Visual Abstract
Abstract
Novel agents, including Bruton’s tyrosine kinase inhibitors (BTKi; ibrutinib, acalabrutinib), venetoclax, and phosphatidylinositol 3-kinase inhibitors (PI3Ki; idelalisib, duvelisib), have fundamentally changed the chronic lymphocytic leukemia (CLL) treatment landscape, allowing for a chemotherapy-free paradigm for many. Randomized trials that demonstrated efficacy of these agents in the relapsed/refractory setting rarely included patients with prior novel agent exposure. Herein, we review available data, including single-arm prospective studies and retrospective cohorts, on outcomes for novel agent approaches after novel agent exposure. We examine data for subsequent treatment options in 3 specific scenarios: (1) progression of disease while receiving BTKi, (2) progression of disease after discontinuation of BTKi for intolerance, and (3) after treatment with venetoclax. Data are most robust for venetoclax-based regimens after progression on BTKi. For patients who experience progression of disease after discontinuation of BTKi for intolerance, venetoclax-based regimens and retreatment with BTKi (depending on severity of initial intolerance) are 2 data-driven options. After frontline venetoclax/obinutuzumab, subsequent treatment approaches depend on whether patients experience progression of disease during or after discontinuation of their fixed duration frontline regimen and whether venetoclax/obinutuzumab was discontinued for intolerance. After progression of disease while on venetoclax, we recommend BTKi as second-line therapy. For patients who experience progression after completion or premature discontinuation (because of intolerance) of fixed duration venetoclax/obinutuzumab, either BTKi or retreatment with venetoclax (with aggressive supportive care if prior intolerance) are reasonable considerations. Subsequent lines of therapy in these scenarios include PI3Ki and consideration of cellular therapies. Finally, clinical trial enrollment for interested patients in any line of therapy is recommended.
Learning Objectives
Compare treatment strategies for patients with CLL requiring therapy after progression on BTK inhibitor or discontinuation for toxicity
Understand data for efficacy of novel agents in the treatment of CLL after discontinuation of venetoclax.
Introduction
Based on promising results from trials examining chemotherapy-free regimens in the front-line setting, Bruton’s tyrosine kinase inhibitors (BTKi) with or without anti-CD20 monoclonal antibodies and venetoclax/obinutuzumab are increasingly being used as first chronic lymphocytic leukemia (CLL)-directed therapy.1-3 This paradigm shift in first-line treatment has altered the therapeutic landscape. As such, optimal sequencing of therapies within a chemotherapy-free paradigm has and will continue to become a pressing issue in the care of patients with relapsed/refractory (R/R) CLL.
Novel agents approved in the R/R setting include ibrutinib, acalabrutinib, idelalisib + rituximab, duvelisib, and venetoclax ± rituximab. Although these agents have demonstrated efficacy in R/R cohorts, the studies that led to their approvals largely examined patients who had received prior chemoimmunotherapy and rarely prior novel agents (Table 1).4-10 As patients receiving novel agents often do well for extended periods of time, data regarding efficacy of novel agents in exclusively novel agent-treated patient populations is limited. Therefore, the sequences being explored are partially a consequence of the order in which agents were approved rather than intrinsic tumor biology. How readily data from chemoimmunotherapy exposed patient cohorts can be extrapolated to patients exclusively treated with novel agents remains to be seen, particularly given potential for differences in accumulated toxicity and resistance mechanisms.
Table 1.
Completed phase 3 clinical trials examining chemotherapy-free regimens vs standard of care in relapsed/refractory CLL
| Novel agent | Control arm | Prior lines of therapy in novel agent arm, median (range) | Patients with prior novel agent exposure | Outcomes (novel agent vs control arm) |
|---|---|---|---|---|
| Ibrutinib5 | Ofatumumab | 3 (1-12) | Not reported | ORR: 91% for ibrutinib |
| Median PFS: 44.1 vs 8.1 mo | ||||
| Median OS: 67.7 vs 65.1 mo | ||||
| Acalabrutinib6 | Investigator’s choice: bendamustine or idelalisib/rituximab | 1 (1-8) | Patients with prior BCRi or venetoclax were excluded | ORR: 81 vs 76% |
| 1-y PFS: 88% vs 68% vs 69% | ||||
| Idelalisib/rituximab7 | Placebo/rituximab | 3 (1-12) | None; prior BTK or PI3Ki as exclusion criteria | ORR: 81% vs 13% |
| 6-mo PFS: 93% vs 46% | ||||
| 1-y OS: 92% vs 80% | ||||
| Duvelisib8 | Ofatumumab | 2 (1-10) | None; prior BTK or PI3Ki as exclusion criteria | ORR: 74% vs 45% |
| Median PFS: 13.3 vs 9.9 mo | ||||
| Venetoclax/rituximab9,10 | Bendamustine | 1 (1->3) | BCRi in 5 patients (2.6%) | ORR 92% vs 72% |
| 3-y PFS: 71% vs 15% | ||||
| 3-y OS: 88% vs 80% |
Although many questions regarding the optimal sequence of novel agents in a chemotherapy-free treatment sequence remain, we will review the available data regarding treatment approaches for patients based on first novel agent exposure and outline potential therapeutic pathways.
Clinical case 1
A 74-year-old man with CLL presents for follow-up. He was diagnosed at 70 years of age after presenting to his primary care physician for fatigue, at which time his complete blood count (CBC) showed lymphocytosis with a white blood cell count of 18.8 × 109/L, absolute B-lymphocyte count (ALC) of 11.6 × 109/L, hemoglobin of 9.4 g/dL, and platelet count of 89 × 109/L. He was noted to have firm, discrete, nontender, and freely mobile lymph nodes ranging from 1 to 4 cm (bidimensional) in the cervical, supraclavicular, and axillary chains and splenomegaly to 3 cm below the costal margin. Prognostic testing at the time of diagnosis showed unmutated immunoglobulin heavy chain variable region gene (IGVH), fluorescence in situ hybridization (FISH) with deletion of chromosome 11q [del(11q)], and no TP53 mutation by next-generation sequencing. Given that he had both anemia and thrombocytopenia, he met criteria for therapy per the International Workshop on Chronic Lymphocytic Leukemia11 and was started on ibrutinib 420 mg oral once daily.
He achieved partial remission (PR) with lymphocytosis after 6 months and PR after 15 months of ibrutinib therapy with reduced size of lymph nodes in all chains and normalization of hemoglobin and platelet count. At today’s visit, he has been on ibrutinib 420 mg daily for 48 months and is tolerating treatment well. However, over the past 6 weeks, he has noted reappearance of lymph nodes in the cervical, axillary, and inguinal chains with development of fatigue. CBC today shows white count of 79.4 × 109/L, ALC of 73.6 × 109/L, hemoglobin of 9.8 g/dL, and platelet count of 80 × 109/L. Lactate dehydrogenase is within the normal range. There is no evidence of autoimmune hemolytic anemia.
Treating relapsed disease after progression on BTKi
For patients experiencing progression while on BTKi therapy, discontinuation of drug can lead to tumor flare, which can be difficult to control and life threatening.12 As such, BTKi can be continued despite progression until the next therapy is ready to be administered. In some scenarios, a brief period of overlapping therapy or bridging to next therapy with steroids may also be warranted.13 If CLL is behaving aggressively or Richter’s transformation is suspected, positron emission tomography (PET)/computed tomography (CT) is recommended for initial evaluation. However, in the setting of progression on B-cell receptor inhibitor (BCRi), sensitivity and specificity of PET is diminished (71% and 50% for lesions with standardized uptake value ≥ 10, respectively), and biopsy is warranted if suspicious lesions are present.14 Characterization of Richter’s transformation through pathologic features is not impacted by prior chemoimmunotherapy vs novel agents.15
For all patients in whom therapy is anticipated in the R/R setting, assessment of clonal evolution through FISH and TP53 mutational testing is indicated. Resistance mutations, including mutations in BTK and 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase γ-2 (PLCγ2) for patients on BTKi and BCL2 for patients on venetoclax, have been described in patients progressing on targeted agents, although testing for these mutations is not routinely performed at this time,16-21 likely because of limited availability. Furthermore, selection of the subsequent line of therapy is not affected by results of these tests based on currently approved agents, although understanding mutational profile may eventually inform treatment selection as we further understand efficacy of new agents in the setting of these mutations.
Although initial reports of progression on BTKi described a poor prognosis,12,22 use of BTKi in earlier lines of therapy and development of additional classes of effective novel agents have significantly improved the outlook for patients experiencing progression of CLL while on BTKi.23,24 Selection of agents after progression on frontline ibrutinib is informed by a series of prospective and retrospective real-world studies of patients treated with ibrutinib in the R/R setting and subsequently treated with novel agents (Table 2). Robust data on selection of therapy in a non–chemotherapy-exposed cohort is not available at this time.
Table 2.
Available evidence for standard of care treatment strategies after BTKi discontinuation for CLL progression or intolerance
| Subsequent therapy | Study design | Number of patients in group of interest | Clinical setting | Prior therapies, median (range) | ORR | Progression data on subsequent therapy | Survival data on subsequent therapy |
|---|---|---|---|---|---|---|---|
| Venetoclax | Prospective24 | 91 | Progression on ibrutinib (n = 50), progression following ibrutinib discontinuation (n = 41) reasons for ibrutinib discontinuation: intolerance (n = 30), achievement of maximal benefit on ibrutinib (n = 6), completion of defined ibrutinib course (n = 3), unspecified (n = 2) | 4 (1-15) | 65% (63% in patients with prior ibrutinib intolerance, 54% for progression on ibrutinib) | 1-y PFS: 75% median PFS: 24.7 mo | 1-y OS: 91% |
| Retrospective26 | 13 | KI discontinuation (progression or intolerance) | Not reported | 76% | Not reported | Not reported | |
| Retrospective27 | Not reported | BCRi discontinuation (progression or intolerance) | Not reported | 74% | 24-mo PFS: 75% | Not reported | |
| Retrospective | 115 | Prior ibrutinib | 3 (0-11) | 69% | 12-mo PFS: 68% For entire cohort of 141 venetoclax treated patients, prior BTKi was not associated with inferior PFS | 12-mo OS: 88% For entire cohort of 141 venetoclax treated patients | |
| Retrospective43 | 62 post-BTKi alone, 10 post-BTKi and PI3Ki | BTKi discontinuation (progression or intolerance) | 3 (1-15) post BTKi alone, 5 (3-15) post BTKi and PI3Ki | 85% in post-BTKi alone, 80% in post BTKi and PI3Ki | 1-y PFS 65% Estimated for entire cohort, prior exposure to BTKi was not significantly associated with inferior PFS | 1-y OS 75% | |
| Median OS 61% Estimated for entire cohort, prior exposure to BTKi was not significantly associated with inferior OS | |||||||
| Acalabrutinib | Prospective23 | 33 | Ibrutinib intolerance | 4 (2-13) | 76% | 1-y PFS: 83% | Not reported |
| Idelalisib | Retrospective26 | 16 | Ibrutinib discontinuation (progression or intolerance) | Not reported | 28% | Not reported | Not reported |
| Retrospective27 | Not reported | Ibrutinib discontinuation (progression or intolerance) | Not reported | 46% | Median PFS: 9 mo | Not reported | |
| Umbralisib | Prospective38 | 44 | BTKi intolerant | 2 (1-7) | Not reported | Median PFS: 23.5 mo For entire cohort of 51 patients, including 7 with prior PI3Ki intolerance | Not reported |
With a median of 4 prior lines of therapy (range, 1-15), a phase 2 clinical trial examined efficacy of venetoclax as a continuous monotherapy in 91 patients who had been previously treated with ibrutinib and progressed before the start of venetoclax. Venetoclax monotherapy was associated with an overall response rate (ORR) of 65%. Regarding outcomes, 1-year progression-free survival (PFS) and overall survival (OS) were estimated to be 75% and 91%, respectively.24 Venetoclax in combination with rituximab for a 2-year fixed duration was studied in the phase 3 MURANO study, which demonstrated the efficacy of this regimen in patients who had received 1 to 3 prior therapies with an ORR of 92% and 3-year PFS and OS of 71% and 88%, respectively. Although only 5 patients (2.6%) in this study had received prior BCRi, results of this study have been applied widely to patients progressing on ibrutinib in clinical practice, and therefore the combination of venetoclax and rituximab after progression on BTKi is also a reasonable choice.9,10 In patients treated with venetoclax and rituximab, achieving deep responses with undetectable minimal residual disease (U-MRD) at the completion of therapy is associated with improved PFS.9 The optimal approach for patients with residual detectable disease at the end of planned fixed-duration therapy requires further investigation.
A pooled analysis of 4 clinical trials examining venetoclax with or without rituximab in the R/R setting included 436 patients, of whom 149 were BCRi exposed (115 refractory, 34 nonrefractory). ORR for the entire cohort was 75% with a complete remission (CR) rate of 22% and median PFS of 30.2 months. Refractoriness to BCRi was associated with increased risk of failure to respond (odds ratio, 2.3; 95% confidence interval [CI], 1.4-3.7), failure to achieve CR (odds ratio, 4.8; 95% CI, 2.3-9.9), and relapse (hazard ratio, 1.9; 95% CI, 1.2-3.1). Although duration of response was shorter for BCRi-refractory patients independent of depth of response, many of these patients were heavily pretreated (median number of prior therapies, 3; range, 1-15) and had additionally received chemoimmunotherapy.25
A retrospective cohort study described 187 patients who had discontinued a BCRi (143 ibrutinib and 35 idelalisib), of whom 114 required subsequent therapy. This included 13 patients treated with venetoclax after discontinuation of either ibrutinib or idelalisib, in whom ORR was 76%. The study further describes that patients treated with idelalisib after ibrutinib discontinuation (n = 16) had an ORR of 28%.26 In a cohort of 683 patients treated with BCRi (91% ibrutinib, 9% idelalisib) or venetoclax, 167 (24%) received a subsequent therapy after BCRi discontinuation. ORR to venetoclax after BCRi discontinuation (for progression or toxicity) was 74%, whereas ORR to idelalisib after ibrutinib discontinuation was 46%. Both of these approaches were associated with superior PFS compared with chemoimmunotherapy in this setting (median PFS, 5.1 months; P < .001; ORR, 50%).27
After progression on venetoclax, retrospective data suggest low response rates when retreating with BTKi if patients have previously experienced progression on a BTKi.28 Additional US Food and Drug Administration (FDA)-approved novel agents for the treatment of R/R CLL are idelalisib with rituximab and duvelisib. These agents were approved based on studies that did not include BCRi- or venetoclax-treated patients.7,8 Therefore, all data regarding their efficacy in novel agent exposed patients come from retrospective studies. In a phosphatidylinositol 3-kinase inhibitor (PI3Ki)-naïve but BTKi-exposed (intolerant or resistant) patient population who discontinued venetoclax, ORR to PI3Ki was 47% with short median PFS (6 months).28 Based on current limited retrospective data, absence of prospective studies, and hypothesized overlapping mechanisms of resistance, responses to PI3Ki are not expected to be durable in patients who are double refractory to BTKi and venetoclax. Patients who are BTKi and venetoclax exposed are considered high risk, and therefore, consideration of cellular therapy (allogeneic stem cell transplant or chimeric antigen receptor T [CAR-T] cell therapy) or enrollment in a clinical trial is appropriate for these patients.
Although data on allogeneic stem cell transplant in patients previously exposed to novel agents are limited,29 current consensus guidelines recommend consideration of cellular immunotherapy (transplant or CAR-T) for patients considered high risk. This is defined as any patient with R/R CLL after chemoimmunotherapy, responding to BTKi or venetoclax with high risk features (TP53 aberration, complex karyotype, multiple lines) and low cellular immunotherapy risk; with nonresponse to first novel agent; or refractory to 2 novel agents.30 In a cohort of 48 patients with CLL previously treated with a median of 2 (range, 1-9) lines of therapy before ibrutinib who subsequently underwent allogeneic stem cell transplant, 12-month PFS was 60% and OS was 72%. Compared with series of ibrutinib-naïve patients undergoing transplant, prior ibrutinib did not appear to adversely affect the safety or efficacy of transplant. Given that many patients with CLL have advanced age and comorbidities, CAR-T therapy, often associated with less morbidity and mortality, provides an appealing cellular immunotherapy option. Twenty-four patients with CLL resistant to ibrutinib subsequently received CD19-directed CAR-T cells and experienced an ORR of 71% and CR rate of 17%.31 TRANSCEND CLL 004, an ongoing study, has reported an ORR of 87% with CR rate of 47% and U-MRD rate of 47% in 16 patients treated with the CD19-directed CAR-T lisocabtagene maraleucel in patients with R/R CLL previously exposed to BTKi.32 Ibrutinib has further been studied as a tool for enhanced response to or persistence of CAR-T cells given its immune modulatory effects. Ibrutinib exposure appears to result in greater ex vivo T-cell expansion and higher ORR.33 Despite the risks associated with cellular therapy, the potential for durable remission may outweigh risks for patients with high-risk disease and should be considered. Current studies are examining CD19-directed CAR-T cells in patients with failure of or incomplete response to ibrutinib and/or other novel agents (NCT03331198, NCT03960840, NCT03676504, NCT03085173) and CD19 CAR-T cells, CD19/CD20 CAR-T cells, CD19/CD20 or CD22, CD19/CD28 CAR-T cells, CD20 CAR-T cells, or γδT cells in patients with R/R CLL with or without prior novel agent exposure. Allogeneic cell sources are also being explored in patients with R/R CLL with or without prior novel agent exposure (NCT03881774, NCT03056339).
Agents with alternate mechanisms of action are currently under investigation with promising preliminary data. Two promising noncovalent reversible BTKi (LOXO-305 and ARQ 531) have reported data from small cohorts treated in early-phase clinical trials. The phase 1 clinical trial of LOXO-305 has reported outcomes of 9 patients with CLL, of whom 7 had received prior ibrutinib and 5 had prior PI3Ki. All CLL patients with available response assessments had documented response, including 1 with BTK C481S mutation.34 Results from the phase 1 study of ARQ 531 demonstrated acceptable safety and evidence of efficacy with PR in 7 of 26 (27%) patients who had CLL, of whom 85% had documented BTK C481S mutations.35 Enrolling clinical trials are additionally examining other potential mechanisms for treating CLL, including inhibition of Syk, ATR, MALT1, STAT3, CDK, JAK1, MELK, PKC-B, XPO1, NEDD8-activating enzyme, and checkpoints; monoclonal antibodies targeting CD38, CD32-b, ROR1, FcγRIIB, Mcl-1, PSMB5, and B-cell activating factor; bispecific T-cell engagers; peptide vaccination; and combination therapies, among others.
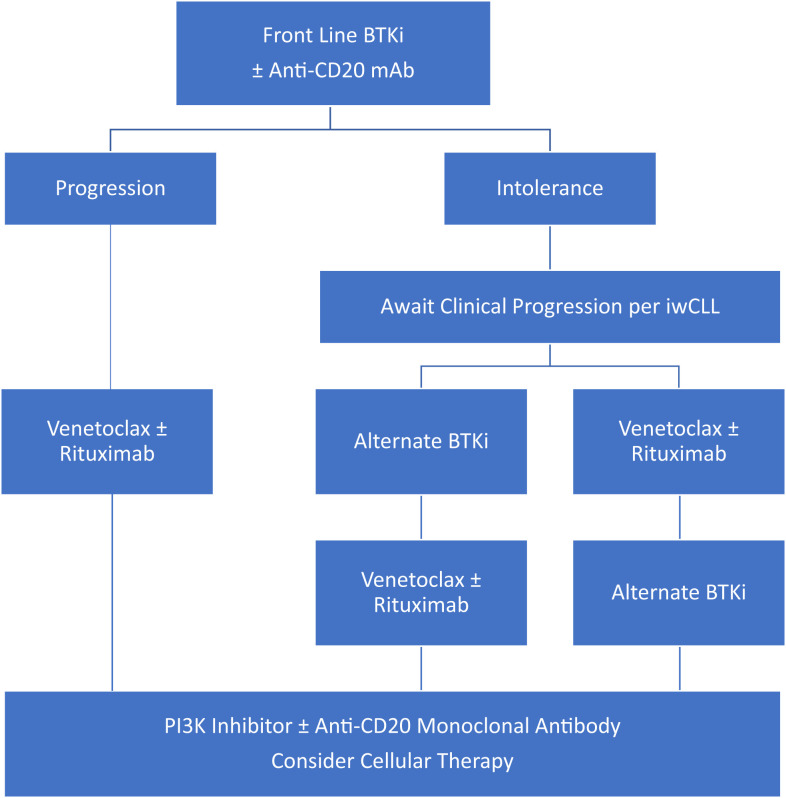
Recognizing limitations of applying available data to patients who have received only 1 prior line of therapy, venetoclax ± rituximab after BTKi failure appears to produce higher response rates and improved outcomes than other standard of care options (PI3Ki, anti-CD20 monoclonal antibodies, or chemoimmunotherapy). For patients treated with a BTKi in the frontline setting, we recommend second-line treatment with venetoclax as a continuous therapy or venetoclax with rituximab as a 2-year fixed duration therapy as standard of care options, with selection between these regimens dependent on patient preference. Alternately, enrollment in clinical trials should be considered if available and of interest to the patient. Subsequent lines of therapy may include PI3K inhibitors (FDA approved but associated with lower response rates, shorter durations of response, significant risk of adverse effects, and limited data in patients previously exposed to novel agents), cellular therapies (depending on availability and patient age/comorbidities), and clinical trial enrollment (Figure 1).
Figure 1.
Proposed treatment algorithms after frontline BTK inhibitor for CLL.
Treating relapsed disease after intolerance to BTKi
Although there is no standard definition of intolerance, real-world evidence suggests that intolerance, rather than progression, is the most common reason for ibrutinib discontinuation.36 With 6 years of follow-up for patients treated in the RESONATE trial, 16% had discontinued ibrutinib because of an adverse event, whereas retrospective series have suggested 20% discontinued for intolerance.5,36 These data suggest that discontinuation of ibrutinib for adverse event is a commonly encountered clinical scenario.
For all patients in whom therapy for R/R CLL is being considered, it is first important to recognize that CLL may not require therapy immediately on progression. Instead, therapy should be initiated when CLL becomes symptomatic, including development of disease-related symptoms, anemia, thrombocytopenia, or massive or symptomatic splenomegaly, lymphadenopathy, or extranodal involvement.11
Patients with PR or CR may be able to discontinue therapy without immediate or significant disease progression, thus allowing for a treatment-free interval. For instance, among 354 patients who received ibrutinib/rituximab through the E1912 trial, 95 have discontinued ibrutinib (51% for adverse effects, 24% for progression, 25% for other reasons). For patients who had discontinued ibrutinib, a median of 23 months elapsed from discontinuation to disease progression.37
Once a patient requires treatment, providers must first determine their comfort with retreating patients with an alternate BTKi dependent on the reason for initial intolerance. Although some patients discontinue BTKi for persistently bothersome, but not life-threatening, adverse events, others have more substantial adverse events (ie, major bleeding, cardiac arrythmia) in which retreatment with an alternate BTKi is not deemed safe.
A study of 33 patients with ibrutinib intolerance examined the efficacy of acalabrutinib (a second-generation, highly specific BTKi), which results in an ORR of 76% with estimated 1- and 2-year PFS of 83% and 76%, respectively.23 In this series, the overall discontinuation rate of acalabrutinib was 12%. For patients who experienced nonsevere toxicity with their initial BTKi, changing to an alternate BTKi is likely to produce response, and toxicity may not recur. For patients in whom BTKi associated toxicity was severe, venetoclax-based regimens are often a more appropriate second-line therapy.24 Additionally, PI3Ki have been examined in BTKi intolerant patients. A phase 2 trial examined the safety and efficacy of the PI3Ki umbralisib in patients with BCRi intolerance (44 with ibrutinib intolerance, 7 with idelalisib intolerance) and demonstrated minimally overlapping toxicity profile (4 patients had recurrent toxicity, 1 required dose modification) and median PFS of 23.5 months.38 Retrospective cohorts examining idelalisib after ibrutinib discontinuation (for intolerance or progression) have reported an ORR of 28% to 46%.26,27 Thus, PI3Ki have data to support their use in this setting as well.
Clinical case 2
A 57-year-old man was diagnosed with CLL after presenting to his primary care physician with cervical lymphadenopathy and cough. A CBC showed a white blood cell count of 32.7 × 109/L, ALC of 31.1 × 109/L, hemoglobin of 11.0 g/dL, and platelet count of 125 × 109/L. Flow cytometric analysis of peripheral blood confirmed the diagnosis of CLL. Given that cough was a presenting symptom, a CT scan was obtained and demonstrated bulky hilar adenopathy, as well as bulky adenopathy throughout his abdomen and pelvis, up to 12 cm. IGVH was unmutated, FISH was without abnormalities, and next-generation sequencing revealed TP53 mutation. Given the desire to pursue time-limited therapy, the decision was made to treat with a 1-year fixed-duration of venetoclax and obinutuzumab.3 He was hospitalized for dose escalation per the venetoclax FDA package insert and tolerated therapy well without any complications.
At the end of 1 year of therapy, he had achieved CR with detectable MRD (0.45% of the peripheral blood). CBC at completion of therapy was consistent with CR, showing a white blood cell count of 6.4 × 109/L, ALC of 1.3 × 109/L, hemoglobin of 15.4 g/dL, and platelet count of 165 × 109/L. A CT scan showed resolution of all prior lymphadenopathy.
Twenty-eight months after completion of therapy, he returned to clinic with fatigue and night sweats. CBC showed development of anemia with hemoglobin of 9.6 g/dL. PET was performed and showed bulky adenopathy above and below the diaphragm, although no lesion had a standardized uptake value greater than 4.0. He presents to discuss therapeutic options.
Treating relapsed disease after progression on or after a venetoclax-based regimen
Venetoclax and obinutuzumab as a 1-year fixed duration combination regimen was FDA approved as a first-line regimen for CLL in 2019 based on findings from the CLL14 study.3 Notably, this regimen appears to produce less durable remission for those with detectable MRD (vs U-MRD), and 6 of 14 early relapses after venetoclax discontinuation in CLL14 had aberration in TP53. Even with this in mind, the number of patients who have received 1 year of fixed duration therapy and subsequently progressed to the point of requiring therapy is low at this time. Thus, data regarding efficacy of novel agents in this setting are limited, particularly in a population who had not previously received chemoimmunotherapy. To inform decision making about treatment in this case, data are extrapolated from data regarding patients who have progressed on venetoclax-based regimens in R/R settings.
The initial phase 1b study examining the safety of venetoclax/rituximab allowed for protocol-guided drug discontinuation for patients who achieved CR or U-MRD in the bone marrow compartment. This study included 49 patients, of whom 13 stopped therapy (2 with CR but detectable MRD, 11 with U-MRD). Both patients with detectable MRD experienced progression of disease after 24 months and subsequently responded to venetoclax retreatment.39 Of the 194 patients treated with venetoclax-rituximab in the phase 3 MURANO study, data regarding response to subsequent therapy are available for 22 patients who have experienced progression of disease and required retreatment. Of 14 patients who received subsequent venetoclax-based therapy (8 with venetoclax/rituximab for 2-year fixed duration, 3 with venetoclax monotherapy, 2 with venetoclax/rituximab continuous therapy, and 1 with venetoclax/ibrutinib), the ORR was 55%.40 Notably, the patients who have relapsed at this time had relatively short treatment-free intervals, likely because of the lack of deep response to venetoclax/rituximab or more aggressive disease biology. Retreatment with venetoclax may be more favorable for patients who derive more benefit (ie, deeper response or longer treatment-free interval) from their first course of venetoclax-based therapy. Of 8 patients who were subsequently treated with ibrutinib, the ORR was 100% in a heavily pretreated cohort.41
Retrospective studies have examined various therapeutic strategies after venetoclax discontinuation (Table 3). In a cohort of 326 patients who had discontinued venetoclax for any reason, 74 were subsequently treated with BTKi (44 BTKi naïve, 10 BTKi intolerant, 20 BTKi refractory). The ORR to BTKi in a BTKi naïve population was 84%, whereas BTKi-exposed patients had an ORR of 54%. Treatment with BTKi after venetoclax discontinuation was associated with a median PFS of 32 months in BTKi-naïve patients. For BTKi-exposed patients, the setting of prior BTKi failure (progression vs intolerance) significantly impacted PFS (median, 4 months vs not reached).28 In a cohort of 23 patients treated with BTKi after venetoclax discontinuation in the setting of disease progression (median prior lines of therapy, 4; range, 2-9), ORR was 91%, median PFS was estimated to be 34 months, and median OS was 42 months.42
Table 3.
Available evidence for standard of care treatment strategies after venetoclax discontinuation for progression or intolerance
| Subsequent therapy | Study design | Number of patients in group of interest | Clinical setting | Prior therapies, median (range) | ORR | Progression data on subsequent therapy | Survival data on subsequent therapy |
|---|---|---|---|---|---|---|---|
| Venetoclax or venetoclax/rituximab | Prospective9 | 14 | Progression after fixed-duration venetoclax/rituximab (13 completed MURANO regimen, 1 discontinued early) | Not reported | 55% (of evaluable patients) | Not reported | Not reported |
| Ibrutinib | Prospective41 | 8 | Progression after fixed-duration venetoclax/rituximab | 1 (1-4) | 100% | 4 on treatment, 3 with PD (median time on ibr 15 mo (3-48)) | No deaths reported |
| Retrospective44 | 27 | Venetoclax discontinuation (18 with PD, 9 for other reasons) | 2 (0-9) | 56% | 9 patients progressed on ibrutinib, time on ibrutinib 3-53 mo | Not reported | |
| Retrospective27 | 6 | Progression on venetoclax | 4 (1-7) | 5/6 with PR | 3 of 6 remain on therapy (6, 13, and 16 mo on therapy) | 3 deaths (2 of toxicity, 1 due to progression) | |
| BTKi | Retrospective28 | 44 | Venetoclax discontinuation (progression, toxicity), BTK naïve | 2 (0-8) | 84% | Median PFS 32 mo | Not reported |
| Retrospective28 | 30 | Venetoclax discontinuation (progression, toxicity), BTK exposed (33% intolerant, 66% resistant) | 4 (1-11) | 53% | Median PFS 12 mo | Not reported | |
| Retrospective42 | 23 | Venetoclax resistance, BTK naïve | 4 (2-9) | 91% | Median PFS 34 mo | ||
| PI3Ki | Retrospective28 | 17 | Venetoclax discontinuation (progression, toxicity), BTK exposed, PI3K naive | 4 (1-6) | 47% | Median PFS 5 mo | Not reported |
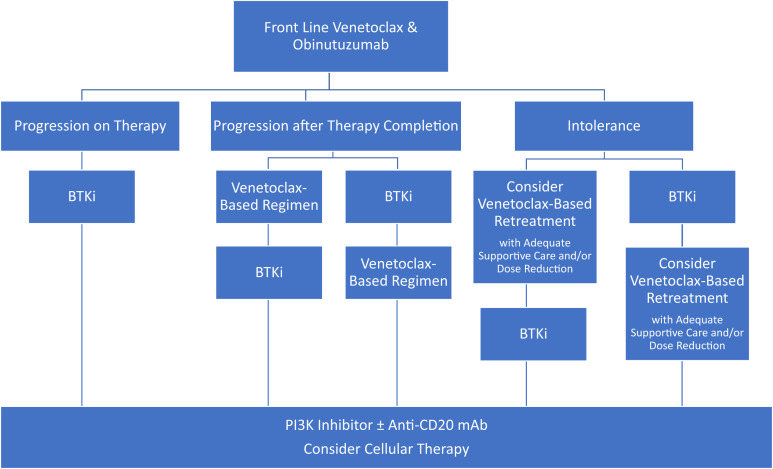
For patients who have progressed after frontline venetoclax and obinutuzumab, initial data suggest that BTKi is likely to produce high response rates and durable remissions. For patients who had deep responses and/or prolonged treatment-free intervals after fixed duration venetoclax therapy, retreatment with a venetoclax-based regimen is an appealing option, although additional data on the efficacy of this approach are needed. Subsequent lines of therapy may include PI3Ki, cellular immunotherapy, and treatment in a clinical trial as described previously (Figure 2).
Figure 2.
Proposed treatment algorithms after frontline venetoclax and obinutuzumab for CLL.
Summary
We have examined the available data guiding treatment sequence decisions in a chemotherapy-free paradigm and noted gaps in the current literature. Decision making is largely extrapolated from studies that included heavily pretreated patients. As such, it is likely that response rates and outcomes will be improved when these agents are used in earlier lines of therapy. As CLL is a chronic disease in which we aim to sequence many therapies to extend survival, future clinical trials should include long-term follow-up to observe subsequent therapies and incorporate sequencing decisions in their design.
Several novel agent combination therapies are currently being studied (NCT03755947, NCT03836261, NCT03580928, NCT03824483). Although these regimens are designed to induce high rates of response and deep remissions with the goal of extending PFS, the optimal approach to therapy for patients who are treated with multiple novel agents simultaneously in the R/R setting remains entirely unexplored and will present an opportunity for active investigation in the future.
Review of this data further highlights the need for studies examining sequencing in a chemotherapy-free paradigm. Because these agents tend to be highly effective with extended periods of PFS, examining these sequences in retrospective, real-world studies is likely to provide data that will be difficult to capture in a prospective fashion.
Acknowledgments
This research was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748. L.R. recognizes support from the American Society of Hematology Research Training Award for Fellows outside of the submitted work.
References
- 1.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharman JP, Banerji V, Fogliatto LM, et al. . ELEVATE TN: phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood. 2019;134(suppl 1):31. [Google Scholar]
- 3.Fischer K, Al-Sawaf O, Bahlo J, et al. . Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Brown JR, O’Brien S, et al. ; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munir T, Brown JR, O’Brien S, et al. . Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghia P, Pluta A, Wach M, et al. . Acalabrutinib vs rituximab plus idelalisib (IdR) or bendamustine (BR) by investigator choice in relapsed/refractory (RR) chronic lymphocytic leukemia: phase 3 ASCEND study. Hematol Oncol 2019;37(suppl 2):86-87. [Google Scholar]
- 7.Furman RR, Sharman JP, Coutre SE, et al. . Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flinn IW, Hillmen P, Montillo M, et al. . The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kater AP, Seymour JF, Hillmen P, et al. . Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269-277. [DOI] [PubMed] [Google Scholar]
- 10.Seymour JF, Kipps TJ, Eichhorst B, et al. . Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. [DOI] [PubMed] [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, et al. . iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745-2760. [DOI] [PubMed] [Google Scholar]
- 12.Jain P, Keating M, Wierda W, et al. . Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierda WG, Tambaro FP. How I manage CLL with venetoclax-based treatments. Blood. 2020;135(17):1421-1427. [DOI] [PubMed] [Google Scholar]
- 14.Mato AR, Wierda WG, Davids MS, et al. . Utility of PET-CT in patients with chronic lymphocytic leukemia following B-cell receptor pathway inhibitor therapy. 2019;104(11): 2258-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131(25):2761-2772. [DOI] [PubMed] [Google Scholar]
- 16.Woyach JA, Furman RR, Liu TM, et al. . Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau DA, Tausch E, Taylor-Weiner AN, et al. . Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burger JA, Landau DA, Taylor-Weiner A, et al. . Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat Commun. 2016;7(1):11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn IE, Underbayev C, Albitar A, et al. . Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017;129(11):1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blombery P, Anderson MA, Gong JN, et al. . Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342-353. [DOI] [PubMed] [Google Scholar]
- 21.Tausch E, Close W, Dolnik A, et al. . Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica. 2019;104(9):e434-e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddocks KJ, Ruppert AS, Lozanski G, et al. . Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awan FT, Schuh A, Brown JR, et al. . Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones JA, Mato AR, Wierda WG, et al. . Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AW, Ma S, Kipps TJ, et al. . Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mato AR, Nabhan C, Barr PM, et al. . Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199-2205. [DOI] [PubMed] [Google Scholar]
- 27.Mato AR, Hill BT, Lamanna N, et al. . Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050-1056. [DOI] [PubMed] [Google Scholar]
- 28.Mato AR, Roeker LE, Jacobs R, et al. . Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreger P, Michallet M, Bosman P, et al. . Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT Chronic Malignancies and Lymphoma Working Parties. Bone Marrow Transplant. 2019;54(1):44-52. [DOI] [PubMed] [Google Scholar]
- 30.Dreger P, Ghia P, Schetelig J, et al. ; European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT). High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132(9):892-902. [DOI] [PubMed] [Google Scholar]
- 31.Turtle CJ, Hay KA, Hanafi LA, et al. . Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi T, Dorritie KA, Soumerai JD, et al. . TRANSCEND CLL 004: minimal residual disease (MRD) negative responses after lisocabtagene maraleucel (Liso-Cel; JCAR017), a CD19-directed CAR T cell product, in patients (pts) with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL). Hematol Oncol. 2019; 37: 109-110. [Google Scholar]
- 33.Geyer MB, Park JH, Riviere I, et al. . Implications of concurrent ibrutinib therapy on CAR T-cell manufacturing and phenotype and on clinical outcomes following CD19-targeted CAR T-cell administration in adults with relapsed/refractory CLL. Blood. 2016;128(22):58-58. [Google Scholar]
- 34.Mato AR, Flinn IW, Pagel JM, et al. . Results from a first-in-human, proof-of-concept phase 1 trial in pretreated B-cell malignancies for Loxo-305, a next-generation, highly selective, non-covalent BTK inhibitor. Blood. 2019;134(suppl 1):501.31395584 [Google Scholar]
- 35.Woyach J, Stephens DM, Flinn IW, et al. . Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood. 2019;134(suppl 1):4298. [Google Scholar]
- 36.Mato AR, Nabhan C, Thompson MC, et al. . Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanafelt TD, Wang V, Kay NE, et al. . Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 trial. Blood. 2019;134(suppl 1):33. [Google Scholar]
- 38.Mato AR, Schuster SJ, Lamanna N, et al. . A phase 2 study to assess the safety and efficacy of umbralisib in patients with chronic lymphocytic leukemia (CLL) who are intolerant to prior BTK or PI3K delta inhibitor therapy. Hematol Oncol. 2019;37(S2):88-89. [Google Scholar]
- 39.Seymour JF, Ma S, Brander DM, et al. . Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seymour JF, Kipps TJ, Eichhorst BF, et al. . Four-year analysis of murano study confirms sustained benefit of time-limited venetoclax-rituximab (venr) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood. 2019;134(suppl 1):355. [Google Scholar]
- 41.Greil R, Fraser G, Leber B, et al. . Efficacy and safety of ibrutinib in relapsed/refractory chronic lymphocytic leukemia patients previously treated with venetoclax in the murano study. HemaSphere. 2019; 3(S1): 527. [Google Scholar]
- 42.Lin VS, Lew TE, Handunnetti SM, et al. . BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135(25):2266-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyre TA, Kirkwood AA, Gohill S, et al. ; the UK CLL Forum. Efficacy of venetoclax monotherapy in patients with relapsed chronic lymphocytic leukaemia in the post-BCR inhibitor setting: a UK wide analysis. Br J Haematol. 2019;185(4):656-669. [DOI] [PubMed] [Google Scholar]
- 44.Brown JR, Davids MS, Chang JE, et al. . Outcomes of ibrutinib (Ibr) therapy in Ibr-naïve patients (pts) with chronic lymphocytic leukemia (CLL) progressing after venetoclax (Ven). Blood. 2019; 2019;134(suppl 1):4320. [Google Scholar]