ABSTRACT
Background
Our prior studies revealed that infant somatic growth is influenced by fructose in breast milk, and fructose in breast milk is increased in response to maternal sugar-sweetened beverage (SSB) intake in lactation. It is unknown whether infant neurodevelopmental outcomes are also influenced by maternal SSBs in lactation.
Objectives
To determine whether infant cognitive development at 24 postnatal months was influenced by maternal fructose consumption during lactation, and whether this relation persisted after accounting for maternal SSB and juice (SSB + J) intake.
Methods
Hispanic mother–infant pairs (n = 88) were recruited across the spectrum of prepregnancy BMI. Mothers completed two 24-h dietary recalls at 1 and 6 postnatal months, and reported breastfeedings per day. The Bayley-III Scales of Infant Development were administered at 24 postnatal months to assess infant cognition. Linear regressions were used to examine associations, reported as unstandardized (B) coefficients, 95% CIs, and P values.
Results
Mothers consumed 1656 ± 470 kcal, 21.8 ± 12 g fructose, and 2.5 ± 2.6 servings SSBs + J, and reported 6.9 ± 2.1 breastfeedings per day at 1 postnatal month. Controlling for maternal age, prepregnancy BMI, education level, kilocalories, infant age, sex, and birthweight revealed that infant cognitive development scores at 24 postnatal months correlated inversely with maternal fructose consumption at 1 postnatal month (B = −0.08; 95% CI = −0.13, −0.03; P < 0.01). The association of infant cognitive development scores with maternal fructose consumption was no longer significant after adjustment for maternal SSB + J intake (B = −0.05; 95% CI = −0.10, 0.00; P = 0.07), whereas maternal SSB + J intake was significant in the same model (B = −0.29; 95% CI = −0.52, −0.05; P = 0.02). Infant cognitive development scores were not associated with maternal fructose and SSB + J consumption at 6 postnatal months.
Conclusions
Our findings suggest that infant neurodevelopmental outcomes at 24 postnatal months can be adversely influenced by maternal fructose intake in early lactation, and this could be attributed to maternal SSB + J intake.
Keywords: sugar-sweetened beverages, maternal nutrition, lactation, infant, cognitive development, brain
Introduction
The current food environment has widespread availability of added sugar. Indeed, there has been a surge in sugar-sweetened beverage (SSB) and juice (SSB + J) consumption (1, 2) that can pose a risk to health outcomes, including obesity, type 2 diabetes, and brain-based outcomes that span self-regulation of food intake to cognitive function (3–5). This could be attributed to several factors: 1) SSBs + J do not support satiety and can be consumed in excess (6); 2) SSBs + J displace nutritious foods and beverages in the diet that are beneficial (7, 8); and 3) SSBs + J contain high-fructose corn syrup (HFCS) or are high in fructose (9, 10), and fructose metabolism contributes to adverse outcomes at the organ and metabolic levels (11). Though the influence of SSB + J intake has been examined in children and adults (12, 13), data are limited earlier in life.
Our research team has particular interest in the influence of maternal SSBs + J on fructose in breast milk, and its effects on infant outcomes. Berger et al. (14) revealed that mothers who consumed a beverage sweetened with HFCS had increased fructose in breast milk. Moreover, Goran et al. (15) revealed that fructose in breast milk was positively associated with infant somatic growth. There is evidence that maternal SSB consumption can alter additional infant outcomes. Cohen et al. (5) revealed that maternal SSB intake during pregnancy correlated inversely with intelligence test scores in children. Though this suggests that acute exposure in utero can alter cognitive development, no studies have examined maternal SSBs + J in lactation, when fructose can conceivably be transmitted via breast milk to affect infant brain function.
Animal studies offer evidence that acute exposure to maternal SSBs + J underlies the association of maternal fructose consumption with infant brain development, which supports cognitive development. That is, maternal SSBs + J are a main source of free fructose in the diet that can be an adverse influence on infant cognition. Studies have shown that rats fed a fructose-sweetened solution had altered brain structure and function (16–19). This has been attributed to several mechanisms: 1) fructose induces oxidative stress in the frontal cortex (18); 2) fructose interferes in actions of neurotrophins that regulate brain maturation (18, 20); and 3) fructose increases inflammatory mediators in the hippocampus (17, 19). These results imply that acute exposure to fructose during critical periods of brain development can compromise maturation of neural systems in centers of the brain that foster cognitive development (18, 21). On this basis, we hypothesized that infant exposure to fructose from maternal SSB + J intake in lactation could alter neurodevelopmental outcomes in a similar manner.
To expand on prior studies in pregnancy (5) and build on our work in lactation (14, 15), the aim of this study was to determine whether infant cognitive development at 24 postnatal months was associated with maternal fructose consumption in lactation, and whether this relation persisted after accounting for maternal SSB + J intake.
Methods
Subjects
Participants were 88 mother–infant pairs recruited from maternity clinics in Los Angeles County for a prospective observational study. As described in Berger et al. (22, 23), mothers were included based on these criteria: 1) self-identified Hispanic ethnicity; 2) ≥18 y of age at delivery; 3) gave birth to a term, singleton newborn; 4) enrolled within 1 postnatal month; 5) intended to breastfeed for 6 postnatal months; and 6) able to read English or Spanish at a fifth-grade level. Mothers reported prepregnancy weight and height so as to recruit uniformly across the spectrum of prepregnancy BMI status (24). Mothers were excluded based on these criteria: 1) reported medications or medical conditions that could affect physical/mental health or nutrition/metabolism; 2) used tobacco or recreational drugs; or 3) had a clinical diagnosis of fetal abnormalities. The Institutional Review Board of Children's Hospital Los Angeles and the University of Southern California approved all procedures. Participants provided written informed consent prior to data collection (22, 23).
Study design
Mother–infant pairs completed 3 visits for the purposes of this study at 1, 6, and 24 postnatal months. At 1 postnatal month, mothers completed historical health-related information and reported infant characteristics at birth. At 1 and 6 postnatal months, mothers completed 24-h dietary recalls and information on breastfeeding practices. At 24 postnatal months, the Bayley-III Scales of Infant Development were administered to all infants to assess cognitive development.
Dietary intake
At 1 and 6 postnatal months, daily maternal consumption of kilocalories, total sugar, added sugar, fructose, and SSBs + J was assessed. In addition, daily maternal consumption of whole fruits (as opposed to SSBs + J) was assessed. Whole fruits are also a source of fructose (bound to fiber), and we wanted to better determine whether maternal SSB + J consumption was the main contributor of fructose (free fructose) that influenced our outcome. A trained registered dietitian conducted two 24-h dietary recalls at each time point using the Nutrition Data System for Research software (version 2018) from the National Coordinating Center at the University of Minnesota with the multiple-pass technique. The first recall was performed in person at our laboratory with the use of food models, portion booklets, or serving containers to assist in estimating serving sizes. The remaining recalls were conducted by telephone. To minimize the potential for undereating or underreporting in the time frame for subsequent recalls, participants were not aware of the telephone recall schedule. Mothers also reported breastfeeding frequency at 1 and 6 postnatal months, as well as infant dietary intake at 24 postnatal months using the same protocol described above.
Cognitive developmental assessment
As described in Berger et al. (23), the Bayley-III Scales of Infant Development (third edition) were administered by a trained and supervised research assistant to assess cognitive, language, and motor capacities at 24 postnatal months. The cognitive scale assesses sensorimotor integration, concept formation, attention, habituation, and memory. Based on the premise of this study (5, 19), analyses were limited to the cognitive scale from this assessment. Scaled (age-standardized) scores, rather than raw scores, were used as the primary outcome variable. The scaled score is a transformation of the raw score to the average performance of a normative sample at a given age. It corresponds to a set position on a normal distribution curve. The internal consistency of the Bayley-III cognitive scale is 0.91 (25).
Statistical analysis
Descriptive statistics are presented as mean ± SD for continuous variables, and as frequency (percentage) for categorical variables. Normal distribution and homogeneity of variances were confirmed by Shapiro–Wilk W and Levene tests, respectively. Because mothers were recruited uniformly across the spectrum of prepregnancy BMI status, differences between maternal prepregnancy BMI status groups (normal weight compared with overweight compared with obese) were tested using an ANOVA with polynomial contrast for continuous variables and a Mantel–Haenszel linear-by-linear association χ2 test for categorical variables. Also, differences between maternal dietary intake variables and reported breastfeedings per day at 1 and 6 postnatal months were assessed using paired samples t tests.
We conducted a set of linear regression models to test the associations of maternal fructose consumption during lactation with infant cognitive development scores at 24 postnatal months, and to determine whether these relations persisted after adjustment for maternal SSB + J intake during lactation. Model I included only maternal fructose consumption at 1 postnatal month as an exposure variable. Model II included maternal age, prepregnancy BMI, education level, kilocalories, infant age, sex, and birthweight as control variables. Model III included maternal SSB + J intake at 1 postnatal month as an additional exposure variable. The same set of linear regression models was used substituting maternal fructose and SSB + J consumption at 1 postnatal month with maternal fructose and SSB + J consumption at 6 postnatal months.
In addition, we conducted exploratory analyses to test associations of other explanatory variables of interest with infant cognitive development scores. This was done to help confirm that maternal fructose from SSB + J consumption in lactation was associated with infant cognitive development scores, and not driven or mediated by related factors that could be on the causal pathway, including maternal whole fruit intake at 1 and 6 postnatal months, and infant fructose and SSB + J consumption at 24 postnatal months. Analyses were conducted with SPSS software (version 25; IBM SPSS Statistics). Statistical significance was set at P < 0.05.
Results
The sample was composed of 88 Hispanic mother–infant pairs (Supplemental Figure 1). Mothers were 29.9 ± 6.7 y old at delivery, had a prepregnancy BMI (kg/m2) of 27.6 ± 5.7 and a 1 postnatal month BMI of 29.5 ± 5.0, and 69.6% were high-school graduates. Mothers consumed 1656 ± 470 kcal/d, of which 22.6% was total sugar and 13.4% was added sugar. Mothers also consumed 21.8 ± 12 g fructose/d and 2.5 ± 2.6 servings SSB + J/d at 1 postnatal month. There was a positive association between maternal fructose consumption and maternal SSB + J intake at 1 postnatal month (B = 2.83; 95% CI = 2.21, 3.45; P < 0.01), because maternal fructose in the diet was likely derived from maternal SSB + J intake during early lactation. There were no differences in reported intake at 6 postnatal months (all P values ≥ 0.10). This indicated that maternal consumption of kilocalories, fructose, and SSBs + J were consistent over time. In contrast, mothers reported a decrease in breastfeeding frequency from 1 to 6 postnatal months (6.9 ± 2.1 compared with 3.6 ± 3.3 breastfeedings/d; P < 0.01).
Characteristics of the mother–infant dyads grouped by maternal prepregnancy BMI status are shown in Table 1. The percentages of mothers classified as normal weight, overweight, and obese were 31.8%, 38.6%, and 29.6%. Mean values of maternal kilocalories, fructose, and SSBs + J did not differ between groups, nor did mean values of infant age, sex, birthweight, or breastfeedings per day and other dietary variables. Similar to findings reported in Berger et al. (23), infant cognitive development scores were lower in those born to mothers classified as obese (P ≤ 0.01).
TABLE 1.
Characteristics of the Hispanic mother–infant pairs1
| Maternal prepregnancy BMI group | |||||
|---|---|---|---|---|---|
| Characteristic | Total | Normal | Overweight | Obese | P 2 |
| n | 88 | 28 | 34 | 26 | |
| Mothers | |||||
| Age at delivery, y | 29.9 ± 6.7 | 29.5 ± 6.7 | 29.3 ± 6.9 | 31.2 ± 6.6 | 0.36 |
| Prepregnancy BMI, kg/m2 | 27.6 ± 5.7 | 21.6 ± 1.5 | 27.5 ± 1.3 | 34.7 ± 4.2 | <0.01 |
| BMI at 1 mo | 29.5 ± 5.0 | 24.9 ± 2.9 | 29.4 ± 2.2 | 34.6 ± 4.4 | <0.01 |
| Education level,3 % | 0.26 | ||||
| Less than eighth grade | 7.8 | 3.4 | 14.3 | 5.3 | |
| Completed eighth grade | 2.9 | 0.0 | 5.7 | 2.7 | |
| Some high school | 19.6 | 22.0 | 17.1 | 20.0 | |
| Completed high school | 26.5 | 30.5 | 22.9 | 26.7 | |
| Some college | 28.4 | 16.9 | 28.6 | 37.3 | |
| Completed college | 10.3 | 18.6 | 8.6 | 5.3 | |
| Completed graduate school | 4.4 | 8.5 | 2.9 | 2.7 | |
| Kilocalories per day at 1 mo | 1656 ± 470 | 1753 ± 508 | 1595 ± 468 | 1629 ± 427 | 0.34 |
| Fructose per day at 1 mo, g | 21.8 ± 12 | 24.4 ± 11 | 18.6 ± 9.0 | 23.1 ± 14 | 0.84 |
| SSBs + J per day at 1 mo (servings) | 2.5 ± 2.6 | 2.7 ± 2.8 | 2.1 ± 1.9 | 2.8 ± 3.0 | 0.73 |
| Whole fruits per day at 1 mo (servings) | 1.5 ± 1.6 | 2.0 ± 1.6 | 1.2 ± 1.2 | 1.4 ± 1.9 | 0.19 |
| Infants | |||||
| Female,3 % | 54.1 | 51.6 | 57.1 | 53.2 | 0.80 |
| Age, d | 730 ± 42 | 724 ± 72 | 734 ± 10 | 731 ± 9.3 | 0.55 |
| Birthweight, kg | 3.4 ± 0.4 | 3.4 ± 0.4 | 3.4 ± 0.4 | 3.4 ± 0.4 | 0.97 |
| Breast feedings per day at 1 mo (number) | 6.9 ± 2.1 | 7.5 ± 1.2 | 6.6 ± 2.2 | 6.6 ± 2.6 | 0.12 |
| Breast feedings per day at 6 mo (number) | 3.6 ± 3.3 | 3.9 ± 3.4 | 3.2 ± 3.5 | 3.9 ± 3.1 | 0.99 |
| Kilocalories per day at 24 mo | 1097 ± 335 | 1098 ± 346 | 1154 ± 288 | 1020 ± 376 | 0.32 |
| Fructose per day at 24 mo, g | 11.3 ± 6.9 | 11.0 ± 7.3 | 11.4 ± 7.3 | 11.6 ± 6.2 | 0.94 |
| SSBs + J per day at 24 mo (servings) | 1.2 ± 1.4 | 1.0 ± 1.1 | 1.1 ± 1.1 | 1.6 ± 1.9 | 0.28 |
| Cognitive development at 24 mo (score) | 9.2 ± 2.4 | 9.4 ± 2.0 | 10.0 ± 2.3 | 7.9 ± 2.6 | 0.02 |
Values are mean ± SD or %. Normal weight, overweight, and obese groups based on maternal BMI at prepregnancy. SSBs + J, sugar-sweetened beverages and juice.
Tests of significance between groups calculated with ANOVA.
Tests of significance between groups were based on χ2 test.
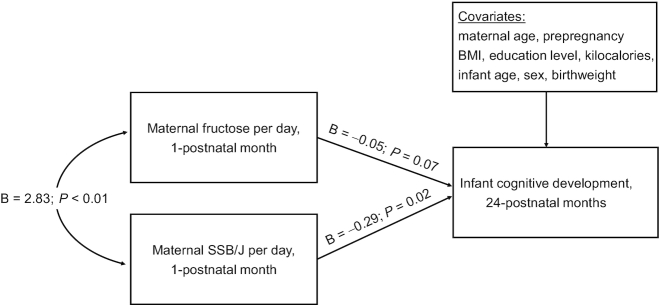
Coefficients from linear regression models are presented in Table 2. Maternal fructose consumption at 1 postnatal month was negatively associated with infant cognitive development scores at 24 postnatal months in the unadjusted model (model I) (B = −0.06; 95% CI = −0.10, −0.02; P < 0.01) and adjusted model that controlled for maternal age, prepregnancy BMI, education level, kilocalories, infant age, sex, and birthweight (model II) (B = −0.08; 95% CI = −0.13, −0.03; P < 0.01). The association of maternal fructose consumption at 1 postnatal month with infant cognitive development scores was no longer significant when maternal SSB + J intake at 1 postnatal month was added to the model (model III) (B = −0.05; 95% CI = −0.10, 0.00; P = 0.07), whereas the association of maternal SSB + J intake at 1 postnatal month with infant cognitive development scores was significant (B = −0.29; 95% CI = −0.52, −0.05; P = 0.02) (Figure 1). In contrast, maternal fructose consumption at 6 postnatal months was not associated with infant cognitive development scores in the unadjusted model or adjusted model (B = −0.05; 95% CI = −0.10, 0.01; P = 0.09), nor was maternal SSB + J intake at 6 postnatal months (B = 0.05; 95% CI = −0.32, 0.43; P = 0.78).
TABLE 2.
Associations of maternal fructose consumption at 1 postnatal month with infant cognitive development scores at 24 postnatal months1
| Model I | Model II | Model III | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r 2 = 0.10 | r 2 = 0.24 | r 2 = 0.30 | |||||||
| B | 95% CI | P | B | 95% CI | P | B | 95% CI | P | |
| Fructose per day at 1 mo | −0.06 | −0.10, −0.02 | <0.01 | −0.08 | −0.13, −0.03 | <0.01 | −0.05 | −0.10, 0.00 | 0.07 |
| SSBs + J per day at 1 mo | — | — | — | — | −0.29 | −0.52, −0.05 | 0.02 | ||
| Kilocalories per day at 1 mo | — | — | 0.00 | 0.00, 0.00 | 0.69 | 0.00 | 0.00, 0.00 | 0.56 | |
| Age at delivery | — | — | −0.01 | −0.09, 0.07 | 0.82 | −0.02 | −0.10, 0.05 | 0.55 | |
| Prepregnancy BMI | — | — | −0.13 | −0.21, −0.04 | <0.01 | −0.13 | −0.21, −0.05 | <0.01 | |
| Education level | — | — | 0.32 | −0.04, 0.68 | 0.08 | 0.29 | −0.06, 0.64 | 0.11 | |
| Infant sex | — | — | −0.27 | −1.25, 0.72 | 0.59 | −0.36 | −1.31, 0.59 | 0.45 | |
| Infant age | — | — | −0.02 | −0.07, 0.03 | 0.41 | −0.01 | −0.06, 0.03 | 0.56 | |
| Infant birthweight | — | — | −0.22 | −1.40, 0.96 | 0.71 | −0.27 | −1.42, 0.88 | 0.65 | |
1Linear regression analyses were conducted to obtain unstandardized (B) coefficients, 95% CIs, and P values. Model I includes only maternal fructose consumption at 1 postnatal month as an exposure variable; model II includes maternal age, prepregnancy BMI, education level, kilocalories, infant age, sex, and birthweight as covariates; model III includes maternal SSBs + J at 1 postnatal month as an additional exposure variable (n = 88). SSBs + J, sugar-sweetened beverages and juice.
Figure 1.
Model of the pathway from maternal fructose consumption at 1 postnatal month to infant cognitive development scores at 24 postnatal months after adjustment for maternal SSBs + J and covariates. Unstandardized (B) coefficients and P values are presented, and were obtained using linear regression analyses (n = 88). SSBs + J, sugar-sweetened beverages and juice.
In a separate set of linear regression analyses, we found that other explanatory variables of interest were not associated with infant cognitive development scores at 24 postnatal months. Maternal whole fruit consumption at 1 postnatal month (B = 0.20; 95% CI = −0.15, 0.54; P = 0.26) and 6 postnatal months (B = −0.01; 95% CI = −0.40, 0.38; P = 0.95) were not associated with infant cognitive development scores. Moreover, infant fructose (B = −0.05; 95% CI = −0.12, 0.03; P = 0.22) and SSB + J intake (B = −0.35; 95% CI = −0.82, 0.12; P = 0.14) at 24 postnatal months were not associated with infant cognitive development scores.
Discussion
In this study of Hispanic mothers, we found that maternal fructose consumption at 1 postnatal month correlated inversely with infant neurodevelopmental outcomes at 24 postnatal months. We also found that maternal SSB + J intake at 1 postnatal month partially accounted for the association between maternal fructose consumption and infant outcomes. Maternal SSB + J intake was therefore determined to be the source of maternal fructose intake responsible for the effect on infant neurodevelopmental outcomes, and not maternal whole fruits. In contrast, maternal fructose and SSB + J consumption at 6 postnatal months and infant fructose and SSB + J intake at 24 postnatal months were not associated with infant cognition. This suggests the presence of a critical window of vulnerability during early lactation when maternal nutrition can be most influential in shaping infant cognitive capacities.
Mothers make mindful decisions about when and how much to feed their infants (26–28). Mothers are less aware, however, that their own nutritional intake also affects their infant's nutritional status, both during pregnancy and lactation. Indeed, others have examined maternal fructose consumption in pregnancy and cognitive capacities in childhood. Cohen et al. (5) found that there was no association of maternal fructose intake during gestation with intelligence test scores at age 7 y, but that maternal SSB intake correlated inversely with cognitive outcomes in children. In contrast, our results are to our knowledge, the first to reveal that maternal fructose consumption during lactation correlated inversely with neurodevelopmental outcomes at 24 postnatal months, and that this association was attributed to maternal SSB + J intake. That is, the link between maternal fructose consumption and infant neurodevelopmental outcomes in our cohort was maternal SSBs + J, and not maternal whole fruits. Maternal SSBs + J are a source of free fructose, whereas whole fruits contain fructose bound to fiber and in lower amounts. We posit that free fructose can be more detrimental to infant neurodevelopmental outcomes than fructose bound to fiber due to differences in the rate of metabolism and potential for adverse health events (5, 29, 30).
There are several differences between the described studies that could explain inconsistent observations. These include timing and duration of exposure to maternal nutrition, mode of transmission to infant, and age at cognitive assessment. Cohen et al. (5) measured maternal fructose consumption and SSBs separate from juice during pregnancy. The authors therefore hypothesized that exposure to maternal nutrition in utero was what influenced cognitive outcomes in children. In contrast, the present study measured maternal fructose consumption and SSBs with juice during lactation. We posit that exposure to maternal nutrition through breast milk was what influenced outcomes in infants (14, 15). This premise is bolstered by our finding that infant SSB + J intake was not associated with cognitive development, which lends support to a direct influence of maternal SSBs + J. However, this is speculative, because maternal fructose and SSB + J consumption could have also persisted from pregnancy to lactation. We are not able to distinguish between pre- and postnatal effects on infant neurodevelopmental outcomes, and acknowledge the potential for a cumulative effect.
These new findings build on our prior work. We previously found that maternal SSB consumption in lactation increased fructose in breast milk within 3 h, and concentrations remained elevated for 5 h (14). It is conceivable that mothers who are habitual consumers of SSBs + J are at risk of elevated fructose in breast milk before and during breastfeeds, because SSBs tend to be made with HFCS (a mixture of free fructose and free glucose), and juice is a natural source of free fructose that contains more of this simple sugar than a commercial soft drink (31). Moreover, we previously found that fructose in breast milk was positively associated with infant somatic growth, including weight, fat mass, and lean mass, in an apparently healthy cohort of infants born at term (15). Because infants tend to feed every 2 to 3 h in the first postnatal month (26), they are well within the window for multiple exposures to fructose from breast milk, the likely result of maternal SSB + J consumption (14).
Though this is a concern for infant somatic growth, it could be an even greater concern for brain development, with the potential for lasting effects. Early postnatal life is a critical period for neural circuit formation (32). Construction and use of new brain circuits creates a high metabolic demand that requires a continual drawing from the available pool of nutrients in breast milk (33, 34). This pool can be compromised with transmission of fructose from the maternal diet, which is likely derived from maternal SSB + J consumption (17–19). This premise is strengthened by our findings: 1) maternal fructose and SSB + J intakes were similar at 1 and 6 postnatal months; 2) breastfeedings per day decreased from 1 to 6 postnatal months; and 3) maternal fructose and SSB + J consumption at 1 postnatal month correlated inversely with infant neurodevelopmental outcomes in our models, whereas maternal fructose and SSB + J consumption at 6 postnatal months were not associated.
Animal studies have shown that early exposure to a fructose-sweetened solution (similar to SSB + J) can interfere in systems that support early neural circuit formation. Rodents fed a fructose-sweetened solution had increased oxidative stress, increased advanced glycation end products, and decreased antioxidant enzymes in the frontal cortex (18). Animals exposed to a fructose-sweetened solution also had decreased brain-derived neurotrophic factor, a protein that supports brain development and circuit formation (18, 20). Moreover, rodents fed a fructose-sweetened solution had increased mediators of inflammation, including IL-6 and IL-1β in the hippocampus (17, 19). These studies suggest that fructose alters the development of neural systems that support cognitive development (18, 21). These findings also indicate that the source of fructose (as free fructose in solution) is important. This is in line with our findings that the link between maternal fructose consumption and infant outcomes was maternal SSB + J consumption and not whole fruits.
This study has several limitations. The prospective observational design cannot be used to establish causality, and randomized controlled trials that manipulate maternal SSB + J consumption would be required to yield that level of causal inference. Though multiple covariates were carefully adjusted for in our analyses, residual confounding caused by unmeasured factors could exist. Information regarding maternal and infant nutrition was based on self-report, not direct observation/measurement. We did not assess maternal intake in pregnancy, and we cannot determine whether intrauterine exposure influenced infant outcomes. We did not assess concentrations of simple sugars in breast milk (e.g., fructose), and so we cannot be certain that exposure through breastfeeds influenced infant outcomes. Our findings are also limited to a relatively small sample of Hispanic mothers and infants in the Southwestern United States; differences in built environment, food choice, and other characteristics might limit generalizability of overall findings (22, 23).
In conclusion, our findings revealed that maternal fructose consumption at 1 postnatal month correlated inversely with infant cognitive development at 24 postnatal months, and this was partially attributed to maternal SSB + J consumption at 1 postnatal month. The absence of the same significant associations at 6 postnatal months raises 2 potential hypotheses: 1) there is a diminished influence of maternal SSB + J consumption on infant brain development as breastfeeding frequency decreases and in turn the extent to which infants are exposed to maternal fructose consumption transmitted through breast milk; and 2) there is a window of temporal vulnerability when maternal nutrition during lactation can pose a risk to infant brain development. Although there is ample evidence that breast milk is beneficial for infant brain development (23, 35), our data indicate that this can be compromised when mothers eat a lower-quality diet during lactation that is high in free fructose from SSBs + J. Our findings can be used to guide interventions to improve maternal nutrition to better complement the timing of breastfeeds and thereby optimize infant cognitive capacities.
Supplementary Material
Acknowledgments
We thank Carla Flores, Danielle Garcia, Rosa Rangel, and Elizabeth Campbell for coordination of this project; and our research collaborators, Natalia Luna, Bernice Flores, Kelsey McAlister, Manuel Muniz Ortiz, Monica Zaldivar, and Andrea Goreti Martinez Sanchez, for administering the Bayley-III Scales of Infant Development.
The authors’ responsibilities were as follows—PKB, LB, BSP, MIG: designed research; PKB, JFP, RBJ, TLA, CR: conducted research; PKB, TAP: performed statistical analysis; PKB, DAF, LB, BSP, MIG: interpreted results; PKB: wrote the paper; PKB, JFP, RBJ, TLA, CR, TAP, DAF, LB, BSP, MIG: revised the paper; MIG: had primary responsibility for final content; and all authors: read and approved the final manuscript.
MIG receives book royalties from Penguin Random House and is a scientific advisor for Yumi. All other authors report no conflicts of interest.
Notes
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K99 HD098288), the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK110793), and the National Center for Advancing Translational Science (UL1TR001855 and UL1TR000130). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also funded by the Gerber Foundation (15PN-013). This funding source had no role in the design, execution, analyses, interpretation of data, writing of the report, or decision to submit the report for publication.
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval from the authors.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: HFCS, high-fructose corn syrup; SSB, sugar-sweetened beverage; SSB + J, sugar-sweetened beverage and juice.
Contributor Information
Paige K Berger, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Jasmine F Plows, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Roshonda B Jones, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Tanya L Alderete, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Claudia Rios, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Trevor A Pickering, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
David A Fields, Department of Pediatrics, The University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Lars Bode, Department of Pediatrics and Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, La Jolla, CA, USA.
Bradley S Peterson, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Michael I Goran, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
References
- 1. Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121(6):e1604–14. [DOI] [PubMed] [Google Scholar]
- 2. An R. Beverage consumption in relation to discretionary food intake and diet quality among US adults, 2003 to 2012. J Acad Nutr Diet. 2016;116(1):28–37. [DOI] [PubMed] [Google Scholar]
- 3. Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, Havel PJ, Laugero KD. Excessive sugar consumption may be a difficult habit to break: a view from the brain and body. J Clin Endocrinol Metab. 2015;100(6):2239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen JFW, Rifas-Shiman SL, Young J, Oken E. Associations of prenatal and child sugar intake with child cognition. Am J Prev Med. 2018;54(6):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care. 2011;14(4):385–90. [DOI] [PubMed] [Google Scholar]
- 7. Bailey RL, Fulgoni VL, Cowan AE, Gaine PC. Sources of added sugars in young children, adolescents, and adults with low and high intakes of added sugars. Nutrients. [Internet]2018;10(1):102 doi:10.3390/nu10010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patetta MA, Pedraza LS, Popkin BM. Improvements in the nutritional quality of US young adults based on food sources and socioeconomic status between 1989–1991 and 2011–2014. Nutr J. [Internet]2019;18(1):32 doi:10.1186/s12937-019-0460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. [DOI] [PubMed] [Google Scholar]
- 10. Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity. 2011;19(4):868–74. [DOI] [PubMed] [Google Scholar]
- 11. Rodrigues DF, Henriques MC, Oliveira MC, Menezes-Garcia Z, Marques PE, Souza Dda G, Menezes GB, Teixeira MM, Ferreira AV. Acute intake of a high-fructose diet alters the balance of adipokine concentrations and induces neutrophil influx in the liver. J Nutr Biochem. 2014;25(4):388–94. [DOI] [PubMed] [Google Scholar]
- 12. Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger PK, Fields DA, Demerath EW, Fujiwara H, Goran MI. High-fructose corn syrup-sweetened beverage intake increases 5-hour breast milk fructose concentrations in lactating women. Nutrients. [Internet]2018;10(6):669 doi:10.3390/nu10060669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients. [Internet]2017;9(2):146 doi:10.3390/nu9020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noble EE, Hsu TM, Liang J, Kanoski SE. Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr Neurosci. 2019;22:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bukhari SHF, Clark OE, Williamson LL. Maternal high fructose diet and neonatal immune challenge alter offspring anxiety-like behavior and inflammation across the lifespan. Life Sci. 2018;197:114–21. [DOI] [PubMed] [Google Scholar]
- 18. Sanguesa G, Cascales M, Grinan C, Sanchez RM, Roglans N, Pallas M, Laguna JC, Alegret M. Impairment of novel object recognition memory and brain insulin signaling in fructose- but not glucose-drinking female rats. Mol Neurobiol. 2018;55(8):6984–99. [DOI] [PubMed] [Google Scholar]
- 19. Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, Kanoski SE. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2015;25(2):227–39. [DOI] [PubMed] [Google Scholar]
- 20. Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez-Pinilla F. Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J Cereb Blood Flow Metab. 2016;36(5):941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noble EE, Kanoski SE. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr Opin Behav Sci. 2016;9:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger PK, Plows JF, Jones RB, Pollock NK, Alderete TL, Ryoo JH, Goran MI. Maternal blood pressure mediates the association between maternal obesity and infant weight gain in early postpartum. Pediatr Obes. [Internet]2019;14:e12560 doi:10.1111/ijpo.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Poulsen M, Ryoo JH, Peterson BS, Bode L, Goran MI. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One. [Internet]2020;15(2):e0228323 doi:10.1371/journal.pone.0228323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–81. [DOI] [PubMed] [Google Scholar]
- 25. Bayley N. Bayley scales of infant and toddler development: administration manual. 3 ed San Antonio, TX: Harcourt Assessment; 2005. [Google Scholar]
- 26. Berger PK, Lavner JA, Smith JJ, Birch LL. Differences in early risk factors for obesity between African American formula-fed infants and White breastfed controls. Pilot Feasibility Stud. [Internet]2017;3:58 doi:10.1186/s40814-017-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Savage JS, Hohman EE, Marini ME, Shelly A, Paul IM, Birch LL. INSIGHT responsive parenting intervention and infant feeding practices: randomized clinical trial. Int J Behav Nutr Phys Act. [Internet]2018;15(1):64 doi:10.1186/s12966-018-0700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr. 2014;99(3):723S–8S. [DOI] [PubMed] [Google Scholar]
- 29. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. [DOI] [PubMed] [Google Scholar]
- 30. Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, Liu W, Tesz GJ, Birnbaum MJ, Rabinowitz JD. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018;27(2):351–61. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker RW, Le KA, Davis J, Alderete TL, Cherry R, Lebel S, Goran MI. High rates of fructose malabsorption are associated with reduced liver fat in obese African Americans. J Am Coll Nutr. 2012;31(5):369–74. [DOI] [PubMed] [Google Scholar]
- 32. Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacol. 2010;35(1):147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deoni S, Dean D, Joelson S, O'Regan J, Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage. 2018;178:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr. 2012;3(3):465S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, Crume T, Dabelea D, Donovan SM, Forman N et al. Lactation and neonatal nutrition: defining and refining the critical questions. J Mammary Gland Biol Neoplasia. 2012;17(2):167–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.