Abstract
Background:
Abnormalities in fronto-striatal-thalamic (FST) sub-circuits are present in schizophrenia and are associated with cognitive impairments. However, it remains unknown whether abnormalities in FST sub-circuits are present before psychosis onset. This may be elucidated by investigating 22q11.2 deletion syndrome (22q11DS), a genetic syndrome associated with a 30% risk for developing schizophrenia in adulthood and a decline in Verbal IQ (VIQ) preceding psychosis onset. Here we examined white matter (WM) tracts in FST sub-circuits, especially those in the dorsolateral (DLPFC) and ventrolateral prefrontal cortex (VLPFC) sub-circuits, and their associations with VIQ in young adults with 22q11DS.
Methods:
Diffusion MRI scans were acquired from 21 individuals with 22q11DS with prodromal symptoms of schizophrenia, 30 individuals with 22q11DS without prodromal symptoms, and 30 healthy controls (mean age: 21±2 years). WM tracts were reconstructed between striatum and thalamus with rostral middle frontal gyrus (rMFG) and inferior frontal gyrus (IFG), representing DLPFC and VLPFC respectively. Fractional anisotropy (FA) and radial diffusivity (RD) were used for group comparisons. VIQ was assessed and associations with the diffusion measures were evaluated.
Results:
FA was significantly increased and RD decreased in most tracts of the DLPFC and VLPFC sub-circuits in 22q11DS. Verbal IQ scores correlated negatively with FA and, at trend level, positively with RD in the right thalamus-IFG tract in 22q11DS with prodromal symptoms.
Conclusions:
While abnormalities in FST sub-circuits are associated with schizophrenia, we observed that these abnormalities are also present in 22q11DS individuals with prodromal symptoms and are associated with verbal performance in the right thalamus-IFG tract.
Keywords: Schizophrenia, Prodromal Symptoms, 22q11.2 Deletion Syndrome, Verbal IQ, Diffusion MRI, White Matter
1. Introduction
Fronto-striatal-thalamic (FST) sub-circuits are neuronal pathways that connect several frontal lobe regions with the striatum and the thalamus. They originate in the prefrontal cortex (PFC) and project to the striatum followed by the thalamus. Feedback loops project from the thalamus back to the PFC, forming the closed cortico-striato-thalamo-cortical loop (Alexander and Crutcher, 1990; Parent and Hazrati, 1995). Originally, FST sub-circuits, especially those involving the striatum, were best known to be involved in motor functions and were associated in the neuropathology of neurodegenerative disorders. Today we know that these sub-circuits are more complex. They are involved in a wide range of mental functions, including, motor, limbic, and cognitive projections (Haber, 2016, 2003; Haber and Calzavara, 2009). FST sub-circuits are of special interest in neuropsychiatric conditions, as they modulate cognitive functions (Alexander et al., 1986; Pauli et al., 2016). Abnormalities or lesions in either gray matter structures, or white matter (WM) tracts of the FST sub-circuits may result in disrupted cognitive functionality (Cummings, 1993).
Cognitive decline is a fundamental hallmark symptom in schizophrenia. Premorbid IQ deficits support a neurodevelopmental model of schizophrenia (Murray and Lewis, 1987; Weinberger, 1987). In the past 30 years, this theory has matured into the developmental risk factor model of psychosis (Murray et al., 2017), incorporating new evidence regarding dysregulated striatal dopamine (Egerton et al., 2017) linking risk factors, such as heavy cannabis use (Murray et al., 2014) and adversity in childhood (Egerton et al., 2016), to psychotic symptoms. Moreover, according to this theory, lower premorbid IQs are associated with an earlier onset of schizophrenia and cognitive deficits may predict social impairment and future functional and treatment outcome (Fett et al., 2011; Gold, 2004; Green et al., 2000; Keefe, 2007; Meier et al., 2014). The risk for a diagnosis in schizophrenia is more than two times higher in individuals with a premorbid Full Scale IQ (FSIQ) lower than 85 (Khandaker et al., 2011; Reichenberg et al., 2010). In addition, Verbal IQ (VIQ) is significantly lower in individuals who later develop schizophrenia (Khandaker et al., 2011).
Abnormalities in FST sub-components and their association with cognitive functions have also been associated with schizophrenia (Li et al., 2020; Quan et al., 2013). Moreover, several neuroimaging studies have explored the connectivity between the PFC and thalamus (Anticevic et al., 2015; Buchsbaum et al., 2006; Hamoda et al., 2018; Levitt et al., 2012), and connections between the PFC and striatum in chronic and first-episode schizophrenia (de Leeuw et al., 2015; Quan et al., 2013). Findings here demonstrated that FST components and their association with cognition is not only disrupted in chronic phases of illness, but also in early phases. However, it is not known whether or not such changes are present prior to the onset of psychotic symptoms.
The PFC is functionally segregated. Two important functional subregions in the PFC are the dorsolateral (DLPFC) and the ventrolateral prefrontal cortex (VLPFC), serving different cognitive processes, including higher cognitive and social cognitive domains. The DLPFC is involved in executive functioning, such as working memory and inhibition (Blumenfeld and Ranganath, 2006; Lara and Wallis, 2015), while the VLPFC is, among other functions, known for processing of language (Rygula et al., 2010). DLPFC and VLPFC have been shown to mediate higher cognitive and social cognitive functions (Shallice and Cipolotti, 2018) which are also abnormal in schizophrenia (Boettiger and D’Esposito, 2005; Manoach et al., 2000; Weinberger et al., 2001). Individuals with first-episode schizophrenia demonstrate an increased activation in the right DLPFC (Zheng et al., 2017). Functional and structural changes in the VLPFC are associated with impulsivity in individuals with schizophrenia (Kaladjian et al., 2011; van Erp et al., 2020). Moreover, during social cognition tasks, the activation of the right VLPFC is significantly decreased (Vucurovic et al., 2020) while during emotion regulation tasks individuals with schizophrenia demonstrated a hyper-activation between the VLPFC and brain motor areas (Zhang et al., 2020). Thus, measuring prefrontal projections separately, from specific cortical source regions of interest, such as the DLPFC and VLPFC, to the striatum and to and from the thalamus, is important as it yields information about specific FST sub-circuits and their associations with psychosis.
Fiber tracts of the FST sub-circuits can be assessed in vivo using diffusion MRI (dMRI). The tractography technique, derived from dMRI, makes it possible to model, visualize, and quantify fiber tracts. The reconstruction of fiber tracts between subcortical and cortical regions, such as those in the FST sub-circuits, pose a challenge to the traditional single-tensor tractography approaches due to the presence of crossing and fanning fibers. This challenge can be overcome by using an Unscented Kalman Filter (UKF) based two-tensor whole brain tractography algorithm (Malcolm et al., 2010), and is thus optimal for studying complex connections such as FST-tracts. This advanced approach makes it possible to reconstruct tracts connecting DLPFC and VLPFC with the striatum and thalamus. DMRI provides insight into pathological changes that affect tissue microstructure (Beaulieu, 2002; Song et al., 2002). Fractional Anisotropy (FA), which measures the extent to which diffusion is directionally restricted, is the most commonly used dMRI parameter. To further understand the microstructural changes of the tissue it is recommended to use additional diffusion measures, such as Axial Diffusivity (AD) and Radial Diffusivity (RD), which demonstrate more specific relationships with WM pathology. AD appears to be more specific to axonal degeneration, whereas RD seems to be modulated by myelin in WM (Alexander et al., 2007). Using the two-tensor tractography technique, our group was the first to look at the DLPFC and VLPFC connections to the striatum and thalamus and reported abnormalities in FA (Levitt et al., 2017) and RD (Quan et al., 2013) in both chronic and first-episode schizophrenia. These connections in individuals at risk to develop schizophrenia are yet to be investigated.
Here, we explore FST sub-components in young adults with 22q11.2 deletion syndrome (22q11DS), also known as Velo-Cardio-Facial (VCFS) or DiGeorge syndrome, who are at increased risk for developing schizophrenia. This disorder is caused by a microdeletion at the q11.2 locus of the chromosome 22 (Carlson et al., 1997). Cognitive delays and psychiatric disorders, including schizophrenia, are present in adolescence and adulthood in 22q11DS. Based on a 30% prevalence of schizophrenia in adult life (Schneider et al., 2014), young adults with 22q11DS may provide valuable insight into the neuropathology and changes in WM preceding the disease (Zinkstok et al., 2019). Cognitive decline, most pronounced in VIQ, representing verbal comprehension and working memory, precedes the onset of psychosis in 22q11DS, and those who develop psychosis diverge more strongly from a typical cognitive trajectory (Vorstman et al., 2015). Patients with 22q11DS with psychosis perform worse on verbal memory and spatial working memory tests compared to patients with 22q11DS without psychosis (Amelsvoort et al., 2004; Chow et al., 2006). Neuroimaging studies provide strong evidence that the microdeletion at the q11.2 locus of the chromosome 22 effects the neurodevelopment of the brain in children and young adults (Zinkstok et al., 2019). Reductions in gyrification (Bakker et al., 2016; Kunwar et al., 2012; Schmitt et al., 2015), cortical thinning (Schaer et al., 2009; Sun et al., 2018), and changes in volumetric measures (Ching et al., 2020) were reported in children and adolescents with 22q11DS. These findings could be indicative of an early neurodevelopmental pathology (Zinkstok et al., 2019). Moreover, studies reported WM abnormalities in individuals with 22q11DS. These studies suggest altered myelin, lower diffusivity, and smaller axonal diameter in multiple tracts in individuals with 22q11DS (Villalón-Reina et al., 2019). However, inconsistent results of both decreases as well as increases in FA in several parts of the brain were found (Bakker et al., 2016; Olszewski et al., 2017; Perlstein et al., 2014; Villalón-Reina et al., 2019). Decreases in AD and RD were reported consistently (Jalbrzikowski et al., 2014; Villalon-Reina et al., 2013). Indeed, cognitive deficits in individuals with 22q11DS have been associated with alterations in WM microstructure. More precisely, FA was significantly increased both globally and locally in patients with 22q11DS with cognitive decline throughout different tracts of the brain overlapping with WM tracts found to be affected in schizophrenia and individuals with 22q11DS with psychosis (Nuninga et al., 2018). Based on these findings, studies of young adults with 22q11DS without overt psychosis but with prodromal symptoms may increase our understanding of cognitive manifestations and early pathology in FST sub-circuits relevant to schizophrenia.
The aim of this study was to test whether components of FST sub-circuits are disrupted in young adults with 22q11DS with and without prodromal symptoms. Here, we explored subcortical-cortical WM connections from DLPFC and VLPFC to the striatum and thalamus. First, we hypothesized that young adults with 22q11DS and prodromal symptoms will have abnormalities in one or more WM tracts in FST sub-circuits. We expected WM abnormalities in the 22q11DS group with prodromal symptoms as compared to individuals with 22q11DS without prodromal symptoms and to healthy controls. To address this, we reconstructed WM connections in the DLPFC- and VLPFC-loop in the FST-circuits using dMRI and two-tensor tractography. Second, we hypothesized an association between dMRI measures in these tracts and performance in intellectual measures, especially VIQ, in young adults with 22q11DS with prodromal symptoms. Given that changes in WM microstructure of the right hemisphere have been associated with prodromal symptoms in adolescents with 22q11DS (Kikinis et al., 2017), we predicted that changes in WM would be associated with cognitive measures in the right brain hemisphere in our young adult sample.
2. Methods
2.1. Participants
We assessed imaging data from 21 participants with 22q11DS with prodromal symptoms (22q11DS+PS), 30 participants with 22q11DS without prodromal symptoms (22q11DS−PS), and 30 healthy controls. The prodromal symptoms were assessed using the Structured Interview for Prodromal Symptoms (SIPS; Miller et al., 2003). Individuals with prodromal symptoms were those who had scores ≥ 3 on at least one item of the positive symptom subscale plus/or scores ≥ 3 on at least two items of the negative or disorganized symptom subscale. This operationalization is consistent with previously published papers in this field (Tang et al., 2014; Weisman et al., 2017). None of the participants were diagnosed with schizophrenia. The participants were matched on age, gender, and handedness (see Table 1). A medical history questionnaire was completed for all participants, to list all of the current medications the participant was taking, the reason for each medication, and age at which the participant began to take each medication. The questionnaire was either completed by the participant her- or himself, or by the parents. An overview of the medication use at the time of the scan is given in Table 1. All details of the study, including exclusion criteria, are described in Kates et al. (2011). Participants were recruited from the International Center for Evaluation, Treatment, and Study of Velo-Cardio-Facial Syndrome at SUNY Upstate Medical University, Syracuse, NY, from parent support groups, and from the surrounding community by the team directed by Dr. Wendy Kates. The presence of the microdeletion was confirmed with fluorescence in situ hybridization (FISH) for 22q11DS participants. All participants provided written informed consent.
Table 1.
Demographic characteristics and medicanon of participants (N = 81).
| 22q11DS+PS (n = 21) | 22q11DS−PS (n = 30) | HC (n = 30) | 22q11DS+PS vs. 22q11DS−PS (p-Value) | 22q11DS+PS vs. HC (p-Value) | 22q11DS−PS vs. HC (p-Value) | |
|---|---|---|---|---|---|---|
| Age (y) | 21.20 ± 2.43a | 20.73 ± 2.17a | 20.89 ± 1.49a | 0.302 | 0.627 | 0.813 |
| Range | 18–26 | 18–26 | 20–24 | – | – | – |
| Gender (male/female) | 11/10 | 17/13 | 16/14 | 0.783b | 1.000b | 1.000b |
| Handedness (right/left) | 14/7 | 21/9 | 23/7 | 1.000b | 0.529b | 0.771b |
| FSIQ | 72.00 ± 10.20a | 78.07 ± 11.97a | 109.47 ± 16.02a | 0.065 | <0.001 | <0.001 |
| Range | 52–97 | 62–103 | 72–142 | – | – | – |
| VIQ | 74.71 ± 10.41a | 79.77 ± 11.99a | 105.70 ± 16.08a | 0.125 | <0.001 | <0.001 |
| Range | 57–100 | 62–103 | 68–134 | – | – | – |
| Scale of prodromal symptoms (SIPS) | ||||||
| Positive symptoms | 5.14 ± 5.05a | 0.30 ± 0.70a | 0.30 ± 1.15a | <0.001 | <0.001 | 1.000 |
| Negative symptoms | 9.29 ± 7.19a | 3.97 ± 4.44a | 1.53 ± 3.82a | 0.005 | <0.001 | 0.027 |
| Disorganization symptoms | 6.43 ± 2.99a | 1.00 ± 1.02a | 0.90 ± 2.02a | <0.001 | <0.001 | 0.810 |
| General symptoms | 6.43 ± 5.05a | 2.23 ± 2.52a | 1.30 ± 2.68a | <0.001 | <0.001 | 0.170 |
| Medication | ||||||
| Anti-depressant/anti-anxiety | 8 (38%) | 7 (23%) | 2 (7%) | – | – | – |
| Stimulants | 4 (19%) | 2 (7%) | 2 (7%) | – | – | – |
| Mood stabilizers | 1 (5%) | 1 (3%) | 0 | – | – | – |
| Antipsychotic medications | 2 (10%) | 0 | 0 | – | – | – |
Note: 22q11DS+PS = individuals with 22q11DS with prodromal symptoms. 22q11DS−PS = individuals with 22q11DS without prodromal symptoms. HC = healthy controls. FSIQ = full scale IQ. VIQ = verbal IQ.
= mean ± standard deviation.
= Fisher’s exact test. Statistically significant differences indicated in bold.
2.2. Image Acquisition and Postprocessing
MRI images were acquired on a 3T Siemens Magnetom Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany), using an ultrafast gradient echo 3D sequence (MPRAGE) with a PAT k-space-based algorithm called GRAPPA. Diffusion and structural MRI were obtained. Structural MRI scans were acquired with T1 and T2 sequences. Scan parameters for structural MRI were: echo time (TE)=3.31 ms, repetition time (TR)=2530 ms, matrix size=256 x 256, field of view (FOV)=256 mm, and slice thickness=1 mm. The dMRI sequence consisted of 64 transverse slices with no gaps and 2.0 mm nominal isotropic resolution acquired along 64 directions with a b-value=900 s/mm2. The parameters included TE=93 ms, TR=8600 ms, matrix size=96 x 96 (zero-filled and reconstructed to 256 x 256), FOV=244 mm. One minimally weighted volume (b0) was acquired within each dMRI dataset.
An in-house pipeline (https://github.com/pnlbwh/pnlpipe) was used to postprocess the MRI data of each participant. The MRI images were aligned, centered, and corrected for eddy current distortions and head motion. The parameters used to acquire the images (e.g., sizes, space directions, space origin), and the quality of the images (e.g., motion artifacts, ringing, ghosting of the skull or eyeballs, cut-offs, signal drops, and other artifacts), were also visually checked.
2.3. FreeSurfer Parcellations and Registration to DTI Space
Brain masks were made for structural (T1and T2 images) and diffusion images for each individual (del Re et al., 2016) using 3DSlicer software, Version 4.5 (Surgical Planning Laboratory, Brigham and Women’s Hospital, Boston, MA, USA; http://www.slicer.org). The masks for T1 were applied to generate a label map for white and gray matter parcellation using FreeSurfer software, Version 4.4 (http://surfer.nmr.mgh.harvard.edu). This software parcellates the brain into 34 cortical, 35 WM, and 40 subcortical regions for each participant (Fischl et al., 2004). We then registered the FreeSurfer label map to the masks of the diffusion images for each participant. This was done by using the symmetric diffeomorphic image registration algorithm of the ANTs software (Klein et al., 2009).
2.4. Two-Tensor Whole Brain Tractography
Tractography was performed to reconstruct fiber tracts of the whole brain for each study participant from the dMRI data. A two-tensor whole brain tractography (Malcolm et al., 2010) was conducted using the Unscented Kalman Filter (UKF). The two-tensor tractography is suitable for tracing neural fiber bundles of the brain connecting cortical and subcortical regions. Every voxel was seeded ten times with a minimum FA of 0.18 to generate tractography streamlines.
2.5. White Matter Query Language (WMQL)
To extract specific fiber tracts from the whole brain tractography, a technique called White Matter Query Language (WMQL) was utilized, which automatically extracts WM tracts from the two-tensor whole brain tractography using formally described neuroanatomical definitions, e.g., FreeSurfer generated brain parcellations (Wassermann et al., 2012). WMQL defines tracts of interest based on cortical and subcortical regions where the fibers begin and end, and also on WM regions where the fiber tract is expected to project. For this study, tracts of interest were the tracts connecting the thalamus and the striatum to the DLPFC and to the VLPFC. The DLPFC was represented by the rostral middle frontal gyrus (rMFG). The VLPFC was represented by the inferior frontal gyrus (IFG) that was constructed from FreeSurfer parcellations of Pars opercularis, Pars triangularis, and Pars orbitalis. The number of streamlines for each fiber tract of interest and each subject was calculated and only tracts with >10 streamlines were included. This resulted in two tracts of interest for the DLPFC sub-circuit (thalamus-rMFG tract, striatum-rMFG tract) and two tracts for the VLPFC sub-circuit (thalamus-IFG tract, striatum-IFG tract) in each hemisphere (see Figure 1). Finally, the diffusion metrics, FA, RD, and AD were extracted from each tract.
Figure 1: Tracts of the ventrolateral prefrontal cortex (VLPFC) loop and the dorsolateral prefrontal cortex (DLPFC) loop of the fronto-striatal-thalamic (FST) sub-circuits are represented.
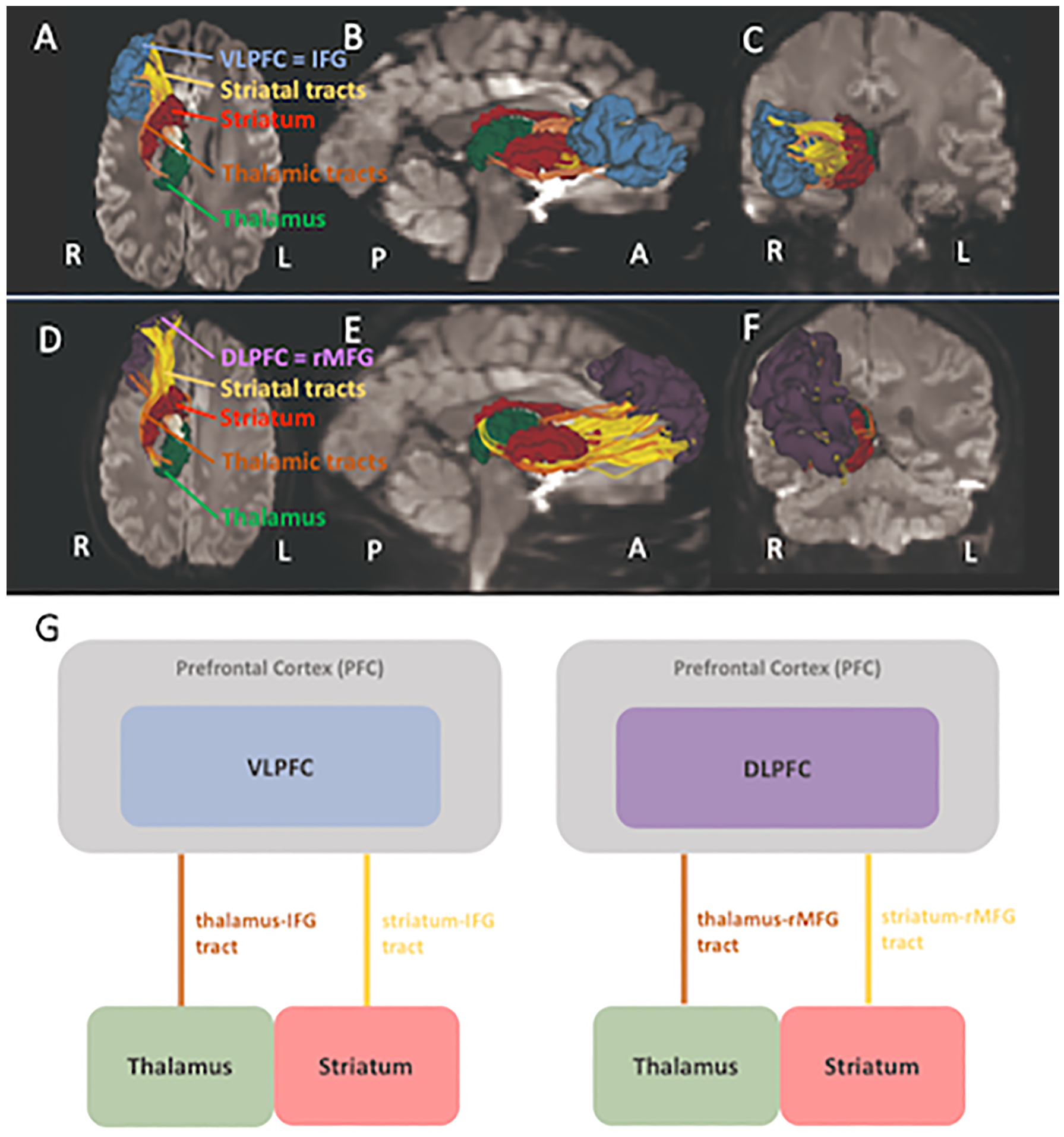
Panel A, B, C show the VLPFC sub-circuit in the inferior, right and frontal view. Panel C, D, E show the DLPFC sub-circuit in the inferior, right and frontal view. Inferior frontal gyrus (IFG, corresponds to VLPFC) is in blue. Rostral middle frontal gyrus (rMFG, corresponds to DLPFC) in purple. Striatum in crimson red. Thalamus in green. Tracts connecting the frontal lobe with striatum are colored yellow, tracts connecting the frontal lobe with thalamus are colored orange. Panel G shows the VLPFC loop on the left and the DLPFC loop on the right.
2.6. Cognitive Measure
VIQ was assessed using the Wechsler Adult Intelligence Scale-3rd edition (WAIS-III; Wechsler, 1997). VIQ measures verbal comprehension and working memory. The cognitive measure was administered by a trained psychologist and double scored by a trained research assistant.
2.7. Statistical Approach
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 24, and the freely available R software (https://www.r-project.org). Demographic and clinical characteristics were compared using independent sample t-tests for continuous variables and Fisher’s exact test for categorial variables.
Normal distribution of the data was assessed using the Kolmogorov-Smirnov test. Homogeneity of variance was tested using the Levene’s test. Linear mixed-effect (LME) modeling was used to examine differences in FA, AD, and RD in FST tracts between three groups, namely 22q11DS+PS, 22q11DS−PS and healthy controls. Linear mixed models have several advantages over traditional repeated-measures methods, such as repeated measures analysis of variance (ANOVA): LME analysis is able to handle unequal numbers of within-subject measurements and it accounts for interindividual variability (Gueorguieva and Krystal, 2004). Our model included Group (3 levels: 22q11DS+PS, 22q11DS−PS, healthy controls) and Hemisphere (2 levels: left, right) as the fixed-effects factors, Subject as a random-effects factor, and FA, AD, and RD as the dependent variables. We conducted four separate models (one for each FA, AD, and RD in each tract). In case of a significant group effect, post-hoc t-tests were calculated between each group. Level of statistical significance was set at p<0.05 (two tailed) and the False Discovery Rate (FDR) was used to correct for multiple comparisons per sub-circuit (i.e., the DLPFC- and VLPFC-loops) and dMRI indices, separately (Benjamini and Hochberg, 1995). Accordingly, the correction for multiple comparison was made for two tracts within each sub-circuit. Standardized effect size, Cohen’s d, was calculated based on the mean and standard deviation.
Finally, Pearson correlations were applied to evaluate the relationship between diffusion metrics and cognitive test performance in VIQ for each sub-circuit and hemisphere separately. This was performed based on lateralization effects reported in previous studies in first-episode schizophrenia (Bleich-Cohen et al., 2012; Sheng et al., 2013). We ran correlations separately for individuals with 22q11DS with and without prodromal symptoms and healthy controls to determine whether the prodromal symptoms drove the correlation in the patient groups.
3. Results
3.1. Demographic Data
Mean age in years was 21 and did not differ significantly between patients with 22q11DS with and without prodromal symptoms and the healthy control group. The age range was 18 to 26 years. There were no significant differences in gender or handedness between the three groups. As predicted, there was a significant difference in FSIQ between the two 22q11DS groups and the healthy group (see Table 1). Although early cognitive decline is a robust indicator for the risk of developing psychosis in individuals with 22q11DS, we did not find significant differences in VIQ in individuals with 22q11DS with and without prodromal symptoms. We did observe, however, a difference at trend level (p=.065) between these subgroups in FSIQ. Because a reduced IQ is not unusual in schizophrenia (Kahn and Keefe, 2013), as well as in 22q11DS (Vorstman et al., 2015), IQ was not included as a covariate in the following statistical analyses.
3.2. Comparison of Tracts between the Groups
The LME analysis revealed significant main effects for group in FA and RD in the thalamus-rMFG tract (FA: p=.017, FDR=.029; RD: p=.029, FDR=.044), the striatum-rMFG tract (FA: p>.001, FDR>.001; RD: p>.001, FDR>.001), the thalamus-IFG tract (FA: p>.001, FDR>.001; RD: p=.001, FDR=.003), and the striatum-IFG tract (FA: p=.005, FDR=.010; RD: p=.004, FDR=.009). No main effect for group in AD was found in any of the tracts. A main effect for hemisphere was revealed in AD in the striatum-IFG tract (p=.003, FDR=.036). No Group x Hemisphere interaction was found to be significant in any of the four tracts. In case of a significant group effect, post-hoc analysis was conducted between each group. In 22q11DS+PS the analysis revealed significant FA increases and RD decreases in the striatum-rMFG tract (FA: d=0.867; RD: d=0.905), the thalamus-IFG tract (FA: d=0.792; RD: d=0.707), and the striatum-IFG tract (FA: d=0.767; RD: d=0.707) compared to healthy controls. In the 22q11DS−PS group FA was significantly increased while RD was decreased in the striatum-rMFG tract (FA: d=0.767; RD: d=0.700) and the thalamus-IFG tract (FA: d=0.509; RD: d=0.566) compared to the healthy control group. Moreover, FA only was found to be significantly increased in the thalamus-rMFG tract (d=0.622). All differences remained statistically significant when FDR correction for multiple testing was performed (see Table 2). There was no significant difference in either FA nor in RD in any of the tracts between 22q11DS+PS and 22q11DS−PS.
Table 2.
Fronto-striatal-thalamic (FST) sub-circuits, FDR-significant group differences between patients with 22q11DS with and without prodromal symptoms and healthy controls for FA, RD, and AD in tracts of the DLPFC and VLPFC sub-circuits.
| 22q11DS+PS (n = 21) |
22q11DS−PS (n = 30) |
HC (n = 30) |
22q11DS+PS vs. 22q11DS−PS |
22q11DS+PS vs. HC |
22q11DS−PS vs. HC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FST sub-circuits | Tract | dMRI indices | M ± SD | M ± SD | M ± SD | p-Value | FDR | d | p-Value | FDR | d | p-Value | FDR | d |
| DLPFC sub-circuit | Thalamus-rMFG | FA | 0.613 ± 0.03 | 0.616 ± 0.03 | 0.594 ± 0.04 | 1.000 | n.s. | 0.100 | 0.087 | n.s. | 0.537 | 0.020 | 0.027 | 0.622 |
| RD | 0.430 ± 0.03 | 0.432 ± 0.04 | 0.452 ± 0.04 | 1.000 | n.s. | 0.057 | 0.061 | n.s. | 0.622 | 0.057 | n.s. | 0.500 | ||
| AD | 1.333 ± 0.05 | 1.341 ± 0.06 | 1.337 ± 0.05 | 1.000 | n.s. | 0.145 | 1.000 | n.s. | 0.080 | 1.000 | n.s. | 0.072 | ||
| Striatum-rMFG | FA | 0.577 ± 0.03 | 0.574 ± 0.03 | 0.551 ± 0.03 | 1.000 | n.s. | 0.100 | <0.001 | <0.001 | 0.867 | <0.001 | <0.001 | 0.767 | |
| RD | 0.467 ± 0.03 | 0.471 ± 0.04 | 0.499 ± 0.04 | 1.000 | n.s. | 0.113 | <0.001 | <0.001 | 0.905 | <0.001 | <0.001 | 0.700 | ||
| AD | 1.312 ± 0.04 | 1.312 ± 0.04 | 1.323 ± 0.04 | 1.000 | n.s. | 0.000 | 0.531 | n.s. | 0.275 | 0.459 | n.s. | 0.275 | ||
| VLPFC sub-circuit | Thalamus-IFG | FA | 0.640 ± 0.03 | 0.630 ± 0.03 | 0.612 ± 0.04 | 0.538 | n.s. | 0.333 | <0.001 | <0.001 | 0.792 | 0.012 | 0.024 | 0.509 |
| RD | 0.404 ± 0.03 | 0.409 ± 0.03 | 0.429 ± 0.04 | 1.000 | n.s. | 0.167 | 0.003 | 0.004 | 0.707 | 0.011 | 0.022 | 0.566 | ||
| AD | 1.346 ± 0.04 | 1.333 ± 0.03 | 1.333 ± 0.03 | 0.192 | n.s. | 0.368 | 0.200 | n.s. | 0.368 | 1.000 | n.s. | 0.000 | ||
| Striatum-IFG | FA | 0.608 ± 0.03 | 0.596 ± 0.03 | 0.585 ± 0.03 | 0.281 | n.s. | 0.400 | 0.004 | 0.005 | 0.767 | 0.240 | n.s. | 0.367 | |
| RD | 0.422 ± 0.04 | 0.433 ± 0.03 | 0.447 ± 0.03 | 0.452 | n.s. | 0.311 | 0.003 | 0.004 | 0.707 | 0.119 | n.s. | 0.467 | ||
| AD | 1.290 ± 0.05 | 1.283 ± 0.04 | 1.294 ± 0.04 | 1.000 | n.s. | 0.155 | 1.000 | n.s. | 0.088 | 1.000 | n.s. | 0.275 | ||
Note: The connections between thalamus-rMFG and striatum-rMFG represent the DLFPC sub-circuit of the fronto-striatal circuit, whereas thalamus-IFG and striatum-IFG represent the VLPFC sub-circuit. FA = Fractional Anisotropy. RD = radial diffusivity. AD = axial diffusivity. 22q11DS+PS = individuals with 22q11DS and prodromal symptoms. 22q11DS−PS = individuals with 22q11DS without prodromal symptoms. HC = healthy controls. M ± SD = mean ± standard deviation. FDR = false discovery rate-adjusted p. d = Cohen’s d. Statistically significant differences after FDR-correction are given in bold.
3.3. Correlations with Cognitive Measure
All variables were normally distributed. Pearson correlations were conducted for each group per sub-circuit and hemisphere, separately, in tracts showing significant differences between groups. VIQ correlated negatively with FA in the right thalamus-IFG tract (r=−0.491, p=.024), while RD correlated positively with VIQ in the right thalamus-IFG tract (r=0.487, p=.025) in individuals with 22q11DS+PS, such that increased FA and decreased RD were associated with a lower VIQ. FA remained significant (FDR-adjusted p=.049) and RD remained significant at trend level (FDR-adjusted p=.050) after FDR correction for multiple comparisons per sub-circuit in each hemisphere and per each dMRI measure was performed. No significant correlations were found in individuals with 22q11DS−PS in the VLPFC or the DLPFC sub-circuits in either hemisphere (see Table 3). Furthermore, there were no significant correlations in the healthy control group. Figure 2 provides scatterplots of the significant correlations in the 22q11DS+PS group.
Table 3.
Pearson correlations for FA and RD in significant tracts and verbal IQ in 22q11DS with and without prodromal symptoms.
| 22q11DS−PS (n = 30) |
22q11DS+PS (n = 21) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sub-circuit | Tract | dMRI indices | r | p-Value | FDR | r | p-Value | FDR |
| Left DLPFC sub-circuit | Thalamus-rMFG | FA | 0.298 | n.s. | n.s. | – | – | – |
| Striatum-rMFG | FA | 0.219 | n.s. | n.s. | −0.242 | 0.391 | n.s. | |
| Striatum-rMFG | RD | −0.284 | n.s. | n.s. | 0.240 | 0.323 | n.s. | |
| Left VLPFC sub-circuit | Thalamus-IFG | FA | 0.330 | n.s. | n.s. | −0.391 | 0.080 | n.s. |
| Striatum-IFG | FA | – | – | – | −0.353 | 0.151 | n.s. | |
| Thalamus-IFG | RD | −0.366 | n.s. | n.s. | 0.398 | 0.074 | n.s. | |
| Striatum-IFG | RD | – | – | – | 0.403 | 0.097 | n.s. | |
| Right DLPFC sub-circuit | Thalamus-rMFG | FA | 0.296 | n.s. | n.s. | – | – | – |
| Striatum-rMFG | FA | 0.690 | n.s. | n.s. | −0.241 | 0.293 | n.s. | |
| Striatum-rMFG | RD | −0.114 | n.s. | n.s. | 0.149 | 0.520 | n.s. | |
| Right VLPFC sub-circuit | Thalamus-IFG | FA | −0.193 | n.s. | n.s. | −0.491 | 0.024 | 0.048 |
| Striatum-IFG | FA | – | – | – | −0.250 | 0.301 | n.s. | |
| Thalamus-IFG | RD | 0.129 | n.s. | n.s. | 0.487 | 0.025 | 0.050 | |
| Striatum-IFG | RD | – | – | – | 0.151 | 0.537 | n.s. | |
Note: Pearson correlation coefficients r are given. Numbers of participants range from 51 to 45. 22q11DS−PS = individuals with 22q11DS without prodromal symptoms. 22q11DS+PS = individuals with 22q11DS with prodromal symptoms. L = left. R = right. FA = fractional anisotropy. RD = Radial Diffusivity. FDR = false discovery rate-adjusted p. The connections between thalamus-rMFG and striatum-rMFG represent the DLFPC sub-circuit of the fronto-striatal circuit, whereas thalamus-IFG and striatum-IFG represent the VLPFC sub-circuit. Results of p ≤ 0.05 are given in bold.
FDR was used to correct for multiple comparisons per sub-circuit (i.e., the DLPFC- and VLPFC-loops) and dMRI indices separately. Accordingly, the correction for multiple comparisons was made for two tracts within each sub-circuit.
Figure 2: Correlations between Verbal IQ and diffusion measures of the thalamus-VLPFC tract in individuals with 22q11DS with prodromal symptoms.
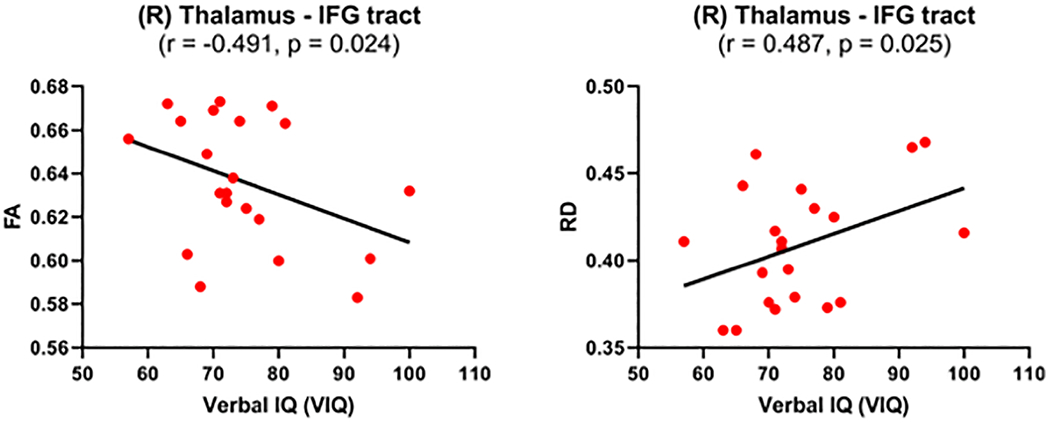
Significant correlations between fractional anisotropy (FA) and radial diffusivity (RD) in the right (R) thalamus-IFG tract and the Verbal IQ (VIQ) were present in 22q11DS group with prodromal symptoms. This tract is part of the VLPFC loop of the FST sub-circuit. Pearson coefficient r and significance value p are given. The black line represents the best-linear-fit for the data.
4. Discussion
This study is, to the best of our knowledge, the first study to report abnormalities in FST sub-circuits, specifically the DLPFC and VLPFC sub-circuits, in a sample of individuals with 22q11DS. Some, but not all, WM tracts of the DLPFC and VLPFC sub-circuits were abnormal in young adults with 22q11DS with or without prodromal symptoms when compared to healthy controls. In addition, the microstructural abnormalities in the VLPFC sub-circuit correlated with VIQ in individuals with 22q11DS with prodromal symptoms, suggesting that the tract connecting the thalamus and IFG is associated with verbal performance.
WM abnormalities were present in the FST tracts of the DLPFC and the VLPFC sub-circuits in both 22q11DS groups, with and without prodromal symptoms. FA was increased, while RD was decreased in all WM tracts that were significantly different in individuals with 22q11DS with and without prodromal symptoms in comparison to healthy controls. Changes in WM tracts connecting PFC with thalamus (Kikinis et al., 2017; Perlstein et al., 2014; Radoeva et al., 2012) have been published earlier (Olszewski et al., 2017; Tylee et al., 2017). The present results are consistent with the results by Olszewski et al. on the same cohort who also found increased FA and decreased RD in thalamic-frontal tracts (Olszewski et al., 2017). In this study, we expand the previous findings by exploring not only the thalamic-frontal tracts, but also striatal-frontal tracts. Furthermore, we report WM abnormalities in subdivisions of prefrontal regions, namely the DLPFC and the VLPFC, as this yields more detailed information about the FST sub-circuits. Interestingly, FA was found to be significantly increased while RD was significantly decreased in the striatum-VLPFC sub-circuits (thalamus-IFG, and striatum-IFG tract) in individuals with 22q11DS with prodromal symptoms only. No such difference was found in individuals with 22q11DS without prodromal symptoms compared to healthy controls. This suggests the importance of striatal projections to the PFC in the development of psychotic symptoms in individuals with 22q11DS.
Increased FA may seem counter-intuitive at first, as reductions in FA have been reported in most studies in patients in the chronic phase of schizophrenia (Grazioplene et al., 2018; Levitt et al., 2012; Mamah et al., 2019). Although inconsistent findings of reduced and increased FA in cerebral WM of the 22q11DS population have been published (Kochunov et al., 2020; Scariati et al., 2016; Villalón-Reina et al., 2019), studies involving only adolescents and young adults with 22q11DS have reported increased FA (Kates et al., 2015; Nuninga et al., 2018). Nuninga et al. reported increased FA in adolescents with 22q11DS and cognitive decline, suggesting that increased FA reflects a higher vulnerability to develop schizophrenia (Nuninga et al., 2018). The authors further speculated that this increase in FA in adolescents with 22q11DS will be followed by a decrease in FA in individuals diagnosed with schizophrenia (accelerated ageing/early maturation theory in schizophrenia). Our findings of increased FA in young adults with 22q11DS and prodromal symptoms in comparison to healthy controls are consequently in line with the findings published by Nuninga et al. We also note that besides of increased FA, we find reduced RD in a cohort of 22q11DS within the age range of 18 to 26 years. Increased FA and decreased RD seem to be a common microstructural characteristic of changes in WM in 22q11DS (Villalón-Reina et al., 2019), although neurobiological interpretations of such changes should be made with caution as FA and RD are not only sensitive to changes in myelin (Beaulieu, 2002). Moreover, changes in FA and RD also depend on extracellular water and axonal density (Schwartz et al., 2005; Takahashi et al., 2000). We were not able to reconfirm that FA is increased in tracts of the VLPFC and the DLPFC sub-circuits in the 22q11DS+PS in comparison to the 22q11DS−PS group. While we observe slightly more robust effect sizes in the 22q11DS+PS subgroup, hinting that the WM pattern in the FST tracts might be more affected in individuals with 22q11DS and prodromal symptoms, this needs to be reconfirmed in larger samples in future studies.
The relationship between tract microstructure and verbal performance of participants was explored by performing correlations between FA, RD and VIQ. Pearson correlations were conducted for each group separately as cognitive decline, most pronounced in VIQ, precedes the onset of psychosis in 22q11DS (Vorstman et al., 2015). We found significant negative associations between FA in the right VLPFC sub-circuit (thalamus-IFG tract) and VIQ in the 22q11DS group with prodromal symptoms. Thus, increased FA in the right VLPFC sub-circuit was associated with lower VIQ. Moreover, decreased RD in the right VLPFC sub-circuit (thalamus-IFG tract) was associated at trend level with lower VIQ. We find these results are noteworthy for the following reasons. First, associations between thalamic-prefrontal WM microstructure and abnormalities in cognitive functions were reported in patients with first-episode psychosis (Cho et al., 2016). Our study demonstrates associations of cognitive functions and WM in the VLPFC sub-circuit in the 22q11DS population with prodromal symptoms and extends our knowledge of abnormalities in WM early in the trajectory of schizophrenia. Second, it has been published that in patients with 22q11DS cognitive decline, most pronounced in VIQ, precedes the onset of psychosis by several years (Vorstman et al., 2015). However, it has not been investigated yet whether the decline in VIQ is associated with any changes in WM in the FST sub-circuits. In this study we demonstrated that abnormalities in the tract of the VLPFC sub-circuit are associated with VIQ. The correlations between VIQ and the dMRI indices of the thalamus-IFG tract were found exclusively in 22q11DS with prodromal symptoms. Third, the correlations were found in the right hemisphere only. This lateralization could be attributed to the early stages of the disease as changes in cortico-cortical connections in the right hemisphere have been reported to be associated with prodromal symptoms in adolescents with 22q11DS (mean age: 18 +/− 2 years) (Kikinis et al., 2017). It is possible that changes in the right hemisphere are present early on and that with disease progression, the disruptions become more pervasive and spread to both hemispheres. Our findings just suggest that a disrupted connectivity within the right FST sub-circuit is present before psychosis onset and is associated with verbal performance.
This study has potential limitations. The UKF based two-tensor tractography algorithm is an advanced method for reconstructing connections between subcortical and cortical regions of the brain and it is more powerful than single-tensor tractography. However, with its use, some of the streamlines might not be anatomically correct. False positive errors are likely (Jones et al., 2013), which is why a rigorous quality control of the reconstructed tracts was performed for each participant. Anatomically incorrect tracts were eliminated, reducing the number of study participants by up to 12% for some tracts. That said, the UKF is a robust method to reconstruct fibers of intersecting and fanning fibers connecting the cortical and subcortical areas. Furthermore, it would have been ideal, besides comparing individuals with 22q11DS with and without prodromal symptoms to a healthy control group, to have another group of individuals with 22q11DS with schizophrenia. Unfortunately, our study did not have sufficient participants with schizophrenia and as such performing meaningful statistical analyses with individuals with schizophrenia were not possible. To summarize, the strength of this study is the large sample size of participants of a rare disorder that can be a genetic model for schizophrenia, a narrow age range amongst participants (18 to 26 years of age), and the application of advanced MRI techniques.
In summary, microstructural abnormalities in brain WM tracts connecting the thalamus and the striatum with prefrontal cortices are present in young adults with 22q11DS with and without prodromal symptoms compared to healthy controls. These abnormalities are associated with the individuals’ cognitive performance in VIQ in the 22q11DS with prodromal symptoms and therefore emphasize the potential involvement of the FST sub-circuits in schizophrenia. While changes in FST circuitry have been reported in patients with schizophrenia, we observed that changes in FST circuitry are also present in young adults with 22q11DS at risk for but without psychotic symptoms. Our results suggest that psychosis onset in 22q11DS may be associated with a complex pattern of WM alterations in the FST sub-circuits.
Acknowledgments:
Role of the Funding Source
This work was supported by funding from the National Institutes of Health grants (MH064824 to WRK, MH106793 to ZK, MH121704 to JJL, MH102377, MH110807 and AG042512 to MK), PROMOS (German Academic Exchange Service; DAAD) to CH, and DAAD to SS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Compliance with Ethical Standards:
The authors declare that they have no conflict of interest in relation to the subject of this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants signed an Informed Consent form, and the study was approved by the institutional review board at SUNY Upstate Medical University, Syracuse, NY and by the Partners Human Research Commission, Boston, MA.
References:
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–29. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, 1990. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. 10.1016/0166-2236(90)90107-L [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL, 1986. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annu. Rev. Neurosci. 9, 357–381. 10.1146/annurev.ne.09.030186.002041 [DOI] [PubMed] [Google Scholar]
- Amelsvoort T van, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D, 2004. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophr. Res. 70, 223–232. 10.1016/J.SCHRES.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD, 2015. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry 72, 882 10.1001/jamapsychiatry.2015.0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker G, Caan MWA, Schluter RS, Bloemen OJN, da Silva- Alves F, de Koning MB, Boot E, Vingerhoets WAM, Nieman DH, de Haan L, Booij J, van Amelsvoort TAMJ, 2016a. Distinct white-matter aberrations in 22q11.2 deletion syndrome and patients at ultra-high risk for psychosis. Psychol. Med. 46, 2299–2311. 10.1017/S0033291716000970 [DOI] [PubMed] [Google Scholar]
- Bakker G, Caan MWA, Vingerhoets WAM, Da Silva-Alves F, De Koning M, Boot E, Nieman DH, De Haan L, Bloemen OJ, Booij J, Van Amelsvoort TAMJ, 2016b. Cortical morphology differences in subjects at increased vulnerability for developing a psychotic disorder: A comparison between subjects with ultra-high risk and 22q11.2 deletion syndrome. PLoS One 11 10.1371/journal.pone.0159928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15, 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 10.2307/2346101 [DOI] [Google Scholar]
- Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M, Faragian S, Hendler T, 2012. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr. Res. 134, 131–136. 10.1016/j.schres.2011.10.011 [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C, 2006. Dorsolateral Prefrontal Cortex Promotes Long-Term Memory Formation through Its Role in Working Memory Organization. J. Neurosci. 26, 916–925. 10.1523/JNEUROSCI.2353-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, D’Esposito M, 2005. Frontal networks for learning and executing arbitrary stimulus-response associations. J. Neurosci. 25, 2723–32. 10.1523/JNEUROSCI.3697-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu K-W, Mitelman S, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, Ahmed S, Tang C, 2006. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann. Gen. Psychiatry 5, 19 10.1186/1744-859X-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C, Papolos D, Pandita RK, Faedda GL, Veit S, Goldberg R, Shprintzen R, Kucherlapati R, Morrow B, 1997. Molecular analysis of velo-cardio-facial syndrome patients with psychiatric disorders. Am. J. Hum. Genet. 60, 851–9. [PMC free article] [PubMed] [Google Scholar]
- Ching CRK, Gutman BA, Sun D, Villalon Reina J, Ragothaman A, Isaev D, Zavaliangos-Petropulu A, Lin A, Jonas RK, Kushan L, Pacheco-Hansen L, Vajdi A, Forsyth JK, Jalbrzikowski M, Bakker G, van Amelsvoort T, Antshel KM, Fremont W, Kates WR, Campbell LE, McCabe KL, Craig MC, Daly E, Gudbrandsen M, Murphy CM, Murphy DG, Murphy KC, Fiksinski A, Koops S, Vorstman J, Crowley TB, Emanuel BS, Gur RE, McDonald-McGinn DM, Roalf DR, Ruparel K, Schmitt JE, Zackai EH, Durdle CA, Goodrich-Hunsaker NJ, Simon TJ, Bassett AS, Butcher NJ, Chow EWC, Vila-Rodriguez F, Cunningham A, Doherty J, Linden DE, Moss H, Owen MJ, van den Bree M, Crossley NA, Repetto GM, Thompson PM, Bearden CE, 2020. Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness. Am. J. Psychiatry 177, appi.ajp.2019.1. 10.1176/appi.ajp.2019.19060583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KIK, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun J-Y, Kim SN, Kwon JS, 2016. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr. Bull. 42, 723–31. 10.1093/schbul/sbv169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EWC, Watson M, Young DA, Bassett AS, 2006. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr. Res. 87, 270–278. 10.1016/J.SCHRES.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, 1993. Frontal-Subcortical Circuits and Human Behavior. Arch. Neurol. 50, 873–880. 10.1001/archneur.1993.00540080076020 [DOI] [PubMed] [Google Scholar]
- de Leeuw M, Bohlken MM, Mandl RCW, Kahn RS, Vink M, 2015. Reduced frontostriatal white matter integrity in schizophrenia patients and unaffected siblings: a DTI study. NPJ Schizophr. 1, 15001 10.1038/npjschz.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re EC, Gao Y, Eckbo R, Petryshen TL, Blokland GAM, Seidman LJ, Konishi J, Goldstein JM, McCarley RW, Shenton ME, Bouix S, 2016. A New MRI Masking Technique Based on Multi-Atlas Brain Segmentation in Controls and Schizophrenia: A Rapid and Viable Alternative to Manual Masking. J. Neuroimaging 26, 28–36. 10.1111/jon.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Howes OD, Houle S, Mckenzie K, Valmaggia LR, Bagby MR, Tseng H-H, Bloomfield MAP, Kenk M, Bhattacharyya S, Suridjan I, Chaddock CA, Winton-Brown TT, Allen P, Rusjan P, Remington G, Meyer-Lindenberg A, Mcguire PK, Mizrahi R, 2017. Elevated Striatal Dopamine Function in Immigrants and Their Children: A Risk Mechanism for Psychosis. Schizophr. Bull. 43, 293–301. 10.1093/schbul/sbw181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, Winton-Brown TT, Bloomfield MAP, Bhattacharyya S, Chilcott J, Lappin JM, Murray RM, McGuire P, 2016. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr. Res. 176, 171–176. 10.1016/j.schres.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez M de G, Penn DL, van Os J, Krabbendam L, 2011. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev. 10.1016/j.neubiorev.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 14, 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Gold JM, 2004. Cognitive deficits as treatment targets in schizophrenia. Schizophr. Res. 72, 21–28. 10.1016/J.SCHRES.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Grazioplene RG, Bearden CE, Subotnik KL, Ventura J, Haut K, Nuechterlein KH, Cannon TD, 2018. Connectivity-enhanced diffusion analysis reveals white matter density disruptions in first episode and chronic schizophrenia. NeuroImage. Clin. 18, 608–616. 10.1016/j.nicl.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive Deficits and Functional Outcome in Schizophrenia: Are We Measuring the "Right Stuff"? Schizophr. Bull 26, 119–136. 10.1093/oxfordjournals.schbul.a033430 [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH, 2004. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch. Gen. Psychiatry 61, 310 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- Haber SN, 2016. Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7–21. 10.1007/978-1-4614-6434-1_135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, 2003. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 26, 317–330. 10.1016/J.JCHEMNEU.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R, 2009. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res. Bull. 78, 69–74. 10.1016/j.brainresbull.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoda HM, Makhlouf AT, Fitzsimmons J, Rathi Y, Makris N, Mesholam-Gately RI, Wojcik JD, Goldstein J, McCarley RW, Seidman LJ, Kubicki M, Shenton ME, 2018. Abnormalities in thalamo-cortical connections in patients with first-episode schizophrenia: a two-tensor tractography study. Brain Imaging Behav. 10.1007/s11682-018-9862-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, Senturk D, Chow C, Thompson PM, Bearden CE, 2014. Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front. Behav. Neurosci. 8, 393 10.3389/fnbeh.2014.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R, 2013. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 73, 239–254. 10.1016/J.NEUROIMAGE.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RSE, 2013. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry 70, 1107–12. 10.1001/jamapsychiatry.2013.155 [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P, 2011. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol. Med. 41, 291–299. 10.1017/S0033291710000796 [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, Botti J-A, Kelchner L, McCarthy C, 2011. Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biol. Psychiatry 69, 945–52. 10.1016/j.biopsych.2010.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Olszewski AK, Gnirke MH, Kikinis Z, Nelson J, Antshel KM, Fremont W, Radoeva PD, Middleton FA, Shenton ME, Coman IL, 2015. White matter microstructural abnormalities of the cingulum bundle in youths with 22q11.2 deletion syndrome: Associations with medication, neuropsychological function, and prodromal symptoms of psychosis. Schizophr. Res. 10.1016/j.schres.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, 2007. Cognitive deficits in patients with schizophrenia: effects and treatment. J. Clin. Psychiatry 68 Suppl 14, 8–13. [PubMed] [Google Scholar]
- Khandaker GM, Barnett JH , White IR, Jones PB, 2011. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr. Res. 132, 220–227. 10.1016/J.SCHRES.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis Z, Cho KIK, Coman IL, Radoeva PD, Bouix S, Tang Y, Eckbo R, Makris N, Kwon JS, Kubicki M, Antshel KM, Fremont W, Shenton ME, Kates WR, 2017. Abnormalities in brain white matter in adolescents with 22q11.2 deletion syndrome and psychotic symptoms. Brain Imaging Behav. 11, 1353–1364. 10.1007/s11682-016-9602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV, 2009. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802. 10.1016/j.neuroimage.2008.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Hong LE, Dennis EL, Morey RA, Tate DF, Wilde EA, Logue M, Kelly S, Donohoe G, Favre P, Houenou J, Ching CRK, Holleran L, Andreassen OA, Velzen LS, Schmaal L, Villalón- Reina JE, Bearden CE, Piras F, Spalletta G, Heuvel OA, Veltman DJ, Stein DJ, Ryan MC, Tan Y, Erp TGM, Turner JA, Haddad L, Nir TM, Glahn DC, Thompson PM, Jahanshad N, 2020. ENIGMA- DTI: Translating reproducible white matter deficits into personalized vulnerability metrics in cross- diagnostic psychiatric research. Hum. Brain Mapp hbm.24998 10.1002/hbm.24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar A, Ramanathan S, Nelson J, Antshel KM, Fremont W, Higgins AM, Shprintzen RJ, Kates WR, 2012. Cortical gyrification in velo-cardio-facial (22q11.2 deletion) syndrome: A longitudinal study. Schizophr. Res. 137, 20–25. 10.1016/j.schres.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AH, Wallis JD, 2015. The Role of Prefrontal Cortex in Working Memory: A Mini Review. Front. Syst. Neurosci. 9, 173 10.3389/fnsys.2015.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Alvarado JL, Nestor PG, Rosow L, Pelavin PE, McCarley RW, Kubicki M, Shenton ME, 2012. Fractional anisotropy and radial diffusivity: Diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr. Res. 136, 55–62. 10.1016/j.schres.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Nestor PG, Levin L, Pelavin P, Lin P, Kubicki M, McCarley RW, Shenton ME, Rathi Y, 2017. Reduced Structural Connectivity in Frontostriatal White Matter Tracts in the Associative Loop in Schizophrenia. Am. J. Psychiatry 174, 1102–1111. 10.1176/appi.ajp.2017.16091046 [DOI] [PubMed] [Google Scholar]
- Li P, Jing R, Zhao R, Shi L, 2020. Association between functional and structural connectivity of the corticostriatal network in people with schizophrenia and unaffected first-degree relatives 1–11. 10.1503/jpn.190015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JG, Shenton ME, Rathi Y, 2010. Filtered multitensor tractography. IEEE Trans. Med. Imaging 29, 1664–75. 10.1109/TMI.2010.2048121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Ji A, Rutlin J, Shimony JS, 2019. White matter integrity in schizophrenia and bipolar disorder: Tract- and voxel-based analyses of diffusion data from the Connectom scanner. NeuroImage. Clin. 21, 101649 10.1016/j.nicl.2018.101649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL, 2000. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol. Psychiatry 48, 99–109. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RSE, Fisher H, Harrington H, Houts R, Poulton R, Moffitt T, 2014. Neuropsychological Decline in Schizophrenia from the Premorbid to Post-Onset Period: Evidence from a Population-Representative Longitudinal Study. Am J Psychiatry 171, 91–101. 10.1176/appi.ajp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 29, 703–15. 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Murray RM, Bhavsar V, Tripoli G, Howes O, 2017. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed Into the Developmental Risk Factor Model of Psychosis. Schizophr. Bull. 43, 1190–1196. 10.1093/schbul/sbx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Lewis SW, 1987. Is schizophrenia a neurodevelopmental disorder? Br. Med. J. (Clin. Res. Ed). 295, 681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Mehta M, Di Forti M, 2014. Different Dopaminergic abnormalities underlie cannabis dependence and cannabis-induced psychosis. Biol. Psychiatry. 10.1016/j.biopsych.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Nuninga JO, Bohlken MM, Koops S, Fiksinski AM, Mandl RCW, Breetvelt EJ, Duijff SN, Kahn RS, Sommer IEC, Vorstman JAS, 2018. White matter abnormalities in 22q11.2 deletion syndrome patients showing cognitive decline. Psychol. Med. 48, 1655–1663. 10.1017/S0033291717003142 [DOI] [PubMed] [Google Scholar]
- Olszewski AK, Kikinis Z, Gonzalez CS, Coman IL, Makris N, Gong X, Rathi Y, Zhu A, Antshel KM, Fremont W, Kubicki MR, Bouix S, Shenton ME, Kates WR, 2017. The social brain network in 22q11.2 deletion syndrome: A diffusion tensor imaging study. Behav. Brain Funct. 10.1186/s12993-017-0122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N, 1995. The cortico-basal ganglia-thalamo-corticalloop. Abstr. Brain Res. Rev. 20, 91–127. [DOI] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD, 2016. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. U. S. A. 113, 1907–1912. 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein MD, Chohan MR, Coman IL, Antshel KM, Fremont WP, Gnirke MH, Kikinis Z, Middleton FA, Radoeva PD, Shenton ME, Kates WR, 2014. White matter abnormalities in 22q11.2 deletion syndrome: preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr. Res. 152, 117–23. 10.1016/j.schres.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan M, Lee S-H, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, Mesholam-Gately RI, Goldstein JM, McCarley RW, Shenton ME, Levitt JJ, 2013. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr. Res. 145, 1–10. 10.1016/j.schres.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR, 2012. Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings. Behav. Brain Funct. 8, 38 10.1186/1744-9081-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, Poulton R, Moffitt TE, 2010. Static and Dynamic Cognitive Deficits in Childhood Preceding Adult Schizophrenia: A 30-Year Study. Am. J. Psychiatry 167, 160–169. 10.1176/appi.ajp.2009.09040574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC, 2010. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J. Neurosci. 30, 14552–9. 10.1523/JNEUROSCI.2631-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scariati E, Padula MC, Schaer M, Eliez S, 2016. Long-range dysconnectivity in frontal and midline structures is associated to psychosis in 22q11.2 deletion syndrome. J. Neural Transm. 10.1007/s00702-016-1548-z [DOI] [PubMed] [Google Scholar]
- Schaer M, Debbané M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, Eliez S, 2009. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): A cross-sectional and longitudinal study. Schizophr. Res. 115, 182–190. 10.1016/j.schres.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, Whinna D, Souders MC, Satterwaite TD, Prabhakaran K, Mcdonald-Mcginn DM, Zackai EH, Gur RC, Emanuel BS, Gur RE, 2015. Aberrant Cortical Morphometry in the 22q11.2 Deletion Syndrome. Biol. Psychiatry 78, 135–143. 10.1016/j.biopsych.2014.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree M, Owen M, Murphy KC, Niarchou M, Kates WR, Antshel KM, Fremont W, McDonald-McGinn DM, Gur RE, Zackai EH, Vorstman J, Duijff SN, Klaassen PWJ, Swillen A, Gothelf D, Green T, Weizman A, Van Amelsvoort T, Evers L, Boot E, Shashi V, Hooper SR, Bearden CE, Jalbrzikowski M, Armando M, Vicari S, Murphy DG, Ousley O, Campbell LE, Simon TJ, Eliez S, 2014. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 171, 627–39. 10.1176/appi.ajp.2013.13070864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ED, Cooper ET, Fan Y, Jawad AF, Chin CL, Nissanov J, Hackney DB, 2005. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport 16, 73–76. 10.1097/00001756-200501190-00017 [DOI] [PubMed] [Google Scholar]
- Shallice T, Cipolotti L, 2018. The Prefrontal Cortex and Neurological Impairments of Active Thought. Annu. Rev. Psychol. 69, 157–180. 10.1146/annurev-psych-010416-044123 [DOI] [PubMed] [Google Scholar]
- Sheng J, Zhu Y, Lu Z, Liu N, Huang N, Zhang Z, Tan L, Li C, Yu X, 2013. Altered volume and lateralization of language-related regions in first-episode schizophrenia. Schizophr. Res. 148, 168–174. 10.1016/j.schres.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH, 2002. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage 17, 1429–1436. 10.1006/NIMG.2002.1267 [DOI] [PubMed] [Google Scholar]
- Sun D, Ching CRK, Lin A, Forsyth JK, Kushan L, Vajdi A, Jalbrzikowski M, Hansen L, Villalon-Reina JE, Qu X, Jonas RK, van Amelsvoort T, Bakker G, Kates WR, Antshel KM, Fremont W, Campbell LE, McCabe KL, Daly E, Gudbrandsen M, Murphy CM, Murphy D, Craig M, Vorstman J, Fiksinski A, Koops S, Ruparel K, Roalf DR, Gur RE, Schmitt JE, Simon TJ, Goodrich-Hunsaker NJ, Durdle CA, Bassett AS, Chow EWC, Butcher NJ, Vila-Rodriguez F, Doherty J, Cunningham A, van Den Bree MBM, Linden DEJ, Moss H, Owen MJ, Murphy KC, McDonald-McGinn DM, Emanuel B, van Erp TGM, Turner JA, Thompson PM, Bearden CE, 2018. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol. Psychiatry 25, 1–13. 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ono J, Harada K, Maeda M, Hackney DB, 2000. Diffusional anisotropy in cranial nerves with maturation: Quantitative evaluation with diffusion MR imaging in rats. Radiology 216, 881–885. 10.1148/radiology.216.3.r00se41881 [DOI] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, Souders MC, Zackai EH, McDonald-McGinn DM, Emanuel BS, Bilker WB, Gur RC, Gur RE, 2014. Subthreshold Psychotic Symptoms in 22q11.2 Deletion Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 53, 991–1000.e2. 10.1016/J.JAAC.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee DS, Kikinis Z, Quinn TP, Antshel KM, Fremont W, Tahir MA, Zhu A, Gong X, Glatt SJ, Coman IL, Shenton ME, Kates WR, Makris N, 2017. Machine-learning classification of 22q11.2 deletion syndrome: A diffusion tensor imaging study. NeuroImage Clin. 10.1016/j.nicl.2017.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Baker RA, Cox K, Okame T, Kojima Y, Eramo A, Potkin SG, 2020. Effect of brexpiprazole on control of impulsivity in schizophrenia: A randomized functional magnetic resonance imaging study. Psychiatry Res. - Neuroimaging 301, 111085 10.1016/j.pscychresns.2020.111085 [DOI] [PubMed] [Google Scholar]
- Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ, 2013. White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage 81, 441–454. 10.1016/J.NEUROIMAGE.2013.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón-Reina JE, Martínez K, Qu X, Ching CRK, Nir TM, Kothapalli D, Corbin C, Sun D, Lin A, Forsyth JK, Kushan L, Vajdi A, Jalbrzikowski M, Hansen L, Jonas RK, van Amelsvoort T, Bakker G, Kates WR, Antshel KM, Fremont W, Campbell LE, McCabe KL, Daly E, Gudbrandsen M, Murphy CM, Murphy D, Craig M, Emanuel B, McDonald-McGinn DM, Vorstman JAS, Fiksinski AM, Koops S, Ruparel K, Roalf D, Gur RE, Eric Schmitt J, Simon TJ, Goodrich-Hunsaker NJ, Durdle CA, Doherty JL, Cunningham AC, van den Bree M, Linden DEJ, Owen M, Moss H, Kelly S, Donohoe G, Murphy KC, Arango C, Jahanshad N, Thompson PM, Bearden CE, 2019. Altered white matter microstructure in 22q11.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol. Psychiatry. 10.1038/s41380-019-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, Armando M, Vicari S, Shashi V, Hooper SR, Chow EWC, Fung WLA, Butcher NJ, Young DA, McDonald-McGinn DM, Vogels A, Van Amelsvoort T, Gothelf D, Weinberger R, Weizman A, Klaassen PWJ, Koops S, Kates WR, Antshel KM, Simon TJ, Ousley OY, Swillen A, Gur RE, Bearden CE, Kahn RS, Bassett AS, Emanuel BS, Zackai EH, Kushan L, Fremont W, Schoch K, Stoddard J, Cubells J, Fu F, Campbell LE, Fritsch R, Vergaelen E, Neeleman M, Boot E, Debbané M, Philip N, Green T, Van DenBree MBM, Murphy D, Canyelles JM, Arango C, Murphy KC, Pontillo M, 2015. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 10.1001/jamapsychiatry.2014.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucurovic K, Caillies S, Kaladjian A, 2020. Neural correlates of theory of mind and empathy in schizophrenia: An activation likelihood estimation meta-analysis. J. Psychiatr. Res. 10.1016/j.jpsychires.2019.10.018 [DOI] [PubMed] [Google Scholar]
- Wassermann D, Makris N, Rathi Y, Shenton M, Kikinis R, Kubicki M, Westin C-F, 2012. On Describing Human White Matter Anatomy: The White Matter Query Language 40, 1301–1315. 10.1007/s10439-011-0452-9.Engineering [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Adult Intelligence Scale - Third Edition, 3rd ed The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Weinberger DR, 1987. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–9. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE, 2001. Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry 50, 825–44. [DOI] [PubMed] [Google Scholar]
- Weisman O, Guri Y, Gur RE, McDonald-McGinn DM, Calkins ME, Tang SX, Emanuel B, Zackai EH, Eliez S, Schneider M, Schaer M, Kates WR, Antshel KM, Fremont W, Shashi V, Hooper SR, Armando M, Vicari S, Pontillo M, Kushan L, Jalbrzikowski M, Bearden CE, Cubells JF, Ousley OY, Walker EF, Simon TJ, Stoddard J, Niendam TA, van den Bree MBM, Gothelf D, 2017. Subthreshold Psychosis in 22q11.2 Deletion Syndrome: Multisite Naturalistic Study. Schizophr. Bull. 43, 1079–1089. 10.1093/schbul/sbx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ai H, Opmeer EM, Marsman JBC, Van Der Meer L, Ruhé HG, Aleman A, Van Tol MJ, 2020. Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychol. Med. 50, 413–421. 10.1017/S0033291719000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Liu W, He W, Yu S, Zhong G, 2017. Altered effective brain connectivity at early response of antipsychotics in first-episode schizophrenia with auditory hallucinations. Clin. Neurophysiol. 128, 867–874. 10.1016/j.clinph.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Zinkstok JR, Boot E, Bassett AS, Hiroi N, Butcher NJ, Vingerhoets C, Vorstman JAS, van Amelsvoort TAMJ, 2019. Neurobiological perspective of 22q11.2 deletion syndrome. The Lancet Psychiatry. 10.1016/S2215-0366(19)30076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


