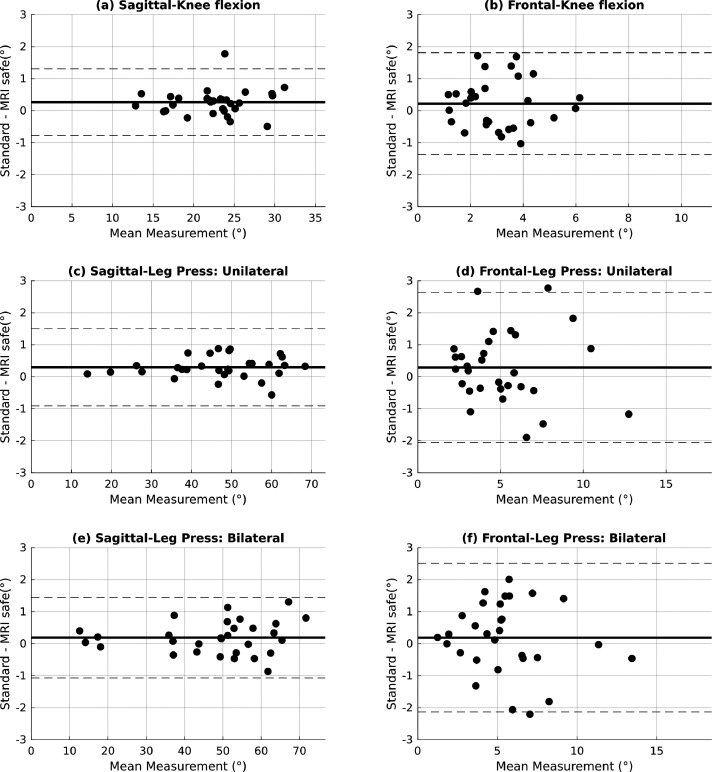
Mean difference between measurements from the standard motion capture system and MRI-compatible system, associated standard deviations and 95% CI, ICCs and corresponding 95% CI lower and upper limits for ICCs are presented in Table 1. The largest mean difference was observed for sagittal plane knee angle during the unilateral leg press at an error of 0.295 °, and the largest variability was observed in the frontal plane knee angle with a standard deviation of 1.196 °. All ICCs calculated were >0.99 for sagittal plane angles and >0.84 for frontal plane angles indicating a high level of agreement between the measurements obtained from the two motion capture systems. Bland-Altman plots for knee flexion, unilateral leg press and bilateral leg press exercises are presented in Figure 3. The plots show absence of magnitude dependent bias in the measurement difference.
Table 1.
Mean differences and standard deviations in degrees between standard motion capture and MRI compatible motion capture system for each leg task along with intraclass correlation coefficient (ICC) and corresponding 95% confidence interval.
| Task | Plane of Motion | Mean Difference | Standard Deviation | 95% CI (Mean Difference) | ICC | 95% CI (ICC) | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Knee Flexion | Sagittal | 0.263 | 0.532 | 0.065 | 0.462 | 0.994 | 0.981 | 0.998 |
| Frontal | 0.220 | 0.811 | -0.083 | 0.523 | 0.841 | 0.693 | 0.921 | |
| Leg Press: Unilateral | Sagittal | 0.295 | 0.614 | 0.066 | 0.524 | 0.999 | 0.996 | 1.000 |
| Frontal | 0.291 | 1.196 | -0.156 | 0.738 | 0.907 | 0.814 | 0.954 | |
| Leg Press: Bilateral | Sagittal | 0.188 | 0.642 | -0.052 | 0.428 | 0.999 | 0.999 | 1.000 |
| Frontal | 0.186 | 1.185 | -0.256 | 0.629 | 0.916 | 0.833 | 0.959 | |
Figure 3.
Bland-Altman plots of knee sagittal and frontal plane angles for all exercises. Mean of the measured angles by both systems is plotted along x-axis and the respective differences along y-axis. The bold horizontal line represents the mean of the difference between the two systems and the dashed lines represent 95% LoA.
Discussion
With fMRI-based examination of brain function as it relates to movement-based motor tasks, kinematic quantification of the motor tasks being studied is crucial to enhance mechanistic linkages between the brain and body. Though previous studies have succeeded in associating brain function with injury- and/or pathology-related movement characteristics (or just for differentiating brain function for populations known to elicit dysfunctional biomechanics [e.g., ACLR]), they have been limited by modalities that preclude whole-brain, ecologically valid examination of brain activity during actual lower extremity movement assessment (e.g., resting-state EEG/fMRI, transcranial magnetic stimulation function).43-52 There is limited knowledge related to the potentially unique subcortical and cortical neural correlates important for eliciting differential movement characteristics – even in task-based lower-extremity fMRI paradigms for which movement is theoretically standardized (i.e., subtle intra- and inter-subject movement reasonably exist). Such cohesive quantification of movement kinematics during fMRI could facilitate the improvement of proposed and existing injury prevention and rehabilitation programs aimed to simultaneously elicit injury resistant movement and enhanced brain function.1,48,53,54 Integrated assessment tools provide the next fundamental step in bridging the gap between traditional lab-based biomechanical observations for quantifying motor performance simultaneously with brain function. These tools could allow for the discovery of the most salient neural mechanisms underlying movement dysfunction to supplement recent recommendations calling for clinicians to leverage neuroplasticity to enhance movement-based, musculoskeletal rehabilitation.55-58 The current data indicate that the evaluated MRI-compatible motion capture affords the possibility to bridge these gaps by simultaneously quantifying movement kinematics with associated brain activity.
The goal of this study was to evaluate the utility and validity of the MRI-compatible motion capture system by testing it against a gold standard, the 3D Mo Cap Motion Analysis system (i.e. a multi-camera motion capture system). The results of the present study revealed excellent agreement for sagittal plane measurements (ICC > 0.99) and good to excellent agreement for frontal plane angle measurements (0.84 < ICC < 0.92). Average mean differences in both planes of movements were less than one degree between the two systems. The MRI-compatible measurement system tracked joint movements throughout the range of movement in these tasks, the highest of which was measured to be ∼72 ° in the sagittal plane during bilateral leg press as seen in Figure 3(e). The high level of agreements in Table 1 imply high consistency between the system's measurements, with mean differences between the two systems measuring less than one degree. Clinically, concurrent quantification of lower extremity kinematics and brain activity could reveal new and unique neural correlates of movements and pathologies – possibly more so than previous, isolated approaches that do not provide simultaneous data collection. For example, simply differences in time of day, testing location, participant motivation, and differential rates of movement versus neurologic recovery could all be considered confounds. The MRI-compatible motion capture system could be a significant tool for assessing the neural mechanistic drivers associated with adaptations from injury prevention training and rehabilitation programs by quantifying the effectiveness of these methods with simultaneous brain function assessment. Further, clinicians could customize training programs to target specific neurologic dysfunction to illicit a further optimized behavioral response, particularly to address neuroplasticity associated with injury.1,53,54
A previous study with a similar system and motion tracking technology (i.e. Moiré phase tracking) but different camera unit36 was validated against a standard motion capture system. With a camera positioned parallel to sagittal plane knee flexion, analysis of maximum and minimum kinematic angles during single leg landing and cutting tasks produced similar, valid findings to the present study. The current data extend these findings by further demonstrating the MRI-compatible measurement system's ability to track multiplanar motion from a single camera oriented towards the frontal plane. The current study validated the use of four markers simultaneously and showed minimal differences in measurements from the gold standard, despite a difference in the location of the plane of movement of the markers relative to the camera (perpendicular to frontal plane knee flexion).
One limitation of the experiment was that the exercises by design did not accentuate large frontal plane displacement during the task. However, from an kinematic perspective during functional tasks, a mere 8 ° differentiates high vs low risk for future ACL injury when assessed during a dynamic landing task13 and 4.1 ° frontal plane increased frontal plane knee motion differentiated those at high risk for secondary injury.59 Thus, even relatively, small changes in frontal knee ROM could be associated with increased susceptibility to movement error and are highly clinically relevant in ranges captured in this investigation. While the mean frontal and sagittal plane measurement error between the two systems were comparable for each task, sagittal plane measurements produced relatively higher agreement (Figure 3). As the included exercises were primarily in the sagittal plane (supported by relatively larger ROM kinematics), agreement data indicate that the MRI compatible motion capture system may be more accurate for tracking the plane of movement with greater relative excursion. Though the present exercises still produced clinically meaningful frontal plane movement, smaller excursions may have precluded more accurate tracking by the MRI compatible system (albeit agreement was still ‘good’). It is reasonable to hypothesize that exercises engaging greater frontal plane motion would be tracked with even further accuracy by the MRI compatible system. As kinematic data were not collected during frontal plane exercises due to the majority of lower extremity neuroimaging paradigms engaging sagittal plane motion, future research that tests different planar movements is warranted to further validate the MRI compatible system. Another limitation of the MRI-compatible system is that it can track a maximum of four markers and as such only four segments per frame. Therefore, concurrent bilateral measurements of hip or foot movement in addition to knee movement would not be directly possible. Also, occasionally the range of movement of the markers was more than +/- 60 °, sometimes resulting in lost tracking due to limited field of view of the camera, and required a repetition of the trial, a scenario less likely with multi-camera MRI compatible systems. Markerless motion tracking systems, though not available for MRI compatible applications, could overcome limitations of tracking markers, though most current systems struggle with tracking individual segments when all body segments are not in view,60 which is the case for fMRI as an individual's torso is inside the bore of the scanner and thus occluded from a camera, while individual markers for a segment may still be visible. Future work to develop multi marker units are warranted to expand this line of research to more functional movement assessments. Last, we collected kinematic data using different sampling rates between the two systems (240Hz for standard motion capture vs. 85 Hz for MRI-compatible motion capture). However, as the frequency of subjects’ movements were less than 2 Hz and the kinematic variable of interest was range of motion, one would expect any differences to be insignificantly small as this would only trivially affect the minimum and maximum values used for calculation (i.e., a large range of motion would still be a large range of motion). While validation using matched or derivative-based sampling rates between the two systems may have been ideal, the present data still indicated that the MRI-compatible system provided accurate and valid kinematic measurements. This system will be valuable to accelerate future research aiming to quantify lower extremity movement during neuroimaging to better understand the neural substrates of movement and pathology.
CONCLUSION
The results of the current investigation demonstrate that the single-camera MRI-compatible motion tracking system provides highly consistent and valid results compared to the gold standard, a multi-camera motion analysis system. These data provide assurance this MRI-compatible motion tracking system will produce valid kinematic results with high fidelity to that of standard biomechanical assessments. As this custom system is also MRI-safe, these data further support its integration with lower-extremity neuroimaging paradigms to discover how brain function contributes to dysfunctional human movement.
REFERENCES
- 1.Grooms DR Kiefer AW Riley MA, et al. Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: Initial findings from the train the brain project. J Sport Rehabil. 2018;27(5):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grooms DR Page SJ Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: Neuroimaging in musculoskeletal medicine. J Athl Train. 2015;50(10):1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grooms DR Page SJ Nichols-Larsen DS Chaudhari AMW White SE Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017;47(3):180-189. [DOI] [PubMed] [Google Scholar]
- 4.Raisbeck LD Diekfuss JA Grooms DR Schmitz R. The effects of attentional focus on brain function during a gross motor task. J Sport Rehabil. 2020;29(4):441-447. [DOI] [PubMed] [Google Scholar]
- 5.Lepley AS Grooms DR Burland JP Davi SM Kinsella-Shaw JM Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res. 2019;237(5):1267-1278. [DOI] [PubMed] [Google Scholar]
- 6.Grooms DR Diekfuss JA Ellis JD, et al. A novel approach to evaluate brain activation for lower extremity motor control. J Neuroimaging. 2019;29(5):580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeger L Marchal-Crespo L Wolf P Riener R Kollias S Michels L. Test-retest reliability of fMRI experiments during robot-assisted active and passive stepping. J Neuroeng Rehabil. 2015;12(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeger L Marchal-Crespo L Wolf P Riener R Michels L Kollias S. Brain activation associated with active and passive lower limb stepping. Front Hum Neurosci. 2014;8: 828. 10.3389/fnhum.2014.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontes EB Okano AH De Guio F, et al. Brain activity and perceived exertion during cycling exercise: an fMRI study. Br J Sports Med. 2015;49(8):556-560. [DOI] [PubMed] [Google Scholar]
- 10.Shanahan CJ Hodges PW Wrigley T V. Bennell KL Farrell MJ. Organisation of the motor cortex differs between people with and without knee osteoarthritis. Arthritis Res Ther. 2015;17(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacIntosh BJ Mraz R Baker N Tam F Staines WR Graham SJ. Optimizing the experimental design for ankle dorsiflexion fMRI. Neuroimage. 2004;22(4):1619-1627. [DOI] [PubMed] [Google Scholar]
- 12.Bohannon RW. Gait performance of hemiparetic stroke patients: selected variables. Arch Phys Med Rehabil. 1987;68(11):777-781. [PubMed] [Google Scholar]
- 13.Hewett TE Myer GD Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. Am J Sports Med. 2005;33(4):492-501. [DOI] [PubMed] [Google Scholar]
- 14.Blin O Ferrandez AM Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci. 1990;98(1):91-97. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff JM Cudkowicz ME Firtion R Wei JY Goldberger AL. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in parkinson's disease and Huntington's disease. Mov Disord. 1998;13(3):428-437. [DOI] [PubMed] [Google Scholar]
- 16.Scarvell JM Smith PN Refshauge KM Galloway H Woods K. Comparison of kinematics in the healthy and ACL injured knee using MRI. J Biomech. 2005;38(2):255-262. [DOI] [PubMed] [Google Scholar]
- 17.Feiring DC Ellenbecker TS. Single versus multiple joint isokinetic testing with ACL reconstructed patients. Isokinet Exerc Sci. 1996;6(2):109-115. [Google Scholar]
- 18.Esfandiarpour F Shakourirad A, Talebian Moghaddam S Olyaei G Eslami A Farahmand F. Comparison of kinematics of ACL-deficient and healthy knees during passive flexion and isometric leg press. Knee. 2013;20(6):505-510. [DOI] [PubMed] [Google Scholar]
- 19.Mirek E Rudzin´ska M Szczudlik A. The assessment of gait disorders in patients with Parkinson's disease using the three-dimensional motion analysis system Vicon. Neurol Neurochir Pol. 41(2):128-133. [PubMed] [Google Scholar]
- 20.Maclachlan L White SG Reid D. Observer rating versus three-dimensional motion analysis of lower extremity kinematics during functional screening tests: a systematic review. Int J Sports Phys Ther. 2015;10(4):482-492. [PMC free article] [PubMed] [Google Scholar]
- 21.Claiborne TL Armstrong CW Gandhi V Pincivero DM. Relationship between hip and knee strength and knee valgus during a single leg squat. J Appl Biomech. 2006;22(1):41-50. [DOI] [PubMed] [Google Scholar]
- 22.Herrington L. Knee valgus angle during single leg squat and landing in patellofemoral pain patients and controls. Knee. 2014;21(2):514-517. [DOI] [PubMed] [Google Scholar]
- 23.Salem GJ Salinas R Harding FV. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84(8):1211-1216. [DOI] [PubMed] [Google Scholar]
- 24.Bonnette S Dicesare CA Kiefer AW, et al. A technical report on the development of a real-time visual biofeedback system to optimize motor learning and movement deficit correction. J Sport Sci Med. 2020;(19):84-94. [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnette S DiCesare CA Kiefer AW, et al. Injury risk factors integrated into self-guided real-time biofeedback improves high-risk biomechanics. J Sport Rehabil. Published online January 30, 2019:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericksen HM Thomas AC Gribble PA Armstrong C Rice M Pietrosimone B. Jump-landing biomechanics following a 4-week real-time feedback intervention and retention. Clin Biomech. 2016;32:85-91. [DOI] [PubMed] [Google Scholar]
- 27.Ericksen HM Thomas AC Gribble PA Doebel SC Pietrosimone BG. Immediate effects of real-time feedback on jump-landing kinematics. J Orthop Sports Phys Ther. 2015;45(2):112-118. [DOI] [PubMed] [Google Scholar]
- 28.Ford KR DiCesare CA Myer GD Hewett TE. Real-time biofeedback to target risk of anterior cruciate ligament injury: a technical report for injury prevention and rehabilitation. J Sport Rehabil. 2015;Technical:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Widmer M Ziegler N Held J Luft A Lutz K. Rewarding Feedback Promotes Motor Skill Consolidation via Striatal Activity. Vol 229 1st ed. Elsevier B.V.; 2016. [DOI] [PubMed] [Google Scholar]
- 30.Widmer M Lutz K Luft AR. Reduced striatal activation in response to rewarding motor performance feedback after stroke. NeuroImage Clin. 2019;24:102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widmer M Stulz S Luft AR Lutz K. Elderly adults show higher ventral striatal activation in response to motor performance related rewards than young adults. Neurosci Lett. 2017;661(September):18-22. [DOI] [PubMed] [Google Scholar]
- 32.Herbst M Maclaren J Lovell-Smith C, et al. Reproduction of motion artifacts for performance analysis of prospective motion correction in MRI. Magn Reson Med. 2014;71(1):182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst M Zahneisen B Knowles B Zaitsev M Ernst T. Prospective motion correction of segmented diffusion weighted EPI. Magn Reson Med. 2015;74(6):1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krug JW Lüsebrink F Speck O Rose G. Optical ballistocardiography for gating and patient monitoring during MRI: An initial study. Comput Cardiol (2010). 2014;41(January):953-956. [Google Scholar]
- 35.Singh A Zahneisen B Keating B, et al. Optical tracking with two markers for robust prospective motion correction for brain imaging. Magn Reson Mater Physics, Biol Med. 2015;28(6):523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinhandl JT Armstrong BSR Kusik TP Barrows RT O’Connor KM. Validation of a single camera three-dimensional motion tracking system. J Biomech. 2010;43(7):1437-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grood ES Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136-144. [DOI] [PubMed] [Google Scholar]
- 38.Umberger BR Caldwell GE. Research Methods in Biomechanics: Musculoskeletal Modeling; 2014.
- 39.Bland JM Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England). 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 40.Zou GY. Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat Methods Med Res. 2013;22(6):630-642. [DOI] [PubMed] [Google Scholar]
- 41.McGraw KO Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30-46. [Google Scholar]
- 42.Koo TK Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumeister J Reinecke K Weiss M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: An EEG study. Scand J Med Sci Sport. 2008;18(4):473-484. [DOI] [PubMed] [Google Scholar]
- 44.Baumeister J Reinecke K Schubert M Weiß M. Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Res. 2011;29(9):1383-1389. [DOI] [PubMed] [Google Scholar]
- 45.Bonnette S Diekfuss JA Grooms DR, et al. Electrocortical dynamics differentiate athletes exhibiting low-and high-ACL injury risk biomechanics. Psychhophysiology. 2020;(June 2019):15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diekfuss JA Grooms DR Yuan W et al. Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. J Sci Med Sport. 2019;22(2):169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diekfuss JA Grooms DR Nissen KS, et al. Alterations in knee sensorimotor brain functional connectivity contributes to ACL injury in male high-school football players: a prospective neuroimaging analysis. Brazilian J Phys Ther. Published online 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diekfuss JA Grooms DR Bonnette S, et al. Real-time biofeedback integrated into neuromuscular training reduces high-risk knee biomechanics and increases functional brain connectivity: A preliminary longitudinal investigation. Psychophysiology. 2020;57(5):2325967119S0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepley AS Ly MT Grooms DR Kinsella-Shaw JM Lepley LK. Corticospinal tract structure and excitability in patients with anterior cruciate ligament reconstruction: A DTI and TMS study. NeuroImage Clin. 2020;25:102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrosimone BG Lepley AS Ericksen HM Clements A Sohn DH Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(6):665-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers CM Fisher B. Mechanisms underlying ACL injury-prevention training: The brain-behavior relationship. J Athl Train. 2010;45(5):513-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miao X Huang H Hu X Li D Yu Y Ao Y. The characteristics of EEG power spectra changes after ACL rupture. PLoS One. 2017;12(2):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armijo-Olivo S. A new paradigm shift in musculoskeletal rehabilitation: Why we should exercise the brain? Brazilian J Phys Ther. 2018;22(2):95-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silfies SP Vendemia JMC Beattie PF Stewart JC Jordon M. Changes in brain structure and activation may augment abnormal movement patterns: An emerging challenge in musculoskeletal rehabilitation. Pain Med. 2017;18(11):2051-2054. [DOI] [PubMed] [Google Scholar]
- 55.Gokeler A Neuhaus D Benjaminse A Grooms DR Baumeister J. Principles of motor learning to support neuroplasticity after ACL injury: Implications for optimizing performance and reducing risk of second ACL injury. Sport Med. 2019;49(6):853-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faltus J Criss CR Grooms DR. Shifting Focus: A cinician's guide to understanding neuroplasticity for anterior cruciate ligament rehabilitation. Curr Sports Med Rep. 2020;19(2):76-83. [DOI] [PubMed] [Google Scholar]
- 57.Diekfuss JA Grooms DR Hogg JA, et al. Targeted application of motor learning theory to leverage youth neuroplasticity for enhanced injury-resistance and exercise performance: OPTIMAL PREP. J Sci Sport Exerc. 2020. 10.1007/s42978-020-00085-y. [Google Scholar]
- 58.Diekfuss JA Bonnette S Hogg JA, et al. Practical Training Strategies to Apply Neuro-Mechanistic Motor Learning Principles to Facilitate Adaptations Towards Injury-Resistant Movement in Youth. J Sci Sport Exerc. 2020. 10.1007/s42978-020-00083-0. [Google Scholar]
- 59.Paterno M V. Schmitt LC Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfister A West AM Bronner S Noah JA. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J Med Eng Technol. 2014;38(5):274-280. [DOI] [PubMed] [Google Scholar]