Abstract
Background
Fear memory is a fundamental capability for animals and humans to survive. Its impairment results in the disability to avoid danger. When memory is reactivated, a reconsolidation process, which can be disrupted by various stimuli, including inflammation, is required to become permanent. Nicotine has been shown to improve cognitive deficits induced by inflammation and other stimuli. Therefore, in the present study, we investigated the effect of nicotine on lipopolysaccharide (LPS)-induced impairment of fear memory reconsolidation and the underlying mechanism.
Methods
Step-through inhibitory avoidance task was recruited to study fear memory of rat, i.p. LPS (0.5 mg/kg) treatment was used to induce inflammation, and western blot and immunostaining were applied to detect protein expression and distribution in medial prefrontal cortex and hippocampus.
Results
Our data showed that LPS induced fear memory reconsolidation impairment without affecting retrieval. In addition, LPS significantly increased inflammation factors tumor necrosis factor-α and interleukin-1 beta and decreased CREB-regulated transcription coactivator 1 (CRTC1) expression and adenosine monophosphate-activated protein kinase (AMPK) activation in hippocampus. More importantly, LPS significantly decreased CRTC1 expression and AMPK activation in neurons by activating microglia cells. Of note, either nicotine treatment or activation of AMPK by intracerebroventricular infusion of metformin reduced LPS-induced impairment of fear memory reconsolidation and ameliorated inflammation factor tumor necrosis factor-α and interleukin-1 beta as well as the expression of CRTC1.
Conclusions
In conclusion, our results showed that acute nicotine treatment alleviates LPS-induced impairment of fear memory reconsolidation through activation of AMPK and upregulation of CRTC1 in hippocampus.
Keywords: AMPK, CRTC1, fear memory, reconsolidation, nicotine, rat
Significance Statement.
Accurate prediction of environmental danger is a fundamental capability for animals and humans to survive; they would lose the ability to avoid danger if fear memory is impaired. Various stimuli, including inflammation, could impair fear memory. Nicotine has shown anti-inflammation effects, and nicotine-derived compounds could work as therapeutic tools against fear memories and anxiety as well as posttraumatic stress disorder. Our study can provide valuable information on improving inflammation-induced impairment of fear memory reconsolidation.
Introduction
Accurate prediction of environmental danger is a fundamental capability for animals and humans to survive; they would lose the ability to avoid danger if fear memory is impaired. When a fear memory is acquired and consolidated, it becomes stable. When it is reactivated, the established memory becomes unstable again and temporarily sensitive to various stimuli, and a reconsolidation stage is required to become permanent (Jin et al., 2007; Tronel and Alberini, 2007; Besnard et al., 2012; Radiske et al., 2017). Reconsolidation of fear memories has been applied for the treatment of posttraumatic stress disorder, a psychiatric disorder associated with memories of traumatic experiences (Kida, 2019).
High rates of smoking in posttraumatic stress disorder patients (45.3%) may be partly due to nicotine’s function to improve cognitive deficits associated with this disorder (Ziedonis et al., 2008), which is confirmed by another study showing that nicotine could modulate the long-lasting storage of fear memory (Lima et al., 2013) and enhance contextual fear memory reconsolidation in rats (Tian et al., 2011). Fear memory could be impaired by various stimuli, including inflammation (Kranjac et al., 2012), and neuroinflammation plays an essential role in the pathogenesis of cognitive dysfunction after lipopolysaccharide (LPS) exposure (Wei et al., 2015; Skelly et al., 2019). Since nicotine could ameliorate schizophrenia-like cognitive deficits induced by maternal LPS exposure (Waterhouse et al., 2016, 2018) and attenuate LPS-induced cognitive dysfunction by inhibiting neuroinflammation in the rat hippocampus (Wei et al., 2015), we speculate that nicotine may alleviate LPS-induced fear memory reconsolidation impairment.
CREB-regulated transcription coactivator 1 (CRTC1) is a potent modulator of cAMP response element (CRE)-driven gene transcription (Xue et al., 2015). CRTC1 plays essential roles in fear memory (Nonaka et al., 2014) and memory reconsolidation (Sekeres et al., 2012). CRTC1 dysfunction is involved in memory impairment in Alzheimer’s disease (Parra-Damas et al., 2017) and ischemic stroke-induced memory impairment (Zhang et al., 2019). It has been reported that CRTC1 could be regulated by adenosine monophosphate-activated protein kinase (AMPK) (Mair et al., 2011; Burkewitz et al., 2015). AMPK plays an essential role in memory (Kobilo et al., 2014) and is involved in the anti-inflammatory effect of nicotine in vivo and in vitro (Cheng et al., 2007). Therefore, nicotine might alleviate LPS-induced impairment of fear memory reconsolidation by activating AMPK.
One-trial step-through inhibitory avoidance task is an ideal behavioral paradigm for studying hippocampus-dependent fear memory (Radiske et al., 2017). In our current study, we will use an inhibitory avoidance task to test our hypothesis that nicotine could alleviate LPS-induced fear memory reconsolidation through activation of AMPK and upregulation of CRTC1.
Materials and Methods
Animals
Sprague-Dawley rats (male, approximately 240–260 g at the time of surgery, 6–7 weeks old) were purchased from SLAC Company (Shanghai, China). They were housed 2 to 3 per cage under constant temperature (23°C ± 1°C) and light-controlled vivarium (12-hour light/-dark cycle). The rats were housed with free access to water and food. All animal procedures were approved by the University Committee on Animal Care of Soochow University and performed according to the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and reduce the number of animals.
Surgery
Rats were implanted with a stainless-steel guide cannula (23 gauge) under anesthesia with 2% pentobarbital sodium (5 mL/kg, i.p.). The coordinates taken from the atlas of Paxinos and Watson 1986 used for intracerebroventricular (ICV) cannulation were 0.3 mm posterior to bregma, 1.4 mm lateral to the midline, and 4.5 mm below the skull surface. Stainless-steel screws and dental cement were used to anchor the cannula to the skull. A dummy cannula was inserted into the guide cannula to alleviate the infection risk. Rats were given 5 to 7 days to recover.
Drug Treatment
LPS (Sigma Chemical Company, St. Louis, MO) was dissolved in saline and injected i.p. at a dose of 0.5 mg/kg 4 hours before the first testing (Rostami et al., 2012). This dose of LPS does not affect locomotor activity, anxiety levels, or general hippocampal function (Shen et al., 2018).
Nicotine hydrogen tartrate (Sigma Chemical Company) was dissolved in saline and adjusted to pH 7.4 with NaOH. Nicotine at a dose of 0.2 or 1 mg/kg was s.c. administered 30 minutes before the injection of LPS (Shu et al., 2015). All nicotine doses are expressed as tartrate. Neither dose of nicotine affects locomotor activity, anxiety levels, or general hippocampal function (Tian et al., 2011; Shu et al., 2015).
Metformin was dissolved in artificial cerebrospinal fluid and infused at a dose of 0.1 mg/5 µL or 0.2 mg/5 µL (Stevanovic et al., 2012). Metformin or artificial cerebrospinal fluid ICV injection was administered 30 minutes before LPS injection. Neither dose of metformin affected locomotor activity, anxiety levels, or general hippocampal function.
Infusion was done by inserting an injection needle (30 gauge, attached to a 5-µL Hamilton syringe via polyethylene tube) into the guide cannula with its tip 1.5 mm beyond the cannula, reaching a total depth of 4.5 mm in ICV. Metformin solution or vehicle (5 µL) was infused into the ICV at a rate of 0.25 µL/min. After infusion, the injection needle stayed in the cannula for another minute (Jin et al., 2007).
Behavioral Test
Apparatus
Rats were trained on a 1-trial step-through inhibitory avoidance task. Behavioral training and testing were conducted in a light-attenuated and silent room. The training apparatus was a trough-shaped alley and consisted of a light safe compartment (30 cm × 30 cm × 30 cm) illuminated by a 40-W lamp and a dark shock compartment (30 cm × 30 cm × 30 cm) connected with a shock generator. The floor of the apparatus consisted of copper rods (0.6 cm in diameter, spaced 1.5 cm apart). Two compartments were separated by a retractable door.
Training Procedure
Before training, each rat was placed in the light compartment and allowed to explore the compartments 2 min/d for 3 consecutive days. For normal training, each rat was placed in the light compartment with its head facing away from the door. When the rat stepped into the dark compartment with all 4 paws, the latency was recorded, the retractable door was closed, and the animal was given an electric foot shock for 3 seconds (0.6 mA). Then the retractable door was raised again, and the rat was allowed to run back into the light compartment. Because of the experience of receiving the foot shock, the rat developed a fear of the dark compartment and avoided stepping into it again. If the rat kept staying in the light compartment for 5 minutes, it was returned to the home cage and the training was over.
Testing Procedure
Memory testing was carried out 24 hours (Testing 1, test for memory retrieval) and 48 hours (Testing 2, test for memory reconsolidation) after training (Fukushima et al., 2014). Each rat was placed in the light compartment and allowed to explore the compartments freely. The floor of the dark compartment was not electrified. If an animal entered the dark compartment within 5 minutes, the latency to step into the dark compartment was recorded, and if not, its latency was assigned 5 minutes.
Experimental Procedures
Experiment 1
This experiment examined the effect of systemic administration of nicotine on LPS-induced memory impairment. Rats were randomly assigned to 6 groups: vehicle, LPS, LPS+nicotine low dose (0.2 mg/kg), LPS+nicotine high dose (1 mg/kg), nicotine low dose (0.2 mg/kg), and nicotine high dose (1 mg/kg).
Experiment 2
This experiment aimed to explore the roles of interleukin-1 beta (IL-β), tumor necrosis factor-α (TNF-α), AMPK activation, and CRTC1 expression in nicotine’s effect on LPS-induced impairment of fear memory reconsolidation.
Experiment 3
This experiment investigated the interaction of LPS-induced inflammation in microglial cells and downregulation of AMPK and CRTC1 in neurons. Primary cultured microglia was treated with LPS at a concentration of 0, 2.5, 5, or 10 μg/mL, respectively, for 24 hours. LPS-conditioned medium (LCM) was collected and applied to SH-SY5Y cells for 48 hours followed by detection of AMPK and CRTC1 in SH-SY5Y cells. As a control, the same concentration of LPS was directly added to the medium of SH-SY5Y cells followed by protein detection of AMPK and CRTC1.
Experiment 4
This experiment investigated whether AMPK was involved in nicotine’s improvement on LPS-induced reconsolidation impairment. The procedure was similar to experiment 1 except that metformin was infused into the ICV 30 minutes before the LPS treatment. Rats were randomly assigned to 6 groups: vehicle, LPS, LPS+metformin low dose (0.1 mg/5 µL), LPS+metformin high dose (0.2 mg/5 µL), metformin low dose (0.1 mg/5 µL), and metformin high dose (0.2 mg/5 µL) group.
Cell Culture
Primary Microglia Cell Culture
Primary microglial cultures were established from the brains of newborn mice (C57BL/6J) at postnatal day 1–2 as we described (Shen et al., 2018). The purity of microglia was confirmed by immunofluorescence (>95% Iba-1+ cells, verified by its colocalization with 4’,6-diamidino-2-phenylindole [DAPI]). Isolated microglia cells were plated into 6-well plate at a density of 5 × 105 cells per well.
SH-SY5Y Cell Culture
SH-SY5Y cells (American Type Culture Collection) were plated onto 6-well plate at the desired density and grown as a monolayer in Dulbecco’s Modified Eagle Media (DMEM)-high glucose medium with 10 % fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator with 5% CO2 and 95% room air (Sun et al., 2017). Then SH-SY5Y cells were treated with 0, 2.5, 5, and 10 μg/mL LPS, respectively, for 24 hours.
LPS-Induced Microglial Activation and Conditioned Media Treatment
Primary microglia cells were plated onto a 6-well plate and treated with10 μg/mL LPS, respectively, for 24 hours, and LCM was collected and applied to SH-SY5Y cells for 48 hours. After the LCM treatment, the cells were washed twice with cold phosphate buffered saline (PBS) and collected for western-blot analysis.
Primary Neurons Culture
Primary neurons were obtained from the cortical of embryo brains at day 17/18 (E17/E18) from pregnant C57 mice, as reported previously (Nuzzo et al., 2019). Primary neurons were incubated for 6 hours with or without 2.5 mM metformin to activate AMPK (Barini et al., 2016).
Histological and Biochemical Analysis
Immunofluorescence for CRTC1
After the behavioral test, rats were perfused with PBS followed by 4% paraformaldehyde (PFA). The 20-μm-thick cryosection was fixed with 4% PFA for analysis of CRTC1 as we previously described (Liu et al., 2017). Briefly, blocking buffer containing 0.3% Triton X-100 and 10% goat serum was used to block nonspecific binding. The hippocampus sections were incubated overnight at 4°C with primary antibodies against CRTC1 (1:200, CST) followed by incubation with 488-conjugated secondary antibody (anti-rabbit, 1:800) for 2 hours at room temperature. Immunofluorescence was visualized under a LSM 700 microscope (Zeiss), and images were taken from the hippocampus.
To evaluate the expression of CRTC1 in SH-SY5Y cells or primary neurons, cells were fixed with 4% PFA for 15 minutes (Lv et al., 2017). Unspecific binding was blocked by 10% goat serum and 0.3% Triton X-100 solution at room temperature for 2 hours. Cells were incubated with rabbit anti-CRTC1 (1:200, CST) or anti-p-AMPK (1:200, CST) in the blocking solution at 4°C overnight. After 3 washes with PBS, the cells were incubated with corresponding 488-conjugated goat anti-rabbit (1:800) at 37°C for 2 hours, and the nuclei were stained with DAPI. Fluorescent images were acquired using a confocal microscope.
Western Blot
After the behavioral test, rats were anesthetized with isoflurane and transcardially perfused with PBS. After decapitation, the brains were removed quickly. The hippocampus, amygdala, and prefrontal cortex were isolated in ice and kept at −80°C for western blot as described before (Luo et al., 2019).
Equal amounts of protein (30 μg) for each sample were loaded into sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. The membranes were blocked with 5% nonfat milk at room temperature for 2 hours. They were then incubated overnight at 4°C with anti-p-AMPK antibody (1:1000; CST), anti-AMPK antibody (1:1000; CST), and anti-CRTC1 antibody (1:2000; Abcam) in tris-buffered saline tween-20 (TBST). The immunoblots were then screened using the ChemiDoc MP System (Bio-Rad).
Real-Time Reverse-Transcribed-PCR
Total RNA was isolated using TRIzol reagents (Invitrogen) according to the manufacturer’s protocol as we described previously (Jin et al., 2015). RNA (3 μg) was reverse-transcribed with random primers in a 20-μL final reaction volume using TaqMan Reverse Transcription Kits (Applied Biosystems). Primers (Integrated DNA Technologies) for IL-1β, TNF-α, and β-actin were designed against known rat sequences: IL-1β forward: 5’- GCTGTGGCAGCTACC TATGTCTTG -3’, reverse: 5’- AGGTCG TCATCATCCCACGAG -3’; TNF-α forward: 5’- GTGATCGGTCCC AACAAGGA -3’, reverse: 5’- CTCCCACCCTACTTTGCTTGTG -3’; β-actin forward: 5’- ACTATCGGCAATGAGCGGTTCC -3’, reverse: 5’- AGCACTGTGTTGGCATAGAGGTC -3’. The fluorescence threshold value was calculated using the SDS Enterprise Database software (Applied Biosystems). The relative value of mRNA expression was calculated by the comparative ∆∆ Ct method described in our previous publication (Liu et al., 2007).
ELISA Assay for IL-1β and TNF-α
IL-1β and TNF-α contents from rats were assessed using an ELISA kit from Boster (Wuhan, China). Aliquots of 100 μL of samples were added to the appropriate micro-titer wells provided by the kit. After discarding the liquid from each well (no wash), 100 μL of biotinylated anti-rat IL-1β or TNF-α antibody solution was added to each well, except the chromogen blanks, and incubated at 37°C for 1 hour. After washing 3 times with the wash buffer, 100 μL avidin-peroxidase complex working solution was added to each well, except the chromogen blanks, and incubated for 30 minutes at 37°C. Lastly, 100 μL of stabilized chromogen was added to each well and incubated for 30 minutes at 37°C in the dark before the addition of 100 μL of stop solution. The absorbance at 450 nm was recorded on a plate reader.
Statistical Analysis
Data were analyzed using 1-way or 2-way ANOVA. P < 0.05 was accepted as statistical significance. All statistical analysis was conducted in SPSS 18.0 statistical programs (SPSS, Chicago, IL). Data in the text and figures are expressed as means ± SEM.
Results
Nicotine Prevents LPS-Induced Fear Memory Reconsolidation Impairment
LPS and cytokine impaired memory consolidation and reconsolidation (Kranjac et al., 2012), and acute nicotine could attenuate LPS-induced cognitive dysfunction by inhibiting neuroinflammation in the rat hippocampus (Wei et al., 2015). Here, we investigated the effect of systemic nicotine administration on LPS-induced impairment of fear memory reconsolidation. The detailed experimental procedure is described in Figure 1A. As shown in Figure 1B, LPS treatment 4 hours before the test had no significant effect on fear memory retrieval (F[1,63] = 0.069, P > .05, Testing 1); however, LPS significantly impaired fear memory reconsolidation (F[1,63] = 6.906, P < .05, Testing 2). Importantly, systemic administration of nicotine significantly prevented LPS-induced impairment of fear memory reconsolidation (F[1,63] = 2.977, P < .05, Testing 2) (Figure 1). Although there is a trend, nicotine did not significantly increase fear memory retrieval (P > .05).
Figure 1.
Nicotine prevents lipopolysaccharide (LPS)-induced impairment of fear memory reconsolidation. (A) Diagram shows the procedure of the experiment. (B) LPS treatment significantly impaired reconsolidation without affecting retrieval of fear memory (*P < .05 vs the vehicle group). Acute subcutaneous nicotine treatment significantly inhibited this effect. #P < .05 vs the LPS group, n = 8–16 for each group.
Nicotine Prevents LPS-Induced Increase of IL-1β and TNF-α in Hippocampus
It has been reported that levels of cytokines and chemokine were significantly upregulated in 4 hours after LPS treatment (Kranjac et al., 2012). In addition, IL-1β has been shown to impair memory reconsolidation (Machado et al., 2015). Here, we examined the expression of IL-1β and TNF-α in hippocampus after the behavioral test. Our results showed that LPS significantly increased the mRNA of IL-1β (F[1,8] = 13.990, P = .006; Figure 2A, right) and TNF-α (F[1,10] = 6.449, P = .029; Figure 2B, right) as well as the protein level of IL-1β (F[1,8] = 10.842, P = .011; Figure 2A, left) and TNF-α (F[1,8] = 10.299, P = .012; Figure 2B, left). Nicotine at a dose of 0.2 mg/kg significantly alleviated the increase of mRNA of IL-1β (F[1,8] = 11.709, P = .009; Figure 2A, right) and TNF-α (F[1, 10] = 5.175, P = .046; Figure 2B, right) as well as the protein level of IL-1β (F[1,8] = 11.461, P = .01; Figure 2A, left) and TNF-α (F[1,8] = 27.155, P = .001; Figure 2B, left). Nicotine at a dose of 1 mg/kg also alleviated the mRNA increase of IL-1β (F[1,8] = 19.019, P = .002; Figure 2A, right) and TNF-α (F[1,10] = 5.947 P = .035; Figure 2B, right) as well as the protein level of IL-1β (F[1,8] = 24.786 P = .001; Figure 2A, left) and TNF-α (F[1,8] = 16.732, P = .003; Figure 2B, left).
Figure 2.
Nicotine prevents lipopolysaccharide (LPS)-induced increase of interleukin-1 beta (IL-1β) and tumor necrosis factor-α (TNF-α) and decrease of adenosine monophosphate-activated protein kinase (AMPK) activation in hippocampus. (A) LPS treatment induced a significant increase of protein (left) and mRNA (right) of IL-1β, and nicotine inhibited such effect. *P < .05 vs the vehicle group, #P < .05 vs the LPS group, n = 5 per group. (B) LPS treatment induced a significant increase of protein (left) and mRNA (right) of TNF-α and nicotine inhibited such effect. *P < .05 vs the vehicle group, #P < .05 vs the LPS group, n = 5 per group. Representative western blot showed the band of AMPK (left). The band intensity of AMPK was quantitated after normalization to the β-actin (right). LPS significantly inhibited the activation of AMPK in hippocampus (C), but not in medial prefrontal cortex (mPFC) (D) or amygdala (E). *P < .05 vs the vehicle group, #P < .05 vs the LPS group, n = 4 per group.
Nicotine Alleviates LPS-Induced Inhibition of AMPK Activation in Hippocampus
Several studies showed that AMPK activity is involved in learning and memory processes (Han et al., 2016). Therefore, we tested if LPS treatment impaired memory reconsolidation through regulating AMPK activation in hippocampus, amygdala, or medial prefrontal cortex (mPFC). As shown in Figure 2, LPS inhibited activation of AMPK in hippocampus (F[1, 7] = 19.235, P = .005; Figure 2C) but not in mPFC (F[1, 11] = 0.941, P = .355; Figure 2D) or amygdala (F[1,11] = 0.085, P = .776; Figure 2E). Nicotine at a dose of 0.2 mg/kg (F[1, 11] = 5.633, P = .039; Figure 2C), but not 1 mg/kg (F[1, 10] = 2.213, P = .171; Figure 2C), significantly reduced LPS-induced inhibition of AMPK activation in hippocampus. In addition, in PFC, although LPS did not significantly decrease AMPK activation (P > .05), nicotine significantly increased the activation of AMPK (P < .05), suggesting that nicotine could activate AMPK (Wu et al., 2015).
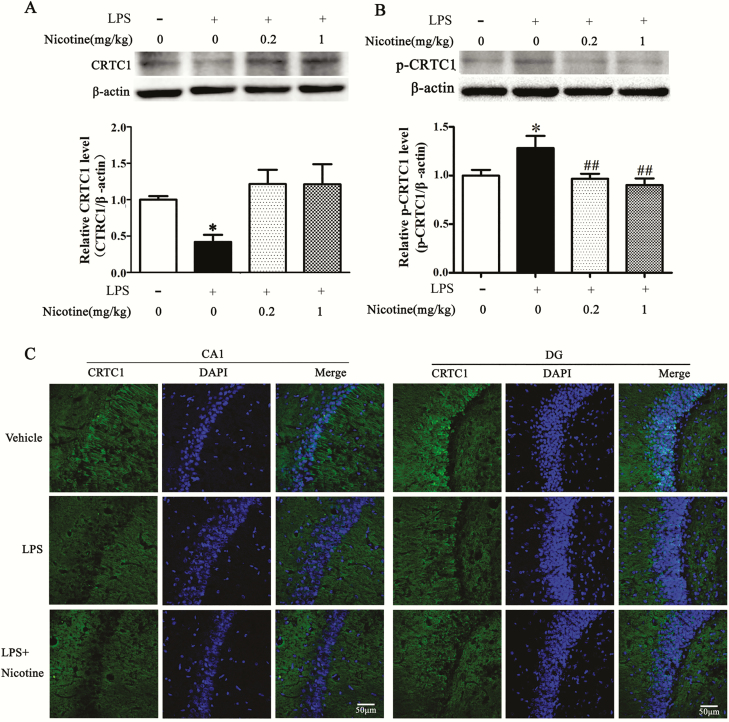
Nicotine Prevents LPS-Induced Downregulation of CRTC1 Expression in Hippocampus
CRTC1, a target of AMPK, has been shown to play a critical role in memory (Nonaka et al., 2014). Phosphorylation status of CRTC1 determines the function of CRTC1 in memory (Parra-Damas et al., 2017). p-CRTC1 is bound to the cytoplasm, and on dephosphorylation it is translocated to the nucleus to affect synaptic plasticity (Ch’ng et al., 2012). Since LPS inhibited AMPK activation in hippocampus but not in amygdala or mPFC, we examined CRTC1 expression in the hippocampus. As shown in Figure 3, LPS downregulated CRTC1 expression (F[1,8] = 27.637, P = .001; Figure 3A) and upregulated p-CRTC1 (Figure 3B) in hippocampus, and nicotine inhibited such effect at a dose of 0.2 mg/kg (F[1, 8] = 10.455, P = .012) or 1 mg/kg (F[1,8] = 5.842, P = .042). Immunofluorescence results confirmed that LPS induced a CRTC1 decrease in CA1 and dental gyrus regions of the hippocampus, and acute nicotine treatment inhibited this effect (Figure 3C).
Figure 3.
Nicotine prevents lipopolysaccharide (LPS)-induced downregulation of CREB-regulated transcription coactivator 1 (CRTC1) expression in hippocampus. (A) Representative western blot showed decreased CRTC1 expression after LPS treatment, and nicotine inhibited such effect (upper). The relative band intensity was quantitated (lower). *P < .05 vs vehicle group, #P < .05 vs the LPS group, n = 3 per group. (B) Representative western blot showed increased p-CRTC1 (top). The band intensity of p-CRTC1 was quantitated (bottom). *P < .05 vs vehicle group, #P < .05 vs the LPS group, n = 3 per group. (C) Immunostaining results revealed that LPS significantly decreased CRTC1 level in CA1 and dental gyrus regions, and nicotine treatment could inhibit the CRTC1 downregulation. n = 3 per group, scale bar = 100 µm.
Interaction of Microglia-Induced Inflammation and Downregulation of AMPK and CRTC1 in Neurons
It has been reported that LPS induced memory deficit by producing the pro-inflammatory cytokines in the central nervous system (Sparkman et al., 2006). Thus, LPS-induced downregulation of AMPK and CRTC1 in neurons may be the result of microglia cell activation. To investigate the relationship between LPS-induced inflammation in microglia cells and downregulation of AMPK and CRTC1 in neurons, we applied LPS (10 μg/mL) in microglia cells for 24 hours, then the cultured media was transferred to the culture medium of SH-SY5Y cells for 48 hours followed by detection of AMPK and CRTC1 with western blot. As shown in Figure 4A, AMPK in SH-SY5Y cells was significantly downregulated when exposed to LCM (F[1, 8] = 83.511, P = .001). A similar effect was seen in the level of CRTC1 (F[1, 11] = 28.309, P = .002; Figure 4B). When LPS was directly added to the culture medium of SH-SY5Y cells, the expression level of CRTC1 was not significantly altered (F[3, 9] = 0.111, P = .951; Figure 4C). It is noteworthy that the dose of LPS did not affect the viability of neurons or microglia cells (Shen et al., 2018).
Figure 4.
Interaction of microglia-induced inflammation and inactivation of adenosine monophosphate-activated protein kinase (AMPK) and downregulation of CREB-regulated transcription coactivator 1 (CRTC1) in neurons. (A) LPS-conditioned medium (LCM) but not LPS significantly inhibited AMPK activation in neurons. (B) LCM but not LPS significantly decreased the expression of CRTC1 in neurons. (C) LPS did not affect CRTC1 expression. (D) Immunostaining results revealed that LCM but not LPS significantly decreased the expression of CRTC1 in neurons. n = 3 per group, scale bar = 50 µm (left), scale bar = 20 µm (right).
To further clarify the interaction between microglia cells and neurons, we exposed neurons to the conditioned media from LPS-treated microglial cells for 24 hours. The immunostaining results showed that LPS had no significant effect on the protein level of CRTC1 in neurons (Figure 4D), while pretreatment neurons with LPS-conditioned media significantly decreased the protein level of CRTC1 in neurons (Figure 4D).
AMPK Activation by Metformin Upregulates CRTC1 Expression in Primary Neurons
To investigate the relationship between AMPK activation and CRTC1 expression, primary neurons were incubated for 6 hours with or without 2.5 mM metformin. The expression of p-AMPK and CRTC1 was examined by immunostaining (Figure 5A, C) and western blot (Figure 5B, D). Immunostaining results showed that metformin increased the p-AMPK/AMPK (Figure 5A) level accompanied by CRTC1 upregulation (Figure 5C) in primary neurons and the results were further confirmed by western blot. Treatment with metformin significantly upregulated the expression of p-AMPK (Figure 5B) and CRTC1 (Figure 5D).
Figure 5.
Effect of metformin (Met) on adenosine monophosphate-activated protein kinase (AMPK) activation and CREB-regulated transcription coactivator 1 (CRTC1) expression in neurons. Immunostaining (A) and western blot (B) results revealed that Met increased the p-AMPK/AMPK level, n = 3 per group. Immunostaining (C) and western blot (D) results showed that Met increased CRTC1 expression in primary neurons, n = 3 per group.
Metformin Treatment Prevents LPS-Induced Fear Memory Impairment
AMPK has been reported to be involved in the reconsolidation of fear memory. We intra-ICV infused an AMPK activator, metformin to investigate if metformin affects LPS-induced impairment of fear memory reconsolidation. The detailed experimental procedures were described in Figure 6A. As shown in Figure 6B, LPS impaired fear memory reconsolidation (F[1,55] = 2.507, P = .042, Testing 2) but not retrieval (F[1,55] = 1.595, P = .213, Testing 1). Furthermore, metformin significantly prevented LPS-induced impairment of fear memory reconsolidation (F[1,55] = 4.656, P = .014, Testing 2). In addition, metformin did not have a significant effect on fear memory retrieval (P > .05).
Figure 6.
Metformin prevented lipopolysaccharide (LPS)-induced fear memory impairment. (A) Diagram shows the experimental procedure. (B) LPS treatment significantly impaired fear memory reconsolidation (*P < .05 vs vehicle group), and intra-ICV administration of metformin significantly inhibited this effect (#P < .05 vs LPS group), n = 9–11 per group.
Metformin Prevents LPS-Induced Increase of IL-1β and TNF-α in Hippocampus
We next checked the expression of IL-1β and TNF-α after LPS and metformin treatment. Our results showed that LPS significantly increased the mRNA of IL-1β (F[1,17] = 4.870, P = .042; Figure 7A, right) and TNF-α (F[1,17] = 4.821, P = .043; Figure 7B, right) as well as the protein level of IL-1β (F[1,17] = 6.329, P = .023; Figure 7A, left) and TNF-α (F[1,17] = 4.650, P = .047; Figure 7B, left). Metformin could significantly alleviate the mRNA increase of IL-1β (F[1,17] = 12.366, P = .003; Figure 7A, right) and TNF-α (F[1,17] = 5.539, P = .032; Figure 7B, right) as well as the protein level of IL-1β (F[1,17] = 9.079, P = .008; Figure 7A, left) and TNF-α (F[1,17] = 11.567, P = .004; Figure 7B, left).
Figure 7.
Metformin prevented lipopolysaccharide (LPS)-induced increase of interleukin-1 beta (IL-1β) and tumor necrosis factor-α (TNF-α) and decrease of CRTC1 in hippocampus. (A) LPS-induced protein (left) and mRNA (right) increase of IL-1β and metformin significantly inhibited such effect, *P < .05 vs the vehicle group, #P < .05 vs the LPS group, n = 5 per group. (B) LPS-induced protein (left) and mRNA (right) increase of TNF-α and Met inhibited such effect. *P < .05 vs the vehicle group, #P < .05 vs the LPS group, n = 5 per group. (C) Representative western blot showed decreased CRTC1 expression after LPS treatment and metformin inhibited such effect (upper). *P < .05 vs vehicle group, #P < .05 vs LPS group, n = 5 per group, scale bar = 100 µm.
Metformin Prevents LPS-Induced Downregulation of CRTC1 Expression in Hippocampus
We also examined CRTC1 expression in hippocampus after LPS and metformin treatment. As shown in Figure 7C, LPS significantly downregulated CRTC1 expression in hippocampus (F[1,17] = 9.139, P = .008), and metformin inhibited such effect (F[1,17] = 4.965, P = .041).
Discussion
In the present study, we showed that acute nicotine treatment significantly decreased LPS-induced impairment of fear memory reconsolidation and significantly alleviated LPS-induced increase of IL-1β and TNF-α in hippocampus. Worthy of note, LPS decreased the activation of AMPK and level of CRTC1 in hippocampus but not amygdala or mPFC, and acute nicotine treatment or AMPK activation by ICV infusion of metformin significantly prevented this change, suggesting that nicotine protects against LPS-induced fear memory reconsolidation deficit by activating AMPK and upregulating CRTC1 in hippocampus (Figure 8).
Figure 8.
A schema for the proposed mechanism underlying nicotine’s effect on lipopolysaccharide (LPS)-induced impairment of fear memory reconsolidation. LPS impaired fear memory reconsolidation accompanied by increased interleukin-1 beta (IL-1β) and tumor necrosis factor-α (TNF-α), inhibition of adenosine monophosphate-activated protein kinase (AMPK) activation, and decreased CREB-regulated transcription coactivator 1 (CRTC1). Nicotine treatment improved fear memory reconsolidation through the activation of AMPK and upregulation of CRTC1.
Memory undergoes extinction or reconsolidation after retrieval. Extinction and reconsolidation are 2 competitive processes, both requiring protein synthesis and dissociable from each other: which one prevails depends on reminder duration (Nader, 2003; Pedreira and Maldonado, 2003). In our study, the maximum duration of retrieval was 300 seconds, so most likely reconsolidation but not extinction will happen after retrieval. In addition, it was demonstrated that inhibitory avoidance memory reconsolidation requires prior learning of relevant nonaversive information (Radiske et al., 2017). There is a habituation stage before training in our protocol; therefore, our testing would produce a reconsolidation but not an extinction process after memory retrieval.
Appropriate fear responses are adaptive, and disruption of fear memory can lead to disability to avoid danger (Flores et al., 2018). LPS administered 4 hours before training impaired spatial memory in the water maze task (Zarifkar et al., 2010). Our study revealed that LPS impaired fear memory reconsolidation but had no effect on memory retrieval. This is consistent with previous studies showing that immune activation induced by LPS did not impair the retrieval of spatial memory (Huang et al., 2010) but impaired fear memory reconsolidation (Kranjac et al., 2012). In addition, i.p. LPS administration given after conditioning impaired contextual but not auditory-cue fear conditioning (Pugh et al., 1998), suggesting a selective disruption of specific hippocampus-dependent memory function during acute neuroinflammation (Czerniawski et al., 2015). One may argue that if LPS only impairs reconsolidation, then there should be no deficit on day 9 in the absence of recall on day 8. We did not perform this experiment as it has been shown that LPS impaired memory consolidation processes when it was administered immediately or 2 hours, but not 12 hours after training (Kranjac et al., 2012).
Our study demonstrated that acute nicotine treatment alleviated LPS-induced impairment of fear memory reconsolidation, which is consistent with a previous study showing that acute nicotine treatment attenuated LPS-induced cognitive dysfunction by inhibiting neuroinflammation in hippocampus (Wei et al., 2015). In addition, nicotine could decrease the fear-related remote emotional memories (Cambiaghi et al., 2015), ameliorate ethanol-induced deficit in contextual fear conditioning (Gould and Lommock, 2003), attenuate the effect of HIV-1 proteins on the neural circuits of working and contextual memories (Nesil et al., 2015), and alleviate schizophrenia-like cognitive deficits induced by maternal LPS exposure (Waterhouse et al., 2016; Waterhouse et al., 2018). These results suggested that nicotine-derived compounds could work as therapeutic tools against fear memories and anxiety (Kutlu and Gould, 2015) as well as posttraumatic stress disorder (Barreto et al., 2015).
LPS has been shown to inhibit the activation of AMPK (Zhou et al., 2014; Wang et al., 2017), and AMPK has been a target to reduce neuroinflammation (Wang et al., 2018; Zhang et al., 2018). Our results showed that LPS decreased the level of AMPK in hippocampus but not mPFC or amygdala, and acute nicotine treatment inhibited this change accompanied by memory improvement, indicating that decreased AMPK in hippocampus may be involved in LPS-induced impairment of fear memory reconsolidation. This is in agreement with a previous study showing that inflammatory cytokine receptors are highly concentrated in areas associated with learning and memory, particularly in hippocampal regions (Shaw et al., 2001), and LPS-induced neuroinflammation in the adult brain may disrupt hippocampus-dependent learning and memory (Sparkman et al., 2006; Russo et al., 2011).
Our results showed that acute nicotine treatment increased the activation of AMPK, which is consistent with a previous study showing that AMPK was involved in the anti-inflammatory effect of nicotine in vivo and in vitro (Cheng et al., 2007). Nicotine has been shown to instigate the formation of abdominal aortic aneurysms (Wang et al., 2012) and induce insulin resistance (Wu et al., 2015) via activation AMPK in mice, indicating that nicotine may alleviate LPS-induced impairment of fear memory reconsolidation through activating AMPK.
LPS-induced inflammation has been demonstrated to cause memory impairment (Chen et al., 2008; Murray et al., 2012; Dehkordi et al., 2015) through pro-inflammatory cytokines (Kranjac et al., 2012; Machado et al., 2015). Here we showed that the increase in IL-1β and TNF-α is still present 24 hours after the recall on day 9; one may argue that the inflammation on day 9 may impair the memory recall. It has been shown that both central cytokine and peripheral cytokine and chemokine levels were heightened in LPS-treated animals 4 hours after LPS injection (Kranjac et al., 2012). However, LPS was given 4 hours before memory retrieval, and it did not affect memory retrieval (Testing 1). Furthermore, 24 hours after LPS treatment, IL-1β was significantly increased in hippocampus, suggesting that LPS-induced upregulation of IL-1β may play a role in AMPK decrease following LPS treatment and nicotine may regulate AMPK through affecting IL-1β.
Consistent with our previous study (Ni et al., 2019), our results showed that LPS induced a significant decrease of CRTC1 in hippocampus. It has been reported that increasing CRTC1 function in the dentate gyrus during memory formation or reactivation could increase memory strength (Sekeres et al., 2012). Reconsolidation is a process that requires gene expression and protein synthesis after the destabilization of the memory trace. CREB-mediated expression of proteins is central to both consolidation and reconsolidation of several types of memory (Alberini and Chen, 2012), the downstream mechanisms in the context of gene expression and protein synthesis relevant for synaptic plasticity that would be the result of impaired CRTC1 function mediated by LPS or neuroinflammation in general. In addition, function of CRTC1 during memory encoding is disrupted in neurodegeneration (Parra-Damas et al., 2017). Since CRTC1 has also been shown to play an important role in fear memory (Nonaka et al., 2014) and memory disorder in Alzheimer’s disease (Parra-Damas et al., 2017) and ischemic stroke-induced memory impairment (Zhang et al., 2019), CRTC1 could be a target to reduce LPS-induced fear memory impairment.
LPS-activated microglia has been shown to induce apoptosis (Dai et al., 2015), neurotoxicity (Alhadidi and Shah, 2018) in PC 12 cells, and neuroinflammatory toxicity in primary cortical neurons and Neuro-2A cells (Pan et al., 2008). In addition, LPS induced memory deficit by producing pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α, which directly affect synaptic plasticity and memory in passive avoidance and context discrimination in the central nervous system (Sparkman et al., 2006; Chen et al., 2008; Murray et al., 2012; Dehkordi et al., 2015). Thus, the downregulation of AMPK and CRTC1 by LPS may account for the activation of microglia. AMPK and CRTC1 in neurons were significantly downregulated when neurons were exposed to LPS-treated, microglia-cultured medium, indicating that LPS may induce memory deficit by activating the microglia cells.
Conclusion
Taken together, this study provides the first demonstration, to our knowledge, that acute nicotine treatment alleviates LPS-induced impairment of fear memory reconsolidation through activation of AMPK and upregulation of CRTC1 in hippocampus.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81870973, 81671145, 81701316, 81371224), by Jiangsu Provincial College of Natural Science research project (17KJB180012), by Suzhou Science and Technology for People’s Livelihood (sys2018025), by the Natural Science Foundation of Ningbo (2018A610288), by Natural Science Foundation of Zhejiang Province (LY19H090003), and by Natural Science Foundation of Zhejiang Province (LY19H090003).
Author Contributions
This work was performed and accomplished by all authors. H. Shu, M. Wang, M. Song, Y. Sun, and X. Shen contributed to the execution of the entire research project and the statistical analyses. H. Shu, Y. Sun, J. Zhang, and X. Jin wrote the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no competing interest.
Statement of Interest
None.
References
- Alberini CM, Chen DY (2012) Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci 35:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadidi Q, Shah ZA (2018) Cofilin mediates LPS-induced microglial cell activation and associated neurotoxicity through activation of NF-κB and JAK-STAT pathway. Mol Neurobiol 55:1676–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barini E, Antico O, Zhao Y, Asta F, Tucci V, Catelani T, Marotta R, Xu H, Gasparini L (2016) Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto GE, Yarkov A, Avila-Rodriguez M, Aliev G, Echeverria V (2015) Nicotine-derived compounds as therapeutic tools against post-traumatic stress disorder. Curr Pharm Des 21:3589–3595. [DOI] [PubMed] [Google Scholar]
- Besnard A, Caboche J, Laroche S (2012) Reconsolidation of memory: a decade of debate. Prog Neurobiol 99:61–80. [DOI] [PubMed] [Google Scholar]
- Burkewitz K, Morantte I, Weir HJM, Yeo R, Zhang Y, Huynh FK, Ilkayeva OR, Hirschey MD, Grant AR, Mair WB (2015) Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 160:842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambiaghi M, Grosso A, Renna A, Concina G, Sacchetti B (2015) Acute administration of nicotine into the higher order auditory Te2 cortex specifically decreases the fear-related charge of remote emotional memories. Neuropharmacology 99:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Uzgil B, Lin P, Avliyakulov NK, O’Dell TJ, Martin KC (2012) Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell 150:207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW (2008) Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Lee YM, Law KK, Lin CW, Yen MH (2007) The involvement of AMP-activated protein kinases in the anti-inflammatory effect of nicotine in vivo and in vitro. Biochem Pharmacol 74:1758–1765. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF (2015) Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun 44:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X, Zhang YM (2015) Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 20:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkordi NG, Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M (2015) Omega-3 fatty acids prevent LPS-induced passive avoidance learning and memory and CaMKII-α gene expression impairments in hippocampus of rat. Pharmacol Rep 67:370–375. [DOI] [PubMed] [Google Scholar]
- Flores A, Fullana MA, Soriano-Mas C, Andero R (2018) Lost in translation: how to upgrade fear memory research. Mol Psychiatry 23:2122–2132. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Zhang Y, Archbold G, Ishikawa R, Nader K, Kida S (2014) Enhancement of fear memory by retrieval through reconsolidation. Elife 3:e02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA (2003) Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci 117:1276–1282. [DOI] [PubMed] [Google Scholar]
- Han Y, Luo Y, Sun J, Ding Z, Liu J, Yan W, Jian M, Xue Y, Shi J, Wang JS, Lu L (2016) AMPK signaling in the dorsal hippocampus negatively regulates contextual fear memory formation. Neuropsychopharmacology 41:1849–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZB, Wang H, Rao XR, Liang T, Xu J, Cai XS, Sheng GQ (2010) Effects of immune activation on the retrieval of spatial memory. Neurosci Bull 26:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Sun Y, Xu J, Liu W (2015) Caveolin-1 mediates tissue plasminogen activator-induced MMP-9 up-regulation in cultured brain microvascular endothelial cells. J Neurochem 132:724–730. [DOI] [PubMed] [Google Scholar]
- Jin XC, Lu YF, Yang XF, Ma L, Li BM (2007) Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci 25:3702–3712. [DOI] [PubMed] [Google Scholar]
- Kida S. (2019) Reconsolidation/destabilization, extinction and forgetting of fear memory as therapeutic targets for PTSD. Psychopharmacology (Berl) 236:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H (2014) AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem 21:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranjac D, McLinden KA, Deodati LE, Papini MR, Chumley MJ, Boehm GW (2012) Peripheral bacterial endotoxin administration triggers both memory consolidation and reconsolidation deficits in mice. Brain Behav Immun 26:109–121. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ (2015) Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol 97:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima RH, Radiske A, Köhler CA, Gonzalez MC, Bevilaqua LR, Rossato JI, Medina JH, Cammarota M (2013) Nicotine modulates the long-lasting storage of fear memory. Learn Mem 20:120–124. [DOI] [PubMed] [Google Scholar]
- Liu W, Furuichi T, Miyake M, Rosenberg GA, Liu KJ (2007) Differential expression of tissue inhibitor of metalloproteinases-3 in cultured astrocytes and neurons regulates the activation of matrix metalloproteinase-2. J Neurosci Res 85:829–836. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu WC, Sun Y, Shen X, Wang X, Shu H, Pan R, Liu CF, Liu W, Liu KJ, Jin X (2017) Normobaric hyperoxia extends neuro- and vaso-protection of N-acetylcysteine in transient focal ischemia. Mol Neurobiol 54:3418–3427. [DOI] [PubMed] [Google Scholar]
- Luo H, Liu Z, Liu B, Li H, Yang Y, Xu ZD (2019) Virus-mediated overexpression of ETS-1 in the ventral hippocampus counteracts depression-like behaviors in rats. Neurosci Bull 35:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv G, Sun D, Zhang J, Xie X, Wu X, Fang W, Tian J, Yan C, Wang H, Fu F (2017) Lx2-32c, a novel semi-synthetic taxane, exerts antitumor activity against prostate cancer cells in vitro and in vivo. Acta Pharm Sin B 7:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado I, Gonzalez PV, Vilcaes A, Carniglia L, Schiöth HB, Lasaga M, Scimonelli TN (2015) Interleukin-1β-induced memory reconsolidation impairment is mediated by a reduction in glutamate release and zif268 expression and α-melanocyte-stimulating hormone prevented these effects. Brain Behav Immun 46:137–146. [DOI] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A (2011) Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470:404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C (2012) Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging 33:603–616.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. (2003) Memory traces unbound. Trends Neurosci 26:65–72. [DOI] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, Li MD (2015) Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Huang H, He D, Chen H, Wang C, Zhao X, Chen X, Cui W, Zhou W, Zhang J (2019) Adeno-associated virus-mediated over-expression of CREB-regulated transcription coactivator 1 in the hippocampal dentate gyrus ameliorates lipopolysaccharide-induced depression-like behaviour in mice. J Neurochem 149:111–125. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Kim R, Fukushima H, Sasaki K, Suzuki K, Okamura M, Ishii Y, Kawashima T, Kamijo S, Takemoto-Kimura S, Okuno H, Kida S, Bito H (2014) Region-specific activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron 84:92–106. [DOI] [PubMed] [Google Scholar]
- Nuzzo T, Feligioni M, Cristino L, Pagano I, Marcelli S, Iannuzzi F, Imperatore R, D’Angelo L, Petrella C, Carella M, Pollegioni L, Sacchi S, Punzo D, De Girolamo P, Errico F, Canu N, Usiello A (2019) Free d-aspartate triggers NMDA receptor-dependent cell death in primary cortical neurons and perturbs JNK activation, Tau phosphorylation, and protein SUMOylation in the cerebral cortex of mice lacking d-aspartate oxidase activity. Exp Neurol 317:51–65. [DOI] [PubMed] [Google Scholar]
- Pan XD, Chen XC, Zhu YG, Zhang J, Huang TW, Chen LM, Ye QY, Huang HP (2008) Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochem Pharmacol 76:362–372. [DOI] [PubMed] [Google Scholar]
- Parra-Damas A, Chen M, Enriquez-Barreto L, Ortega L, Acosta S, Perna JC, Fullana MN, Aguilera J, Rodríguez-Alvarez J, Saura CA (2017) CRTC1 function during memory encoding is disrupted in neurodegeneration. Biol Psychiatry 81:111–123. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H (2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38:863–869. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW (1998) Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun 12:212–229. [DOI] [PubMed] [Google Scholar]
- Radiske A, Gonzalez MC, Conde-Ocazionez SA, Feitosa A, Köhler CA, Bevilaqua LR, Cammarota M (2017) Prior learning of relevant nonaversive information is a boundary condition for avoidance memory reconsolidation in the rat hippocampus. J Neurosci 37:9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami F, Oryan S, Ahmadiani A, Dargahi L (2012) Morphine preconditioning protects against LPS-induced neuroinflammation and memory deficit. J Mol Neurosci 48:22–34. [DOI] [PubMed] [Google Scholar]
- Russo I, Barlati S, Bosetti F (2011) Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem 116:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA, Frankland PW, Josselyn SA (2012) Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 32:17857–17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM (2001) Lipopolysaccharide causes deficits in spatial learning in the water maze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res 124:47–54. [DOI] [PubMed] [Google Scholar]
- Shen X, Sun Y, Wang M, Shu H, Zhu LJ, Yan PY, Zhang JF, Jin X (2018) Chronic N-acetylcysteine treatment alleviates acute lipopolysaccharide-induced working memory deficit through upregulating caveolin-1 and synaptophysin in mice. Psychopharmacology (Berl) 235:179–191. [DOI] [PubMed] [Google Scholar]
- Shu H, Zheng GQ, Wang X, Sun Y, Liu Y, Weaver JM, Shen X, Liu W, Jin X (2015) Activation of matrix metalloproteinase in dorsal hippocampus drives improvement in spatial working memory after intra-VTA nicotine infusion in rats. J Neurochem 135:357–367. [DOI] [PubMed] [Google Scholar]
- Skelly DT, Griffin ÉW, Murray CL, Harney S, O’Boyle C, Hennessy E, Dansereau MA, Nazmi A, Tortorelli L, Rawlins JN, Bannerman DM, Cunningham C (2019) Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry 24:1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW (2006) Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci 26:10709–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanovic D, Janjetovic K, Misirkic M, Vucicevic L, Sumarac-Dumanovic M, Micic D, Starcevic V, Trajkovic V (2012) Intracerebroventricular administration of metformin inhibits ghrelin-induced hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology 96:24–31. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen X, Zhang X, Shen X, Wang M, Wang X, Liu WC, Liu CF, Liu J, Liu W, Jin X (2017) β2-adrenergic receptor-mediated HIF-1α upregulation mediates blood brain barrier damage in acute cerebral ischemia. Front Mol Neurosci 10:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Huang F, Li P, Li Z, Zhou S, Deng H, Yang Y (2011) Nicotine enhances contextual fear memory reconsolidation in rats. Neurosci Lett 487:368–371. [DOI] [PubMed] [Google Scholar]
- Tronel S, Alberini CM (2007) Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry 62:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang C, Zhang M, Liang B, Zhu H, Lee J, Viollet B, Xia L, Zhang Y, Zou MH (2012) Activation of AMP-activated protein kinase α2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat Med 18:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xue GX, Liu WC, Shu H, Wang M, Sun Y, Liu X, Sun YE, Liu CF, Liu J, Liu W, Jin X (2017) Melatonin alleviates lipopolysaccharide-compromised integrity of blood-brain barrier through activating AMP-activated protein kinase in old mice. Aging Cell 16:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ruan W, Mi J, Xu J, Wang H, Cao Z, Saavedra JM, Zhang L, Lin H, Pang T (2018) Balasubramide derivative 3C modulates microglia activation via CaMKKβ-dependent AMPK/PGC-1α pathway in neuroinflammatory conditions. Brain Behav Immun 67:101–117. [DOI] [PubMed] [Google Scholar]
- Waterhouse U, Roper VE, Brennan KA, Ellenbroek BA (2016) Nicotine ameliorates schizophrenia-like cognitive deficits induced by maternal LPS exposure: a study in rats. Dis Model Mech 9:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse U, Brennan KA, Ellenbroek BA (2018) Nicotine self-administration reverses cognitive deficits in a rat model for schizophrenia. Addict Biol 23:620–630. [DOI] [PubMed] [Google Scholar]
- Wei P, Liu Q, Li D, Zheng Q, Zhou J, Li J (2015) Acute nicotine treatment attenuates lipopolysaccharide-induced cognitive dysfunction by increasing BDNF expression and inhibiting neuroinflammation in the rat hippocampus. Neurosci Lett 604:161–166. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, Lu Q, Ouyang C, Xie Z, Zhao Z, Zhuo X, Viollet B, Foretz M, Wu J, Yuan Z, Zou MH (2015) Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med 21:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue ZC, Wang C, Wang QW, Zhang JF (2015) CREB-regulated transcription coactivator 1: important roles in neurodegenerative disorders. Sheng Li Xue Bao 67:155–162. [PubMed] [Google Scholar]
- Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, Rastegar K, Moosavi M (2010) Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol 634:84–88. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shen X, Dong J, Liu WC, Song M, Sun Y, Shu H, Towse CL, Liu W, Liu CF, Jin X (2019) Inhibition of reactive astrocytes with fluorocitrate ameliorates learning and memory impairment through upregulating CRTC1 and synaptophysin in ischemic stroke rats. Cell Mol Neurobiol 39:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH (2018) Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immun 70:179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Cao Y, Ao G, Hu L, Liu H, Wu J, Wang X, Jin M, Zheng S, Zhen X, Alkayed NJ, Jia J, Cheng J (2014) CaMKKβ-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid Redox Signal 21:1741–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Breslau N, Brown RA, George TP, Williams J, Calhoun PS, Riley WT (2008) Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res 10:1691–1715. [DOI] [PubMed] [Google Scholar]