Abstract
Background:
Blood flow restriction (BFR) training enhances muscular strength and hypertrophy in several populations including older adults and injured athletes. However, the efficacy of emerging BFR technologies on muscular adaptations, vascular health, and pain is unclear.
Purpose:
The purpose of this study was to examine muscular performance, pain and vascular function in response to eight weeks of BFR compared to traditional resistance training and a control group.
Study Design:
Randomized control trial
Methods:
Thirty-one overtly healthy participants (age: 23 ± 4y, 65% female) underwent eight weeks of supervised high load resistance training (RES), low load resistance training with BFR (BFR) or no training (control, CON). RES and BFR (with pneumatic bands) performed seven upper and lower body exercises, two to three sessions per week at 60% and 30% of one-repetition maximum (1RM), respectively. Twenty-four hours post-exercise, general muscle soreness was assessed via a visual analog scale (VAS) and present pain intensity (PPI) of the McGill Pain Questionnaire. At baseline and after eight weeks, participants underwent one-repetition maximum (1RM), and flow-mediated dilation (FMD) testing.
Results:
At baseline all groups exhibited similar muscle strength and endurance and vascular function. At the end of training, RES and BFR groups significantly increased muscle strength (1RM) to a similar magnitude as compared to the CON group (p < 0.0001), but did not alter body composition. FMD significantly increased in RES and BFR groups compared to CON group (p = 0.006). VAS and PPI were similar between RES and BFR groups throughout the exercise sessions until VAS decreased in the BFR group after the last session compared to the RES group (p = 0.02).
Conclusion:
Compared to RES, BFR resulted in similar muscular performance (strength and endurance) and vascular improvements at a lower exercise intensity, suggesting BFR is an effective alternative to high load resistance training. Further longitudinal studies may gain greater understanding regarding general muscle pain and soreness when using BFR.
Level of Evidence:
Therapy, Level 2
Keywords: Blood flow restriction, Movement system, Pain, Resistance training, Vascular function
INTRODUCTION
Blood flow restriction (BFR) training has been applied in a variety of settings to improve strength and endurance while exercising at lower intensities.1,2 Emerging portable BFR training systems are transforming accessibility and affordability of BFR. The BFR technique includes several modifying factors that can influence hypertrophy and strength (cuff width, cuff pressure, exercise sets, repetitions, and intensity).2 Variance in types of BFR equipment and exercise protocols along with the lack of efficacy and standards related to the development of BFR equipment systems exist throughout the literature, making evidence-based implementation of BFR training difficult. For example, in rehabilitation where patients may better tolerate a lower exercise intensity due to injury or disease, BFR application has included resistance training loads ranging from 10-30% of one-repetition maximum (1RM).1 BFR application is intended to produce strength gains by impeding the venous inflow during lower load exercises, regardless of age, fitness level, or injury status.
Resistance training is often prescribed in rehabilitation settings to prevent muscular atrophy that accompanies orthopedic injuries or aging. BFR training is an appealing alternative method compared to traditional resistance training because high intensity exercise may be contraindicated in older adults with sarcopenia or painful joints where joint compressive forces should be kept at a lower intensity.1,3 Current American College of Sports Medicine guidelines recommend higher training intensities (>60%1RM) for muscular hypertrophy and strength gains in healthy adults, while noting that 60% 1RM is on the low end of the spectrum and more applicable to individuals with a lower training age.4 BFR training improves muscle hypertrophy and strength to a greater extent than low load resistance training alone and produces same or similar gains in hypertrophy and similar gains in strength as moderate to high load resistance training.5,6 BStrong BFR bandsTM are a system that reportedly allows arterial blood flow during BFR resistance training while impeding venous return. The bands are pneumatic and when inflated utilize a “barrel” system allowing more elasticity and non-uniform circumferential pressure which mitigate risks of arterial occlusion such as ischemia-perfusion injury or rhabdomyolysis. During BFR a hypoxic environment generates the accumulation of metabolites, a component of the theorized mechanism encouraging muscle hypertrophy.7 Metabolites such as lactate, promote increased growth hormone production and fast-twitch fiber recruitment allowing for hypertrophy and strength development at intensities as low as 20%1RM.7-9 Thus, low load BFR training may maximize muscular gains and exercise tolerance while minimizing risks of injury associated with high load resistance training.1,3
The effect of BFR training on subjective pain may be of interest for practitioners. Assessing pain is challenging to compare across the literature due to methodological issues including timing of measurements, assessment tools, BFR method and exercise regimens.2,7 Pain response to BFR training has been less commonly studied, as most research has focused on single joint exercises over a limited time frame. Acutely, muscle pain after BFR has been reported to be lower than during resistance training alone, particularly in rehabilitating populations.8,10,11 Evidence suggests lower limb BFR training results in less or similar pain responses in individuals with patellofemoral pain12,13 and knee osteoarthritis.14 Reducing load to prevent stress on joints reduces the potential for pain after exercise, an ideal strategy to improve exercise tolerance in rehabilitating patients.
Previous research on BFR training often focuses on assessment of strength while observing undesirable cardiovascular outcomes such as increased blood pressure, which is also observed in high load resistance training.15,16 Similar to pain, the effect of resistance training with and without BFR on vascular health is controversial.17-20 Endothelial dysfunction, a part of the pathogenesis in atherosclerosis and a marker of vascular health that can be assessed using flow-mediated dilation (FMD). Briefly, after a period of occlusion of a vessel, blood flow and internal wall shear stress increase, in turn stimulating the endothelium to activate nitric oxide production and cause vasodilation. FMD is expressed as the change in brachial artery diameter after reactive hyperemia and has been found to independently predict future cardiovascular events.21 An anticipated range of FMD in healthy adults prior to exercise training would be 7-10%, with a smaller change in diameter in those with known cardiovascular disease (2-3%). Evidence suggests resistance training alone increases FMD in healthy adults22,23 and in diseased populations in a dose response fashion.24 However, few studies have assessed FMD in BFR training. Credeur et al18 performed handgrip training with BFR, resulting in a 30% reduction FMD. Hunt et al19 found no change in FMD (6.5% to 5.7%) also using handgrip training with BFR, but demonstrated adaptations in artery diameter. Both studies were short term (∼4 weeks), used different exercise intensities, and were isolated to upper body resistance training. BFR training may still have a beneficial effect on endothelial function due to greater shear stress during ischemic exercise inducing release of nitric oxide and causing vasodilation.15,25 Therefore, the purpose of this study was to compare the effects of low load, blood flow restriction training to traditional high load resistance training on muscular performance and general muscle pain. In addition, this study sought to evaluate vascular function after the 8-week exercise intervention.
METHODS
Participants
Thirty-one adults (11 males, 20 females) participated in this randomized controlled trial (Table 1). Participants were recruited via printed advertisements on various campus sites and word of mouth. Participants with known cardiovascular disease or significant medical condition, currently taking any chronic medications, blood pressure >160/100mmHg or orthopedic injury deemed unsafe to exercise by the researchers were excluded from the study. Before participating in the study, all participants gave written, informed consent and answered no to all questions on the Physical Activity Readiness Questionnaire (PAR-Q).26 The study was approved by the Institutional Review Board of the Columbus State University.
Table 1.
Baseline characteristics for participants. Most data presented as mean±SD, unless otherwise indicated.*
| Variable | RES (n = 10) | BFR (n = 11) | CON (n = 10) | All (n = 31) |
|---|---|---|---|---|
| Demographic Profile | ||||
| Age (y) | 23 ± 3 | 24 ± 4 | 23 ± 3 | 23 ± 4 |
| Sex (female), n (%) | 5 (50) | 8 (73) | 7 (70) | 20 (65) |
| Anthropometrics | ||||
| Height (m) | 1.70 ± 0.10 | 1.69 ± 0.09 | 1.68 ± 0.08 | 1.69 ± 0.09 |
| Weight (kg) | 79.6 ± 15.9 | 71.7 ± 15.1 | 71.9 ± 15.3 | 74.4 ± 15.4 |
| BMI (kg/m2) | 27.8 ± 7.2 | 24.9 ± 3.9 | 25.1 ± 4.0 | 25.9 ± 5.2 |
| Body Fat (%) | 28.8 ± 16.4 | 24.8 ± 9.9 | 31.2 ± 6.9 | 28.1 ± 11.6 |
| FM (kg) | 24.1 ± 18.8 | 17.5 ± 10.1 | 23.3 ± 8.3 | 21.5 ± 13.1 |
| FFM (kg) | 54.3 ± 10.7 | 54.0 ± 14.1 | 48.9 ± 8.8 | 52.5 ± 11.4 |
| Cardiovascular Measures | ||||
| Resting HR (bpm) | 70 ± 4 | 68 ± 8 | 74 ± 10 | 70 ± 8 |
| Systolic blood pressure (mmHg) | 118 ± 8 | 108 ± 10 | 116 ± 10 | 114 ± 10 |
| Diastolic blood pressure (mmHg) | 76 ± 6 | 76 ± 8 | 78 ± 4 | 76 ± 6 |
| Baseline artery diameter (mm) | 3.93 ± 0.46 | 3.76 ± 0.55 | 3.72 ± 0.51 | 3.81 ± 0.50 |
| Peak artery diameter (mm) | 4.21 ± 0.43 | 4.07 ± 0.63 | 4.11 ± 0.52 | 4.16 ± 0.53 |
| FMD (%) | 9.9 ± 2.9 | 8.1 ± 2.9 | 10.5 ± 1.9 | 9.5 ± 2.8 |
Abbreviations: RES, traditional resistance training; BFR, resistance training with blood flow restriction; CON, control; y, years; %, percent; m, meter; kg, kilogram; BMI, body mass index; FM, fat mass; FFM, fat-free mass; HR, heart rate; mmHg, millimeters of mercury; mm, millimeter; FMD, flow mediated dilation.
NOTE: There were no significant differences observed between groups at baseline (p > 0.05).
Study Design
Data were collected at baseline and after an eight-week resistance training program. Following completion of baseline testing, participants were randomized using a random number generator scheme from a website27 to one of three groups: traditional resistance training (RES), resistance training with blood flow restriction (BFR), or no training (i.e. control, CON). The RES and BFR groups participated in 20 exercise sessions, at the frequency of two to three times per week over the course of eight weeks. The CON group were asked to maintain their current exercise and physical activity regimen. Approximately 48hr after the last exercise session, participants underwent the same data collection procedures performed at baseline.
Procedures
Participants arrived to the laboratory between 8:00 and 10:00AM for testing after an overnight fast of 10-12hr. Participants refrained from alcohol and exercise for 48hrs prior to any testing. Anthropometric data including body weight, body fat, fat-free mass and fat mass were assessed using air displacement plethysmography (BodPod®, Cosmed Inc., Concord, CA) following manufacturer's instructions. Following anthropometric measurements, brachial artery vascular function was assessed using FMD.28 Briefly, participants rested in the supine position while a three-lead electrocardiogram monitored the cardiac cycle. Resting vitals (heart rate and blood pressure) were measured at the end of a 30min rest period. FMD was induced by five minutes of forearm occlusion by inflation of a pneumatic cuff (Hokanson) to 200mmHg, positioned ∼1cm distal to the olecranon process. Using ultrasonography (Logiq e Ultrasound, GE Healthcare, WI), the brachial artery was imaged 30s prior to cuff release and for 3min following return to normal, unrestricted blood flow. FMD was calculated as the percent change in brachial artery diameter. To assess muscular performance, measures of muscular strength and endurance were included. Muscular strength, defined as the external force that can be generated by a specific muscle or muscle group, was assessed using the isotonic 1RM test according to ACSM guidelines.4 The 1RM was assessed unilaterally for arm curl (standing biceps curl) and arm extension (overhead triceps extension), and bilaterally for leg extension (knee extension machine), leg curl (prone knee flexion machine) and heel raise (heel raise machine). The unilateral exercises, the 1RM was averaged across both limbs to determine a bilateral 1RM proxy measurement for strength. Handgrip strength, with the elbow by the side and flexed to 90 degrees, was assessed unilaterally using a handgrip dynamometer (Jamar) adjusted to each individual's hand. The participants were asked to perform a maximal voluntary contraction, with the best of three trials recorded. Muscular endurance was assessed by the number of push-ups able to be completed in one minute.
Exercise Intervention
Exercise intervention was conducted in a health and wellness facility using Cybex resistance equipment for the bilateral exercises between 8:00am and 6:00pm. During the resistance training intervention, all participants refrained from any structured physical activity outside of the study. RES and BFR groups participated in a light warm-up (10-15 min treadmill or cycle) followed by five resistance exercises (arm extension, arm curl, leg extension, leg curl, and heel raise). Handgrip training was performed using the hand dynamometer using the same repetition scheme for the respective group. Pushups were performed without the use of equipment. RES performed three sets of 10 repetitions of each exercise at 60%1RM with a two to three-minute rest between exercises. Researchers were trained in the use of BStrong BandsTM. The BFR group applied a relatively narrow (5.5 cm wide for arms/7.0 cm wide for legs), elastic, pneumatic band (BStrong Training SystemsTM, Park City, UT, USA). Bands were placed on both the upper arms (between deltoid and biceps brachii) and both upper thighs (inferior to gluteal fold), respectively. Bands were inflated to 250mmHg (upper body) or 350mmHg (lower body) for the duration of the exercises. Arm bands were inflated first during upper body exercises and then removed, followed by lower body band inflation and lower body exercises. The bands were not deflated between sets or exercises. BFR exercises were carried out with a format of 3 sets of 30 repetitions (or to fatigue) with 30-60 seconds rest in between sets and two to three minutes rest between exercises, at 30%1RM. While there is no standard BFR training protocol established in the literature, this format was chosen based of the manufacturer's recommendations to achieve the metabolic overload required for the benefits of BFR training. Training load progressed every two weeks by 10%1RM in RES and to a maximum of 50%1RM in BFR.
Approximately 24hrs after each exercise session, participants reported their general perception of muscle soreness and muscular pain using a unidimensional visual analog scale (VAS) and the multidimensional McGill Pain Questionnaire (MPQ).29 The VAS, consisting of a 10-centimeter line with terminal descriptors (no pain, severe pain), was used to measure general muscle pain severity. The MPQ was used to assess present pain intensity (PPI), or magnitude of pain, based on a 0-5 scale that best fit their symptoms at the given time. The levels of PPI included none, mild, discomforting, distressing, horrible and excruciating and a higher score is equivalent to higher pain. Word descriptors were converted to their respective value after each exercise session. The MPQ and VAS is a reliable, valid measure of the quality and quantity of pain and has been previously used to assess delayed onset-muscle soreness (DOMS).29-31
Statistics
All analyses were performed using SPSS Version 23 (IBM Corp., Armork, NY). All data were assessed for normality using the Shapiro-Wilks test and presented as mean and standard deviation (SD). One-way ANOVAs was used to examine differences in outcome variables (body composition, muscular strength and endurance, and vascular measurements) between groups at baseline. A 2x3 (time by group) repeated measure ANOVA was used to determine differences post intervention in outcome variables. A 2x2 (time by group) repeated measure ANOVA was used to determine differences post intervention in muscle pain and soreness of the RES and BFR groups. Post-hoc testing was performed using Bonferroni where appropriate. Significance was set a p < 0.05.
RESULTS
Participant Characteristics
Body composition and vascular measurements were comparable between groups at baseline (Table 1). Males had lower body fat (18.8 ± 10.1 vs. 33.2 ± 9.0%, p < 0.001) and fat mass (15.4 ± 10.2 vs. 24.9 ± 12.2kg, p = 0.03) and higher fat free mass (63.7 ± 8.5 vs. 46.3 ± 7.5kg, p < 0.001) compared to females. Males also had a greater baseline and peak brachial artery diameter compared to females (p < 0.005). No differences were observed between groups in regard to muscular endurance and strength (Table 2). However, males had greater handgrip strength, 1RM for all exercises and completed a greater number of push-ups compared to females (p < 0.005).
Table 2.
Muscular strength and endurance outcomes.
| Variable | Pre-training | Post-training | % Change | Time x group p |
|---|---|---|---|---|
| Handgrip strength (kg) | ||||
| Control | 28 ± 12 (19-36) | 28 ± 11 (20-35) | -0.1 ± 6.5 | 0.05 |
| RES | 32 ± 8 (26-38) | 37 ± 13 (27-46) | 11.8 ± 15.6 | |
| BFR | 31 ± 8 (25-37) | 33 ± 8 (27-39) | 14.3 ± 4.3 | |
| Arm extension 1RM (lb) | ||||
| Control | 15 ± 5 (11-18) | 14 ± 5 (10-17) | -4.5 ± 9.5 | <0.001 |
| RES | 20 ± 6 (16-23) | 28 ± 9 (22-34) | 18.4 ± 5.8 | |
| BFR | 18 ± 8 (12-23) | 24 ± 14 (18-30) | 14.9 ± 4.5 | |
| Arm curl 1RM (lb) | ||||
| Control | 23 ± 10 (15-31) | 24 ± 12 (15-32) | 1.1 ± 3.5 | <0.001 |
| RES | 28 ± 9 (21-34) | 34 ± 10 (26-41) | 22.4 ± 12.9 | |
| BFR | 28 ± 12 (19-36) | 33 ± 13 (23-44) | 20.7 ± 9.7 | |
| Leg curl 1RM (lb) | ||||
| Control | 88 ± 26 (68-107) | 83 ± 25 (65-101) | -5.1 ± 7.9 | <0.001 |
| RES | 106 ± 26 (87-124) | 140 ± 32 (117-162) | 33.2 ± 17.2 | |
| BFR | 105 ± 20 (89-119) | 130 ± 26 (111-148) | 25.6 ± 17.6 | |
| Leg extension 1RM (lb) | ||||
| Control | 162 ± 49 (127-196) | 156 ± 50 (120-191) | -3.7 ± 7.5 | 0.001 |
| RES | 163 ± 44 (131-194) | 197 ± 55 (157-236) | 21.7 ± 14.6 | |
| BFR | 159 ± 34 (132-184) | 191 ± 39 (163-217) | 21.6 ± 18.9 | |
| Heel raise 1RM (lb) | ||||
| Control | 185 ± 76 (130-239) | 183 ± 74 (130-236) | -0.5 ± 1.6 | <0.001 |
| RES | 174 ± 55 (134-213) | 226 ± 74 (172-279) | 29.9 ± 12.0 | |
| BFR | 177 ± 64 (126-223) | 229 ± 64 (182-275) | 35.0 ± 20.6 | |
| Push-ups (reps) | ||||
| Control | 25 ± 11 (17-33) | 25 ± 10 (18-32) | 3.2 ± 15.3 | <0.001 |
| RES | 29 ± 12 (20-38) | 51 ± 19 (36-64) | 91.6 ± 64.1 | |
| BFR | 33 ± 17 (20-46) | 48 ± 20 (33-64) | 51.5 ± 25.7 | |
Abbreviations: RES, traditional resistance training; BFR, resistance training with blood flow restriction; CON, control; 1RM, one-repetition maximum; lbs, pounds; reps, repetitions. Data presented as mean±SD (95% confidence interval).
Exercise Intervention
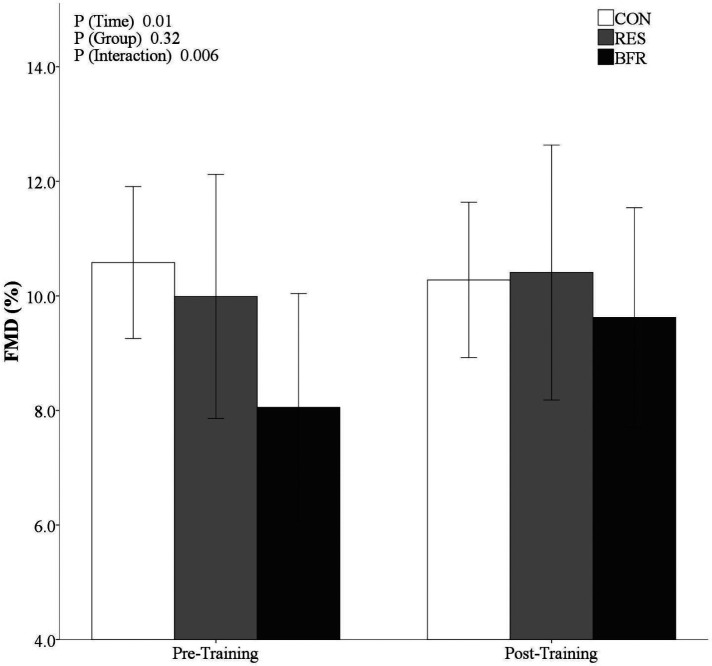
All participants completed the resistance training with the exception of one BFR participant who missed one exercise session due to an injury unrelated to the study. No adverse events occurred during exercise. From pre- to post-training, there were no differences in body composition within or between groups (p > 0.05). Resting HR (p = 0.47), systolic blood pressure (SBP, p = 0.44) and diastolic blood pressure (DBP, p = 0.46) were similar between groups after training. RES and BFR demonstrated a decrease in SBP from pre- to post-training (p = 0.005). There was a significant time by group interaction (p = 0.006) where FMD did not significantly change in the CON (10.5 ± 1.9 to 10.3 ± 1.8%), but did increase in RES (9.9 ± 2.9 to 10.4 ± 3.1%) and BFR (8.1 ± 2.9 to 9.7 ± 2.9%) from pre- to post-training (Figure 1). Baseline and peak artery diameter were not different within or between groups after training. A main effect of time was observed (p = 0.01) and time by group (p = 0.006) for FMD. However, there was no group effect of FMD (p = 0.32).
Figure 1.
Mean (95% confidence interval) flow-mediated dilation (FMD) after eight weeks of intervention.
There were significant time by group interactions (p < 0.001) found between CON and both RES and BFR after training, displayed in Table 2. There was an increase in 1RM in all resistance exercises in RES and BFR post-training. Muscular endurance, assessed using maximal push-up repetitions, exhibited the greatest change from pre- to post-training in RES (91% change) and BFR (51% change). Pre- and post-test 1RM were similar in CON after eight weeks (P > 0.05), and saw slight, insignificant decreases in handgrip, arm extension, leg extension, leg curl, and heel raise 1RM.
Subjective Pain
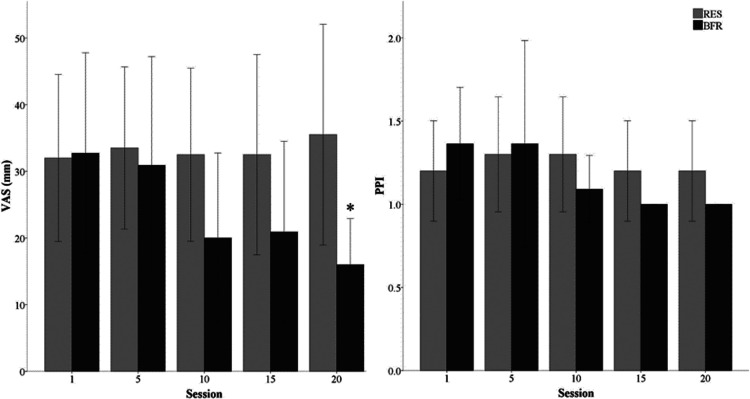
No time by group interaction occurred in VAS (p = 0.22) and PPI (p = 0.23) scales over the course of the training intervention (Figure 2). After the initial exercise session, there were no differences in VAS (RES, 32 ± 18 vs. BFR, 33 ± 22mm, p = 0.93) or PPI (RES, 1.2 ± 0.4 vs. BFR 1.3 ± 0.5, p = 0.43). By session 10, the BFR group was not statistically different for lower VAS (p = 0.14) and PPI (p = 0.24) compared to RES overall, which continued through session 19. At session 20, BFR reported significantly less pain assessed by the VAS (p = 0.02) compared to RES, but PPI remained was not significantly different (p = 0.15). In describing PPI across all exercise sessions, RES (78%) and BFR (91%) most frequently reported pain as “mild”. “Discomforting” PPI was reported less frequently in BFR (7%) compared to RES (20%), and no participants selected PPI as “horrible” throughout the any of the interventions.
Figure 2.
Reported muscle pain 24-hours post-exercise using mean visual analog scale (VAS) and pain intensity index (PPI) across the first, tenth and last exercise session.
*Significant difference between groups (p<0.05).
DISCUSSION
This study investigated effects of low load BFR training (30%1RM) in comparison to traditional high load resistance training (60%1RM) in multiple body regions. The results indicate that eight weeks of BFR training with BStrong Training SystemsTM was as effective improving 1RM as high load, resistance training in healthy adults in the measured muscle performances. The improvements found during BFR were of a similar magnitude as traditional resistance training, but were able to be achieved at lighter loads which may minimize risk of injury and overuse. BFR training was also able to achieve these increases in 1RM and endurance. In the final session, general muscle pain was lower in BFR compared to RES, which may encourage compliance to exercise regimens and minimize likelihood of functional limitations to exercise such as delayed onset muscle soreness.
Participants in this study were of similar body composition and fitness compared to those included in previous BFR research.30,32 Increased 1RM for upper and lower body exercises in both resistance and BFR training was observed in this study. Participants in the RES group showed greater change in 1RM in all exercises to a greater extent than BFR, except for handgrip. Changes in the RES group may have been driven by greater fast-twitch muscle fiber recruitment and neuromuscular adaptation.7 This may also contribute to greater reported general muscle pain compared in RES as there was greater mechanical stress, however not significantly different from BFR. Many of the upper body exercises in the intervention required gripping equipment, which may have contributed to the increase in handgrip strength in BFR. This study may support the theory of mechanism in BFR; lower mechanical stress of exercise in combination with an ischemic environment created by BFR may have enhanced lactate production and growth hormone release. In turn, the lower load training still elicited a strong enough metabolic response to develop adaptations in strength.7
Low load BFR training may allow for resistance exercise that might otherwise be painful for injured or frail patients.8,30 The results of the current study suggest that as BFR training persists, pain may decrease which may be useful motivation to enhance exercise tolerance and compliance. This may be due to the onset of resistance training being light and slow progression, or the effect of BFR training itself. Semi elastic BFR bands can reduce venous inflow to generate muscle fatigue with less mechanical damage. BFR participants may have experienced less delayed onset muscle soreness in the final session as measured by the VAS and MPQ scales, although was not assessed directly.
FMD improves with resistance training in healthy adults and diseased populations in previous studies with similar training characteristics (length, frequency, intensity) as were used the present study.33-35 Kambic et al36 observed improvements in FMD with BFR training of a similar magnitude in patients with coronary artery disease. However, FMD was similar between the control and BFR groups after eight weeks of leg extension exercise.36 This study may have reached statistically significant differences in FMD due to the application of four limb training protocol generating a greater hypoxic environment throughout training. BFR training may create more hypoxic stress due to reduced blood flow,37 which may systemically stimulate the cascading effects of vascular endothelial growth factor (VEGF). VEGF and increased growth hormone may lead to increased release of nitric oxide and vasodilation, driving the adaptations seen in FMD. There were no changes in resting brachial artery diameter, suggesting improvements in function may occur before structural adaptations.20
A limitation of the present study was the use of young, healthy adults who were fairly fit. Such participants may have experienced faster post-exercise recovery, less pain and magnitude of strength development from the training intervention. Previous research has found greater strength adaptations in untrained individuals.2 This in turn may have minimized the differences seen in between group comparisons. The authors also acknowledge the instruments used to assess pain (VAS and MPQ) are self-report tools and subject to the participant's opinion. The control group refrained from structured physical activity, which may have reduced their overall physical activity and therefore fitness during the study. Exercise volumes between RES and BFR were not matched despite yielding similar strength gains, as previous research has shown that low load high repetition training does not demonstrate the same results as BFR training.5 Hypertrophy was not measured in this study, but literature suggests such adaptations may appear as early as two weeks.6 To the authors’ knowledge this is the first study to conduct four limb resistance training with a unique BFR training system, as well as measure perceived pain and vascular function after a longer duration (i.e. at least six months) training intervention.
CONCLUSION
The results of the current study suggest that BFR training was able to produce similar strength gains, as measured by 1RM (in multiple movements), in the trained muscles and elicited a lower pain response compared to resistance training alone at the end of the study. BFR training with BStrong Training SystemsTM is a safe and effective alternative to traditional high load resistance training. Thus, BFR may be an effective tool to be applied and studied in clinical populations, where greater compression on joints may be contraindicated due to health or injury. In addition, BFR and resistance training improved flow-mediated dilation, suggesting both may positively influence vascular health. BFR training may be appealing for practitioners interested in preserving and developing muscular fitness with a safe, unique system.
REFERENCES
- 1.Hughes L Paton B Rosenblatt B Gissane C Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003-1011. [DOI] [PubMed] [Google Scholar]
- 2.Loenneke JP Wilson JM Marin PJ Zourdos MC Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849-1859. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley R. Blood flow restriction training in rehabilitation: A useful adjunct or Lucy's latest trick? J Orthop Sports Phys Ther. 2019;49(5):294-298. [DOI] [PubMed] [Google Scholar]
- 4.American College of Sports Medicine, Riebe D Ehrman JK Liguori G Magal M. ACSM's guidelines for exercise testing and prescription. Tenth Edition ed. Philidelphia: Wolters Kluwer; 2018. [Google Scholar]
- 5.Takarada Y Takazawa H Sato Y Takebayashi S Tanaka Y Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol (1985). 2000;88(6):2097-2106. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda T Fujita S Ogasawara R Sato Y Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: A pilot study. Clin Physiol Funct Imaging. 2010;30(5):338-343. [DOI] [PubMed] [Google Scholar]
- 7.Pearson SJ Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45(2):187-200. [DOI] [PubMed] [Google Scholar]
- 8.Korakakis V Whiteley R Epameinontidis K. Blood flow restriction induces hypoalgesia in recreationally active adult male anterior knee pain patients allowing therapeutic exercise loading. Phys Ther Sport. 2018;32:235-243. [DOI] [PubMed] [Google Scholar]
- 9.Takarada Y Nakamura Y Aruga S Onda T Miyazaki S Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000;88(1):61-65. [DOI] [PubMed] [Google Scholar]
- 10.Hughes L Patterson SD Haddad F, et al. Examination of the comfort and pain experienced with blood flow restriction training during post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: A UK National Health Service trial. Phys Ther Sport. 2019;39:90-98. [DOI] [PubMed] [Google Scholar]
- 11.Korakakis V Whiteley R Giakas G. Low load resistance training with blood flow restriction decreases anterior knee pain more than resistance training alone: A pilot randomised controlled trial. Phys Ther Sport. 2018;34:121-128. [DOI] [PubMed] [Google Scholar]
- 12.Giles L Webster KE McClelland J Cook JL. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: A double-blind randomised trial. Br J Sports Med. 2017;51(23):1688-1694. [DOI] [PubMed] [Google Scholar]
- 13.Giles LV Tebbutt SJ Carlsten C Koehle MS. The effect of low and high-intensity cycling in diesel exhaust on flow-mediated dilation, circulating NOx, endothelin-1 and blood pressure. PLoS One. 2018;13(2):e0192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryk FF Dos Reis AC Fingerhut D, et al. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1580-1586. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi M Okita K. Blood flow restricted exercise and vascular function. Int J Vasc Med. 2012;2012:543218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyachi M Kawano H Sugawara J, et al. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004;110(18):2858-2863. [DOI] [PubMed] [Google Scholar]
- 17.Casey DP Pierce GL Howe KS Mering MC Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol. 2007;100(4):403-408. [DOI] [PubMed] [Google Scholar]
- 18.Credeur DP Hollis BC Welsch MA. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc. 2010;42(7):1296-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt JE Galea D Tufft G Bunce D Ferguson RA. Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol. 2013;115(3):403-411. [DOI] [PubMed] [Google Scholar]
- 20.Rakobowchuk M McGowan CL de Groot PC Hartman JW Phillips SM MacDonald MJ. Endothelial function of young healthy males following whole body resistance training. J Appl Physiol. 2005;98(6):2185-2190. [DOI] [PubMed] [Google Scholar]
- 21.Ras RT Streppel MT Draijer R Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344-351. [DOI] [PubMed] [Google Scholar]
- 22.Badrov MB Freeman SR Zokvic MA Millar PJ McGowan CL. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur J Appl Physiol. 2016;116(7):1289-1296. [DOI] [PubMed] [Google Scholar]
- 23.Getty AK Wisdo TR Chavis LN, et al. Effects of circuit exercise training on vascular health and blood pressure. Prev Med Rep. 2018;10:106-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Early KS Stewart A Johannsen N Lavie CJ Thomas JR Welsch M. The effects of exercise training on brachial artery flow-mediated dilation: A meta-analysis. J Cardiopulm Rehabil Prev. 2017;37(2):77-89. [DOI] [PubMed] [Google Scholar]
- 25.Boeno FP Ramis TR Farinha JB de Lemos LS Medeiros NDS Ribeiro JL. Acute effects of strength exercise with blood flow restriction on vascular function of young healthy males. J Vasc Bras. 2018;17(2):122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warburton D Jamnik S. Shephard R. Gledhill N. The 2019 Physical Activity Readiness Questionnaire for everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit J of Canada. 2018;11(4):80-83. [Google Scholar]
- 27.Randomization. http://randomization.com/. Accessed 09-05-2018.
- 28.Thijssen DH Black MA Pyke KE, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleather DJ Guthrie SR. Quantifying delayed-onset muscle soreness: A comparison of unidimensional and multidimensional instrumentation. J Sports Sci. 2007;25(8):845-850. [DOI] [PubMed] [Google Scholar]
- 30.Knutzen KM Pendergrast BA Lindsey B Brilla LR. The effect of high resistance weight training on reported pain in older adults. J Sports Sci Med. 2007;6(4):455-460. [PMC free article] [PubMed] [Google Scholar]
- 31.Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1(3):277-299. [DOI] [PubMed] [Google Scholar]
- 32.Madarame H Neya M Ochi E Nakazato K Sato Y Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc. 2008;40(2):258-263. [DOI] [PubMed] [Google Scholar]
- 33.Kwon HR Min KW Ahn HJ et al. Effects of aerobic exercise vs. resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes Metab J. 2011;35(4):364-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGowan CL Visocchi A Faulkner M, et al. Isometric handgrip training improves local flow-mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2007;99(3):227-234. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto T Masuhara M Ikuta K. Effect of low-intensity resistance training on arterial function. Eur J Appl Physiol. 2011;111(5):743-748. [DOI] [PubMed] [Google Scholar]
- 36.Kambic T Novakovic M Tomazin K Strojnik V Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: A pilot randomized controlled trial. Front Physiol. 2019;10:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downs ME Hackney KJ Martin D, et al. Acute vascular and cardiovascular responses to blood flow-restricted exercise. Med Sci Sports Exerc. 2014;46(8):1489-1497. [DOI] [PubMed] [Google Scholar]