Visual Abstract
Professional illustration by Patrick Lane, ScEYEnce Studios
Abstract
Venous thromboembolism (VTE; deep vein thrombosis and/or pulmonary embolism) is a well-established cause of morbidity and mortality in the medical and surgical patient populations. Clinical research in the prevention and treatment of VTE has been a dynamic field of study, with investigations into various treatment modalities ranging from mechanical prophylaxis to the direct oral anticoagulants. Aspirin has long been an inexpensive cornerstone of arterial vascular disease therapy, but its role in the primary or secondary prophylaxis of VTE has been debated. Risk-benefit tradeoffs between aspirin and anticoagulants have changed, in part due to advances in surgical technique and postoperative care, and in part due to the development of safe, easy-to-use oral anticoagulants. We review the proposed mechanisms in which aspirin may act on venous thrombosis, the evidence for aspirin use in the primary and secondary prophylaxis of VTE, and the risk of bleeding with aspirin as compared with anticoagulation.
Learning Objectives
Understand the evidence supporting the use of low-dose aspirin in the primary prophylaxis of VTE in specific medical and surgical contexts
Understand the evidence related to the use of low-dose aspirin in the secondary prophylaxis of VTE
Review the safety profile and bleeding risk of aspirin use in comparison with anticoagulation
Clinical case 1
A 65-year-old man with no prior medical history undergoes an elective total knee replacement for chronic degenerative disease. The surgery is uneventful, and he is discharged the next day. He has no personal or family history of venous thromboembolism (VTE) and he is not obese. He is educated on the importance of early mobility and rehabilitation. He is motivated and asks how best to minimize his postoperative risk of VTE.
The physiology behind venous thrombosis
Hemostasis is a balance between clot formation and clot degradation, a tightly regulated system of procoagulant and anticoagulant forces. Thrombosis occurs when this equilibrium is disrupted. Clinicians have historically approached the prevention and treatment of arterial and venous thrombosis somewhat differently, in part because of perceived pathophysiologic differences.
Arterial thrombosis is a platelet-predominant phenomenon, often associated with atherosclerotic damage and inflammation. Ruptured plaque and high shear forces promote the binding and unfolding of von Willebrand factor, inciting platelet aggregation and activation. Histopathology of the arterial clot is characterized by fibrin, leukocytes, and an abundance of platelets, providing a classic “white” appearance.1 Arterial thrombi most often present clinically as acute stroke, myocardial infarction, or peripheral arterial disease.
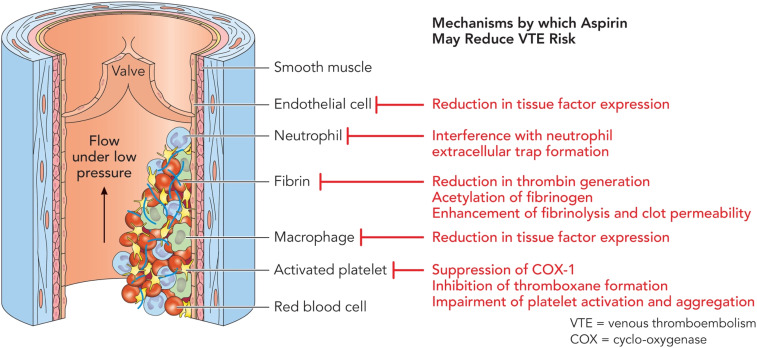
Venous thrombosis, on the other hand, is generally thought of as a disorder in plasma coagulation. Venous thrombi are fibrin-rich, originating in areas of slower blood flow such as the deep veins of the legs. It has been proposed that the endothelium becomes activated and sets off a cascade of inflammation and activation of the coagulation pathway. On histopathology, venous clots are composed of fibrin, leukocytes, and red blood cells, providing a classic “red” appearance; platelets are less prominent than they are in arterial thrombi (Figure 1).1-3
Figure 1.
Composition of venous thrombosis and the antithrombotic effects of aspirin. Venous thrombosis typically originates in areas of slower blood flow, such as the venous anatomy near valves. Venous clots consist primarily of fibrin, red blood cells, and leukocytes. Platelets are involved, but are less prominent in comparison with the platelet-rich arterial thrombus. Aspirin exerts various antithrombotic effects on the participating cells and proteins of thrombus formation, and fibrinolysis via cyclooxygenase (COX) and COX-independent pathways. Professional illustration by Patrick Lane, ScEYEnce Studios.
These distinctions notwithstanding, there is significant mechanistic overlap between arterial and venous thrombosis. The presence of platelets in venous thrombi provides a biologic rationale for the hypothesis that antiplatelet therapy may reduce the risk of VTE in some settings.
Aspirin
Mechanism of action
Acetylsalicylic acid, also known as aspirin, was the first synthetic drug produced, in 1897.4 Cyclooxygenase (COX) isoenzymes, COX-1 and COX-2, catalyze the formation of prostaglandins, thromboxane, and levuloglandins.5 Aspirin inhibits COX activity (mainly COX-1) irreversibly. The suppression of COX-1 decreases the generation of thromboxane A2 (TXA2), an important cofactor for platelet activation and aggregation.6 Aspirin is also suspected to downregulate tissue factor expression, thrombin formation, and downstream thrombin-mediated coagulant reactions. In addition, aspirin may participate in the acetylation of various proteins to catalyze more efficient fibrinolysis (Figure 1).7-9 Aspirin may also exert influence COX-independent pathways to inhibit platelet aggregation and dense granule secretion.8,9
Dosing
Aspirin is absorbed primarily in the stomach and upper small intestine. Doses of 30 to 100 mg of aspirin daily are sufficient to inhibit platelet TXA2 synthesis.10 Paradoxically, higher doses of aspirin appear to have weaker effects on fibrin properties than the lower 75-mg daily dose.11 Low-dose aspirin is typically considered optimal for the primary and secondary prophylaxis of arterial thrombosis.12,13 In the setting of VTE prophylaxis following total joint arthroplasty, a pooled analysis of numerous studies found no significant differences in symptomatic pulmonary embolism (PE), symptomatic deep vein thrombosis (DVT), 90-day mortality, or major bleeding across patient groups receiving low-dose or high-dose aspirin (defined as >162 mg).14
Primary prophylaxis of venous thrombosis
VTE (DVT and PE) is a well-established cause of morbidity and mortality in the medical and surgical patient populations.15,16 The orthopedic surgery community has long embraced aspirin for postsurgical VTE prophylaxis, mainly after total hip arthroplasty [THA] and total knee arthroplasty [TKA].17 Aspirin is widely available and inexpensive, does not require monitoring, and is conventionally thought to confer a lower bleeding risk than anticoagulants in the perioperative period. Many studies support the use of aspirin for primary VTE prophylaxis, but much of the available evidence is considered low quality because it is retrospective and/or subject to selection bias.18 On the other hand, there is a significant amount of high-quality evidence relevant to aspirin use in this postarthroplasty setting; we review this evidence here.
In a meta-analysis of randomized studies by the Antiplatelet Trialists’ Collaboration in 1994, antiplatelet therapy (not exclusive to aspirin) was found to effect a significant reduction in VTE risk and a favorable trend toward mortality benefit (compared with no prophylaxis).19 This finding was reinforced by the multinational and prospective Pulmonary Embolism Prevention (PEP) study. In the PEP study, 17 000 patients undergoing surgery for hip fracture or elective arthroplasty were randomized to either 160 mg of aspirin daily or placebo, starting preoperatively and continued for 35 days. Aspirin reduced the risk of symptomatic VTE by ∼36% when compared with placebo.20 Other forms of thromboprophylaxis were concurrently allowed. The statistical significance of benefit was seen primarily in the hip-fracture group, but not observed in the subgroup receiving low-molecular-weight heparin (LMWH) or in patients who were undergoing elective arthroplasty. There was also a trend toward more major nonfatal bleeding and nonfatal myocardial infarctions with aspirin, practically balancing out the benefit of VTE reduction. However, the evidence of efficacy in the PEP study supported aspirin’s inclusion as an option to consider for postorthopedic surgical VTE prophylaxis, even in the early 2000s (Table 1). Although aspirin may be better than placebo in regard to reducing VTE risk, there was still debate around the overall efficacy and safety of low-dose aspirin when compared with low-dose anticoagulants.
Table 1.
Summary of clinical practice guidelines involving the use of aspirin for the pharmacologic prophylaxis of VTE
| Society/Year VTE indication | Recommendation |
|---|---|
| ACCP24/2016 | |
| Secondary prophylaxis | In patients with an unprovoked proximal DVT or PE who are stopping anticoagulant therapy and do not have a contraindication to aspirin, we suggest aspirin over no aspirin to prevent recurrent VTE |
| AAOS43/2012 | |
| Elective hip or knee arthroplasty | We suggest the use of pharmacologic agents and/or mechanical compressive devices for the prevention of VTE in patients undergoing elective hip or knee arthroplasty, and who are not at elevated risk beyond that of the surgery itself for VTE or bleeding |
| ASH44/2019 | |
| THA or TKA | The ASH guideline panel suggests using aspirin (ASA) or anticoagulants |
| When anticoagulants are used, the panel suggests using DOACs over LMWH | |
| The panel suggests using any of the DOACs approved for use | |
| If a DOAC is not used, the panel suggests using LMWH rather than warfarin and recommends LMWH rather than UFH | |
| ESA45/2018 | |
| Hip fracture, hip arthroplasty, or knee arthroplasty | We recommend using aspirin, considering that it may be less effective than or as effective as LMWH for prevention of DVT and PE after THA, TKA, and hip-fracture surgery |
| Aspirin may be associated with less bleeding after THA, TKA, and hip-fracture surgery than other pharmacological agents | |
| General orthopedic procedures | Aspirin may be less effective than or as effective as LMWHs for prevention of DVT and PE after other orthopedic procedures |
| General surgery | We do not recommend aspirin as thromboprophylaxis in general surgery; however, this type of prophylaxis could be interesting especially in low-income countries and adequate large-scale trials with proper study designs should be carried out |
| NICE46/2018 | |
| Hip arthroplasty | Offer VTE prophylaxis to people undergoing elective hip-replacement surgery whose risk of VTE outweighs their risk of bleeding |
| Choose any 1 of: LMWH (for 10 d) followed by aspirin (75 or 150 mg) for a further 28 d; LMWH (for 28 d) combined with antiembolism stockings (until discharge); rivaroxaban | |
| Knee arthroplasty | Offer VTE prophylaxis to people undergoing elective knee-replacement surgery whose VTE risk outweighs their risk of bleeding |
| Choose any 1 of: aspirin (75 or 150 mg) for 14 d; LMWH (for 14 d) combined with antiembolism stockings (until discharge); rivaroxaban | |
| Multiple myeloma patients on immunomodulator therapy | Consider pharmacological VTE prophylaxis for people with myeloma who are receiving chemotherapy with thalidomide, pomalidomide, or lenalidomide with steroids |
| Choose either: aspirin (75 or 150 mg) or LMWH | |
| SIGN47/2010 | |
| General surgical patient | Aspirin is not recommended as the sole pharmacological agent for VTE prophylaxis in surgical patients, as other available agents are more effective |
| Orthopedic surgical patient | As other agents are more effective for prevention of DVT, aspirin is not recommended as the sole pharmacological agent for VTE prophylaxis in orthopedic patients |
| Medical patient | When the assessment of risk favors use of thromboprophylaxis, UFH, LMWH, or fondaparinux should be administered |
| Aspirin is not recommended as the sole pharmacological agent for VTE prophylaxis in medical patients |
AACP, American College of Chest Physicians; AAOS, American Academy of Orthopaedic Surgeons; ASA, acetylsalicylic acid; ASH, American Society of Hematology; DOAC, direct oral anticoagulant; ESA, European Society of Anaesthesiology; NICE, National Institute for Health and Clinical Excellence; SIGN, Scottish Intercollegiate Guidelines Network; UFH, unfractionated heparin.
In the Extended Prophylaxis Comparing Low-Molecular-Weight Heparin to Aspirin in Total Hip Arthroplasty (EPCAT) trial, patients undergoing hip arthroplasty received 10 days of prophylaxis-dose dalteparin, and then were randomized to 28 days of low-dose aspirin or continued dalteparin. Aspirin was noninferior to dalteparin in the prevention of symptomatic VTE over a 90-day follow-up period; bleeding event rates were similar.21 The study was limited by low adherence in the LMWH group and an imbalance in risk factors for thrombosis between the 2 groups. A second randomized trial, Extended Venous Thromboembolism Prophylaxis Comparing Rivaroxaban to Aspirin Following Total Hip and Knee Arthroplasty II (EPCAT II), established low-dose aspirin as noninferior to rivaroxaban for VTE prevention in patients undergoing hip or knee arthroplasty (all patients received low-dose rivaroxaban for the first 5 postoperative days).22 Patients at high risk for VTE, such as those with known thrombophilia, prior VTE, cancer, or morbid obesity were underrepresented or excluded. Bleeding was uncommon in both groups and usually occurred within 10 days of surgery, possibly because rivaroxaban was used by all patients during the first 5 postoperative days. Addition of mechanical compression to either regimen was optional and relatively uncommon in both groups (approximate rate, 16%).
In a more recent systematic review that pooled data from 13 randomized trials, aspirin was found to be comparable to other antithrombotic agents in preventing postoperative VTE after total joint arthroplasty.23 The safety data from this pooled comparison did not identify a significant difference in major bleeding rates between aspirin and the comparator anticoagulants (∼0.5% of patients in both groups experienced major bleeding). There was a trend toward lower rates of wound hematoma and wound infection in patients receiving aspirin, but the differences were not statistically significant. The findings of the meta-analysis should be interpreted with caution as the event rates were low and there was significant heterogeneity across trials.
With the continued uncertainty about how aspirin compares to anticoagulants, the upcoming Pulmonary Embolism Prevention after Hip and Knee Replacement (PEPPER) and VTE Prevention Following Total Hip and Knee Arthroplasty (EPCAT III) trials (NCT02810704 and NCT04075240, respectively) will be of particular interest. EPCAT III will randomize patients undergoing hip and knee arthroplasty to receive either aspirin alone or aspirin and rivaroxaban for the prevention of VTE, using similar inclusion and exclusion criteria as EPCAT II; patients with metastatic cancer, existing need for long-term anticoagulation, and previously documented VTE will be excluded. The PEPPER trial will randomize a similar patient population to 4 weeks of VTE prophylaxis with either rivaroxaban, aspirin, or warfarin. PEPPER appears to have less stringent exclusion criteria than EPCAT II and will not explicitly exclude patients with prior VTE or cancer. However, patients who are already on chronic anticoagulation will not be eligible to enroll. All patients will receive in-hospital pneumatic compression (along with modern surgical techniques and postoperative care); this design should provide insight into the possibility that combination mechanical prophylaxis and aspirin may lead to even lower VTE rates.
In summary, all low-risk patients in the postarthroplasty surgical setting are candidates for thromboprophylaxis with aspirin. Whether a few days of an anticoagulant prior to low-dose aspirin has benefit, and whether prolonged administration of a low-dose anticoagulant may be the best choice for high-risk patients, remain unanswered questions. For many patients, the addition of mechanical VTE prophylaxis (eg, with sequential compression devices) effectively closing any efficacy gap between low-dose aspirin and a low-dose anticoagulant (if such a gap exists) is a possibility.
The patient in our case is at low risk for VTE following TKA. We would recommend the use of a hybrid strategy with 10 mg of rivaroxaban daily for 5 days followed by low-dose aspirin for an additional 9 days, as was done in EPCAT II. Pending the results of the upcoming PEPPER and EPCAT-3 trials, available evidence would also support low-dose direct oral anticoagulants (DOACs), low-dose LMWH, or, because he is low risk, low-dose aspirin for an entire 14-day course.
Clinical case 2
A 58-year-old woman with a history of coronary artery disease, morbid obesity, and a prior unprovoked proximal DVT 3 years ago presents to her primary care office prior to embarking on a long international flight to a low-resource setting. For her past unprovoked DVT, she completed 6 months of warfarin and decided to forgo further anticoagulation. She had no bleeding complications while on therapy. She takes 81 mg of aspirin daily as recommended by her cardiologist. She is concerned about her risk to develop DVT or PE, either while traveling or at some point later in life. She wonders whether she should consider adding 1 of the new anticoagulants to her medication regimen.
Aspirin
Secondary prophylaxis of venous thrombosis
If anticoagulant therapy is stopped 6 to 12 months after a first unprovoked VTE, the 5-year risk of recurrence is ∼30%.24 Whereas aspirin is widely accepted as a secondary prevention strategy for stroke and myocardial infarction, the role of aspirin to prevent recurrent VTE is less defined.
The International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism (INSPIRE) investigators pooled analyses from 2 underpowered trials: the Warfarin and Aspirin (WARFASA) and the Aspirin to Prevent Recurrent Venous Thromboembolism (ASPIRE) studies.25-27 Across the WARFASA and ASPIRE trials, the combined study population completed anywhere from 6 weeks to 24 months of initial anticoagulation therapy before randomization to low-dose aspirin or placebo. The median follow-up time was 30.4 months, in which there was a statistically significant 32% reduction in VTE recurrence with aspirin when compared with placebo. The authors also suspect, through refined estimate modeling, that with full medical adherence, aspirin would prevent closer to 40% of recurrent events. The major bleeding rate was low (0.5%) and essentially identical between the aspirin and placebo groups.
In the Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN CHOICE) trial, patients who completed 6 to 12 months of anticoagulation for VTE were randomized to rivaroxaban (either 10 mg or 20 mg daily) or aspirin (up to 100 mg daily) to prevent recurrence.28 With a mean follow-up of 1 year, VTE occurred in 1.5% of patients receiving 20 mg of rivaroxaban, 1.2% of patients receiving 10 mg of rivaroxaban, and 4.4% of patients receiving aspirin. Whether DOACs and/or warfarin reduce the risk of myocardial infarction or noncardioembolic stroke as effectively as aspirin is not yet known.
The results of these trials suggest that aspirin has some efficacy in preventing VTE recurrence; patients who use aspirin as a long-term secondary prevention strategy can expect a VTE recurrence risk lower than if they took no medication but higher than if an anticoagulant was used instead. However, the safety benefit of aspirin (vs anticoagulant therapy), if there is one, may not offset the lower efficacy of aspirin. Aspirin, though easily acquired over the counter, can cause serious adverse effects including renal dysfunction, gastrointestinal pathology, and, most importantly, serious bleeding.6
The risks
Long-term stroke-prevention trials in patients with atrial fibrillation provide an excellent assessment of the relative bleeding risk with aspirin compared with the anticoagulants. In review of recent randomized controlled trials (RCTs) and systematic reviews of RCTs in which aspirin was compared with placebo, the major bleeding rate seen with aspirin is unsurprisingly higher than that seen with no antithrombotic therapy (Table 2). When aspirin is compared with warfarin or the DOACs across various indications, there is a trend suggesting that aspirin may cause less bleeding than anticoagulants, but in most studies, the difference fails to achieve statistical significance (Table 3). In a systematic review of patients older than 65 years on antiplatelet therapy, the risk of major hemorrhage associated with chronic antiplatelet drug use is very close to the risk associated with the oral anticoagulants.29 Overall, major bleeding was as frequent among patients taking antiplatelet therapy as among patients taking warfarin in RCTs. Of course, the difficulty in establishing a safety benefit from aspirin (vs anticoagulants) may be due to a lack of power to detect a difference; however, excellent safety profiles of modern anticoagulant strategies (at least within randomized trials) casts some doubt on the assumption that aspirin is a much safer long-term alternative to anticoagulant therapy.
Table 2.
Summary of major bleeding outcomes across major SRs and RCTs comparing aspirin and placebo
| Study reference | Indication | Aspirin dose compared with placebo | No. of major bleeding events: aspirin vs placebo | RR/HR |
|---|---|---|---|---|
| ECLAP34 | Primary thromboprophylaxis for polycythemia vera | Aspirin 100 mg daily | 3 vs 2 | RR, 1.62 (0.27-9.71) |
| RCT, 2004; n = 518 | ||||
| INSPIRE25 | Recurrent VTE prevention | Aspirin 100 mg daily | 9 vs 7 | HR, 1.31 (0.48-3.53) |
| RCT, 2014; n = 1224 | ||||
| POISE-248 | Perioperative administration of aspirin in patients undergoing noncardiac surgery | Aspirin 200 mg daily | 230 vs 188 | HR, 1.23 (1.01-1.49) |
| RCT, 2014; n = 10 010 | ||||
| Mahmoud et al, 201949 | Primary cardiovascular prevention | Aspirin mostly, 75-100 mg | 1301 vs 901 | RR, 1.47 (1.31-1.65) |
| SR, 11 RCTs; n = 157 248 | ||||
| Zheng et al, 201950 | Primary cardiovascular prevention | Aspirin ≤100 mg daily | 1195 vs 834 | HR, 1.54 (1.35-1.76) |
| SR, 11 RCTs; n = 134 470 |
Major bleeding as defined by criteria set in each individual randomized control trial or systematic review.
HR, hazard ratio; INSPIRE, International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism; n, number of patients; POISE-2, Perioperative Ischemic Evaluation 2; RR, relative risk; SR, systematic review and meta-analysis.
Table 3.
Summary of major bleeding outcomes across major SRs and RCTs comparing anticoagulants and aspirin
| Study reference | Indication | Comparators | No. of major bleeding events | RR/HR |
|---|---|---|---|---|
| AVERROES51 | Stroke prophylaxis in atrial fibrillation | Apixaban 5 mg BID vs aspirin 81 to 324 mg daily | 44 vs 39 | HR, 1.13 (0.74-1.75) |
| RCT, 2011; n = 5599 | ||||
| Warkentin et al, 201252 | Any indication for long-term antithrombotic therapy | Warfarin vs aspirin 75 to 300 mg daily | 69 vs 54 | OR, 1.27 (0.83-1.94) |
| SR, 8 RCTs; n = 2904 | ||||
| COMPASS53 | Secondary cardiovascular prevention | Rivaroxaban 5 mg BID vs aspirin 100 mg daily | 288 vs 170 | HR, 1.51 (1.25-1.84) |
| RCT, 2017; n = 27 395 | ||||
| EINSTEIN-CHOICE28 | Extended treatment of VTE | Rivaroxaban 10 mg daily vs aspirin 81 mg daily Rivaroxaban 20 mg daily vs aspirin 81 mg daily |
5 vs 3 6 vs 3 |
HR, 1.64 (0.39-6.84) HR, 2.01 (0.50-8.04) |
| RCT, 2017; n = 3365 | ||||
| EPCAT II22 | Post-joint arthroplasty extended VTE prophylaxis | Rivaroxaban 10 mg daily vs aspirin 81 mg | 5 vs 8 | RR, 0.62 (0.20-1.90) |
| RCT, 2018; n = 3424 | ||||
| NAVIGATE ESUS54 | Secondary stroke prophylaxis | Rivaroxaban 15 mg daily vs aspirin 100 mg daily | 62 vs 13 | HR, 2.72 (1.68-4.39) |
| RCT, 2018; n = 7213 | ||||
| Xie et al, 201955 | Prevention of VTE | Rivaroxaban mostly 10 mg daily vs aspirin mostly 100 mg daily or less | 16 vs 11 | RR, 0.81 (0.42-1.55) |
| SR, 9 RCTs; n = 7656 | ||||
| Ng et al, 202056 | Stroke prophylaxis in atrial fibrillation | Aspirin (subgroup, dose not specified) vs warfarin | Not provided | RR, 0.63 (0.41-0.96)* |
| SR, 37 RCTs; n = 100 142 | ||||
| Matharu et al, 202023 | Post-joint arthroplasty VTE prophylaxis | Low- and high-dose aspirin (subgroup) vs comparator anticoagulants | 11 vs 10 | RR, 1.11 (0.47-2.59)* |
| SR, 13 RCTs; n = 6060 |
Major bleeding as defined by criteria set in each individual randomized control trial or systematic review.
AVERROES, Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; BID, twice daily; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; EINSTEIN-CHOICE, Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism; EPCAT II, Extended Venous Thromboembolism Prophylaxis Comparing Rivaroxaban to Aspirin Following Total Hip and Knee Arthroplasty II; NAVIGATE ESUS, New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source; OR, odds ratio. See Table 2 for expansion of other abbreviations.
Note relative risk ratio is reported with respect to aspirin in contrast to the format in the rest of the table.
Primary VTE prevention in selected medical populations
In specific medical contexts, such as in some patients with myeloproliferative neoplasms (MPNs) and in some patients with multiple myeloma, aspirin is widely used to reduce the risk of both VTE and arterial thrombosis. There is evidence that supports platelet activation and dysfunction in both MPNs and in multiple myeloma.30,31 In newly diagnosed multiple myeloma, the VTE rate is estimated to be at least 10% with the majority of events occurring in the first 6 months of induction.32 A systematic review of 6 studies, encompassing 1125 patients receiving lenalidomide-based therapy, demonstrated a 10.7% total risk of VTE with aspirin compared with 1.4% with LMWH in multiple myeloma patients.33 More evidence is needed to determine the best strategy to reduce the risk of arterial and venous thrombosis in myeloma patients starting immunomodulatory drugs.
In the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) trial, low-dose aspirin (100 mg per day), when compared with placebo, reduced a composite end point of thrombotic complications without a significantly increased incidence of major bleeding.34 On the other hand, in essential thrombocythemia, a systematic review of 24 observational studies concluded that patients who received antiplatelet therapy (mainly low-dose aspirin) derived a modest relative risk reduction of 26% with a median increase in major bleeding of 30%.35 Unfortunately, with a lack of randomized trial data, these observational studies were deemed to have a high risk of bias and the evidence was rated very uncertain.
The use of aspirin for secondary VTE prevention is perhaps best reserved for situations in which antiplatelet therapy is already strongly recommended for another indication (eg, coronary stent placement), or anticoagulation is contraindicated or simply cannot be acquired due to cost or logistics. For example, in clinical case 2, aspirin could be an option for secondary thromboprophylaxis because she already has an existing cardiac indication for aspirin. Although the combination of antiplatelet and anticoagulant therapy would likely reduce the risk for VTE compared with antiplatelet therapy alone, the marginal benefit in this patient (who had 1 unprovoked DVT 3 years ago) would likely not offset the bleeding risk.36-42
There are other intriguing hypotheses that would be interesting to test. For example, might primary VTE prophylaxis with aspirin be of benefit to some patients during and/or after hospitalization for acute medical illness? Would selected persons undertaking long-distance travel benefit from taking low-dose aspirin? Which patients would benefit and for how long would such treatment be recommended? For now, these questions remain unanswered.
Conclusion
For many patients, aspirin is an inexpensive, safe and effective VTE-prevention strategy following total joint arthroplasty. Although ongoing clinical trials (EPCAT III and PEPPER) will further clarify the roles of low-dose aspirin and low-dose anticoagulants after joint replacement surgery, there is already robust evidence to support low-dose aspirin as part of a hybrid strategy after an initial period of low-dose anticoagulant administration.
For secondary VTE prophylaxis, aspirin is less effective than anticoagulants but more effective than placebo. Establishing that long-term aspirin use is clearly safer than long-term anticoagulant exposure (especially compared with low-dose, oral factor Xa inhibitors) has been surprisingly difficult. Unless a safety benefit from aspirin can be established in well-designed prospective studies, patients who need long-term antithrombotic therapy for VTE will often choose a low-dose factor Xa inhibitor, once presented with the risk-benefit tradeoffs.
References
- 1.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017;38(11):785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28(3):387-391. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S, Johnson TA, Duru N, et al. . Fibrinolysis and inflammation in venous thrombus resolution. Front Immunol. 2019;10:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sneader W. The discovery of aspirin: a reappraisal. BMJ. 2000;321(7276):1591-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004;10(6):577-588. [DOI] [PubMed] [Google Scholar]
- 6.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206-1218. [DOI] [PubMed] [Google Scholar]
- 7.Ajjan RA, Standeven KF, Khanbhai M, et al. . Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol. 2009;29(5):712-717. [DOI] [PubMed] [Google Scholar]
- 8.Undas A, Brummel-Ziedins KE, Mann KG. Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007;109(6):2285-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Undas A, Brummel-Ziedins K, Mann KG. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J Thromb Haemost. 2014;12(11):1776-1787. [DOI] [PubMed] [Google Scholar]
- 10.Undas A, Undas R, Musiał J, Szczeklik A. A low dose of aspirin (75 mg/day) lowers thrombin generation to a similar extent as a high dose of aspirin (300 mg/day). Blood Coagul Fibrinolysis. 2000;11(3):231-234. [PubMed] [Google Scholar]
- 11.Antovic A, Perneby C, Ekman GJ, et al. . Marked increase of fibrin gel permeability with very low dose ASA treatment. Thromb Res. 2005;116(6):509-517. [DOI] [PubMed] [Google Scholar]
- 12.Collaboration AT; Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297(18):2018-2024. [DOI] [PubMed] [Google Scholar]
- 14.Azboy I, Groff H, Goswami K, Vahedian M, Parvizi J. Low-dose aspirin is adequate for venous thromboembolism prevention following total joint arthroplasty: a systematic review. J Arthroplasty. 2020;35(3):886-892. [DOI] [PubMed] [Google Scholar]
- 15.Spyropoulos AC, Hussein M, Lin J, Battleman D. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102(5):951-957. [DOI] [PubMed] [Google Scholar]
- 16.Heit JA, O’Fallon WM, Petterson TM, et al. . Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248. [DOI] [PubMed] [Google Scholar]
- 17.Azboy I, Barrack R, Thomas AM, Haddad FS, Parvizi J. Aspirin and the prevention of venous thromboembolism following total joint arthroplasty: commonly asked questions. Bone Joint J. 2017;99-B(11):1420-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegrini VD Jr, Eikelboom J, McCollister Evarts C, et al. ; Steering Committee of The PEPPER Trial. Selection bias, orthopaedic style: knowing what we don’t know about aspirin. J Bone Joint Surg Am. 2020;102(7):631-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative overview of randomised trials of antiplatelet therapy--III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6923):235-246. [PMC free article] [PubMed] [Google Scholar]
- 20.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000; 355(9212):1295-1302. [PubMed] [Google Scholar]
- 21.Anderson DR, Dunbar MJ, Bohm ER, et al. . Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800-806. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DR, Dunbar M, Murnaghan J, et al. . Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378(8):699-707. [DOI] [PubMed] [Google Scholar]
- 23.Matharu GS, Kunutsor SK, Judge A, Blom AW, Whitehouse MR. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180(3):376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016;150(4):988]. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]
- 25.Simes J, Becattini C, Agnelli G, et al. ; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062-1071. [DOI] [PubMed] [Google Scholar]
- 26.Becattini C, Agnelli G, Schenone A, et al. ; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967. [DOI] [PubMed] [Google Scholar]
- 27.Brighton TA, Eikelboom JW, Mann K, et al. ; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987. [DOI] [PubMed] [Google Scholar]
- 28.Weitz JI, Lensing AWA, Prins MH, et al. ; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. [DOI] [PubMed] [Google Scholar]
- 29.Melkonian M, Jarzebowski W, Pautas E, Siguret V, Belmin J, Lafuente-Lafuente C. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(7):1500-1510. [DOI] [PubMed] [Google Scholar]
- 30.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176-2184. [DOI] [PubMed] [Google Scholar]
- 31.Kristinsson SY. Thrombosis in multiple myeloma. Hematology Am Soc Hematol Educ Program. 2010;2010:437-444. [DOI] [PubMed] [Google Scholar]
- 32.Bradbury CA, Craig Z, Cook G, et al. . Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood. 2020;136(9):1091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Ani F, Bermejo JM, Mateos MV, Louzada M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide - a systematic review. Thromb Res. 2016;141:84-90. [DOI] [PubMed] [Google Scholar]
- 34.Landolfi R, Marchioli R, Kutti J, et al. ; European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114-124. [DOI] [PubMed] [Google Scholar]
- 35.Chu DK, Hillis CM, Leong DP, Anand SS, Siegal DM. Benefits and risks of antithrombotic therapy in essential thrombocythemia: a systematic review. Ann Intern Med. 2017;167(3):170-180. [DOI] [PubMed] [Google Scholar]
- 36.Lopes RD, Heizer G, Aronson R, et al. ; AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509-1524. [DOI] [PubMed] [Google Scholar]
- 37.Cannon CP, Bhatt DL, Oldgren J, et al. ; RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513-1524. [DOI] [PubMed] [Google Scholar]
- 38.Dewilde WJ, Oirbans T, Verheugt FW, et al. ; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura-Nakano Y, Shizuta S, Komasa A, et al. ; OAC-ALONE Study Investigators. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139(5):604-616. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda S, Kaikita K, Akao M, et al. ; AFIRE Investigators. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103-1113. [DOI] [PubMed] [Google Scholar]
- 41.van Rein N, Heide-Jørgensen U, Lijfering WM, Dekkers OM, Sørensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139(6):775-786. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg BA, Kim S, Piccini JP, et al. ; ORBIT-AF Investigators and Patients. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128(7):721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs JJ, Mont MA, Bozic KJ, et al. . American Academy of Orthopaedic Surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Bone Joint Surg Am. 2012;94(8):746-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson DR, Morgano GP, Bennett C, et al. . American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenny JY, Pabinger I, Samama CM; ESA VTE Guidelines Task Force. European guidelines on perioperative venous thromboembolism prophylaxis: aspirin. Eur J Anaesthesiol. 2018;35(2):123-129. [DOI] [PubMed] [Google Scholar]
- 46.National Institute for Health and Clinical Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. https://www.nice.org.uk/guidance/ng89. Accessed 31 May 2020. [PubMed]
- 47.Scottish Intercollegiate Guidelines Network (SIGN). Prevention and Management of Venous Thromboembolism. SIGN publication no. 122. Edinburgh, United Kingdom: SIGN; 2010. [Google Scholar]
- 48.Devereaux PJ, Mrkobrada M, Sessler DI, et al. ; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. [DOI] [PubMed] [Google Scholar]
- 49.Mahmoud AN, Gad MM, Elgendy AY, Elgendy IY, Bavry AA. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J. 2019;40(7):607-617. [DOI] [PubMed] [Google Scholar]
- 50.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connolly SJ, Eikelboom J, Joyner C, et al. ; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817. [DOI] [PubMed] [Google Scholar]
- 52.Warkentin AE, Donadini MP, Spencer FA, Lim W, Crowther M. Bleeding risk in randomized controlled trials comparing warfarin and aspirin: a systematic review and meta-analysis. J Thromb Haemost. 2012;10(4):512-520. [DOI] [PubMed] [Google Scholar]
- 53.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. [DOI] [PubMed] [Google Scholar]
- 54.Hart RG, Sharma M, Mundl H, et al. ; NAVIGATE ESUS Investigators. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201. [DOI] [PubMed] [Google Scholar]
- 55.Xie J, Jiang M, Lin Y, Deng H, Xie X, Li L. Rivaroxaban versus aspirin in prevention of venous thromboembolism: a meta-analysis of 9 randomized controlled trials comprising 7,656 patients. Thromb Haemost. 2019;119(9):1517-1526. [DOI] [PubMed] [Google Scholar]
- 56.Ng SS, Lai NM, Nathisuwan S, et al. . Comparative efficacy and safety of warfarin care bundles and novel oral anticoagulants in patients with atrial fibrillation: a systematic review and network meta-analysis. Sci Rep. 2020;10(1):662. [DOI] [PMC free article] [PubMed] [Google Scholar]