Abstract
Autoinflammatory diseases are conditions in which pathogenic inflammation arises primarily through antigen-independent hyperactivation of immune pathways. First recognized just over two decades ago, the autoinflammatory disease spectrum has expanded rapidly to include more than 40 distinct monogenic conditions. Related mechanisms contribute to common conditions such as gout and cardiovascular disease. Here we review the basic concepts underlying the “autoinflammatory revolution” in the understanding of immune-mediated disease and introduce major categories of monogenic autoinflammatory disorders recognized to date, including inflammasomopathies and other IL-1-related conditions; interferonopathies; and disorders of NFκB and/or aberrant TNF activity. We highlight phenotypic presentation as a reflection of pathogenesis and outline a practical approach to the evaluation of patients with suspected autoinflammation.
Keywords: autoinflammation, innate immunity, inflammasome, interferon, NFκB
Introduction: Autoimmunity vs. Autoinflammation
In 1901, the German immunologist Paul Ehrlich recognized an important theoretical downside to the immune system’s capacity to recognize specific targets – namely, the possibility that errors could translate into immune attack on self. He employed the now-famous phrase “horror autotoxicus” to describe what he presumed to be an absolute aversion to such self-targeting.1 However, evidence in favor of immune mistakes emerged rapidly, and by the 1950s disease-causing autoantibodies had been clearly demonstrated.2–4 Autoimmunity is now recognized as a core mechanism of human disease, arising when antigen-specific components of adaptive immunity – T cells, B cell and antibodies – mistakenly target autologous tissues as though they were foreign. Hundreds of autoimmune diseases are now recognized, impacting every organ system and affecting at least 3% of the population.5 Allergic diseases similarly reflect aberrant antigen-directed immunity, albeit directed against innocuous targets from the environment.
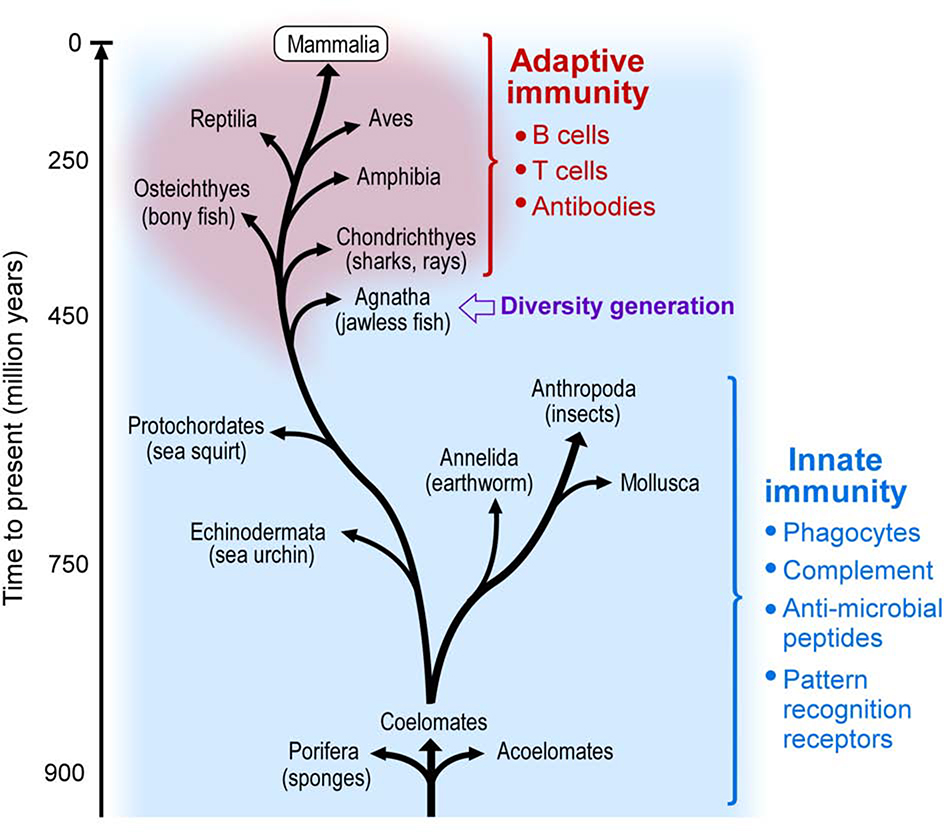
However, adaptive immunity is only one arm of immune defense. From an evolutionary point of view, the ability to generate antigen specificity through genetic recombination was a late development, originating in distinct but related systems in the jawless and jawed vertebrates approximately 500 million years ago (Figure 1).6 For the vast majority of species, immunity against pathogens is “hard-wired” into the organism’s genetic code. In humans, innate immune mechanisms include lineages such as neutrophils, macrophages, mast cells, NK cells, and innate-like lymphocytes; cell-surface and intracellular pattern recognition mechanisms such as Toll-like receptors; and soluble defensive proteins such as C reactive protein and complement. If inflammatory disease can arise through adaptive immune mistakes, it is plausible to suppose that it might also develop though dysfunction in these antigen-independent pathways.
Figure 1. Innate and adaptive immunity in evolution.
Simplified evolutionary tree of animal phyla depicting the development of adaptive immunity at the stage of jawless fishes. Adapted from Porcelli SA, “Innate immunity” in Kelley and Firestein’s Textbook of Rheumatology, Eds: Firestein GS, Budd R, Gabriel SE, McInnes IB, O’Dell JR,10th ed. 2017 with permission.
Obvious as this possibility appears in retrospect, it is only in the last two decades that diseases originating though excesses of innate immunity have been recognized. This new understanding began with the discovery that familial Mediterranean fever (FMF) arose from mutations in MEFV, encoding pyrin, a protein expressed predominately by innate lineages.7, 8 Subsequent genetic dissection of a second heritable inflammatory disease, TNF Receptor Associated Periodic Syndrome (TRAPS) arising from mutant TNFRSF1A, enabled the insight that FMF and TRAPS represented a new category of disease.9 Daniel Kastner and colleagues originated the concept of autoinflammatory disease to denote inflammatory disorders that arise through mechanisms distinct from autoimmunity and distinguished by features such as absence of autoantibodies.9
Since its introduction in 1999, the term “autoinflammation” has been employed widely but variably. Here we define an autoinflammatory disease as one in which pathogenic inflammation arises primarily through antigen-independent hyperactivation of immune pathways. The monogenic autoinflammatory diseases represent loss-of-function mutations in genes that suppress inflammation or gain-of-function mutations in genes that propagate inflammation, resulting in immune activation spontaneously or with minimal triggering. Broadly, autoinflammatory diseases reflect disorders of innate immunity while autoimmune and allergic diseases represent disorders of adaptive immunity. However, this simplistic division is at best a first approximation. Innate and adaptive immunity are densely interconnected, and dysfunction in one often disturbs function in the other. For example, some diseases now considered autoinflammatory feature autoantibodies. T and B cells may mediate and theoretically even initiate autoinflammatory diseases, as long as activation does not reflect antigen-targeted misrecognition of self. While the clearest examples of autoinflammation so far have been monogenic, it is likely that there are also autoinflammatory diseases that arise through defects in many genes (polygenic).
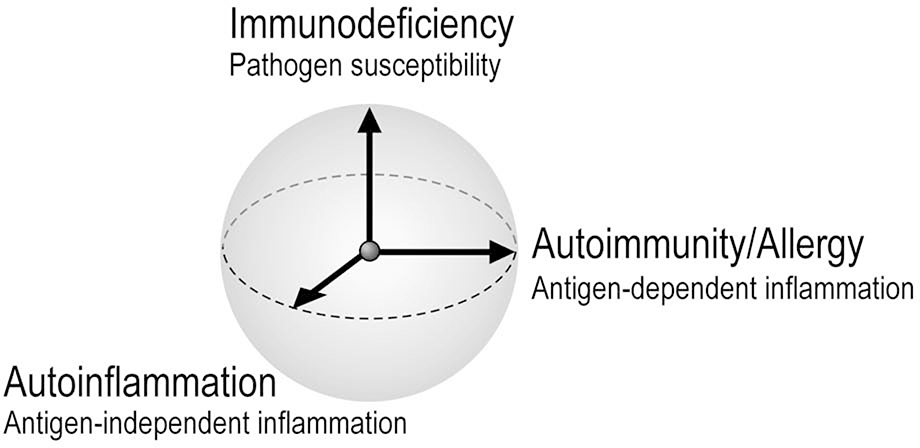
Autoinflammation represents one axis of immune dysfunction, together with autoimmunity/allergy and immunodeficiency (Figure 2). Allergists/immunologists are likely to encounter autoinflammation in the course of the evaluation of patients with fever, rash, lung disease, and other disease manifestations that can reflect aberrant immunity. Patients with primary immunodeficiencies may exhibit autoinflammation in addition to impaired immunocompetence, and patients with autoinflammation may paradoxically experience recurrent infections. For these reasons, as specialists in immune dysfunction, allergists and immunologists need to be prepared to provide a medical home to these complex patients.
Figure 2. Three axes of immune dysfunction.
Schematic representation of conceptually orthogonal ways in which dysregulated immunity leads to human disease: autoinflammation (aberrant antigen-independent immune activation), autoimmunity / allergy (aberrant antigen-dependent immune activation), and immunodeficiency (defects in innate or adaptive immunity resulting in inadequate defense against pathogens). For simplicity, the axes are depicted as beginning at a common origin reflecting normal immune function. However, some immune states might potentially cross this origin, as for example enhanced resistance to plague in individuals bearing mutant MEFV.
Categories of autoinflammatory diseases
Theoretically, there could be as many autoinflammatory diseases as there are immune pathways. Indeed, the last decade has witnessed an explosion of new autoinflammatory disorders, and more remain to be discovered. Many of these conditions engage multiple pathways and fit into several pathogenic categories.10 The first autoinflammatory disease recognized, FMF, is an inflammasomopathy; other autoinflammatory diseases arise through defects affecting interferon, NFκB and/or aberrant TNF activity, and miscellaneous mechanisms (Table 1). New categories of autoinflammatory disease will no doubt emerge over time.
Table 1. The monogenic autoinflammatory diseases.
Presented are a simplified representation of disease mechanism classification, causative gene, heritability, major clinical manifestations, and typical treatment options for a representative range of monogenic autoinflammatory diseases.
| Mechanism | Disease | Gene | Inheritance | Clinical presentation | Targeted therapy | |
|---|---|---|---|---|---|---|
| Inflammasomopathies and other IL-1 family conditions | Pyrin activation | FMF | MEFV | AR or AD | fever, pain, (abdominal, chest, joint), rash | IL-1, colch. |
| PAAND | MEFV | AD | fever, myalgia, myositis, rash, abscesses | IL-1, colch. | ||
| MKD | MVK | AR | fever, pain (abdominal, extremity), vomiting, rash | IL-1 | ||
| PAPA | PSTPIP1 | AD | pyoderma gangrenosum, arthritis | IL-1, TNF | ||
| Hz/Hc127 | PSTPIP1 | AD | rash, FTT, hepatosplenomegaly, neutropenia | IL-1, TNF | ||
| PFIT128 | WDR1 | AR | fever, infection, oral inflammation, perianal ulceration | IL-18 | ||
| Cryopyrin activation | FCAS | NLRP3 | AD | cold urticaria, extremity pain, conjunctivitis, fever | IL-1 | |
| MWS | NLRP3 | AD | urticarial rash, extremity pain, hearing loss, conjunctivitis, fever | IL-1 | ||
| NOMID | NLRP3 | AD | CNS inflammation, urticaria, knee arthropathy, fever | IL-1 | ||
| Majeed’s129 | LPIN2 | AR | osteomyelitis, fevers, rash, dyserythropoietic anemia | IL-1 | ||
| NLRC4 activation | AIFEC | NLRC4 | AD | enterocolitis, rash, arthritis, fever | IL-1, IL-18 | |
| FCAS/NOMID | NLRC4 | AD | cold urticaria, extremity pain, fever, CNS disease | IL-1 | ||
| NLRP12 activation | FCAS | NLRP12 | AD | cold urticaria, extremity pain, fever | TNF, IL-1 | |
| NLRP1 activation | NAIAD130 | NLRP1 | AD | Ocular, laryngeal, skin dyskeratosis, fever, arthritis | IL-1, TNF | |
| Receptor antagonist deficiency | DIRA | IL1RN | AR | pustular rash, osteomyelitis, periostitis, fever, | IL-1 | |
| DITRA | IL36RN | AR | pustular psoriasis, fever, malaise | TNF, IL- 17/12/23? | ||
| Type I Interferonopathies | Nucleic acid processing and degradation | Aicardi-Goutières syndrome | TREX1, ADAR1, RNASEH2A/B/C SAMHD1, IFIH1 | AR (AD: IFIH1) | fever, neurologic decline, encephalopathy, cerebral calcification, chilblains, autoantibodies | JAK, RTI? |
| monogenic SLE complements | DNASE1/2/1L3, | AR (AD: DNASE1) | autoantibodies, cytopenias, glomerulonephritis, skin cytopenias, glomerulonephritis, skin rash, oral ulcers, arthritis | JAK? | ||
| Nucleic acid sensing | SMS | IFIH1, DDX58a | AD | calcification of aorta / cardiac valves, osteopenia, acro-osteolysis, dental anomalies | JAK? | |
| SAVI | TMEM137 | AD | Chilblain’s rash, small vessel vasculitis, arthritis, ILD | JAK | ||
| Proteasome | CANDLE / PRAAS, PRAID131 | PSMB4, PSMA3, PSMB8, POMP, PSMG2,PSMB9, PSMB10 | Digenic, AR (AD: POMP) | fever, joint contractures, annular plaques, eyelid swelling, hepatosplenomegaly, lipodystrophy, FTT, developmental delay, anemia | JAK | |
| IFN signaling | AGS-like | USP18, ISG15, STAT2 | AR | skin ulcerations, seizures, hydrocephalus, cerebral calcifications, respiratory failure | JAK | |
| other | SPENCD132 | ACP5 | AR | skeletal dysplasia, short statue, cerebral calcification, cytopenias, autoantibodies | ? | |
| NF-kB and/or aberrant TNF activity | dysregulation of NFkB signaling | HA20 | TNFAIP3 | AD | oral, gastrointestinal and genital ulcerations, fever, arthritis, recurrent infection | TNF, IL-1, JAK? |
| RELA haploinsuf.133 | RELA | AD | oral and gastrointestinal ulcerations, cytopenias, lymphoproliferative disease | TNF | ||
| ORAS | OTULIN | AR | fever, panniculitis, diarrhea, arthritis, FTT | TNF | ||
| LUBAC deficiency134,135 | HOIL1, HOIP | AR | fever, recurrent infection, FTT, hepatosplenomegaly, amylopectin-like deposits in muscles | TNF? | ||
| Dysregulation of TNF | Blau | NOD2 | AD | granulomatous dermatitis, uveitis, polyarticular arthritis | TNF | |
| TRAPS | TNFRSF1A | AD | episodic fever, abdominal pain, headache, conjunctivitis, painful centrifugal rash | IL-1, TNF | ||
| DADA2 | ADA2 | AR | systemic vasculitis, fever, rash, stroke, cytopenias, hypogammaglobulinemia | TNF, HSCT | ||
| CRIA136,137 | RIPK1 | AD | fever, lymphadenopathy, hepatosplenomegaly | IL-6? | ||
| Other mechanisms | Golgi-ER Transport | COPA | COPA | AD | arthritis, ILD, diffuse alveolar hemorrhage, autoantibodies | IL-17? JAK? |
| Intracellular calcium signaling | PLAID | PLCG2 | AD | cold urticaria, atopy, granulomatous dermatitis, hypogammaglobulinemia, infection, autoantibodies | ? | |
| APLAID | PLCG2 | AD | blistering skin lesions, ILD, bronchiolitis, eye inflammation, enterocolitis, immunodeficiency | ? | ||
| tRNA biogenesis | SIFD138 | TRNT1 | AR | fever, developmental delay, seizures, microcytic anemia hypogammaglobulinemia | TNF | |
| Lipid metabolism? ER stress? | LACC1 deficiency139,140 | LACC1/FAMIN | AR | fever, systemic JIA, oligoarticular/polyarticular JIA | ? | |
| Cytokine dysregulation | VEO-IBD141,142 | IL-10, IL10RA, IL10RB | AR | Early-onset colitis, FTT | HSCT, IL-1? | |
| Actin Assembly | ARPC1B deficiency143,144 | ARPC1B | AR | platelet abnormalities, bleeding, recurrent infection, small vessel vasculitis, eczema, arthritis | ? | |
| Actin polymerization | CDC42 deficiency145,146 | CDC42 | AR | Neurodevelopmental defects, facial dysmorphism cytopenias, recurrent infection, fever, rash | IL-1 | |
1. Inflammasomopathies and other diseases arising through IL-1-family cytokines
The inflammasomes are a family of protein complexes that activate caspase-1, also called IL-1 converting enzyme, leading to proteolytic activation of IL-1β and IL-18. Caspase-1 also cleaves gasdermin D, which then forms membrane pores that release these cytokines into the extracellular milieu and allow solute entry to trigger a pro-inflammatory form of cell death termed pyroptosis. The inflammasome forms when a core nucleating protein changes conformation in response to a cytoplasmic danger signal, resulting in prion-like assembly of ASC (Apoptosis-associated Speck like protein containing a Caspase recruitment domain) proteins that in turn coordinate reciprocal activation of multiple caspase-1 molecules. Multiple inflammasomes are well-established, defined by their nucleating proteins including pyrin, cryopyrin (NLRP3/NALP3), NLRC4, NLRP1, and AIM2. Inflammasomopathies arise from mutations in these genes or in genes encoding direct or indirect inflammasome regulators, leading to inappropriate nucleation of the inflammasome complex.11 The molecular mechanisms underlying the activation of different inflammasomes in the setting of disease may include cytoskeletal dysregulation for pyrin or redox stress or phosphorylation for cryopyrin.12–14 Clinical differences among the inflammasomopathies reflect the nature and severity of the genetic defect, as well as the cellular distribution of a particular inflammasome and its substrates, helping to determine the predominant downstream mediator. For example, pyrin and cryopyrin are expressed widely in innate immune lineages, as is their substrate pro-IL-1β. Overactivity of these inflammasomes therefore manifests as widespread immunopathology mediated primarily through IL-1β. By contrast, the NLRC4 inflammasome is expressed in the gut lining (among other locations), where its substrate pro-IL-18 predominates over pro-IL-1β, contributing to severe colitis mediated in part by IL-18, although IL-1β also plays a role. Mutations affecting the NLRP3 inflammasome inhibitor CARD8 (also called Cardinal) can manifest as inflammatory bowel disease.15 NLRP1 is expressed mainly in skin, such that overactivity presents as primarily skin pathology (Multiple Self-healing Palmoplantar Carcinoma and Familial Keratosis Lichenoides Chronica, not discussed further here).16 No AIM2-driven inflammasomopathy has yet been reported.
Pyrin inflammasomopathies.
Familial Mediterranean fever is the most common of the monogenic autoinflammatory disorders, due to the high carrier frequency of MEFV mutations in specific populations from the Mediterranean region. FMF is classically considered autosomal recessive, since most affected patients carry two mutations; however, it is perhaps better regarded as autosomal dominant with limited penetrance, on the basis of families with clear autosomal dominant inheritance, affected patients (up to 30%) with only one detectable mutation, and evidence from Mefv mutant mice indicating that causal mutations are gain-of-function.17, 18 Multiple founder mutations and high prevalence reflect enhanced resistance to pathogens that evolved mechanisms to neutralize conventional pyrin inflammasome assembly, including the agent of plague, Yersinia pestis.19, 20 Patients typically present in childhood with episodes lasting 2–3 days of fever, abdominal and/or chest pain, occasional erysipelas-like lower extremity rash, monoarticular arthritis, neutrophilia and elevated inflammatory markers. The primary long-term morbidity is amyloidosis, most frequently affecting the kidneys. Pyrin Associated Autoinflammation with Neutrophilic Dermatosis (PAAND) is a dominantly-inherited disease due to unique activating mutations in MEFV.21 Patients present in childhood with recurrent episodes lasting weeks and characterized by rashes including sterile skin abscesses, but also fever, myalgia, myositis, and elevated acute phase reactants similar to FMF. Some patients exhibit abdominal pain. Maintenance colchicine therapy is the standard of care for these two related disorders, although PAAND often responds only partially. IL-1-targeted therapies can be effective, and TNF inhibitor response is also reported.21–23
Mevalonate Kinase Deficiency (MKD), also called Hyper IgD Syndrome (HIDS), is classified as a pyrin inflammasomopathy because disease pathophysiology is mediated by dysregulation of the pyrin regulatory factor RhoA.24 MKD is autosomal recessive, consistent with mutations in MVK being loss of function. A founder mutation in northern Europe accounts for its prevalence in the Netherlands and northern France. Patients often present in childhood (usually infancy) with episodes lasting 5–7 days of fever, abdominal pain, vomiting, rash, and elevated inflammatory markers. Vaccines are frequent triggers for these episodes. Serum IgD and IgA levels are often elevated and urine mevalonate may be elevated. IL-1 inhibitors are somewhat effective but may require aggressive dosing.22
Pyogenic Arthritis with Pyoderma gangrenosum and Acne (PAPA) is an autosomal dominant disorder due to mutations in PSTPIP1, encoding a protein that binds and likely activates pyrin.25 PAPA usually presents in childhood with sterile arthritis and systemic inflammation, while cutaneous features develop in adolescence or young adulthood. Patients typically respond to IL-1 or TNF blockade, although some may require aggressive dosing or a combination therapy.26
Cryopyrin (NLRP3) inflammasomopathies.
Cryopyrin Associated Periodic Syndrome (CAPS) is a continuum of previously-defined autoinflammatory syndromes of increasing severity including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal onset multisystem inflammatory disease (NOMID). The lines between these sub-phenotypes are blurry. Most CAPS patients possess heterozygous germline or somatic gain-of-function mutations in NLRP3, with a fairly consistent genotype-phenotype correlation such that specific mutations predict clinical features and severity along the disease continuum. Patients with very similar clinical presentations but without easily-defined NLRP3 mutations may either have NLRP3 mutations that are difficult to detect due to somatic mosaicism or mutations in genes with related function including NLRP12, NLRC4 and F12 (Factor 12).27, 28 For most CAPS patients, symptoms begin within the first year of life, although rarely presentation is delayed until adulthood. Common symptoms include urticaria-like rash, fever, arthralgia, myalgia, headache, and conjunctivitis, usually in the context of persistent systemic inflammation indicated by neutrophilia and elevated acute phase reactants. At the severe end of the spectrum, chronic sterile meningitis results in cognitive impairment and hearing loss. Features that may distinguish each sub-phenotype include cold sensitivity in FCAS, AA amyloidosis in MWS, and significant CNS and bone disease in NOMID. IL-1 blockade is the standard of care and most patients respond when dosed adequately.27
NLRC4 inflammasomopathy:
Patients with NLRC4 gain-of-function mutations may present with CAPS-like symptoms, but the classic presentation is a disease termed Autoinflammation with Infantile Enterocolitis (AIFEC) characterized by early-onset hyperinflammation (discussed elsewhere in this issue) involving rash, joint symptoms, severe intestinal disease, and hepatosplenomegaly.29, 30 Patients exhibit systemic inflammation including hyperferritinemia, cytopenias, and laboratory evidence of liver and kidney injury, resembling macrophage activation syndrome. IL-1 inhibitors may be helpful, but accumulating evidence support a central role for IL-18.31
NLRP12-related disease.
The initial description of patients with heterozygous mutations in NLRP12 resembled the NLRP3-associated FCAS phenotype, resulting in this disease being referred to as familial cold autoinflammatory syndrome 2 (FCAS2).32 Despite similar domain structure and cell expression patterns of NLRP12 and NLRP3, the function of these proteins may be different. While reliable reports suggest that NLRP12 can form an active inflammasome, NLRP12 also regulates NFκB, placing this condition at a border with disorders discussed further below and potentially explaining a lack of complete response to IL-1 blockade, in particular since disease-associated mutations in NLRP12 are generally loss of function.32–34 Expanded access to genetic sequencing has seen the phenotype of NLRP12 mutations broaden to include other autoinflammatory phenotypes as well as immunodeficiency and autoimmunity, implying diverse roles for NLRP12.
Deficiency of IL-1-family cytokine antagonists.
A common feature of the IL-1 cytokine family (including IL-1α, IL-1β, IL-18, and IL-36) is the presence of endogenous circulating antagonists. These include IL-1 receptor antagonist (IL-1ra, available in recombinant form as anakinra) and a similar receptor antagonist for IL-36 termed IL-36ra. Deficiency of either protein results in an autoinflammatory disease. Deficiency of IL-1ra (DIRA), due to biallelic mutation of IL1RN, results in neonatal-onset pustulosis, multifocal osteomyelitis and periostitis due to excess IL-1 signaling.35, 36 As expected, DIRA is managed effectively by anakinra.35, 37 Deficiency of IL-36ra (DITRA) from mutations in IL36RN causes generalized pustular psoriasis, since IL-36 receptor is expressed primarily in the skin and other epithelial cells in contact with the environment.38 Some of the original DIRA patients also had DITRA due to large deletions involving these contiguous genes.35, 36, 39 DITRA flares are accompanied by fever, malaise, neutrophilia and elevated inflammatory markers.40 Although recombinant IL36ra is not yet available, DITRA can be effectively managed by biologics that target TNF, IL-17, or IL-12/23.41 The efficacy of IL-1 inhibition is variable.41, 42 IL-18 has two antagonists, circulating IL-18 binding protein (IL-18bp) and the anti-inflammatory IL-1-family cytokine IL-37; diseases arising from defects in these mechanisms are not yet described.
2. Interferonopathies
Interferons (IFN) are a family of cytokines involved in immune defense. Three IFN families are recognized. Type I interferons encompass IFNα, IFNβ and other members, involved in antiviral defense and signaling through the type I IFN receptor and its signal transduction kinases JAK1 and TYK2. Type II interferons are limited to IFNγ, a cytokine implicated in multiple aspects of adaptive and innate immunity that signals via the type II IFN receptor and its kinases JAK1 and JAK2. Type III interferons, termed IFNλ, are less well understood and signal through a distinct receptor that shares kinases with the type I IFN receptor. To date, autoinflammatory diseases related to interferon – commonly known as the interferonopathies – reflect aberrant activation of type I pathways, though in the future type II and type III interferonopathies may be recognized.
The (type I) interferonopathies represent conditions in which the type I interferon axis is aberrantly activated.43 IFNα/β production is triggered by viral DNA or RNA, and correspondingly interferonopathies often arise through defects in nucleic acid sensing or through accumulation of host nucleic acid or other intracellular debris that mimic chronic viral infection. Alternately, mutations may aberrantly amplify type I interferon receptor signaling. Clinically, patients with interferonopathies often exhibit fever (type I IFNs are potent endogenous pyrogens), rash, and systemic inflammation, frequently together with skin vasculitis and basal ganglia calcifications (Table 1). Interestingly, many of these diseases feature autoantibodies, highlighting the lack of a sharp divide between autoinflammatory and autoimmune disorders.
Disorders of degradation or processing of endogenous nucleic acids.
Recognition of type I IFN-mediated autoinflammation originated with studies of Aicardi-Goutières syndrome (AGS), a group of inherited disorders characterized by early-onset neurologic decline, encephalopathy, cerebral calcification, and sometimes fevers and systemic inflammation.44 Vasculopathy manifesting as cold-induced chilblains and livedo reticularis are common skin manifestations.45 Patients with AGS display variable features of autoimmunity, ranging from low-titer autoantibodies and mild cytopenias to the full clinical spectrum of systemic lupus erythematosus. Seven types of AGS have been defined based on the causative genes: TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1 and IFIH1.46 With the exception of IFIH1, which amplifies IFN signaling (see below), these genes participate in nucleic acid processing, and loss of function translates into accumulation of endogenous nucleic acids. Most AGS patients present early in life, but asymptomatic individuals (incomplete penetrance) and delayed presentation with milder disease are well recognized, suggesting a role for modifier genes and environmental factors. Additional genes implicated in type I interferonopathy through aberrant nucleic acid handling include DNA-degrading enzymes (DNAse1, DNAse2 and DNAse1L3) that are associated with monogenic forms of lupus, potentially reflecting constitutive recognition of accumulated intracellular host DNA triggering pathways engaged by DNA viruses.47
JAK inhibitors (JAKinibs) disrupt JAK signaling downstream of the IFN receptor complex. JAKinibs that preferentially target JAK1/2 (ruxolitinib and baricitinib) or JAK1/3 (tofacitinib) have shown therapeutic efficacy in AGS, with improvement in quality of life, growth, inflammatory markers, autoimmune manifestations and corticosteroid dependency.48–50 Neurologic damage in AGS is likely irreversible but early treatment may attenuate decline. Intriguingly, one source of stimulatory nucleic acid for some AGS subtypes may be activation of endogenous retroviral elements; a recent study demonstrated successful use of nucleoside analogue reverse-transcriptase inhibitors to suppress the IFN signature in AGS patients, especially those with RNASEH-complex mutations.51
Disorders of enhanced nucleic acid sensing.
MDA5 (encoded by IFIH1) and RIG-I (encoded by DDX58) are members of the RIG-I-like receptor family that sense viral nucleic acids and activate type I IFN production.52 Gain-of-function mutations in IFIH1 cause an autosomal dominant form of AGS (Type VII), characterized in some patients by psoriasis and pulmonary hypertension in addition to the classic AGS findings.53, 54 Other gain-of-function mutations in IFIH1 and DDX58 cause Singleton-Merten syndrome, a distinct interferonopathy characterized by aortic and valvular calcification, osteopenia, acro-osteolysis and dental anomalies.55, 56
STING-associated Vasculopathy of Infancy (SAVI) is a syndrome of vasculitis rash, arthritis and interstitial lung disease caused by gain-of-function mutations of TMEM137, which encodes stimulator of interferon genes (STING).57 While the vasculopathy of SAVI can resemble AGS, interstitial lung disease distinguishes STING from most other interferonopathies, likely reflecting tissue-specific effects of STING expression in the lung. JAKinibs can be effective but their impact on the IFN signature is variable, corresponding generally to an incomplete clinical response.49, 58
Disorders of proteasome function.
The proteasome is a multimeric protein complex that degrades ubiquitinated proteins both in steady-state and with cell activation. Production of type I IFN by immune cells can be driven by proteasome dysfunction, as shown by experiments using proteasome inhibitors.59 Mutations that disrupt proteasome subunits or chaperone proteins involved in proteasome assembly, either recessive or digenic affecting different components, are associated with the diseases Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature (CANDLE) syndrome and Proteasome-Associated Autoinflammatory Syndrome (PRAAS).59–61 Patients with CANDLE / PRAAS typically present during infancy with recurrent fever, annular violaceous plaques, violaceous eyelid swelling, hepatosplenomegaly, lipodystrophy and failure to thrive; PRAAS patients may also have panniculitis. A strong type I IFN signature is present in the peripheral blood, but unlike AGS, clinical features of autoimmunity are uncommon.62 The JAKinibs baricitinib and ruxolitinib can partially ameliorate clinical manifestations and laboratory abnormalities; corticosteroids and methotrexate are also employed.49, 63
Disorders of amplified IFN receptor signaling.
Ubiquitin specific protease (USP)-18 is an endogenous inhibitor of type I IFN signaling that binds to one of the type I IFN receptor chains, IFNAR2, to block JAK1 activation. Deficiency of USP18 causes an aggressive interferonopathy with severe brain inflammation.64 ISG15 is a stabilizer of USP18, such that ISG15 deficiency causes a related interferonopathy characterized by brain calcifications and seizures through insufficiency of USP18.65 The recruitment of USP18 to IFNAR2 requires a binding site on STAT2, and severe early-onset interferonopathy arises from STAT2 mutations that disrupt the interaction with USP18.66, 67 JAK inhibition with ruxolitinib led to remarkable improvement in one patient with USP18 deficiency.68
3. Disorders of NFκB and/or aberrant TNF activity
The NFκB complex is a central signaling hub within the cytoplasm, integrating signals from multiple cell surface and intracellular danger sensors, upon which one of several transcription factors is freed to move to the nucleus where it triggers coordinated expression of pro-inflammatory genes. Regulation of NFκB is correspondingly complex, involving a set of sensor proteins, inhibitory proteins, and ubiquitin-dependent functional modifications.69, 70 Defects in any of these pathways can lead to aberrant activation of NFκB. Hallmark clinical features of the “NFκBopathies” (sometimes termed the Relopathies, since RelA and RelB are key components of the NFκB complex) are fever, systemic inflammation, and sometimes granuloma formation.71 Key upstream activators of NFκB activation include receptors in the TNF family, which engage this pathway both via canonical (degradation of the NFκB inhibitor IκBα) and non-canonical (variant NFκB complex formation) pathways.72 NFκB activation also results in TNF production. Thus, NFκB and TNF are closely intertwined, helping to define a subgroup of autoinflammatory diseases that often respond at least partially to TNF blockade (Table 1).
Haploinsufficiency of A20.
TNFAIP3 encodes the protein A20, a ubiquitin-editing enzyme that functions as a negative regulator of NFκB. Haploinsufficiency of A20 (HA20) causes recurrent oral and genital ulcers that mimics Behcet’s disease with relapsing and remitting disease.73, 74 Other phenotypes include early onset autoimmunity and immunodeficiency including an presentation similar to autoimmune lymphoproliferative syndrome.75 Peripheral blood mononuclear cells from HA20 patients show overproduction of TNF and IL-1β, and biologics targeting these cytokines are often used in severe cases. Although NFκB and type I IFN are non-redundant pathways, HA20 patients may exhibit elevated expression of IFN signature genes that parallels disease activity, potentially reflecting a regulatory role of A20 on IFN signaling as well as the induction of IFNβ by TNF.76, 77 Importantly, the presence of a type I IFN signature predicted a good response to JAKinib treatment in HA20 cases that were resistant to anti-cytokine treatment.78
Blau syndrome.
Gain-of-function mutations in the cytoplasmic sensor NOD2 result in Blau syndrome, an autosomal dominant disease characterized by arthritis, uveitis, and granulomatous dermatitis.79 Patients usually present early in life (prior to age 5) with skin rash as the initial manifestation. The arthritis associated with Blau syndrome is typically symmetric, with marked synovitis but not always erosions, and affecting wrists, ankles, knees and fingers.80 Additional manifestations of Blau syndrome are central nervous system inflammation, interstitial pneumonitis, liver inflammation and vasculitis. TNF inhibitors can be highly effective, whereas response to IL-1 blockade is inconsistent.81
Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS).
TRAPS is a dominantly-inherited recurrent fever disorder due to mutations in TNFRSF1A, encoding TNF receptor 1 (TNFR1).9 Usually beginning in childhood, patients experience episodes lasting 2–4 weeks that include fever, abdominal and muscle pain, headache, and conjunctivitis, without response to colchicine. Hallmark features are a tender centrifugal rash and periorbital edema. Chronic elevation of inflammatory markers corresponds to an increased risk of systemic amyloidosis. The mechanism of disease remains incompletely understood. While failure to shed TNF receptor was initially suspected, aberrant trafficking or signaling of mutant TNFR1 leading to inflammasome activation appears to be a more accurate disease mechanism. Etanercept (soluble TNFR1) therapy can be successful in some patients, but efficacy is frequently lost and – paradoxically – anti-TNF antibodies such as infliximab can worsen disease.9, 82, 83 IL-1 blockade is more consistently effective and has become standard of care, suggesting a role for the inflammasome in disease pathophysiology.27
Deficiency of adenosine deaminase 2 (DADA2).
Biallelic mutations in ADA2 result in a syndrome that variably features systemic vasculitis, early-onset stroke, cytopenias and immunodeficiency.84, 85 Vasculitis in DADA2 can resemble the medium vessel vasculitis polyarteritis nodosa. TNF is the predominant driver of inflammation, because TNF inhibitors are strikingly effective at treating vasculitis and preventing stroke.86 Mutations with residual ADA2 function tend to be associated with stroke and other inflammatory manifestations responsive to TNF inhibition, while mutations that abrogate gene function manifest as profound immunodeficiency and hematologic compromise, often not responsive to TNF inhibitors; bone marrow transplant should be considered for this subset of patients.87 The physiologic function of ADA2 is not clear, but appears to be different from that of ADA1 and ADAR (double-stranded RNA-specific adenosine deaminase) based on the unique biochemical properties and clinical consequences of mutations in these genes.88
4. Autoinflammation mediated by other mechanisms
The mechanisms by which disordered immune function can lead to inflammatory pathology are potentially as numerous as the list of genes involved in immune function. Some autoinflammatory diseases have yet to fall into an established pathogenic category, or to establish a category of their own (Table 1).
COPA syndrome.
Coatomer protein subunit α, encoded by COPA, is a part of coat protein complex I that regulates retrograde transport from the Golgi to the endoplasmic reticulum (ER). Mutations in COPA cause an autosomal dominant syndrome of autoimmunity, inflammatory arthritis, interstitial lung disease and diffuse alveolar hemorrhage.89 ER stress from disrupted intracellular trafficking skews effector T cells towards a Th17 phenotype. Patients with COPA may also have a type I IFN signature, raising the possibility that it is an interferonopathy.90 Consistent with this finding, COPA has been found to regulate normal trafficking of STING 91, 92. However, a murine model implicated autoreactive T cells derived through abnormal thymic function, suggesting that COPA might be a monogenic autoimmune disease in addition to (or rather than) a monogenic autoinflammatory condition, illustrating the challenge of distinguishing unambiguously between categories of immune dysfunction.93 Numerous immunosuppressive agents have been trialed in COPA syndrome with variable success.94 JAKinib treatment was effective in one COPA patient with severe arthritis, although the type I IFN signature was not assessed.95
PLAID/APLAID.
Phospholipase C gamma 2 (PLCG2)-associated antibody deficiency and immune dysregulation (PLAID) further exemplifies the intersection of autoinflammation, autoimmunity/allergy, and immunodeficiency (Figure 2). PLCγ2 hydrolyzes phosphatidylinositol-4,5-bisphosphate into diacylglycerol and inositol trisphosphate, triggering calcium release from the ER to mediate cell activation.96 In PLAID, heterozygous genomic deletions in the autoinhibitory domain of PLCG2 cause constitutive enzyme activation and enhanced signaling at cold temperatures. Clinical features include cold urticaria, atopy, granulomatous dermatitis, hypogammaglobulinemia, recurrent sinopulmonary infection, and variable manifestations of autoimmunity.97 A constellation of autoinflammatory features was later found in patients with heterozygous missense mutations in these autoinhibitory regions. This combined phenotype of autoinflammation and PLAID (APLAID) is characterized by recurrent blistering skin lesions, interstitial pneumonitis and bronchiolitis, eye inflammation, enterocolitis, cellulitis and immunodeficiency.98, 99 Treatment for PLAID includes cold avoidance, antihistamines, and antibiotic prophylaxis and/or IVIG for immunodeficiency.100 Experience with APLAID is limited, but corticosteroids, hydroxychloroquine, and IL-1 inhibitors have not shown sustained efficacy.99
Disorders of complement.
Complement is a system of plasma proteins and related cell-surface regulators that serves multiple functions in innate immunity, including recognition and clearance of pathogens. Coordinated closely with adaptive immunity, the complement system plays a key role in antibody-dependent pathogen targeting and in clearance of immune complexes. Defects in complement or its surface inhibitory proteins result in pathogenic conditions including immunodeficiency, early-onset systemic lupus erythematosus, and age-related macular degeneration. In some cases, these conditions represent inflammatory consequences of antigen-independent hyperactivation of immune pathways, fulfilling the definition of autoinflammatory diseases. Complement activation and its implications have been reviewed extensively and are not considered further here.101
The “autoinflammatory penumbra”
Diseases such as FMF, TRAPS, MKD and CAPS seem at this point clearly autoinflammatory, but for some conditions the situation is less straightforward. Antigen-independent immune activation contributes to initiation or propagation of tissue injury in multiple diseases. Some diseases present with clinical features resembling the autoinflammatory diseases but understanding of pathogenesis remains too limited to draw firm conclusions. These conditions belong to the “autoinflammatory penumbra” (Figure 3).
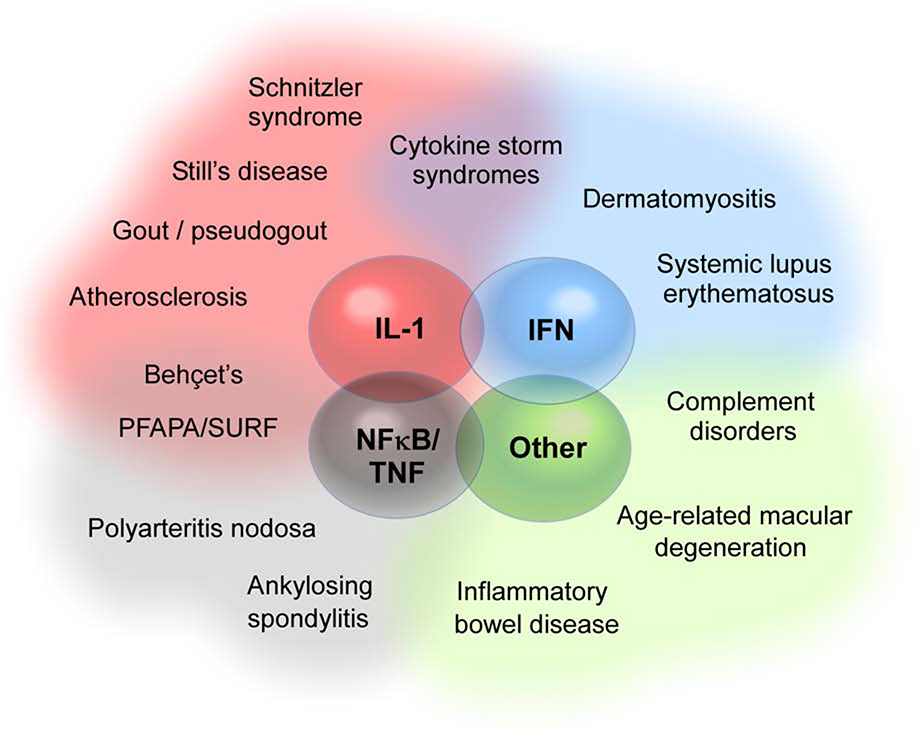
Figure 3. The autoinflammatory penumbra.
Major families of autoinflammatory disorders include the inflammasome/IL-1 diseases, the interferonopathies, the NFκB/TNF disorders, and autoinflammation mediated by other mechanisms. Many other human diseases exhibit clinical and/or mechanistic overlap with these disease families without perhaps being best conceptualized as primarily autoinflammatory; some of these are depicted in a shaded zone (penumbra) surrounding the monogenic autoinflammatory diseases. PFAPA, Periodic Fevers with Aphthous stomatitis, Pharyngitis and Adenopathy; SURF, Syndrome of Undifferentiated Recurrent Fever.
Several examples illustrate the scope of this penumbra. Gout and pseudogout are mediated through activation of the cryopyrin inflammasome by monosodium urate and calcium phosphate dihydrate crystals.102 Cholesterol crystals activate the same inflammasome, conferring an autoinflammatory element to atherosclerosis, consistent with the (modest) efficacy of IL-1β blockade in cardiovascular disease.103–105 Ankylosing spondylitis is strongly associated with the MHC class I allele HLA-B27; this association may reflect the propensity of HLA-B27 to mis-fold and thereby trigger the unfolded protein response rather than its antigen specificity.106 The pediatric disorders, Periodic Fevers with Aphthous stomatitis, Pharyngitis and Adenopathy (PFAPA) and Syndrome of Undifferentiated Recurrent Fever (SURF, an increasingly recognized group of patients who meet some but not all criteria for PFAPA), as well as the adult-onset Schnitzler syndrome are commonly regarded as autoinflammatory due their presentation with episodic fever.107 For these conditions, the lack of a molecular understanding renders classification only provisional. For example, a recent study made the surprising observation that PFAPA exhibits genetic associations with the HLA region as well as other loci associated with T cell function, findings that implicate antigen-directed immunity.108 Systemic juvenile idiopathic arthritis and its adult counterpart adult-onset Still’s disease are characterized by fever, rash, arthritis, and in many patients a brisk response to IL-1β blockade. These features resemble other autoinflammatory diseases, but an HLA association and characteristic changes in T cells suggest that (like PFAPA) this phenotype is unlikely to be “purely” autoinflammatory.109–111 Inflammatory bowel disease likely reflects both immune hyper-responsiveness and defects in mucosal barrier function.112
The autoinflammatory penumbra is to be expected. Immune function is complicated, and it is not surprising that aberrant immune activity can manifest with varying combinations of autoinflammation, autoimmunity/allergy, and immunodeficiency. For example, FMF-associated mutations in MEFV are associated with an increased risk of rheumatoid arthritis and higher prevalence of ankylosing spondylitis and vasculitis.113–115 Patients with CDC42 deficiency, ARPC1B deficiency or homozygous mutations in WDR1 present with recurrent infections but also clinical features of autoinflammation (Table 1). Most primary immune defects can be expected to yield a phenotype that resides somewhere within the autoinflammatory-autoimmunity/allergy-immunodeficiency spectrum rather than adhering tightly to a single axis alone (Figure 2).
Clinical approach to the autoinflammatory diseases
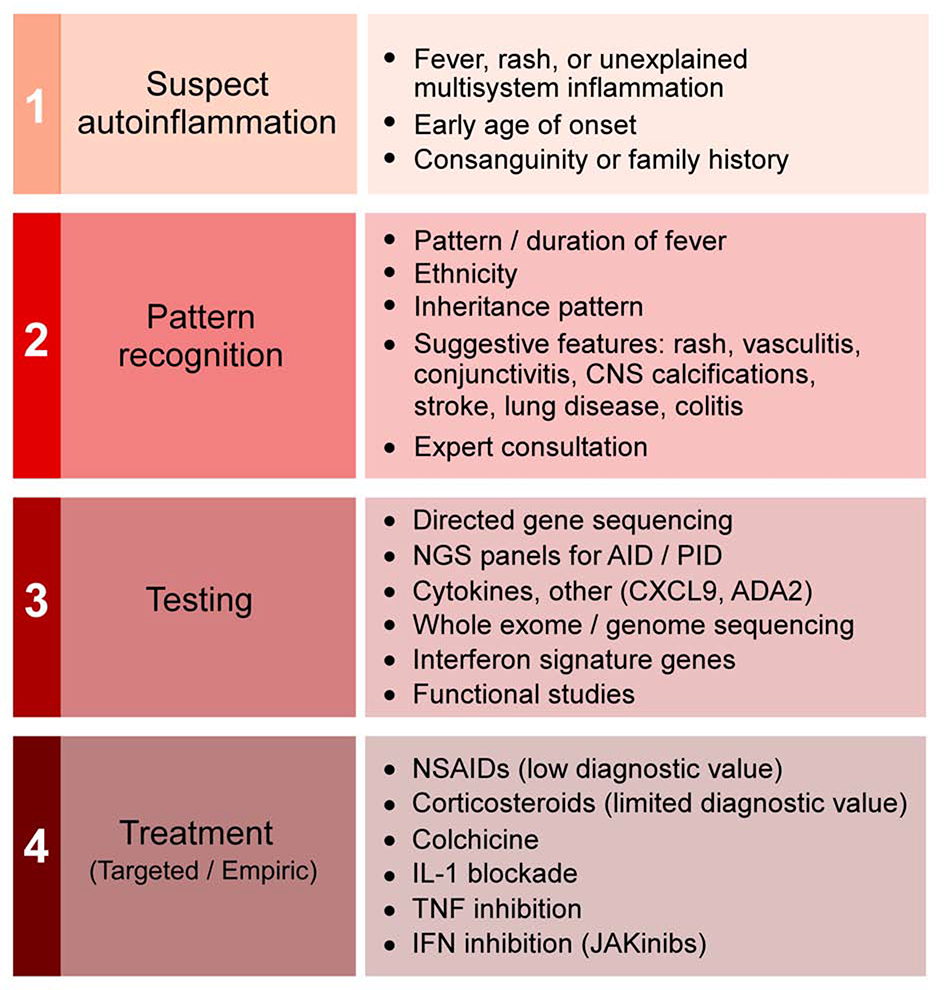
Given the complexity of the autoinflammatory family, how can clinicians proceed to diagnosis and management without having to master each disease? We suggest a four-step approach (Figure 4).
Figure 4. Clinical approach to patients with suspected autoinflammation.
Please see text for discussion of each step.
First, consider autoinflammation in the differential diagnosis.
Several clinical features of inflammation facilitate disease recognition. Fever is common, albeit not invariable, as is elevation in inflammatory markers such as C reactive protein (CRP) and the erythrocyte sedimentation rate (ESR). Many patients have skin involvement, including evanescent or persistent rashes, sometimes with vasculitis. Inflammation typically affects many organ systems, sometimes including lung and the gastrointestinal tract. Disease often begins early in life, and close relatives may have similar disease, suggesting a genetic etiology. Broadly, autoinflammation should be suspected in any patient – especially a child – with persistent or recurrent inflammatory episodes that fail to fit the pattern of other established diseases.
Second, recognize hallmarks of particular autoinflammatory diseases.
Clinical history, physical examination, and laboratory / imaging studies remain essential to diagnosis. For example, recurrent episodes of fevers and abdominal pain lasting less than 48 hours in a patient with Middle Eastern heritage suggests FMF. An acral vasculitic rash and basal ganglia calcifications suggests an interferonopathy. Childhood-onset stroke with livedo suggests DADA2. Where possible, clinicians with limited exposure to this disease family should seek help from colleagues with more experience recognizing clinical patterns within the autoinflammatory spectrum. For the more common periodic fever syndromes, validated classification criteria are available and provide useful guidance for diagnosis.116
Third, cast a broad net.
Not all autoinflammatory diseases manifest in their canonical form. The therapeutic implications of correct diagnosis support a broad screen for autoinflammation-associated genetic mutations early in the evaluation of unexplained multisystem inflammation, using any of a range of commercial services that test a large panel of immune-related genes. Genetic findings require cautious interpretation because many variants of unknown significance will be irrelevant, while some described as likely benign could still represent low-penetrance causal variants. Commercial screens will miss noncoding mutations, copy number variants, complex chromosomal rearrangements, mutations in novel disease genes, and states of mosaicism in which the mutation affects only a subset of cells.117 Genetic testing guidelines for the autoinflammatory diseases are available, and an updated list of variants and associated phenotypes is provided at Infevers, an online database of autoinflammatory mutations at https://infevers.umai-montpellier.fr/.118, 119
Clinical tests can suggest or in some cases establish a diagnosis. Examples include circulating serum cytokines such as IL-6 and IL-18, serum IgD and IgA, or urine organic acids in MKD, ADA2 activity, and stable proxies for cytokines that are difficult to measure directly, such as the chemokine CXCL9 as a proxy for IFNγ.120 Further tests are available largely on a research basis, such as assessment of peripheral blood for cytokine release or expression of type I IFN-stimulated genes.10, 121 Whole-exome or whole-genome sequencing, potentially together with sequencing of affected and unaffected family members, can provide definitive guidance but requires specialized expertise and can miss large deletions and mutations affecting non-coding regions such as promoters and enhancers.
Fourth, consider empiric therapy.
Many autoinflammatory diseases operate through pathways or mediators for which inhibitors are available. NSAIDs and corticosteroids are anti-inflammatory but have little value as therapeutic diagnostics. The exception of PFAPA, in which abrogation of fever with a single dose of corticosteroids strongly supports the diagnosis.122 By contrast, response to colchicine supports an autoinflammatory etiology, since it can attenuate assembly of the pyrin inflammasome and thus treat FMF as well as an appreciable fraction of suspected autoinflammatory syndromes that defy molecular diagnosis.123–125 Response to the recombinant IL-1 receptor antagonist anakinra establishes the role of IL-1 in an inflamed patient. The short half-life of this agent (4–6 hours) and documented safety even in patients with severe bacterial illness render a therapeutic trial feasible and safe in many clinical contexts.126 Another option is empiric canakinumab (anti-IL-1β antibody), although this drug has a considerably longer half-life (26 days).22 JAKinibs such as tofacitinib, baricitinib, and ruxolitinib can be employed to explore the role of interferon signaling, albeit with caution because of their broad immunosuppressive reach and uncertain safety in bacterial and viral infection.10, 49 IL-18 blockade can establish the causative contribution of this cytokine, as exemplified by experience in NLRC4-related disease.31 Careful utilization of targeted immunomodulators provide a diagnostic and therapeutic path forward for the >50% of patients with presumed autoinflammatory disease who defy diagnosis despite genetic testing and expert evaluation.124, 125
Conclusion: autoinflammation in immune-mediated diseases
Like the primary immunodeficiencies, the monogenic autoinflammatory disorders provide a window into human immunity in action, illustrating the degree to which precise regulation of inflammatory pathways is essential to health. Autoinflammation is not all-or-none. Related mechanisms contribute to classic autoimmune diseases as well as to diseases not usually considered primarily immune-mediated, including the crystalline arthropathies and atherosclerosis. Improved understanding of autoinflammation has both illuminated new immune pathways and provided novel diagnostic and therapeutic possibilities for our patients.
Acknowledgements:
PAN is funded by NIAMS awards 2R01AR065538, R01AR075906, R01AR073201, P30AR070253, R21AR076630 and NHLBI award R21HL150575; the Fundación Bechara, and the Arbuckle Family Fund for Arthritis Research. PYL is supported by NIAMS K08-AR074562, a Rheumatology Research Foundation Investigator Award and a Boston Children’s Hospital Faculty Career Development Award. HMH is funded by NIH awards: R01 DK113592, R01 HL140898, R01 R01AI134030 and a UC collaborative grant.
Conflict of interest: PAN has been supported by investigator-initiated research grants from AbbVie, Bristol-Myers Squibb, Novartis, Pfizer, Sobi; consulting from Bristol-Myers Squibb, Cerecor, Miach Orthopedics, Novartis, Pfizer, Quench Bio, Sigilon, Simcere, Sobi, and XBiotech; royalties from UpToDate Inc.; and salary support from the Childhood Arthritis and Rheumatology Research Alliance. PYL has no conflict of interest. HMH has been supported by research grants from Bristol-Myers Squibb, Regeneron IFM, Jecure, and Zomagen; consulting and speaking from Novartis and Sobi.
Abbreviations
- AGS
(Aicardi-Goutières syndrome)
- AR
(Autosomal recessive)
- CAPS
(Cryopyrin-associated periodic syndrome)
- COPA
(COPI Coat Complex Subunit Alpha)
- DITRA
(Deficiency of IL-36 receptor antagonist)
- FMF
(Familial Mediterranean fever)
- HA20
(Haploinsufficiency of A20)
- JAK
(Janus kinase)
- JAKinhibs
(JAK inhibitors)
- MKD
(Mevalonate kinase deficiency)
- NF-κB
(Nuclear factor kappa B)
- PFAPA
(Periodic fever, aphthous stomatitis, pharyngitis, adenitis)
- PLAID
(Phospholipase C gamma 2–associated antibody deficiency and immune dysregulation)
- PRAAS
(PRoteasome-associated autoinflammatory syndrome)
- STING
(Stimulator of interferon genes)
- TRAPS
(TNF receptor–associated periodic syndrome)
- USP18
(Ubiquitin-specific protease 18)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrlich P, Morganroth J. Studies On Haemolysins: Fifth Communication (Berlin. klinischer Wochenschrift 1901) In: Ehrlich P, Bolduin C, editors. Collected Studies on Immunity New York: John WIley & Sons; 1910. p. 71–87. [Google Scholar]
- 2.Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol 2001; 2:279–81. [DOI] [PubMed] [Google Scholar]
- 3.Witebsky E, Rose NR. Studies on organ specificity. IV. Production of rabbit thyroid antibodies in the rabbit. J Immunol 1956; 76:408–16. [PubMed] [Google Scholar]
- 4.Rose NR, Witebsky E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol 1956; 76:417–27. [PubMed] [Google Scholar]
- 5.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003; 2:119–25. [DOI] [PubMed] [Google Scholar]
- 6.Flajnik MF. A cold-blooded view of adaptive immunity. Nat Rev Immunol 2018; 18:438–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consortium TIF. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 1997; 90:797–807. [DOI] [PubMed] [Google Scholar]
- 8.Consortium FF. A candidate gene for familial Mediterranean fever. Nat Genet 1997; 17:25–31. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999; 97:133–44. [DOI] [PubMed] [Google Scholar]
- 10.de Jesus AA, Hou Y, Brooks S, Malle L, Biancotto A, Huang Y, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest 2020; 130:1669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman HM, Broderick L. The role of the inflammasome in patients with autoinflammatory diseases. J Allergy Clin Immunol 2016; 138:3–14. [DOI] [PubMed] [Google Scholar]
- 12.Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Front Immunol 2019; 10:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassi S, Carta S, Delfino L, Caorsi R, Martini A, Gattorno M, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion. Proc Natl Acad Sci U S A 2010; 107:9789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell 2017; 68:185–97 e6. [DOI] [PubMed] [Google Scholar]
- 15.Mao L, Kitani A, Similuk M, Oler AJ, Albenberg L, Kelsen J, et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J Clin Invest 2018; 128:1793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016; 167:187–202 e17. [DOI] [PubMed] [Google Scholar]
- 17.Booty MG, Chae JJ, Masters SL, Remmers EF, Barham B, Le JM, et al. Familial Mediterranean fever with a single MEFV mutation: where is the second hit? Arthritis Rheum 2009; 60:1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 2011; 34:755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung LK, Park YH, Zheng Y, Brodsky IE, Hearing P, Kastner DL, et al. The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome. Cell Host Microbe 2016; 20:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YH, Remmers EF, Lee W, Ombrello AK, Chung LK, Shilei Z, et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat Immunol 2020; 21:857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masters SL, Lagou V, Jeru I, Baker PJ, Van Eyck L, Parry DA, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med 2016; 8:332ra45. [DOI] [PubMed] [Google Scholar]
- 22.De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N Engl J Med 2018; 378:1908–19. [DOI] [PubMed] [Google Scholar]
- 23.Moghaddas F, Llamas R, De Nardo D, Martinez-Banaclocha H, Martinez-Garcia JJ, Mesa-Del-Castillo P, et al. A novel Pyrin-Associated Autoinflammation with Neutrophilic Dermatosis mutation further defines 14–3-3 binding of pyrin and distinction to Familial Mediterranean Fever. Ann Rheum Dis 2017; 76:2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 2016; 17:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzano AV, Borghi A, Meroni PL, Cugno M. Pyoderma gangrenosum and its syndromic forms: evidence for a link with autoinflammation. Br J Dermatol 2016; 175:882–91. [DOI] [PubMed] [Google Scholar]
- 26.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol 2009; 161:1199–201. [DOI] [PubMed] [Google Scholar]
- 27.Booshehri LM, Hoffman HM. CAPS and NLRP3. J Clin Immunol 2019; 39:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheffel J, Mahnke NA, Hofman ZLM, Maat S, Wu J, Bonnekoh H, et al. Cold-induced urticarial autoinflammatory syndrome related to factor XII activation. Nat Commun 2020; 11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 2014; 46:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akula MK, Shi M, Jiang Z, Foster CE, Miao D, Li AS, et al. Control of the innate immune response by the mevalonate pathway. Nat Immunol 2016; 17:922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 2017; 139:1698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A 2008; 105:1614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 2012; 37:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeru I, Hentgen V, Normand S, Duquesnoy P, Cochet E, Delwail A, et al. Role of interleukin-1beta in NLRP12-associated autoinflammatory disorders and resistance to anti-interleukin-1 therapy. Arthritis Rheum 2011; 63:2142–8. [DOI] [PubMed] [Google Scholar]
- 35.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 2009; 360:2426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med 2009; 360:2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Annu Rev Med 2014; 65:223–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 2011; 365:620–8. [DOI] [PubMed] [Google Scholar]
- 39.Brau-Javier CN, Gonzales-Chavez J, Toro JR. Chronic cutaneous pustulosis due to a 175-kb deletion on chromosome 2q13: excellent response to anakinra. Arch Dermatol 2012; 148:301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauber M, Bal E, Pei XY, Madrange M, Khelil A, Sahel H, et al. IL36RN Mutations Affect Protein Expression and Function: A Basis for Genotype-Phenotype Correlation in Pustular Diseases. J Invest Dermatol 2016; 136:1811–9. [DOI] [PubMed] [Google Scholar]
- 41.Hospach T, Glowatzki F, Blankenburg F, Conzelmann D, Stirnkorb C, Mullerschon CS, et al. Scoping review of biological treatment of deficiency of interleukin-36 receptor antagonist (DITRA) in children and adolescents. Pediatr Rheumatol Online J 2019; 17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi-Semerano L, Piram M, Chiaverini C, De Ricaud D, Smahi A, Kone-Paut I. First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with anakinra. Pediatrics 2013; 132:e1043–7. [DOI] [PubMed] [Google Scholar]
- 43.Uggenti C, Lepelley A, Crow YJ. Self-Awareness: Nucleic Acid-Driven Inflammation and the Type I Interferonopathies. Annu Rev Immunol 2019; 37:247–67. [DOI] [PubMed] [Google Scholar]
- 44.Crow YJ. Aicardi-Goutieres Syndrome In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)). Seattle (WA); 1993. [Google Scholar]
- 45.Kolivras A, Aeby A, Crow YJ, Rice GI, Sass U, Andre J. Cutaneous histopathological findings of Aicardi-Goutieres syndrome, overlap with chilblain lupus. J Cutan Pathol 2008; 35:774–8. [DOI] [PubMed] [Google Scholar]
- 46.Crow YJ, Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol 2015; 15:429–40. [DOI] [PubMed] [Google Scholar]
- 47.Alperin JM, Ortiz-Fernandez L, Sawalha AH. Monogenic Lupus: A Developing Paradigm of Disease. Front Immunol 2018; 9:2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kothur K, Bandodkar S, Chu S, Wienholt L, Johnson A, Barclay P, et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology 2018; 90:289–91. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018; 128:3041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briand C, Fremond ML, Bessis D, Carbasse A, Rice GI, Bondet V, et al. Efficacy of JAK1/2 inhibition in the treatment of chilblain lupus due to TREX1 deficiency. Ann Rheum Dis 2019; 78:431–3. [DOI] [PubMed] [Google Scholar]
- 51.Rice GI, Meyzer C, Bouazza N, Hully M, Boddaert N, Semeraro M, et al. Reverse-Transcriptase Inhibitors in the Aicardi-Goutieres Syndrome. N Engl J Med 2018; 379:2275–7. [DOI] [PubMed] [Google Scholar]
- 52.Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity 2011; 34:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adang LA, Frank DB, Gilani A, Takanohashi A, Ulrick N, Collins A, et al. Aicardi goutieres syndrome is associated with pulmonary hypertension. Mol Genet Metab 2018; 125:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Carvalho LM, Ngoumou G, Park JW, Ehmke N, Deigendesch N, Kitabayashi N, et al. Musculoskeletal Disease in MDA5-Related Type I Interferonopathy: A Mendelian Mimic of Jaccoud’s Arthropathy. Arthritis Rheumatol 2017; 69:2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang MA, Kim EK, Now H, Nguyen NT, Kim WJ, Yoo JY, et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 2015; 96:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira CR, Crow YJ, Gahl WA, Gardner PJ, Goldbach-Mansky R, Hur S, et al. DDX58 and Classic Singleton-Merten Syndrome. J Clin Immunol 2019; 39:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014; 371:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volpi S, Insalaco A, Caorsi R, Santori E, Messia V, Sacco O, et al. Efficacy and Adverse Events During Janus Kinase Inhibitor Treatment of SAVI Syndrome. J Clin Immunol 2019; 39:476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brehm A, Liu Y, Sheikh A, Marrero B, Omoyinmi E, Zhou Q, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest 2015; 125:4196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torrelo A CANDLE Syndrome As a Paradigm of Proteasome-Related Autoinflammation. Front Immunol 2017; 8:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendinez F, Hernandez A, et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol 2010; 62:489–95. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, Babay S, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 2012; 64:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jesus AA, Brehm A, VanTries R, Pillet P, Parentelli AS, Montealegre Sanchez GA, et al. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J Allergy Clin Immunol 2019; 143:1939–43 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meuwissen ME, Schot R, Buta S, Oudesluijs G, Tinschert S, Speer SD, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med 2016; 213:1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Bogunovic D, Payelle-Brogard B, Francois-Newton V, Speer SD, Yuan C, et al. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 2015; 517:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duncan CJA, Thompson BJ, Chen R, Rice GI, Gothe F, Young DF, et al. Severe type I interferonopathy and unrestrained interferon signaling due to a homozygous germline mutation in STAT2. Sci Immunol 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gruber C, Martin-Fernandez M, Ailal F, Qiu X, Taft J, Altman J, et al. Homozygous STAT2 gain-of-function mutation by loss of USP18 activity in a patient with type I interferonopathy. J Exp Med 2020; 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alsohime F, Martin-Fernandez M, Temsah MH, Alabdulhafid M, Le Voyer T, Alghamdi M, et al. JAK Inhibitor Therapy in a Child with Inherited USP18 Deficiency. N Engl J Med 2020; 382:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell 2017; 168:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 2017; 17:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steiner A, Harapas CR, Masters SL, Davidson S. An Update on Autoinflammatory Diseases: Relopathies. Curr Rheumatol Rep 2018; 20:39. [DOI] [PubMed] [Google Scholar]
- 72.Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol 2014; 26:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 2016; 48:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 2016; 48:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takagi M, Ogata S, Ueno H, Yoshida K, Yeh T, Hoshino A, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol 2017; 139:1914–22. [DOI] [PubMed] [Google Scholar]
- 76.Saitoh T, Yamamoto M, Miyagishi M, Taira K, Nakanishi M, Fujita T, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol 2005; 174:1507–12. [DOI] [PubMed] [Google Scholar]
- 77.Yarilina A, Ivashkiv LB. Type I interferon: a new player in TNF signaling. Curr Dir Autoimmun 2010; 11:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwartz DM, Blackstone SA, Sampaio-Moura N, Rosenzweig S, Burma AM, Stone D, et al. Type I interferon signature predicts response to JAK inhibition in haploinsufficiency of A20. Ann Rheum Dis 2020; 79:429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wouters CH, Maes A, Foley KP, Bertin J, Rose CD. Blau syndrome, the prototypic autoinflammatory granulomatous disease. Pediatr Rheumatol Online J 2014; 12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rose CD, Pans S, Casteels I, Anton J, Bader-Meunier B, Brissaud P, et al. Blau syndrome: cross-sectional data from a multicentre study of clinical, radiological and functional outcomes. Rheumatology (Oxford) 2015; 54:1008–16. [DOI] [PubMed] [Google Scholar]
- 81.Nagakura T, Wakiguchi H, Kubota T, Yamatou T, Yamasaki Y, Nonaka Y, et al. Tumor Necrosis Factor Inhibitors Provide Longterm Clinical Benefits in Pediatric and Young Adult Patients with Blau Syndrome. J Rheumatol 2017; 44:536–8. [DOI] [PubMed] [Google Scholar]
- 82.Simon A, Park H, Maddipati R, Lobito AA, Bulua AC, Jackson AJ, et al. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc Natl Acad Sci U S A 2010; 107:9801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 2011; 208:519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 2014; 370:911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 2014; 370:921–31. [DOI] [PubMed] [Google Scholar]
- 86.Ombrello AK, Qin J, Hoffmann PM, Kumar P, Stone D, Jones A, et al. Treatment Strategies for Deficiency of Adenosine Deaminase 2. N Engl J Med 2019; 380:1582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee PY, Kellner ES, Huang Y, Furutani E, Huang Z, Bainter W, et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2). J Allergy Clin Immunol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee PY. Vasculopathy, Immunodeficiency, and Bone Marrow Failure: The Intriguing Syndrome Caused by Deficiency of Adenosine Deaminase 2. Front Pediatr 2018; 6:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watkin LB, Jessen B, Wiszniewski W, Vece TJ, Jan M, Sha Y, et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet 2015; 47:654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volpi S, Tsui J, Mariani M, Pastorino C, Caorsi R, Sacco O, et al. Type I interferon pathway activation in COPA syndrome. Clin Immunol 2018; 187:33–6. [DOI] [PubMed] [Google Scholar]
- 91.Deng Z, Chong Z, Law CS, Mukai K, Ho FO, Martinu T, et al. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J Exp Med 2020; 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lepelley A, Martin-Niclos MJ, Le Bihan M, Marsh JA, Uggenti C, Rice GI, et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J Exp Med 2020; 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng Z, Law CS, Ho FO, Wang KM, Jones KD, Shin JS, et al. A Defect in Thymic Tolerance Causes T Cell-Mediated Autoimmunity in a Murine Model of COPA Syndrome. J Immunol 2020; 204:2360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsui JL, Estrada OA, Deng Z, Wang KM, Law CS, Elicker BM, et al. Analysis of pulmonary features and treatment approaches in the COPA syndrome. ERJ Open Res 2018; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krutzke S, Rietschel C, Horneff G. Baricitinib in therapy of COPA syndrome in a 15-year-old girl. Eur J Rheumatol 2019:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 2000; 13:25–35. [DOI] [PubMed] [Google Scholar]
- 97.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med 2012; 366:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet 2012; 91:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neves JF, Doffinger R, Barcena-Morales G, Martins C, Papapietro O, Plagnol V, et al. Novel PLCG2 Mutation in a Patient With APLAID and Cutis Laxa. Front Immunol 2018; 9:2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milner JD. PLAID: a Syndrome of Complex Patterns of Disease and Unique Phenotypes. J Clin Immunol 2015; 35:527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol 2019; 19:503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–41. [DOI] [PubMed] [Google Scholar]
- 103.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 105.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019; 381:2497–505. [DOI] [PubMed] [Google Scholar]
- 106.Navid F, Colbert RA. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat Rev Rheumatol 2017; 13:25–40. [DOI] [PubMed] [Google Scholar]
- 107.Broderick L, Kastner DL, Hoffman HM. Recurrent Fever Syndromes In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases. 3rd ed; 2014. [Google Scholar]
- 108.Manthiram K, Preite S, Dedeoglu F, Demir S, Ozen S, Edwards KM, et al. Common genetic susceptibility loci link PFAPA syndrome, Behcet’s disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U S A 2020; 117:14405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ombrello MJ, Remmers EF, Tachmazidou I, Grom A, Foell D, Haas JP, et al. HLA-DRB1*11 and variants of the MHC class II locus are strong risk factors for systemic juvenile idiopathic arthritis. Proc Natl Acad Sci U S A 2015; 112:15970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nigrovic PA. Autoinflammation and autoimmunity in systemic juvenile idiopathic arthritis. Proc Natl Acad Sci U S A 2015; 112:15785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Henderson LA, Hoyt KJ, Lee PY, Rao DA, Jonsson AH, Nguyen JP, et al. Th17 reprogramming of T cells in systemic juvenile idiopathic arthritis. JCI Insight 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strober W, Asano N, Fuss I, Kitani A, Watanabe T. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s disease. Immunol Rev 2014; 260:249–60. [DOI] [PubMed] [Google Scholar]
- 113.Rabinovich E, Livneh A, Langevitz P, Brezniak N, Shinar E, Pras M, et al. Severe disease in patients with rheumatoid arthritis carrying a mutation in the Mediterranean fever gene. Ann Rheum Dis 2005; 64:1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 2005; 84:1–11. [DOI] [PubMed] [Google Scholar]
- 115.Akkoc N, Sari I, Akar S, Binicier O, Thomas MG, Weale ME, et al. Increased prevalence of M694V in patients with ankylosing spondylitis: additional evidence for a link with familial mediterranean fever. Arthritis Rheum 2010; 62:3059–63. [DOI] [PubMed] [Google Scholar]
- 116.Gattorno M, Hofer M, Federici S, Vanoni F, Bovis F, Aksentijevich I, et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis 2019. [DOI] [PubMed] [Google Scholar]
- 117.Hoffman HM, Broderick L. Editorial: It Just Takes One: Somatic Mosaicism in Autoinflammatory Disease. Arthritis Rheumatol 2017; 69:253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shinar Y, Ceccherini I, Rowczenio D, Aksentijevich I, Arostegui J, Ben-Chetrit E, et al. ISSAID/EMQN Best Practice Guidelines for the Genetic Diagnosis of Monogenic Autoinflammatory Diseases in the Next-Generation Sequencing Era. Clin Chem 2020; 66:525–36. [DOI] [PubMed] [Google Scholar]
- 119.Van Gijn ME, Ceccherini I, Shinar Y, Carbo EC, Slofstra M, Arostegui JI, et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J Med Genet 2018; 55:530–7. [DOI] [PubMed] [Google Scholar]
- 120.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 2017; 76:166–72. [DOI] [PubMed] [Google Scholar]
- 121.Lee PY, Schulert GS, Canna SW, Huang Y, Sundel J, Li Y, et al. Adenosine deaminase 2 as a biomarker of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann Rheum Dis 2020; 79:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ter Haar N, Lachmann H, Ozen S, Woo P, Uziel Y, Modesto C, et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann Rheum Dis 2013; 72:678–85. [DOI] [PubMed] [Google Scholar]
- 123.Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A 2016; 113:E4857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Papa R, Rusmini M, Volpi S, Caorsi R, Picco P, Grossi A, et al. Next generation sequencing panel in undifferentiated autoinflammatory diseases identifies patients with colchicine-responder recurrent fevers. Rheumatology (Oxford) 2020; 59:344–60. [DOI] [PubMed] [Google Scholar]
- 125.Ter Haar NM, Eijkelboom C, Cantarini L, Papa R, Brogan PA, Kone-Paut I, et al. Clinical characteristics and genetic analyses of 187 patients with undefined autoinflammatory diseases. Ann Rheum Dis 2019; 78:1405–11. [DOI] [PubMed] [Google Scholar]
- 126.Fisher CJ Jr., Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. Jama 1994; 271:1836–43. [PubMed] [Google Scholar]
- 127.Holzinger D, Fassl SK, de Jager W, Lohse P, Rohrig UF, Gattorno M, et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. J Allergy Clin Immunol 2015; 136:1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Standing AS, Malinova D, Hong Y, Record J, Moulding D, Blundell MP, et al. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J Exp Med 2017; 214:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lorden G, Sanjuan-Garcia I, de Pablo N, Meana C, Alvarez-Miguel I, Perez-Garcia MT, et al. Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation. J Exp Med 2017; 214:511–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis 2017; 76:1191–8. [DOI] [PubMed] [Google Scholar]
- 131.Poli MC, Ebstein F, Nicholas SK, de Guzman MM, Forbes LR, Chinn IK, et al. Heterozygous Truncating Variants in POMP Escape Nonsense-Mediated Decay and Cause a Unique Immune Dysregulatory Syndrome. Am J Hum Genet 2018; 102:1126–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Briggs TA, Rice GI, Daly S, Urquhart J, Gornall H, Bader-Meunier B, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet 2011; 43:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Badran YR, Dedeoglu F, Leyva Castillo JM, Bainter W, Ohsumi TK, Bousvaros A, et al. Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration. J Exp Med 2017; 214:1937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boisson B, Laplantine E, Dobbs K, Cobat A, Tarantino N, Hazen M, et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J Exp Med 2015; 212:939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol 2012; 13:1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lalaoui N, Boyden SE, Oda H, Wood GM, Stone DL, Chau D, et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 2020; 577:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 2020; 577:109–14. [DOI] [PubMed] [Google Scholar]
- 138.Chakraborty PK, Schmitz-Abe K, Kennedy EK, Mamady H, Naas T, Durie D, et al. Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD). Blood 2014; 124:2867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wakil SM, Monies DM, Abouelhoda M, Al-Tassan N, Al-Dusery H, Naim EA, et al. Association of a mutation in LACC1 with a monogenic form of systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2015; 67:288–95. [DOI] [PubMed] [Google Scholar]
- 140.Huang C, Hedl M, Ranjan K, Abraham C. LACC1 Required for NOD2-Induced, ER Stress-Mediated Innate Immune Outcomes in Human Macrophages and LACC1 Risk Variants Modulate These Outcomes. Cell Rep 2019; 29:4525–39 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009; 361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, et al. Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016; 151:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kahr WH, Pluthero FG, Elkadri A, Warner N, Drobac M, Chen CH, et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nat Commun 2017; 8:14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Volpi S, Cicalese MP, Tuijnenburg P, Tool ATJ, Cuadrado E, Abu-Halaweh M, et al. A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol 2019; 143:2296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lam MT, Coppola S, Krumbach OHF, Prencipe G, Insalaco A, Cifaldi C, et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J Exp Med 2019; 216:2778–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gernez Y, de Jesus AA, Alsaleem H, Macaubas C, Roy A, Lovell D, et al. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1beta inhibition. J Allergy Clin Immunol 2019; 144:1122–5 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]