Abstract
Most myocardial pathologic conditions are associated with cardiac fibrosis, the expansion of the cardiac interstitium through deposition of extracellular matrix (ECM) proteins. Although replacement fibrosis plays a reparative role after myocardial infarction, excessive, unrestrained or dysregulated myocardial ECM deposition is associated with ventricular dysfunction, dysrhythmias and adverse prognosis in patients with heart failure. The members of the Transforming Growth Factor (TGF)-β superfamily are critical regulators of cardiac repair, remodeling and fibrosis. TGF-βs are released and activated in injured tissues, bind to their receptors and transduce signals in part through activation of cascades involving a family of intracellular effectors the receptor-activated Smads (R-Smads). This review manuscript summarizes our knowledge on the role of Smad signaling cascades in cardiac fibrosis. Smad3, the best-characterized member of the family plays a critical role in activation of a myofibroblast phenotype, stimulation of ECM synthesis, integrin expression and secretion of proteases and anti-proteases. In vivo, fibroblast Smad3 signaling is critically involved in scar organization and exerts matrix-preserving actions. Although Smad2 also regulates fibroblast function in vitro, its in vivo role in rodent models of cardiac fibrosis seems more limited. Very limited information is available on the potential involvement of the Smad1/5/8 cascade in cardiac fibrosis. Dissection of the cellular actions of Smads in cardiac fibrosis, and identification of patient subsets with overactive or dysregulated myocardial Smad-dependent fibrogenic responses are critical for design of successful therapeutic strategies in patients with fibrosis-associated heart failure.
1. Introduction:
The term “cardiac fibrosis” describes the expansion of the cardiac interstitium due to net accumulation of extracellular matrix (ECM) proteins [1],[2]. Cardiac fibrosis is not a single disease entity, but rather a pathologic response that can be appropriate or inappropriate, depending on the context. For example, following myocardial infarction, massive loss of cardiomyocytes overwhelms the negligible regenerative capacity of the adult mammalian heart, and activates a fibrogenic response that ultimately results in scar formation. Despite the absence of contractile function, the collagen-based scar plays an important protective role, maintaining the structural integrity of the ventricle and preventing catastrophic mechanical complications, such as cardiac rupture [3],[4],[5],[6],[7]. Formation of scar following myocardial infarction is part of a reparative response and results in “replacement fibrosis”. In many other myocardial pathologic conditions, such as hypertensive heart disease, aortic stenosis, or diabetic cardiomyopathy, cardiac fibrosis develops insidiously in the absence of significant loss of cardiomyocytes and involves predominantly the interstitial and perivascular areas. These changes may contribute to the pathogenesis of heart failure by promoting both systolic and diastolic dysfunction and may be critically implicated in the development of heart failure with preserved ejection fraction (HFpEF) [8],[9].
The members of the TGF-β superfamily play a critical role in regulation of cardiac fibrotic responses [10],[11]. In humans, the TGF-β superfamily is composed of 33 members. that can be subclassified into several subfamilies. The three TGF-β isoforms, TGF-β1, β2 and β3 are the best studied members of the family in myocardial diseases [12]. The superfamily also includes the Growth differentiation factors (GDFs), the activins, the bone morphogenetic proteins (BMPs), the inhibins, the nodal and anti-Mullerian hormone proteins. Following myocardial injury, several members of the TGF-β superfamily are induced and/or activated and modulate the phenotype of both cardiomyocytes and non-cardiomyocytes [13],[14],[15],[16],[17]. The best characterized intracellular effectors of the TGF-β superfamily proteins are the Smads, a group of 8 structurally related proteins in humans with homologues that have been identified in both vertebrates and invertebrates. The founding member of the family is the product of the Drosophila gene Mothers against decapentaplegic (Mad), which was found to mediate signaling through the BMP homologue decapentaplegic (Dpp) [18]. In parallel, the Sma proteins were identified as Mad homologues in nematodes. Thus, the term “Smad”, (combining Sma and Mad) was coined to name the vertebrate members of this family [19]. From a functional perspective, the Smads are classified into 3 groups: a) the Receptor Activated Smads (R-Smads: Smad1, Smad2, Smad3, Smad5 and Smad8), responsible for TGF-β superfamily signaling, b) the common Smad (Co-Smad), Smad4, which binds to the R-Smads forming the signaling complex and c) the inhibitory Smads (I-Smads: Smad6 and Smad7), which are involved in negative regulation of R-Smad-mediated cascades [20].
Fibroblasts, immune cells, vascular cells and cardiomyocytes, the main cellular effectors of cardiac fibrosis are highly responsive to TGF-βs and activate Smad-dependent signaling cascades that play a critical role in regulation of the fibrogenic transcriptional program. This review manuscript discusses the role of Smad-dependent signaling cascades in cardiac fibrotic conditions.
2. The TGF-β signaling cascade: from the cell surface to the nucleus
The heart contains latent stores of TGF-β [21] that can be rapidly activated following injury [22] through interactions of the latent TGF-β complexes with proteases [23],[24], specialized matrix proteins (such as ED-A fibronectin and thrombospondin-1) [25],[26],[27] and cell surface integrins [28]. Moreover, in many fibrosis-associated myocardial conditions, infiltration with platelets capable of releasing large amounts of TGF-β from their granules [29], and de novo synthesis of TGF-β isoforms in cardiomyocytes, fibroblasts, immune cells and vascular cells [30],[17],[16], further increase the amounts of activatable TGF-βs in the site of injury. Several other members of the TGF-β superfamily are also upregulated in the injured and remodeling myocardium, including activins [31], BMPs [15] and GDFs [13].
All TGF-β superfamily members transduce signals through binding to characteristic combinations of type I and type II TGF-β receptors (TβRs) [32]. Humans have 7 type I TβRs, (also known as activin-like receptor kinase (ALK)1–7), and 5 type II TβRs (TβRII, ActRII, ActRIIB, AMHRII and BMPRII) [33]. The three TGF-β isoforms (TGF-β1, -β2, and -β3) act through a single type II receptor (TβRII) that may interact with ALK5 [34], or ALK1 (or both) depending on the cell type and the context [35],[36],[37],[38]. Some studies have suggested that ALK2 and ALK3 may also mediate certain TGF-β-induced actions [39]. Other members of the superfamily use different TGF-β receptor combinations. Activins signal through ALK4, or ALK7 after binding to the ActRII or ActRIIB type II receptors. On the other hand, the members of the BMP family signal through a range of combinations, including one of the type I receptors ALK1 ALK2, ALK3 and ALK6 and one of the ActRII, ActRIIB and BMPRII type II receptors [40] [41].
Binding of a TGF-β superfamily member to its receptors triggers formation of a heterotetrameric complex, composed of two type I and two type II receptor molecules (Figure 1). Subsequently, the phosphorylated type I receptor interacts with and phosphorylates members of the R-Smad family at the carboxyterminal Ser-Ser-X-Ser (SSXS) motif, activating a Smad-dependent (canonical) signaling cascade. Type I receptors have preferred Smad partners: ALK5, ALK4 and ALK7 phosphorylate Smad2 and Smad3, whereas ALK1, ALK2, ALK3 and ALK6 phosphorylate Smad1, Smad5 and Smad8 [42]. After phosphorylation by the type I receptor, the R-Smads dissociate from the receptor and form trimeric complexes with the common Smad, Smad4. These complexes can be either homomeric or heteromeric: one Smad4 molecule can bind to two molecules of the same R-Smad, or may form a mixed complex, composed of two different R-Smads (Smad2 and Smad3, or even Smad1 and Smad3). Subsequently, the R-Smad/Smad4 complex translocates to the nucleus, where it binds to Smad-binding elements or GC-rich sequences in the promoter regions of target genes, regulating their transcription [43].
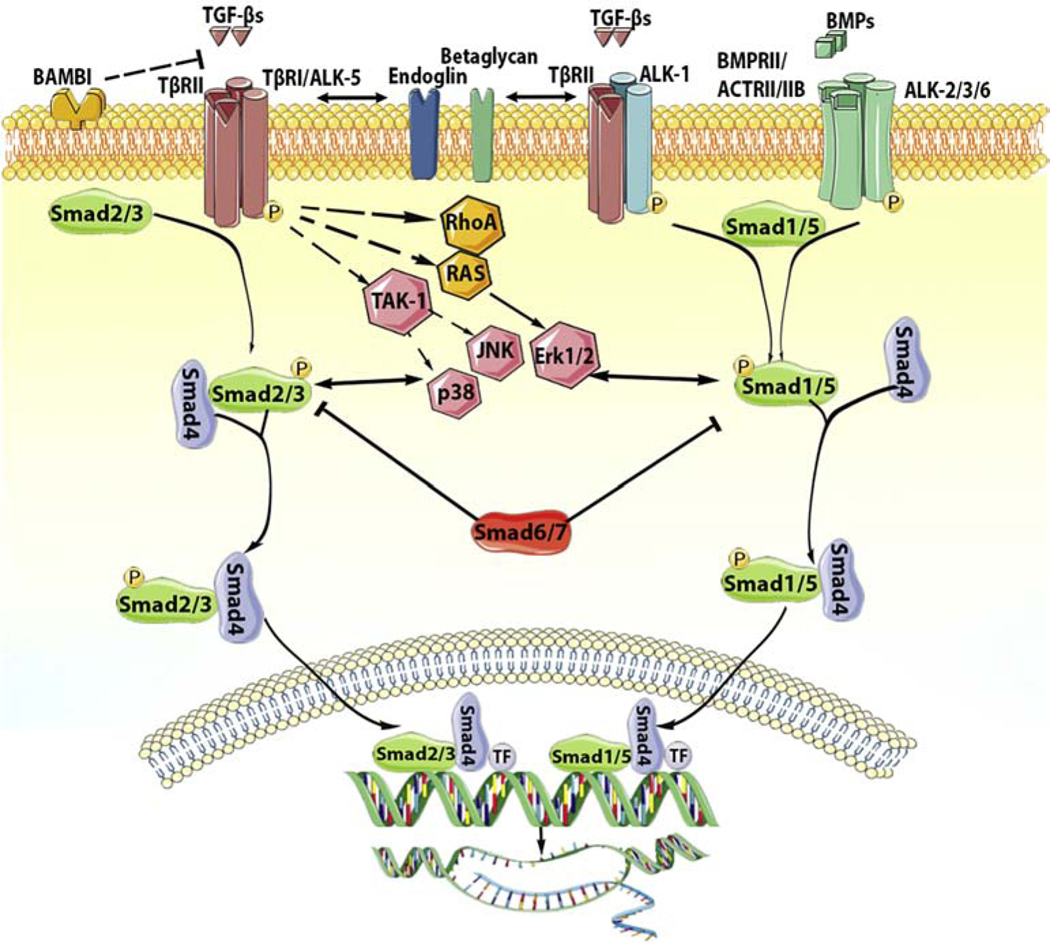
Figure 1: Signaling cascades activated by the TGF-β superfamily.
TGF-β superfamily ligands signal by binding to distinct combinations of two type II, and two type I receptors. TGF-βs bind TβRII inducing transphosphorylation of the type I receptor ALK5. Activated ALK5 phosphorylates the receptor-activated Smads (R-Smads) Smad2 and Smad3, which then form a heterotrimeric complex with the common Smad, Smad4, promoting the translocation of the Smad complex to the nucleus. Interactions between the Smad complex and transcriptional activators or repressors regulate transcription of target genes. In some cell types, TGF-βs may also act through another type I receptor, ALK1, stimulating Smad1/5/8 signaling. BMPs also bind to their specific type II receptors, subsequently activating type I receptors (ALK2, ALK3, ALK6) and phosphorylating Smad1/5. Smad1 and Smad5 then bind to Smad4 and translocate to the nucleus regulating transcription. In addition to the Smad-dependent signaling, TGF-β superfamily members may also signal through non-canonical Smad-independent cascades. The inhibitory Smads (Smad6 and Smad7) negatively regulate TGF-β superfamily cascades. Accessory receptors, such as endoglin and betaglycan, modulate TGF-β signaling. The cartoon was designed using Servier Medical Art (https://smart.servier.com/).
The Smad cascades are modulated through the effects of accessory receptors (such as betaglycan and endoglin) [42], interactions with cytoplasmic proteins, and negative feedback pathways. Endoglin has been suggested to regulate the balance between Smad1/5 and Smad2/3 cascades in response to TGF-βs. It is predominantly expressed in endothelial cells and in activated fibroblasts, and negatively regulated ALK5-Smad2/3 responses, while enhancing ALK1-Smad1/5/8 signaling [44],[45],[46]. Betaglycan, on the other hand can either activate or inhibit TGF-β signaling responses, depending on its expression levels, and on contextual factors [47], [48]. Several other transmembrane molecules (including CD44 and neuropilin-1) [49],[50] have been reported to act as non-selective co-receptors that may modulate TGF-β responses, while interacting with other growth factors and bioactive mediators.
Negative feedback mechanisms act to restrain overactive Smad signaling responses. At the receptor level, TGF-β-induced overexpression of the cell surface pseudo-receptor BAMBI (BMP and activin membrane-bound inhibitor) competes with TβRI for ligand binding [51],[52] and may suppress TGF-β/R-Smad responses[53]. The I-Smads Smad6 and Smad7 are induced following TGF-β stimulation and compete with R-smads for their binding to activated TβRI or Smad4, while also mediating TβRI degradation by recruitment of Smurf ubiquitin ligases [54]. Moreover, the nuclear co-repressors Ski and SnoN can bind to the translocated R-Smad/Smad4 complex promoting its degradation via Smurf2, or directly preventing transcription of Smad-target genes by recruiting histone deacetylases [55].
It should be emphasized that, in addition to Smad signaling cascades, TGF-βs also activate non-canonical signaling pathways, such as mitogen-activated protein kinase (MAPK), TGF-β-activated kinase 1 (TAK1), Rho GTPase, phosphatidylinositol3-kinase/AKT and focal adhesion kinases (FAK) [56] [57] [58]. These non-canonical pathways extensively interact with the Smad-mediated cascades and add additional layers of complexity to the biology of the TGF–β response [59],[60],[61], Moreover, because these non-Smad cascades are also common downstream effector pathways for a wide range of mediators, dissection of their relative role in mediating effects of TGF-β superfamily members is challenging.
3. Can Smads be activated through mechanisms independent of TGF-β superfamily members?
Although the members of the TGF-β superfamily are considered the main activators of Smad signaling, a growing body of evidence suggests that TGF-β-independent mechanisms may also contribute to Smad activation in certain cell types. In epithelial cell lines, viral infection has been suggested to activate Smads in a TGF-β-independent manner [62]. In macrophages, phagocytosis rapidly and consistently activated Smad3 in the absence of TGF-β secretion [30], In NK cells, effects of Smad4 were found to be TGF-β-independent [63]. Experiments in renal mesangial cells suggested that advanced glycation end-products may activate R-Smads through a TβRII-independent mechanism [64]. The significance of TGF-β-independent Smad signaling in cardiac fibrosis has not been documented. Although the fibrogenic actions of angiotensin II are associated with marked activation of Smad2 and Smad3, these effects are likely indirect and involve activation and induction of TGF-β [65],[66]. Thus, Smad activation in fibrotic cardiac conditions should be viewed as the result of induction and/or activation of TGF-β superfamily members.
4. The cellular basis of cardiac fibrosis
As the main matrix-producing cells, activated fibroblasts are the key cellular effectors of myocardial fibrosis, regardless of underlying etiology [67]. In many cardiac fibrotic conditions, fibroblasts convert to secretory myofibroblasts, expressing contractile proteins, such as α-smooth muscle actin (α-SMA), and producing large amounts of structural matrix proteins and fibrogenic matricellular macromolecules [68],[69],[70],[71]. Myofibroblasts contribute to the fibrotic response, not only by secreting matrix proteins, but also by producing proteases, such as matrix metalloproteinases (MMPs), and their inhibitors [72],[73], thus regulating matrix metabolism. Emerging evidence suggests that myofibroblast conversion is not required for fibrogenic activation. In a mouse model of type 2 diabetes, increased interstitial and perivascular collagen deposition was not associated with myofibroblast conversion [74]. Moreover, single cell transcriptomic analysis suggests that in remodeling hearts, high matrix gene synthesis in fibroblast subpopulations may occur in the absence of myofibroblast transdifferentiation [75],[76]. Thus, alternative pathways of fibroblast activation may significantly contribute to cardiac fibrotic responses. The expansion and activation of fibroblasts and myofibroblasts in remodeling hearts may also involve several other cell types, including immune cells, cardiomyocytes and vascular cells. Macrophages and lymphocytes have been implicated in fibroblast activation through secretion of fibrogenic growth factors, cytokines and matricellular proteins [77],[78],[79],[80]. Mast cells may also contribute to fibrotic cardiac remodeling by releasing their fibrogenic granular contents [81],[82]. Under conditions of ischemic, mechanical or metabolic stress, cardiomyocytes are also capable of producing fibrogenic mediators and may contribute to fibroblast activation [83],[84]. Vascular endothelial cells can also produce bioactive mediators that stimulate fibroblasts. Several studies have suggested that endothelial cells may undergo endothelial-to-mesenchymal transition (EndMT) [85],[86],[87] thus directly participating in the myocardial fibrotic response. However, several publications using robust lineage tracing strategies in models of myocardial infarction and pressure overload have challenged the contribution of EndMT in cardiac fibrotic conditions [88],[89],[90].
The bulk of the experimental evidence suggests a major contribution of fibroblast Smad3 signaling in the cellular responses leading to myocardial fibrosis. In contrast, evidence supporting the role of other Smad proteins in fibroblast activation, and data on the potential involvement of Smad cascades in fibrogenic activation of other cell types are limited.
5. Smad signaling cascades in fibroblasts
Activation of Smad2 and Smad3 has been extensively demonstrated in fibroblasts infiltrating fibrotic and remodeling hearts; in contrast, much less evidence is available on Smad1/5/8 activation [91],[17],[92]. In isolated cardiac fibroblasts TGF-β isoforms, but not BMPs, rapidly activate Smad2 and Smad3 signaling [91],[93]. In vitro, Smad3 is a central activating signal for cardiac fibroblasts that regulates several different functions (Table 1, Figure 2) [10]. First, Smad3 induces myofibroblast conversion, increasing α-SMA expression and promoting its incorporation into stress fibers, the hallmark of myofibroblast transition [94],[95]. Smad3-mediated myofibroblast conversion involves direct effects on α-SMA transcription [94], [96], and may be accentuated by indirect actions, such as upregulation of fibronectin [7], a specialized matrix protein that stimulates acquisition of a myofibroblast phenotype [97] or downregulation of the transcription factor Forkhead box protein O3a (FoxO3a) [98], a signal that inhibits myofibroblast conversion [98, 99]. Second, Smad3 stimulates the transcription of structural and matricellular extracellular matrix proteins, including type I and type III collagens, fibronectin, periostin and tenascin-C [7, 94], [100], [101], and induces synthesis of matrix-crosslinking enzymes, such as lysyl-oxidase [102] and tissue transglutaminase [103]. Third, Smad3 promotes a matrix-preserving phenotype characterized by suppressed synthesis and reduced activity of matrix metalloproteinase (MMP)-3 and MMP-8 and induction of anti-proteases, such as Tissue Inhibitor of Metalloproteinases (TIMP)-1 [93],[91]. Fourth, Smad3 stimulates fibroblast expression of α2, α5, β3and α11 integrins, facilitating interactions between the fibroblasts and the extracellular matrix; these interactions may be important for formation of an organized scar [7] [104], Fifth, Smad3 may promote fibroblast migration and may enhance the capacity of fibroblasts to contract free-floating collagen lattices [94] (which is an indicator of fibroblast activation and is independent on myofibroblast conversion) [105]. Sixth, some studies have suggested that Smad3 may increase fibroblast viability under conditions of stress by exerting anti-apoptotic actions [106]. Finally, in addition to all these activating actions, Smad3 inhibits fibroblast proliferation, suggesting that Smad3-dependent myofibroblast activation is accompanied by tight regulation of the density of cardiac fibroblasts. The effects of Smad3 on cardiac fibroblasts are modulated through interactions with Smad-independent pathways [102],[7],[107], or through synergistic interactions with other fibrogenic transcription factors, such as scleraxis [108].
Table 1:
In vitro effects of Smad signaling cascades in regulation of cardiac fibroblast phenotype
| Species | Culture Condition | Smads manipulation | Role of Smad | Reference |
|---|---|---|---|---|
| Human | Collagen pads | Pharmacologic Smad3 inhibition with SIS3. | Smad3 mediates TGF-β1-induced α-SMA and α11 integrin expression. | [104] |
| Rat | Culture plates | Pharmacologic Smad3 inhibition with SIS3. | Smad3 mediates TGF-β1-induced LOX expression. | [102] |
| Rat (neonatal) | Culture plates | Pharmacologic Smad3 inhibition with SIS3. | Smad3 mediates TGFβ1-induced cytoprotective and antiapoptotic effects in fibroblasts exposed to simulated ischemia/reperfusion | [106] |
| Rat (neonatal) | Culture plates | Pharmacologic Smad3 inhibition with SIS3. | Smad3 promotes cardiac myofibroblast conversion by mediating TGF-β1-induced downregulation of FoxO3a. | [98] |
| Mouse | Culture plates | Smad2/Smad3 siRNA knockdown. | • Smad3 mediates baseline collagen I, collagen IV, fibronectin and TSP1 synthesis. • Smad2 mediates baseline collagen V, fibronectin, periostin and versican. |
[109] |
| Mouse | Collagen pads/Culture plates. | Smad2/Smad3 siRNA knockdown. | • Smad3, but not Smad2, mediates α2 and α5 integrin expression. • Both Smad 2 and Smad3 stimulate α-SMA incorporation into stress fibers |
[92] |
| Mouse | Collagen pads | Fibroblasts from global Smad3 null mice | Smad3 mediates TGF-β1-induced transcription of tissue transglutaminase. | [103] |
| Mouse | Culture plates | Fibroblasts from global Smad3 null mice | Smad3 mediates TGFβ–induced collagen III and tenascin-C synthesis. | [100] |
| Mouse | Collagen pads | Fibroblasts from global Smad3 null mice | Smad3 mediates TGF-β-induced TIMP1 upregulation and MMP3/MMP8 suppression, promoting a matrix-preserving phenotype. | [93] |
| Mouse | Collagen pads/Culture plates | Fibroblasts from global Smad3 null mice. | • Smad3 accentuates contraction of fibroblast-populated lattices. • Smad3 mediates expression of Integrins α2, α5 and β3 in pad fibroblasts. • Smad3 mediates α-SMA synthesis only in plated fibroblasts. |
[7] |
| Mouse | Collagen pads/Culture plates | Fibroblasts from global Smad3 null mice. | • Smad3 mediates anti-proliferative and promigratory effects of TGF-β. • Smad3 mediates contraction of fibroblast-populated lattices. • Smad3 stimulates CTGF and extracellular matrix gene synthesis. |
[94] |
| Mouse | Culture plates | Fibroblasts from global Smad3 null mice. | Smad3 mediates angiotensin II-induced upregulation of Collagen I, α-SMA, TNF-α, IL-1β, and MCP-1. | [140] |
| Mouse | Collagen pads/Culture plates | Cre overexpression in fibroblasts from Smad2, Smad3 and Smad2/3 fl/fl mice to delete loxP-targeted Smads. | • Smad3 (but not Smad2) mediates TGF-β1-induced fibroblast-populated pad contraction. • Both Smad2 and Smad3 stimulate expression of extracellular matrix genes (Col1a1, Col1a2, Col3a1, Fn1, Fbln1, Tnc), matrix regulators (Loxl2, Adam12, Adam30, Sparc) and cell adhesion genes (Itga2, Itga8, Itgb3). |
[101] |
Col, collagen; CTGF, connective tissue growth factor; Fbln, fibulin; Fn, fibronectin; IL, interleukin; Itg, integrin; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; LOX, lysyl oxidase; SMA, smooth muscle actin; TIMP, Tissue inhibitor of metalloproteinases; Tnc, tenascin; TNF, tumor necrosis factor; TGF, transforming growth factor.
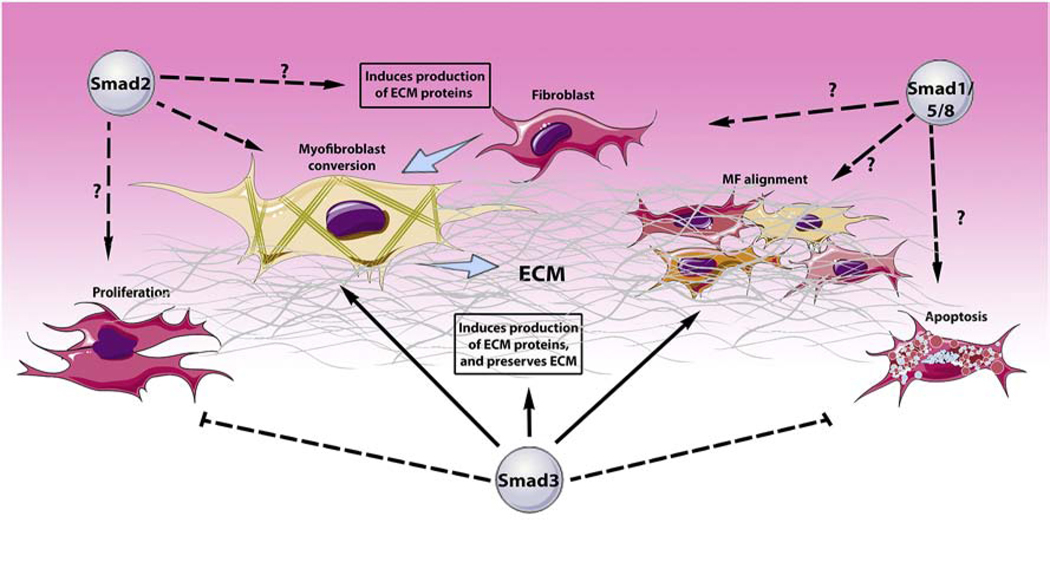
Figure 2: Smad-mediated actions in cardiac fibroblasts.
The Smad proteins are key intracellular effectors of cascades involving TGF-β superfamily signaling. In cardiac fibroblasts, receptor-activated Smads regulate viability, proliferation, migration, transcriptional profile, phenotype and function. Smad3 activates fibroblast synthesis of extracellular matrix (ECM) proteins, while preserving the ECM by reducing protease-mediated matrix degradation. Smad3 also promotes the conversion of cardiac fibroblasts to myofibroblasts, and regulates myofibroblast alignment and formation of an organized scar by regulating integrin expression. Moreover, Smad3 tightly regulates cardiac fibroblast numbers by regulating proliferation and apoptosis. Smad2, has been reported to modulate fibroblast gene expression in vitro; however, its’ in vivo effects are more limited. The role of Smad1/5/8 in regulation of cardiac fibroblast phenotype is not known. The cartoon was designed using Servier Medical Art (https://smart.servier.com/).
In vitro studies using isolated cardiac fibroblasts also suggest a significant role for Smad2 in activation of a fibrogenic transcriptional program (Figure 2), albeit with less consistent effects on functional activity. siRNA knockdown experiments suggested that Smad2 enhances fibronectin, periostin and versican expression by unstimulated cardiac myofibroblasts [109]. Smad2 deletion experiments using Cre-expressing adenovirus transfection in Smad2 fl/fl cardiac fibroblasts suggested broad effects of Smad2 in TGF-β-induced extracellular matrix gene synthesis. Moreover, Smad2 knockdown was found to inhibit incorporation of α-SMA into myofibroblast stress fibers [92]. However, in contrast to the critical effects of Smad3, Smad2 did not mediate integrin synthesis in cardiac fibroblasts [92] and Smad2 loss did not affect fibroblast-mediated contraction of collagen lattices [96]. The distinct effects of Smad2 and Smad3 on fibroblast gene transcription and function may be due to distinct patterns of activation and nuclear translocation, or to different interactions with co-repressors and co-activators that may affect their transcriptional targets.
Very limited information is available regarding the role of the Smad1/5/8 pathway in cardiac fibroblast phenotype and function. Findings from studies examining the role of Smad1 signaling in fibroblast responses in other organs have produced conflicting results. In lung fibroblasts, Smad1 activation was found to be enhanced by the accessory receptor betaglycan and antagonized the stimulatory actions of ALK5/Smad3 signaling [110]. In contrast, in a mouse model of fibrosis due to forced expression of ALK5 that recapitulates features of scleroderma, activation of a fibrogenic program was surprisingly found to be dependent on Smad1 [111], and was attributed to a ALK5/ALK1/Smad1 axis. In cardiac fibroblasts, inhibition of matrix gene expression by BMP7 was attributed to activation of Smad1 signaling [112]. The significance of these observations in regulation of cardiac fibrotic responses in vivo is not known.
6. The inhibitory Smads as negative regulators of fibroblast function
The I-Smads, Smad6 and Smad7 lack the carboxyterminal SSXS motif and cannot be phosphorylated upon binding to type 1 receptors, but act to inhibit signals transduced by TGF-β superfamily ligands through interactions with TβRs or R-Smads [113]. Smad6 preferentially inhibits BMP responses, mediated through ALK3 and ALK6, whereas Smad7 inhibits both TGF-β and BMP-induced cascades [114]. Several molecular mechanisms have been proposed to explain the inhibitory effects of I-Smads on TGF-β superfamily signaling. First, I-Smads may directly associate with TGF-β receptors, inhibiting TβRI kinase activity, or simply interfering with R-Smad:TβR binding [115],[116],[117]. Second, I-Smads may form a complex with the transmembrane pseudoreceptor BAMBI, inhibiting TβR-driven R-Smad activation [52]. Third, I-Smads may interact with the Smurf1 and Smurf2 type E3 ligases promoting degradation of R-Smads [118],[119]. Fourth, I-Smads may interfere with formation of the R-Smad:Smad4 complex [120].
Whether Smad6 is involved in regulation of fibrosis has not been investigated. Smad7 on the other hand has been suggested to act as a negative regulatory signal that may inhibit myocardial fibrosis. In vitro, Smad7 synthesis is induced upon TGF-β stimulation and Smad7 overexpression in cardiac fibroblasts suppresses collagen synthesis [121],[122]. The in vivo role of Smad7 in regulation of cardiac fibrotic responses is supported predominantly through associative data. In models of ventricular remodeling, Smad7 induction may serve as a TGF-β-induced endogenous inhibitory signal that restrains fibrogenic activation [121],[123]. In contrast, in other pathologic conditions, such as atrial fibrillation, atrial fibrosis has been attributed to reduced Smad7 levels in response to neurohumoral activation [124]. Direct in vivo evidence documenting the role of endogenous Smad7 in regulation of fibroblast responses is lacking. Two published studies demonstrated increased cardiac fibrosis in a hypomorphic Smad7 mutant line in which exon 1 of the Smad7 gene was deleted (Smad7Δex1)[125],[126]. Unfortunately, the significance of these findings is unclear, as these animals exhibit preserved Smad7 functions due to the presence of an intact MH2 domain, the key effector domain involved in Smad7 interactions with TβRs and R-Smads [117],[127].
7. Smad activation in immune cells and endothelial cells may trigger fibrogenic responses.
In addition to their effects on fibroblasts, Smad signaling may regulate phenotype and function of other cell types, thus contributing to their fibrogenic actions. In macrophages Smad3 plays an important role in activation of a phagocytic program, but also mediates the transition of macrophages to an anti-inflammatory phenotype in response to phagocytosis [30]. Phagocytic Smad3 null macrophages had impaired capacity to synthesize fibrogenic TGF-βs [30]. Whether this defect has an impact on the fibrogenic potential of macrophages has not been tested. In endothelial cells, Smad3 has been implicated in EndMT in a model of diabetic renal injury [128]. Whether Smad-dependent EndMT contributes to cardiac fibrosis has not been tested. However, this mechanism is unlikely considering the strong evidence from lineage tracing studies suggesting that the majority of activated fibroblasts in cardiac fibrotic conditions are not derived from endothelial cells [88],[89],[90],[129].
8. The role of Smad signaling cascades in homeostasis of the cardiac ECM network.
Smad cascades are critically involved in cardiac development [130]; however, their role in homeostasis of the adult heart is poorly understood. The adult mouse myocardium has high levels of constitutive Smad2 and Smad3 expression; however, baseline Smad2/3 phosphorylation is low [109]. In vivo studies showed that fibroblast-specific Smad3 (but not Smad2) loss modestly but significantly attenuated collagen levels in young adult mouse hearts, without affecting cardiac geometry or function [109]. These findings support the notion that fibroblast Smad3 signaling may play a role in maintaining the basal levels of collagen in the myocardium; however, this effect is not critical for preservation of function. Whether other Smad cascades are involved in homeostasis of the cardiac ECM network has not been investigated.
9. Smad signaling cascades in conditions associated with cardiac fibrosis (Table 2).
Table 2:
In vivo Effects of Smad Signaling in Cardiac Fibrosis
| Model | Intervention | Role of Smad | Proposed Mechanism | Reference |
|---|---|---|---|---|
| Age-associated changes | Cardiomyocyte- specific Smad4 knockout mouse | Cardiomyocyte Smad4 protected the heart from age-associated hypertrophy and fibrosis. | Smad4-mediated protection of cardiomyocytes from injury, or suppression of fibrogenic signals. | [160] |
| Non-reperfused MI | Myofibroblast-specific Smad2 or Smad3 loss. | Myofibroblast Smad3 a. Regulates organization of myofibroblast arrays. b. Mediates alignment of structural collagen fibers in the infarct Myofibroblast Smad2 does not play a major role in infarct healing. |
Smad3-mediated integrin α2 and α5 activation. | [92] |
| Reperfused MI | Global Smad3 null mice | Smad3 mediates cardiac collagen deposition in the infarct, the border zone and the remote remodeling. | Smad3-mediated synthesis of extracellular matrix genes and TIMPs. | [100] |
| Reperfused MI | Global Smad3 null mice | Smad3 mediates deposition in the infarct, promotes myofibroblast conversion, but reduces myofibroblast proliferation. | Smad3-mediated anti-proliferative actions, α-SMA expression, induction of ECM proteins and CTGF. | [94] |
| Reperfused and non-reperfused MI | Myofibroblast-specific Smad3 loss | Myofibroblast Smad3: a. Prevents late cardiac rupture. b. Mediates formation and organization of well-aligned arrays of myofibroblasts. |
Smad3-mediated activation of α5 integrin-NOX2 axis. | [7] |
| Pressure overload (TAC) | Myofibroblast-specific Smad3 KO mice | Myofibroblast Smad3 preserves the extracellular matrix and attenuates collagen degradation | Smad3-mediated inhibition of collagenase (MMP3/8)-driven matrix degradation, | [93] |
| Pressure overload (TAC) Overexpression of a cardiomyocyte-specific, latency-resistant TGF-β mutant transgene. | Conditional Fibroblast and Myofibroblast-specific TβR1, TβR2, Smad2, Smad3 and Smad2/3 null mice. | Fibroblast Smad3, but not Smad2, mediates the fibrotic response in the pressure-overloaded heart and in a genetic model of cardiac TGF-β overactivation. | Smad3-mediated transcription of matrix genes. | [101] |
| Pressure overload (TAC). | Global Smad3 null mice | Smad3 mediates myocardial fibrosis | Smad3-mediated collagen synthesis. | [141] |
| Pressure overload | ALK5 inhibition using a small molecule inhibitor (SM16) | ALK5 mediates fibrosis and diastolic dysfunction. | Smad2/3-mediated expression of pro-fibrotic genes. | [142] |
| Angiotensin II infusion | Global Smad3 null mice | Smad 3 mediates angiotensin II-induced cardiac fibrosis. | Smad 3-mediated induction of TGFβ1, CTGF, collagen I/III and α-SMA. | [140] |
| db/db mouse | Obese diabetic db/db mice with partial or complete Smad3 loss. | Smad3 promotes fibrosis in obese diabetic mice. | Smad3-mediated suppression of MMP activity. | [133] |
| T. Cruzi infection | Pharmacological ALK5 inhibition with GW788388. | ALK5 mediates fibrosis. | Smad2/3-mediated matrix synthesis. | [165] |
TAC, transverse aortic constriction; CTGF, connective tissue growth factor; ECM, extracellular matrix; MI, myocardial infarction; MMP, matrix metalloproteinase; NOX, NADPH Oxidase; SMA, smooth muscle actin; TIMP, Tissue inhibitor of metalloproteinases.
9.1. The role of Smad cascades in reparative fibrosis of the infarcted heart.
Myocardial infarction results in sudden loss of up to a billion cardiomyocytes, activating an inflammatory reaction that clears the infarct from dead cells and matrix debris and sets the stage for fibroblast-driven repair [131],[132]. Formation of a scar is critical for preservation of the structural integrity of the infarcted ventricle; however, excessive, unrestrained or expanded fibrosis may cause adverse remodeling, contributing to systolic and diastolic dysfunction and to the pathogenesis of heart failure. Induction and activation of TGF-βs in the infarcted myocardium is associated with activation of Smad2 and Smad3 in all cell types involved in cardiac repair, including border zone cardiomyocytes, infarct fibroblasts and macrophages [91],[92],[30]. The relative contribution of various members of the TGF-β superfamily in Smad2/3 activation remains unclear. The 3 TGF-β isoforms, the major activators of Smad2 and Smad3 cascades exhibit distinct patterns of induction following myocardial infarction with early upregulation of TGF-β1 and TGF-β2 and delayed increase in TGF-β3 levels [16],[30]. Early studies using mice with global germline loss of Smad3 provided insights into the role of the pathway in fibrosis of the infarcted heart. Complete absence of Smad3 attenuated collagen deposition in the infarcted and remodeling myocardium, despite increased infiltration of the infarct with myofibroblasts [91],[94]. Based on in vitro studies, the effects of global Smad3 loss were attributed to alterations in fibroblast phenotype and function, leading to attenuated expression of structural collagens, tenascin-C and fibronectin, and reducing secretion of Connective Tissue Growth Factor (CTGF)/CCN2, a downstream mediator of TGF-β-induced fibrogenesis [94]. However, considering the broad effects of Smad3 on all cell types involved in cardiac repair and remodeling, and the effects of Smad3 signaling on baseline homeostasis [133], dissection of cellular mechanisms using the global loss-of-function model is challenging.
Investigations using cell-specific loss-of-function models have significantly enhanced our understanding of the role of Smad cascades in myocardial infarction. (Table 2). Studies using cardiomyocyte-specific knockouts suggested that cardiomyocyte Smad3 signaling is not implicated in cardiac homeostasis, but contributes to the pathogenesis of adverse remodeling and dysfunction following reperfused myocardial infarction [7]. The detrimental actions of cardiomyocyte Smad3 in post-infarction ventricular dysfunction were attributed to pro-apoptotic effects that may involve activation of oxidative pathways and to induction and activation of MMP2 that may accentuate matrix degradation, causing adverse dilative remodeling [7].
On the other hand, Smad3 loss in infarct myofibroblasts perturbed repair of the infarcted heart, increasing the incidence of catastrophic late rupture in the model of non-reperfused infarction, and accentuating adverse remodeling in the model of reperfused infarction. Impaired repair in the absence of myofibroblast Smad3 signaling was related to perturbed fibroblast:extracellular matrix interactions that resulted in formation of a disorganized scar [7]. Smad3 plays an important role in induction of fibroblast integrins, the cell surface proteins that link the cells to the extracellular matrix [7]. Smad3-mediated integrin synthesis in fibroblasts is critically involved in activation of an oxidative response, that promotes a reparative program. Moreover, integrin synthesis may be important for scar organization, contributing to myofibroblast alignment in the healing infarct [7], a process that may involve expression of polarity genes [134]. In contrast to the critical role of myofibroblast Smad3 in cardiac repair, Smad2 activation in myofibroblasts did not play a significant role in post-infarction repair [92]. Although both Smad2 and Smad3 are activated in infarct myofibroblasts, only Smad3 is involved in integrin upregulation [92].
The role of the Smad1/5/8 cascade in the pathogenesis of post-infarction fibrosis remains unknown. Smad1 activation in the infarcted ventricle may involve induction of both BMPs and TGF-βs. Cardiomyocyte Smad1 has been suggested to play a protective role following ischemic injury through effects that may involve activation of anti-apoptotic signals [135]. Whether these effects result in reduced fibrosis has not been tested. Moreover, direct effects of Smad1 signaling in fibroblast activation have not been explored.
9.2. Smad signaling in fibrotic remodeling of the pressure-overloaded heart
Myocardial activation of the Smad2/3 pathway has been reported in both adult and pediatric patients with heart failure [136]. Adult patients with dilated cardiomyopathy exhibited higher levels of myocardial Smad2/3 activity; this was associated with a more prominent fibrotic response [136]. Left ventricular pressure overload is the predominant pathophysiologic perturbation responsible for cardiac remodeling and fibrosis in patients with chronic hypertension or aortic stenosis. Studies in animal models of pressure overload induced through transverse aortic constriction or through neurohumoral activation showed robust myocardial activation of Smad2 Smad3 [137],[138] and Smad1 [139]. The molecular links between mechanical stress and activation of Smad signaling cascades remain poorly understood, but may involve angiotensin II-mediated actions and subsequent activation and induction of TGF-βs in the cardiac interstitium. Early studies using global loss-of-function approaches suggested that Smad3 mediates fibrosis and ventricular dysfunction in a model of angiotensin II infusion [140], and may promote fibrosis following pressure overload [141]. Moreover, ALK5 inhibition attenuated dysfunction and inhibited fibrosis in rodent models of left ventricular pressure overload; these protective actions were attributed to inhibition of Smad2/3 signaling [142],[143],[144]. However, considering the broad effects of Smad3 on all cells involved in cardiac remodeling and fibrosis (including cardiomyocytes, vascular cells, fibroblasts and immune cells), dissection of cell biological mechanisms responsible for the effects of R-Smads requires cell-specific interventions.
Experiments in fibroblast and myofibroblast-specific knockout mice have suggested an important role for Smad3 signaling in activation of fibroblasts in failing and remodeling hearts. These actions appear to have a major impact on cardiac function. Deletion of Smad3 (but not Smad2) from cardiac fibroblasts attenuated the cardiac fibrotic response to pressure overload. The fibrogenic actions of Smad3 were attributed to increased transcription of extracellular matrix genes, including type I and type III collagens, periostin, fibronectin, tenascin-C, and to upregulation of integrins [96]. In an independent study focusing on the role of fibroblast Smad3 in pressure overload-induced cardiac remodeling, early activation of Smad3 in myofibroblasts protected from systolic dysfunction by preserving the extracellular matrix through downmodulation of collagenases. The findings suggested that Smad3 signaling in activated myofibroblasts plays a crucial role in TGF-β-mediated suppression of the collagenases MMP3 and MMP8 and in upregulation of TIMP1, inhibiting fragmentation of the matrix under conditions of mechanical stress. In the absence of Smad3 in fibroblasts, increased MMP8-mediated proteolytic activity was associated with collagen denaturation and generation of pro-inflammatory matrix fragments that enhance inflammation, promote cardiomyocyte apoptosis and cause dysfunction. [93]. Thus, activated myofibroblasts may play an important protective role in the early stages of cardiac remodeling.
9.3. Smad signaling in fibrosis of the diabetic heart.
Diabetes, obesity and metabolic dysfunction are associated with an increased incidence of fibrosis that involves not only the myocardium [145],[146],[74] but also other organs, such as the liver and kidney [147]. Cardiac fibrosis may contribute to the pathogenesis of diastolic dysfunction and to the development of Heart Failure with Preserved Ejection Fraction (HFpEF), a common form of heart failure in diabetic and obese subjects [148]. Myocardial induction of TGF-β superfamily members and activation of Smad2 and Smad3 cascades have been consistently reported in animal models of type 1 and type 2 diabetes [149],[150],[133],[151]. In contrast, evidence of activation of the Smad1/5/8 cascade in the diabetic heart is lacking; however, Smad1 activation in the diabetic kidney has been implicated in mesangial expansion [152]. Activation of the TGF-β/Smad2/3 axis in the diabetic myocardium may reflect, at least in part, the effects of hyperglycemia on expression of TGF-β receptors [153]. Moreover, diabetes-associated activation of the renin-angiotensin-aldosterone system (RAAS), oxidative stress, chronic stimulation of inflammatory cytokines and protein kinase C (PKC) activation may induce de novo synthesis of TGF-β, or activate latent stores, leading to stimulation of Smad2 and Smad3 signaling cascades [154],[155],[156].
Our knowledge on the role of Smad3 in diabetic cardiac fibrosis is derived from experiments using a global haploinsufficiency model. In the db/db mouse model of obesity-associated type 2 diabetes, Smad3 heterozygotes had attenuated fibrosis and improved diastolic function, associated with reduced collagen deposition and increased MMP activity. The reduction in interstitial collagen in animals with partial loss of Smad3 was associated with mild ventricular dilatation [133]. The findings are consistent with an important role for Smad3 in mediating fibrosis in diabetic hearts. However, considering the broad effects of Smad3 in all myocardial cells, whether these findings reflect Smad-dependent actions on fibroblast activity remains unknown. Moreover, the role of other R-Smads in diabetes-associated fibrosis is not known.
9.4. Smad signaling in aging-associated fibrosis.
Senescence of the heart is associated with structural and morphological changes as hypertrophy and fibrosis that may lead to increased ventricular stiffness and impaired diastolic function [157]. Loss of one TGF-β1 allele in TGF-β1 heterozygous mice ameliorated age-associated myocardial fibrosis and reduced myocardial stiffness [158]. Whether aging-associated fibrosis is due to activation of Smad signaling pathways in fibroblasts remains unknown. Smad signaling in cardiomyocytes plays an important role in cardiac homeostasis in aging hearts [159]. Mice with cardiomyocyte-specific Smad4 loss exhibited increased hypertrophy associated with reduced cardiomyocyte survival and accentuated fibrosis as they aged [160],[159]. Fibrotic remodeling in the absence of cardiomyocyte Smad4 may be due to cell death and subsequent activation of a reparative program, or may reflect paracrine effects of stressed, or injured cardiomyocytes on interstitial fibroblasts.
Moreover, perturbed Smad signaling may explain the impaired reparative response of the senescent heart to injury. 24-month old mice exhibited increased post-infarction ventricular dilation, associated with markedly reduced deposition of collagen in the infarct zone [161]. The pathologic alterations noted in senescent mouse infarcts resembled the pathology of healing infarcts in mice with myofibroblast-specific Smad3 loss [7]. Moreover, fibroblasts harvested from senescent mouse hearts exhibit blunted Smad2 activation in response to TGF-β stimulation [161]. These observations support the intriguing hypothesis that the aging-associated impairment in repair following myocardial infarction may be related to attenuated TGF-β/Smad2/3 activation [162]. Perturbed Smad3 signaling in senescent fibroblasts may reflect a reduced reparative reserve that may be responsible for formation of a disorganized scar, composed of a fragmented matrix network. These age-associated alterations may reduce the tensile strength of the infarct, accentuating adverse remodeling and promoting post-infarction heart failure.
9.5. Smad signaling in myocarditis-induced cardiac fibrosis
Myocardial fibrosis is an important cellular mechanism responsible for development of dysfunction in patients with myocarditis who develop chronic dysfunction and cardiomyopathy. TGF-βs have been implicated in the pathogenesis of myofibroblast activation and fibrosis in models of autoimmune [163] and viral myocarditis[164]. However, evidence supporting the involvement of Smad signaling cascades in myocarditis-induced fibrosis is scarce. In a mouse model of chronic myocarditis due to Chagas disease, ALK5 inhibition was found to attenuate fibrosis[165]. The protective actions were attributed to inhibition of Smad2/3 activity.
9.6. Smad signaling in valve fibrosis
Patients with mitral valve prolapse exhibit myxomatous degeneration of the valve, associated with activation of valve interstitial cells, matrix remodeling and valve fibrosis. In patients with mitral valve prolapse who underwent repair for severe mitral regurgitation, Smad2/3 activation was noted in fibrotic valve tissue and was associated with extracellular matrix deposition [166],[167]. Smad2/3 signaling was implicated in activation of a fibrogenic phenotype in valve interstitial cells [167]. In addition to fibrotic involvement of the mitral valve, patients with mitral valve prolapse also exhibit myocardial interstitial remodeling that is independent of the volume overload associated with severe mitral regurgitation [168]. These myocardial fibrotic changes may explain the high incidence of arrhythmias in patients with severe mitral valve prolapse. It is tempting to hypothesize that a common Smad-dependent mechanism may be involved in the pathogenesis of valvular and ventricular fibrosis in patients with malignant mitral valve prolapse. However, evidence supporting this notion is lacking.
10. Targeting Smads in cardiac fibrosis
TGF-β signaling pathways are critically involved in cardiac fibrosis, remodeling and dysfunction and represent promising therapeutic targets [12],[169],[170]. Targeting R-Smad signaling is feasible; however, the cell-specific and context-dependent actions of Smad cascades and our limited knowledge on the effects of key members of the Smad family hamper therapeutic translation, posing several major challenges. First, Smad3 activation plays distinct roles in various cell types that greatly affect outcome following myocardial injury. In the infarcted heart, fibroblast and macrophage Smad3 activation serves a reparative role, whereas cardiomyocyte Smad3 activation promotes dysfunction [7],[30]. Thus, development of effective therapeutics targeting the Smad3 cascade would require cell-specific interventions. Second, human patients with heart failure or myocardial infarction exhibit remarkable pathophysiologic heterogeneity. Following myocardial infarction, some patients may have excessive pro-inflammatory activation and defective matrix deposition, thus developing dilative ventricular remodeling and systolic dysfunction, while others have accentuated fibrotic responses and may develop diastolic dysfunction. Accentuated fibrosis may involve overactive Smad3 signaling; however effective therapeutic strategies would require identification of fibrosis-prone patients using biomarkers or imaging strategies[171]. Third, the effects of Smad3 inhibition in patients with heart failure may be dependent on the severity of fibrotic remodeling and the phenotype of fibrotic lesions. In chronic hypertensive heart failure, sustained or overactive Smad3 activation in interstitial cells is likely to be detrimental, promoting matrix deposition and accentuating diastolic dysfunction. However, in the early stages of hypertensive cardiac remodeling, Smad3-dependent activation of myofibroblasts may exert protective matrix-preserving actions that preserve the matrix that surrounds the cardiomyocytes and prevent release of pro-inflammatory matrikines [93]. Third, our current knowledge on the role of Smad cascades in myocardial disease is limited to evidence on the role of Smad3 in cardiomyocytes and fibroblasts. We currently have very limited information regarding the role of Smad3 in regulation of lymphocyte, dendritic cell and vascular cell function in the remodeling heart, and we know very little regarding the potential role of endogenous Smad2 and Smad1/5/8 cascades in myocardial disease. Fourth, considering the role of Smad signaling pathways in cardiac and vascular homeostasis and in tissue repair, chronic therapy to inhibit Smad3 or Smad4 may carry significant risks [133],[159].
11. Conclusions:
Smad signaling cascades play a critical role in cardiac fibrotic responses; however, our current understanding of their actions is not sufficient to design therapeutic interventions. Moreover, the context-dependent and cell-specific actions of receptor-activated Smads pose major challenges in therapeutic implementation. Extensive experimental work is needed to study the patterns and mechanisms of Smad activation in myocardial diseases and the role of specific members of the family in regulating phenotype and function of the cells involved in cardiac remodeling. Moreover, clinical investigations are needed to identify heart failure patient subpopulations with perturbed or overactive Smad responses in order to tailor therapeutic interventions.
HIGHLIGHTS.
TGF-β superfamily members signal through Smad-dependent and non-Smad pathways.
Smad3 signaling plays a crucial role in fibroblast activation following myocardial injury.
Myofibroblast-specific Smad3 activation protects the infarcted heart from rupture and adverse remodeling.
Smad2 does not play a crucial role in activation of cardiac fibroblasts in myocardial injury models.
SOURCES OF FUNDING:
Dr. Frangogiannis’ laboratory is supported by NIH R01 grants HL76246, HL85440, and R01 HL149407 and by Department of Defense grants PR151029, PR151134, and PR181464. Dr. Humeres is supported by an American Heart Association post-doctoral award 19POST34450144.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Frangogiannis NG, Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities, Mol Aspects Med, 65 (2019) 70–99. [DOI] [PubMed] [Google Scholar]
- [2].Berk BC, Fujiwara K, Lehoux S, ECM remodeling in hypertensive heart disease, J Clin Invest, 117 (2007) 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gao XM, White DA, Dart AM, Du XJ, Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions, Pharmacol Ther, 134 (2012) 156–179. [DOI] [PubMed] [Google Scholar]
- [4].Hanna A, Shinde AV, Frangogiannis NG, Validation of diagnostic criteria and histopathological characterization of cardiac rupture in the mouse model of nonreperfused myocardial infarction, Am J Physiol Heart Circ Physiol, 319 (2020) H948–H964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Forte E, Skelly DA, Chen M, Daigle S, Morelli KA, Hon O, Philip VM, Costa MW, Rosenthal NA, Furtado MB, Dynamic Interstitial Cell Response during Myocardial Infarction Predicts Resilience to Rupture in Genetically Diverse Mice, Cell Rep, 30 (2020) 3149–3163 e3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, van den Hoff MJ, Ouchi N, Recchia FA, Walsh K, Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture, EMBO Mol Med, 8 (2016) 949–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, Conway SJ, Graff JM, Frangogiannis NG, Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium, Circulation, 137 (2018) 707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nagueh SF, Heart Failure with Preserved Ejection Fraction: Insights into Diagnosis and Pathophysiology, Cardiovasc Res, July 27;cvaa228. doi: 10.1093/cvr/cvaa228. Online ahead of print. (2020). [DOI] [PubMed] [Google Scholar]
- [9].Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM, Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction, Circulation, 131 (2015) 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frangogiannis NG, Transforming Growth Factor (TGF)-beta in tissue fibrosis, J Exp Med, 217 (2020) e20190103. 10.20190110.20191084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lodyga M, Hinz B, TGF-beta1 - A truly transforming growth factor in fibrosis and immunity, Semin Cell Dev Biol, 101 (2020) 123–139. [DOI] [PubMed] [Google Scholar]
- [12].Hanna A, Frangogiannis NG, The Role of the TGF-beta Superfamily in Myocardial Infarction, Front Cardiovasc Med, 6 (2019) 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC, The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury, Circ Res, 98 (2006) 351–360. [DOI] [PubMed] [Google Scholar]
- [14].Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC, GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice, Nat Med, 17 (2011) 581–588. [DOI] [PubMed] [Google Scholar]
- [15].Sanders LN, Schoenhard JA, Saleh MA, Mukherjee A, Ryzhov S, McMaster WG Jr., Nolan K, Gumina RJ, Thompson TB, Magnuson MA, Harrison DG, Hatzopoulos AK, BMP Antagonist Gremlin 2 Limits Inflammation After Myocardial Infarction, Circ Res, 119 (2016) 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG, Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction, Am J Pathol, 164 (2004) 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM, Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing, J Mol Cell Cardiol, 31 (1999) 667–678. [DOI] [PubMed] [Google Scholar]
- [18].Raftery LA, Twombly V, Wharton K, Gelbart WM, Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila, Genetics, 139 (1995) 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kretzschmar M, Massague J, SMADs: mediators and regulators of TGF-beta signaling, Curr Opin Genet Dev, 8 (1998) 103–111. [DOI] [PubMed] [Google Scholar]
- [20].Hata A, Chen YG, TGF-β Signaling from Receptors to Smads, Cold Spring Harb Perspect Biol, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ghosh S, Brauer PR, Latent transforming growth factor-beta is present in the extracellular matrix of embryonic hearts in situ, Dev Dyn, 205 (1996) 126–134. [DOI] [PubMed] [Google Scholar]
- [22].Annes JP, Munger JS, Rifkin DB, Making sense of latent TGFbeta activation, J Cell Sci, 116 (2003) 217–224. [DOI] [PubMed] [Google Scholar]
- [23].Yao Y, Hu C, Song Q, Li Y, Da X, Yu Y, Li H, Clark IM, Chen Q, Wang QK, ADAMTS16 Activates Latent TGF-beta, Accentuating Fibrosis and Dysfunction of the Pressure-overloaded Heart, Cardiovasc Res, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Taimor G, Schluter KD, Frischkopf K, Flesch M, Rosenkranz S, Piper HM, Autocrine regulation of TGF beta expression in adult cardiomyocytes, J Mol Cell Cardiol, 31 (1999) 2127–2136. [DOI] [PubMed] [Google Scholar]
- [25].Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, Olsen AL, Im M, Lodyga M, Wells RG, White ES, Hinz B, The fibronectin ED-A domain enhances recruitment of latent TGF-beta-binding protein-1 to the fibroblast matrix, J Cell Sci, 131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE, Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions, Biochem Biophys Res Commun, 339 (2006) 633–641. [DOI] [PubMed] [Google Scholar]
- [27].Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML, The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts, Circulation, 111 (2005) 2935–2942. [DOI] [PubMed] [Google Scholar]
- [28].Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B, Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction, Cardiovasc Res, 102 (2014) 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyer A, Wang W, Qu JX, Croft L, Degen JL, Coller BS, Ahamed J, Platelet TGF-beta 1 contributions to plasma TGF-beta 1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload, Blood, 119 (2012) 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen B, Huang S, Su Y, Wu YJ, Hanna A, Brickshawana A, Graff J, Frangogiannis NG, Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation, Circ Res, 125 (2019) 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen Y, Rothnie C, Spring D, Verrier E, Venardos K, Kaye D, Phillips DJ, Hedger MP, Smith JA, Regulation and actions of activin A and follistatin in myocardial ischaemia-reperfusion injury, Cytokine, 69 (2014) 255–262. [DOI] [PubMed] [Google Scholar]
- [32].Massague J, How cells read TGF-beta signals, Nat Rev Mol Cell Biol, 1 (2000) 169–178. [DOI] [PubMed] [Google Scholar]
- [33].Heldin CH, Moustakas A, Signaling Receptors for TGF-β Family Members, Cold Spring Harb Perspect Biol, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rahimi RA, Leof EB, TGF-beta signaling: a tale of two responses, J Cell Biochem, 102 (2007) 593–608. [DOI] [PubMed] [Google Scholar]
- [35].Eickelberg O, Centrella M, Reiss M, Kashgarian M, Wells RG, Betaglycan inhibits TGF-beta signaling by preventing type I-type II receptor complex formation. Glycosaminoglycan modifications alter betaglycan function, J Biol Chem, 277 (2002) 823–829. [DOI] [PubMed] [Google Scholar]
- [36].Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P, Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors, Embo J, 21 (2002) 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang H, Du L, Zhong Y, Flanders KC, Roberts JD Jr., Transforming growth factor-beta stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1, Am J Physiol Lung Cell Mol Physiol, 313 (2017) L615–L627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nurgazieva D, Mickley A, Moganti K, Ming W, Ovsyi I, Popova A, Sachindra K Awad, Wang N, Bieback K, Goerdt S, Kzhyshkowska J, Gratchev A, TGF-beta1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes, J Immunol, 194 (2015) 709–718. [DOI] [PubMed] [Google Scholar]
- [39].Daly AC, Randall RA, Hill CS, Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth, Mol Cell Biol, 28 (2008) 6889–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feng XH, Derynck R, Specificity and versatility in tgf-beta signaling through Smads, Annu Rev Cell Dev Biol, 21 (2005) 659–693. [DOI] [PubMed] [Google Scholar]
- [41].Miyazono K, Maeda S, Imamura T, BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk, Cytokine Growth Factor Rev, 16 (2005) 251–263. [DOI] [PubMed] [Google Scholar]
- [42].Heldin CH, Moustakas A, Signaling Receptors for TGF-beta Family Members, Cold Spring Harb Perspect Biol, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xiao Z, Latek R, Lodish HF, An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity, Oncogene, 22 (2003) 1057–1069. [DOI] [PubMed] [Google Scholar]
- [44].Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P, Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction, Embo J, 23 (2004) 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Leask A, Abraham DJ, Finlay DR, Holmes A, Pennington D, Shi-Wen X, Chen Y, Venstrom K, Dou X, Ponticos M, Black C, Bernabeu C, Jackman JK, Findell PR, Connolly MK, Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts, Arthritis Rheum, 46 (2002) 1857–1865. [DOI] [PubMed] [Google Scholar]
- [46].Rodriguez-Pena A, Eleno N, Duwell A, Arevalo M, Perez-Barriocanal F, Flores O, Docherty N, Bernabeu C, Letarte M, Lopez-Novoa JM, Endoglin upregulation during experimental renal interstitial fibrosis in mice, Hypertension, 40 (2002) 713–720. [DOI] [PubMed] [Google Scholar]
- [47].You HJ, Bruinsma MW, How T, Ostrander JH, Blobe GC, The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation, Carcinogenesis, 28 (2007) 2491–2500. [DOI] [PubMed] [Google Scholar]
- [48].Tazat K, Hector-Greene M, Blobe GC, Henis YI, TbetaRIII independently binds type I and type II TGF-beta receptors to inhibit TGF-beta signaling, Mol Biol Cell, 26 (2015) 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Villalobos E, Criollo A, Schiattarella GG, Altamirano F, French KM, May HI, Jiang N, Nguyen NUN, Romero D, Roa JC, Garcia L, Diaz-Araya G, Morselli E, Ferdous A, Conway SJ, Sadek HA, Gillette TG, Lavandero S, Hill JA, Fibroblast Primary Cilia Are Required for Cardiac Fibrosis, Circulation, 139 (2019) 2342–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG, CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response, J Immunol, 180 (2008) 2625–2633. [DOI] [PubMed] [Google Scholar]
- [51].Sekiya T, Oda T, Matsuura K, Akiyama T, Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by TGF-beta signaling, Biochem Biophys Res Commun, 320 (2004) 680–684. [DOI] [PubMed] [Google Scholar]
- [52].Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, Chen YG, Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling, J Biol Chem, 284 (2009) 30097–30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Villar AV, Garcia R, Llano M, Cobo M, Merino D, Lantero A, Tramullas M, Hurle JM, Hurle MA, Nistal JF, BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-beta signaling, Biochim Biophys Acta, 1832 (2013) 323–335. [DOI] [PubMed] [Google Scholar]
- [54].Miyazawa K, Miyazono K, Regulation of TGF-β Family Signaling by Inhibitory Smads, Cold Spring Harb Perspect Biol, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Luo K, Ski and SnoN: negative regulators of TGF-beta signaling, Curr Opin Genet Dev, 14 (2004) 65–70. [DOI] [PubMed] [Google Scholar]
- [56].Funaba M, Zimmerman CM, Mathews LS, Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase, J Biol Chem, 277 (2002) 41361–41368. [DOI] [PubMed] [Google Scholar]
- [57].Liu S, Xu SW, Kennedy L, Pala D, Chen Y, Eastwood M, Carter DE, Black CM, Abraham DJ, Leask A, FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype, Mol Biol Cell, 18 (2007) 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shi-wen X, Parapuram SK, Pala D, Chen Y, Carter DE, Eastwood M, Denton CP, Abraham DJ, Leask A, Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts, Arthritis Rheum, 60 (2009) 234–241. [DOI] [PubMed] [Google Scholar]
- [59].Hough C, Radu M, Dore JJ, Tgf-beta induced Erk phosphorylation of smad linker region regulates smad signaling, PLoS One, 7 (2012) e42513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Leivonen SK, Hakkinen L, Liu D, Kahari VM, Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts, J Invest Dermatol, 124 (2005) 1162–1169. [DOI] [PubMed] [Google Scholar]
- [61].Dolivo DM, Larson SA, Dominko T, Crosstalk between mitogen-activated protein kinase inhibitors and transforming growth factor-beta signaling results in variable activation of human dermal fibroblasts, Int J Mol Med, 43 (2019) 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gowripalan A, Abbott CR, McKenzie C, Chan WS, Karupiah G, Levy L, Newsome TP, Cell-to-cell spread of vaccinia virus is promoted by TGF-beta-independent Smad4 signalling, Cell Microbiol, 22 (2020) e13206. [DOI] [PubMed] [Google Scholar]
- [63].Wang Y, Chu J, Yi P, Dong W, Saultz J, Wang Y, Wang H, Scoville S, Zhang J, Wu LC, Deng Y, He X, Mundy-Bosse B, Freud AG, Wang LS, Caligiuri MA, Yu J, SMAD4 promotes TGF-beta-independent NK cell homeostasis and maturation and antitumor immunity, J Clin Invest, 128 (2018) 5123–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chung AC, Zhang H, Kong YZ, Tan JJ, Huang XR, Kopp JB, Lan HY, Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling, J Am Soc Nephrol, 21 (2010) 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hao J, Wang B, Jones SC, Jassal DS, Dixon IM, Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro, Am J Physiol Heart Circ Physiol, 279 (2000) H3020–3030. [DOI] [PubMed] [Google Scholar]
- [66].AlQudah M, Hale TM, Czubryt MP, Targeting the renin-angiotensin-aldosterone system in fibrosis, Matrix Biol, 91–92 (2020) 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Humeres C, Frangogiannis NG, Fibroblasts in the Infarcted, Remodeling, and Failing Heart, JACC Basic Transl Sci, 4 (2019) 449–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ, Collagen remodeling after myocardial infarction in the rat heart, Am J Pathol, 147 (1995) 325–338. [PMC free article] [PubMed] [Google Scholar]
- [69].Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA, Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction, J Clin Invest, 98 (1996) 2218–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Komatsubara I, Murakami T, Kusachi S, Nakamura K, Hirohata S, Hayashi J, Takemoto S, Suezawa C, Ninomiya Y, Shiratori Y, Spatially and temporally different expression of osteonectin and osteopontin in the infarct zone of experimentally induced myocardial infarction in rats, Cardiovasc Pathol, 12 (2003) 186–194. [DOI] [PubMed] [Google Scholar]
- [71].Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, Karvonen H, Hinz B, The myofibroblast at a glance, J Cell Sci, 133 (2020). [DOI] [PubMed] [Google Scholar]
- [72].Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT, Regulation of collagen degradation in the rat myocardium after infarction, J Mol Cell Cardiol, 27 (1995) 1281–1292. [DOI] [PubMed] [Google Scholar]
- [73].Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z, Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner, Am J Physiol Cell Physiol, 298 (2010) C679–692. [DOI] [PubMed] [Google Scholar]
- [74].Alex L, Russo I, Holoborodko V, Frangogiannis NG, Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction, Am J Physiol Heart Circ Physiol, 315 (2018) H934–H949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE, Harvey RP, Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury, Elife, 8:e43882. doi: 10.7554/eLife.43882. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McLellan MA, Skelly DA, Dona MSI, Squiers GT, Farrugia GE, Gaynor TL, Cohen CD, Pandey R, Diep H, Vinh A, Rosenthal NA, Pinto AR, High-Resolution Transcriptomic Profiling of the Heart During Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy, Circulation, doi: 10.1161/CIRCULATIONAHA.119.045115. Online ahead of print. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P, Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure, J Exp Med, 214 (2017) 3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Okyere AD, Tilley DG, Leukocyte-Dependent Regulation of Cardiac Fibrosis, Front Physiol, 11 (2020) 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P, Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure, Circ Heart Fail, 8 (2015) 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hartupee J, Mann DL, Role of inflammatory cells in fibroblast activation, J Mol Cell Cardiol, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS, Cardiac mast cells: the centrepiece in adverse myocardial remodelling, Cardiovasc Res, 89 (2012) 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML, Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion, Circulation, 98 (1998) 687–698. [DOI] [PubMed] [Google Scholar]
- [83].Liu Q, Zhu LJ, Waaga-Gasser AM, Ding Y, Cao M, Jadhav SJ, Kirollos S, Shekar PS, Padera RF, Chang YC, Xu X, Zeisberg EM, Charytan DM, Hsiao LL, The axis of local cardiac endogenous Klotho-TGF-beta1-Wnt signaling mediates cardiac fibrosis in human, J Mol Cell Cardiol, 136 (2019) 113–124. [DOI] [PubMed] [Google Scholar]
- [84].Flevaris P, Khan SS, Eren M, Schuldt AJT, Shah SJ, Lee DC, Gupta S, Shapiro AD, Burridge PW, Ghosh AK, Vaughan DE, Plasminogen Activator Inhibitor Type I Controls Cardiomyocyte Transforming Growth Factor-beta and Cardiac Fibrosis, Circulation, 136 (2017) 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R, Endothelial-to-mesenchymal transition contributes to cardiac fibrosis, Nat Med, 13 (2007) 952–961. [DOI] [PubMed] [Google Scholar]
- [86].Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, Hirata K, Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition, Circulation, 121 (2010) 2407–2418. [DOI] [PubMed] [Google Scholar]
- [87].Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK, Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition, Dis Model Mech, 4 (2011) 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R, Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation, Circ Res, 115 (2014) 625–635. [DOI] [PubMed] [Google Scholar]
- [89].Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM, Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis, J Clin Invest, 124 (2014) 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD, Molkentin JD, Genetic lineage tracing defines myofibroblast origin and function in the injured heart, Nat Commun, 7 (2016) 12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG, Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling, Circulation, 116 (2007) 2127–2138. [DOI] [PubMed] [Google Scholar]
- [92].Huang S, Chen B, Su Y, Alex L, Humeres C, Shinde AV, Conway SJ, Frangogiannis NG, Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart, J Mol Cell Cardiol, 132 (2019) 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Russo I, Cavalera M, Huang S, Su Y, Hanna A, Chen B, Shinde AV, Conway SJ, Graff J, Frangogiannis NG, Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program, Circ Res, 124 (2019) 1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG, Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction, Circ Res, 107 (2010) 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cogan JG, Subramanian SV, Polikandriotis JA, Kelm RJ Jr., Strauch AR, Vascular smooth muscle alpha-actin gene transcription during myofibroblast differentiation requires Sp1/3 protein binding proximal to the MCAT enhancer, J Biol Chem, 277 (2002) 36433–36442. [DOI] [PubMed] [Google Scholar]
- [96].Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD, Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis, J Clin Invest, 127 (2017) 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G, The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1, J Cell Biol, 142 (1998) 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vivar R, Humeres C, Anfossi R, Bolivar S, Catalan M, Hill J, Lavandero S, Diaz-Araya G, Role of FoxO3a as a negative regulator of the cardiac myofibroblast conversion induced by TGF-beta1, Biochim Biophys Acta Mol Cell Res, 1867 (2020) 118695. [DOI] [PubMed] [Google Scholar]
- [99].Koo JB, Nam MO, Jung Y, Yoo J, Kim DH, Kim G, Shin SJ, Lee KM, Hahm KB, Kim JW, Hong SP, Lee KJ, Yoo JH, Anti-fibrogenic effect of PPAR-gamma agonists in human intestinal myofibroblasts, BMC Gastroenterol, 17 (2017) 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG, Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling, Circulation, 116 (2007) 2127–2138. [DOI] [PubMed] [Google Scholar]
- [101].Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD, Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis, J Clin Invest, 127 (2017) 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD, Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling, Cytokine, 55 (2011) 90–97. [DOI] [PubMed] [Google Scholar]
- [103].Shinde AV, Dobaczewski M, de Haan JJ, Saxena A, Lee KK, Xia Y, Chen W, Su Y, Hanif W, Kaur Madahar I, Paulino VM, Melino G, Frangogiannis NG, Tissue transglutaminase induction in the pressure-overloaded myocardium regulates matrix remodelling, Cardiovasc Res, 113 (2017) 892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA, α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy, Cardiovasc Res, 96 (2012) 265–275. [DOI] [PubMed] [Google Scholar]
- [105].Shinde AV, Humeres C, Frangogiannis NG, The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling, Biochim Biophys Acta, 1863 (2017) 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Vivar R, Humeres C, Ayala P, Olmedo I, Catalan M, Garcia L, Lavandero S, Diaz-Araya G, TGF-beta1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways, Biochim Biophys Acta, 1832 (2013) 754–762. [DOI] [PubMed] [Google Scholar]
- [107].Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL, Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts, Cardiovasc Res, 91 (2011) 80–89. [DOI] [PubMed] [Google Scholar]
- [108].Bagchi RA, Czubryt MP, Synergistic roles of scleraxis and Smads in the regulation of collagen 1alpha2 gene expression, Biochim Biophys Acta, 1823 (2012) 1936–1944. [DOI] [PubMed] [Google Scholar]
- [109].Huang S, Chen B, Humeres C, Alex L, Hanna A, Frangogiannis NG, The role of Smad2 and Smad3 in regulating homeostatic functions of fibroblasts in vitro and in adult mice, Biochim Biophys Acta Mol Cell Res, 1867 (2020) 118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schwartze JT, Becker S, Sakkas E, Wujak LA, Niess G, Usemann J, Reichenberger F, Herold S, Vadasz I, Mayer K, Seeger W, Morty RE, Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-beta signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts, J Biol Chem, 289 (2014) 3262–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M, Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways, J Biol Chem, 282 (2007) 10405–10413. [DOI] [PubMed] [Google Scholar]
- [112].Chen X, Xu J, Jiang B, Liu D, Bone Morphogenetic Protein-7 Antagonizes Myocardial Fibrosis Induced by Atrial Fibrillation by Restraining Transforming Growth Factor-beta (TGF-beta)/Smads Signaling, Med Sci Monit, 22 (2016) 3457–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Miyazawa K, Miyazono K, Regulation of TGF-beta Family Signaling by Inhibitory Smads, Cold Spring Harb Perspect Biol, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K, The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling, J Cell Biol, 155 (2001) 1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kamiya Y, Miyazono K, Miyazawa K, Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction, J Biol Chem, 285 (2010) 30804–30813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K, Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors, J Biol Chem, 282 (2007) 20603–20611. [DOI] [PubMed] [Google Scholar]
- [117].Mochizuki T, Miyazaki H, Hara T, Furuya T, Imamura T, Watabe T, Miyazono K, Roles for the MH2 domain of Smad7 in the specific inhibition of transforming growth factor-beta superfamily signaling, J Biol Chem, 279 (2004) 31568–31574. [DOI] [PubMed] [Google Scholar]
- [118].Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K, Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts, J Clin Invest, 113 (2004) 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T, Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads, Mol Biol Cell, 14 (2003) 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hata A, Lagna G, Massague J, Hemmati-Brivanlou A, Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor, Genes Dev, 12 (1998) 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM, Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart, Am J Physiol Heart Circ Physiol, 282 (2002) H1685–1696. [DOI] [PubMed] [Google Scholar]
- [122].Wang B, Omar A, Angelovska T, Drobic V, Rattan SG, Jones SC, Dixon IM, Regulation of collagen synthesis by inhibitory Smad7 in cardiac myofibroblasts, Am J Physiol Heart Circ Physiol, 293 (2007) H1282–1290. [DOI] [PubMed] [Google Scholar]
- [123].Yu H, Zhao G, Li H, Liu X, Wang S, Candesartan antagonizes pressure overload-evoked cardiac remodeling through Smad7 gene-dependent MMP-9 suppression, Gene, 497 (2012) 301–306. [DOI] [PubMed] [Google Scholar]
- [124].He X, Gao X, Peng L, Wang S, Zhu Y, Ma H, Lin J, Duan DD, Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7, Circ Res, 108 (2011) 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY, Heuchel R, Yan BP, Yu CM, Lan HY, Deficiency of Smad7 enhances cardiac remodeling induced by angiotensin II infusion in a mouse model of hypertension, PLoS One, 8 (2013) e70195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY, Yan BP, Yu CM, Lan HY, Smad7 inhibits angiotensin II-induced hypertensive cardiac remodelling, Cardiovasc Res, 99 (2013) 665–673. [DOI] [PubMed] [Google Scholar]
- [127].Li R, Rosendahl A, Brodin G, Cheng AM, Ahgren A, Sundquist C, Kulkarni S, Pawson T, Heldin CH, Heuchel RL, Deletion of exon I of SMAD7 in mice results in altered B cell responses, J Immunol, 176 (2006) 6777–6784. [DOI] [PubMed] [Google Scholar]
- [128].Li J, Qu X, Yao J, Caruana G, Ricardo SD, Yamamoto Y, Yamamoto H, Bertram JF, Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy, Diabetes, 59 (2010) 2612–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Quijada P, Misra A, Velasquez LS, Burke RM, Lighthouse JK, Mickelsen DM, Dirkx RA Jr., Small EM, Pre-existing fibroblasts of epicardial origin are the primary source of pathological fibrosis in cardiac ischemia and aging, J Mol Cell Cardiol, 129 (2019) 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Dunker N, Krieglstein K, Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis, Eur J Biochem, 267 (2000) 6982–6988. [DOI] [PubMed] [Google Scholar]
- [131].Prabhu SD, Frangogiannis NG, The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis, Circ Res, 119 (2016) 91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Shinde AV, Frangogiannis NG, Fibroblasts in myocardial infarction: A role in inflammation and repair, J Mol Cell Cardiol, 70C (2014) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG, Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice, Circ Heart Fail, 8 (2015) 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Blankesteijn WM, Essers-Janssen YP, Verluyten MJ, Daemen MJ, Smits JF, A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart, Nat Med, 3 (1997) 541–544. [DOI] [PubMed] [Google Scholar]
- [135].Masaki M, Izumi M, Oshima Y, Nakaoka Y, Kuroda T, Kimura R, Sugiyama S, Terai K, Kitakaze M, Yamauchi-Takihara K, Kawase I, Hirota H, Smad1 protects cardiomyocytes from ischemia-reperfusion injury, Circulation, 111 (2005) 2752–2759. [DOI] [PubMed] [Google Scholar]
- [136].Jana S, Zhang H, Lopaschuk GD, Freed DH, Sergi C, Kantor PF, Oudit GY, Kassiri Z, Disparate Remodeling of the Extracellular Matrix and Proteoglycans in Failing Pediatric Versus Adult Hearts, J Am Heart Assoc, 7 (2018) e010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yagi S, Aihara K, Ikeda Y, Sumitomo Y, Yoshida S, Ise T, Iwase T, Ishikawa K, Azuma H, Akaike M, Matsumoto T, Pitavastatin, an HMG-CoA reductase inhibitor, exerts eNOS-independent protective actions against angiotensin II induced cardiovascular remodeling and renal insufficiency, Circ Res, 102 (2008) 68–76. [DOI] [PubMed] [Google Scholar]
- [138].Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF, Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts, Circ Res, 102 (2008) 185–192. [DOI] [PubMed] [Google Scholar]
- [139].Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG, Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload, Histochem Cell Biol, 131 (2009) 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, Tse HF, Lan HY, Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling, Hypertension, 55 (2010) 1165–1171. [DOI] [PubMed] [Google Scholar]
- [141].Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N, Mann DL, Adaptive and maladptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading, Circ Heart Fail, 2 (2009) 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Engebretsen KV, Skardal K, Bjornstad S, Marstein HS, Skrbic B, Sjaastad I, Christensen G, Bjornstad JL, Tonnessen T, Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5, J Mol Cell Cardiol, 76 (2014) 148–157. [DOI] [PubMed] [Google Scholar]
- [143].Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ, Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction, Am J Physiol Heart Circ Physiol, 298 (2010) H1415–1425. [DOI] [PubMed] [Google Scholar]
- [144].Bjornstad JL, Skrbic B, Marstein HS, Hasic A, Sjaastad I, Louch WE, Florholmen G, Christensen G, Tonnessen T, Inhibition of SMAD2 phosphorylation preserves cardiac function during pressure overload, Cardiovasc Res, 93 (2012) 100–110. [DOI] [PubMed] [Google Scholar]
- [145].Russo I, Frangogiannis NG, Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities, J Mol Cell Cardiol, 90 (2016) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cavalera M, Wang J, Frangogiannis NG, Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities, Transl Res, 164 (2014) 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sharma K, Obesity, oxidative stress, and fibrosis in chronic kidney disease, Kidney Int Suppl (2011), 4 (2014) 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Packer M, Kitzman DW, Obesity-Related Heart Failure With a Preserved Ejection Fraction: The Mechanistic Rationale for Combining Inhibitors of Aldosterone, Neprilysin, and Sodium-Glucose Cotransporter-2, JACC Heart Fail, 6 (2018) 633–639. [DOI] [PubMed] [Google Scholar]
- [149].Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA, alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy, Cardiovasc Res, 96 (2012) 265–275. [DOI] [PubMed] [Google Scholar]
- [150].Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde A, Frangogiannis NG, Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation, Circ Res, 113 (2013) 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ares-Carrasco S, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sanchez-Nino MD, Ortiz A, Egido J, Tunon J, Lorenzo O, Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes, Am J Physiol Heart Circ Physiol, 297 (2009) H2109–2119. [DOI] [PubMed] [Google Scholar]
- [152].Matsubara T, Araki M, Abe H, Ueda O, Jishage K, Mima A, Goto C, Tominaga T, Kinosaki M, Kishi S, Nagai K, Iehara N, Fukushima N, Kita T, Arai H, Doi T, Bone Morphogenetic Protein 4 and Smad1 Mediate Extracellular Matrix Production in the Development of Diabetic Nephropathy, Diabetes, 64 (2015) 2978–2990. [DOI] [PubMed] [Google Scholar]
- [153].Wu L, Derynck R, Essential role of TGF-beta signaling in glucose-induced cell hypertrophy, Dev Cell, 17 (2009) 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Connelly KA, Kelly DJ, Zhang Y, Prior DL, Advani A, Cox AJ, Thai K, Krum H, Gilbert RE, Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy, Circ Heart Fail, 2 (2009) 129–137. [DOI] [PubMed] [Google Scholar]
- [155].Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L, SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart, Cardiovasc Diabetol, 18 (2019) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR, Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females, Hypertension, 66 (2015) 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Biernacka A, Frangogiannis NG, Aging and Cardiac Fibrosis, Aging Dis, 2 (2011) 158–173. [PMC free article] [PubMed] [Google Scholar]
- [158].Brooks WW, Conrad CH, Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice, J Mol Cell Cardiol, 32 (2000) 187–195. [DOI] [PubMed] [Google Scholar]
- [159].Umbarkar P, Singh AP, Gupte M, Verma VK, Galindo CL, Guo Y, Zhang Q, McNamara JW, Force T, Lal H, Cardiomyocyte SMAD4-Dependent TGF-beta Signaling is Essential to Maintain Adult Heart Homeostasis, JACC Basic Transl Sci, 4 (2019) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X, Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure, Circ Res, 97 (2005) 821–828. [DOI] [PubMed] [Google Scholar]
- [161].Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG, Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction, J Am Coll Cardiol, 51 (2008) 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Chen B, Huang S, Frangogiannis NG, Aging, cardiac repair and Smad3, Aging (Albany NY), 10 (2018) 2230–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Blyszczuk P, Muller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, Glatz K, Basler K, Luscher TF, Distler O, Eriksson U, Kania G, Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis, Eur Heart J, 38 (2017) 1413–1425. [DOI] [PubMed] [Google Scholar]
- [164].Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang CB, Yang Y, Zou Y, Ge J, Chen R, Chen H, Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-beta1 signaling in coxsackievirus B3-induced cardiomyopathy, Eur J Pharmacol, 658 (2011) 168–174. [DOI] [PubMed] [Google Scholar]
- [165].Ferreira RR, Abreu RDS, Vilar-Pereira G, Degrave W, Meuser-Batista M, Ferreira NVC, da Cruz Moreira O, da Silva Gomes NL, Mello de Souza E, Ramos IP, Bailly S, Feige JJ, Lannes-Vieira J, de Araújo-Jorge TC, Waghabi MC, TGF-β inhibitor therapy decreases fibrosis and stimulates cardiac improvement in a pre-clinical study of chronic Chagas’ heart disease, PLoS Negl Trop Dis, 13 (2019) e0007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G, Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers, Circulation, 126 (2012) S189–197. [DOI] [PubMed] [Google Scholar]
- [167].Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD, TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves, Cardiovasc Res, 99 (2013) 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, Shah DJ, Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse, J Am Coll Cardiol, 72 (2018) 823–834. [DOI] [PubMed] [Google Scholar]
- [169].Frangogiannis NG, Targeting the transforming growth factor (TGF)-beta cascade in the remodeling heart: benefits and perils, J Mol Cell Cardiol, 76 (2014) 169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Huynh LK, Hipolito CJ, Ten Dijke P, A Perspective on the Development of TGF-beta Inhibitors for Cancer Treatment, Biomolecules, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Frangogiannis NG, The inflammatory response in myocardial injury, repair, and remodelling, Nat Rev Cardiol, 11 (2014) 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]