Visual Abstract
Abstract
Chimeric antigen receptor (CAR) T-cell therapy has changed the landscape of immunotherapy for B-cell malignancies, including mature B-cell lymphomas. Although two CD19 CAR T-cell products have been commercially approved to treat relapsed/refractory B-cell lymphomas, outcomes in these patients remain inferior to those of patients with B-cell leukemia, regardless of therapy. Recent clinical studies and preclinical reports suggest that certain characteristics, such as the suppressive lymphoma tumor microenvironment and inferior endogenous T-cell fitness, may contribute to discrepant responses in these patients. In addition, these studies revealed that limited CAR T-cell persistence and tumor antigen escape, which also impact B-cell acute lymphoblastic leukemia, may play a more prominent role in lymphoma. Multiple promising strategies to overcome these barriers have advanced to clinical trials. In this review, we assess CAR T-cell therapies for pediatric relapsed/refractory mature B-cell lymphomas, potential obstacles diminishing antitumor activity and limiting CAR T-cell persistence, and current strategies to overcome these obstacles.
Learning Objectives
Understand current chimeric antigen receptor T-cell therapy options for mature B-cell and Burkitt lymphomas in pediatric patients
Describe challenges and optimization strategies specific to chimeric antigen receptor T-cell therapy for mature B-cell lymphomas
Introduction
T cells genetically modified to express chimeric antigen receptors (CARs) targeting CD19 have changed how practitioners salvage refractory B-cell acute lymphoblastic leukemia (B-ALL), including use of allogeneic hematopoietic stem cell transplant (allo-HSCT). The widespread expression of CD19 and accessibility of leukemic blasts make B-ALL an ideal target for CAR T cells. Several groups reported remarkable complete remission (CR) rates, up to 90%, in heavily pretreated patients with relapsed/refractory (r/r) disease receiving a single CD19.CAR T-cell infusion preceded by cyclophosphamide and fludarabine (Cy/Flu) lymphodepletion.1-3 On the basis of these successes, in 2017, tisagenlecleucel (tisa-cel; Kymriah [FMC63 single-chain variable fragment, 4-1BBζ]; Novartis) was commercialized for refractory acute lymphoblastic leukemia (ALL) in second or greater relapse.
In adults with B-cell non-Hodgkin lymphoma (B-NHL), CD19.CAR T cells have induced durable responses,4,5 leading to US Food and Drug Administration approval of axicabtagene ciloleucel (axi-cel; Yescarta [FMC63 single-chain variable fragment, CD28ζ]; Kite Pharma/Gilead Sciences) and later tisagenlecleucel. However, these therapies remain limited to investigational trials in pediatric B-cell lymphomas.
CAR T cells are an appealing therapy for B-cell lymphomas due to the widespread expression of targetable antigens, with CD19 being the most frequent. Still, despite the fact that CD19 is uniformly expressed on >95% of B-cell lymphomas/leukemias, response rates to CD19.CAR T cells remain lower for lymphomas. Results in adults with B-NHL have been variable, with CR rates ranging from 52% to 82%.4-6 However, it remains largely unknown why mature CD19+ malignancies appear to be less sensitive to CD19.CAR T cells than their less mature counterparts. Furthermore, Burkitt lymphoma (BL), a predominantly pediatric/young adult malignancy, is the least studied CD19-expressing malignancy in CAR T-cell trials, and results reported to date have varied. Using a patient scenario, we review obstacles that decrease responses to CAR T cells in pediatric mature B-cell lymphomas, as well as strategies under investigation to overcome them, before reviewing ongoing and upcoming clinical trials for this patient population.
Clinical case
A 16-year-old boy presented with a 2-week history of neck and right arm swelling and a large mediastinal mass seen upon imaging. Lymph node biopsy revealed diffuse large B-cell lymphoma (DLBCL) positive for CD19/CD20/BCL-2/BCL-6. After rituximab, cyclophosphamide, vincristine, prednisone, doxorubicin, and high-dose methotrexate, he achieved CR. Eight months after completing treatment, the mediastinal mass recurred. He was treated with 6 cycles of rituximab, ifosfamide, carboplatin, and etoposide, followed by radiation to the mediastinum (achieving CR), followed by high-dose chemotherapy and autologous HSCT. Eight months after HSCT, however, he experienced relapse. Now 18 years old with multiply relapsed disease, he received a commercially available CD19 CAR product after lymphodepletion. Following infusion, he developed grade 2 cytokine release syndrome (CRS) requiring tocilizumab and anakinra. Four weeks after CAR T-cell treatment, positron emission tomography–computed tomography (PET-CT) revealed a partial response.
Understanding the mechanisms of this patient’s PR will inform his treatment. The residual PET avidity may represent disease, though activated T cells at disease sites could cause a “pseudoflare” effect. Ongoing research addresses whether patients with B-NHL have a slower response to CAR T cells than patients with ALL. Also, whether biopsy would be helpful in this situation remains to be seen. Several clinical studies incorporate checkpoint inhibition to improve CAR T-cell persistence and clinical responses, a strategy that might benefit this patient. Below, we explore in detail how the field is addressing these and other pressing questions to optimize CAR T cells in pediatric patients with mature B-cell lymphoma.
Pediatric mature B-cell lymphomas: pathophysiology and treatment
NHL is the fourth most common malignancy in children and adolescents7 and encompasses a heterogeneous group of malignancies that originate from B or T lymphocytes and natural killer (NK) cells. Unlike adult NHL, which typically presents as low- or intermediate-grade disease, mature B-NHLs in children (eg, BL, DLBCL, and primary mediastinal large B-cell lymphoma) often present as aggressive, disseminated disease, sometimes with marrow and central nervous system involvement. BL is the most common aggressive pediatric B-cell lymphoma. Although BL and Burkitt-like NHL predominate among younger children, DLBCL occurs more commonly in adolescents. Despite aggressive phenotypes, most children with BL and DLBCL initially respond to treatment, with >90% 4-year event-free survival.8 These tumors exhibit a mature phenotype, expressing surface immunoglobulin and B-cell markers CD19, CD20, and CD10.7
Adding the anti-CD20 monoclonal antibody rituximab to chemotherapy has improved outcomes in B-NHL.9 However, in the 10% to 20% of patients who experience relapse or have refractory disease, objective response rates (ORRs) are only 20% to 30% after conventional salvage chemotherapy, and 5-year survival rates are dismal (10% to 30%, rising to ∼30% to 50% in patients who qualify for subsequent transplant).10 These statistics and impressive results of CD19.CAR T cells for pre–B-ALL have increased interest in CD19.CAR T cells for pediatric B-NHL.
CAR T cells for mature B-cell lymphomas
In adults (aged ≥18 years), two products—tisagenlecleucel and axi-cel—have been approved by the US Food and Drug Administration for patients with B-cell lymphoma in whom two previous lines of treatment have failed.
The ZUMA-1 phase 2 multicenter trial treated 81 patients with r/r DLBCL with axi-cel preceded by lymphodepletion, reporting an ORR of 82% and CR of 49%.5 OS at 18 months was 52%. Results were similar after 2 years: ORR of 83% and CR of 58%.11 Notably, patients did not receive “bridging chemotherapy” during CAR T-cell manufacture, which averaged 17 days, but only 1 patient died of disease progression before receiving CAR T cells.
In the JULIET phase 2 multicenter study, 93 patients with r/r DLBCL received tisagenlecleucel preceded by Cy/Flu.4 Despite a disease burden similar to that in ZUMA-1, 92% of patients received bridging chemotherapy. CAR T cells were manufactured from fresh as opposed to cryopreserved (as in ZUMA-1) apheresis products. Median time from enrollment/apheresis to infusion was 54 days, and manufacture failure occurred in only 7% of patients.6 ORR was 52%, and 40% of patients achieved CR. Overall relapse-free survival was ∼65% at 12 months.
The TRANSCEND NHL-001 trial tested, in adults with DLBCL after Cy/Flu, a single dose of JCAR017 (lisocabtagene maraleucel [liso-cel]), a CD19-directed 4-1BB.CAR T-cell product with a defined 1:1 ratio of CD4:CD8 T cells in bulk. Bridging chemotherapy was used in 59% of patients. In long-term follow-up of the 255 evaluable patients with DLBCL treated, the ORR was 73% and CR was 53%, with a median duration of response of 13.3 months.12
Differences in CAR engineering and manufacturing among the trials may have contributed to the variation in response rates. Axi-cel includes a CD28 costimulatory domain, whereas tisa-cel and liso-cel use 4-1BB costimulatory domains. Axi-cel and liso-cel both include a CD28 transmembrane domain, whereas tisa-cel includes a CD8-α transmembrane domain. In addition to the CAR construct, the T-cell composition may have influenced outcomes because liso-cel used equal doses of CD4 and CD8 T cells, whereas axi-cel and tisa-cel used bulk T cells for CAR T-cell manufacture.
Furthermore, lengthy manufacture period and bridging chemotherapy may have contributed to the decreased ORR in JULIET compared with ZUMA-1. Although bridging chemotherapy can reduce the number of malignant cells each CAR T-cell must target, one may speculate that these chemotherapy regimens could add toxicity without controlling tumor growth in these heavily pretreated patients with refractory disease. Of note, the TRANSCEND trial also allowed bridging chemotherapy, although a smaller percentage of patients received it. Table 1 compares the efficacy of various CD19.CAR T-cell products in the treatment of B-ALL vs B-NHL, with consistently lower CR rates in the B-NHL group when using the same CD19.CAR T-cell product.
Table 1.
Efficacy of various CD19.CAR T-cell products in r/r B-ALL compared with B-NHL
| CD19.CAR T cells in r/r B-ALL | CD19.CAR T cells in r/r B-NHL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAR T-cell product | Age (y) | No. of pts | Efficacy CR (MRD-neg CR) | Median duration of response | CAR T-cell product | Age (y) | No. of pts | Efficacy CR (PR) | Median duration of response |
| Tisagenlecleucel (Maude, 201841) | 3-21 | 75 | 81% MRD-neg CR | Not reached | Tisagenlecleucel (Schuster et al, 20194) | 22-76 | 93 | 40% (12%) | Not reached |
| Axicabtagene ciloleucel (Wierda, 201842) | 18-69 | 35 | 78% MRD-neg CR | NP | Axicabtagene ciloleucel (Neelapu et al, 20175) | 23-76 | 101 | 54% (28%) | 8.1 mo |
| JCAR014 (Turtle, 201643) | 20-73 | 29 | 93% (86%) | NP | JCAR014 (Turtle, 201644) | 22-70 | 32 | 50% (22%) | NP |
| CD19.CD28ζ (Lee et al, 20152) | 1-30 | 21 | 70% (60%) | NP | CD19.CD28ζ (Kochenderfer, 201745) | 26-67 | 22 | 55% (18%) | 12.5 mo |
MRD-neg, minimal residual disease–negative, NP, not provided; PR, partial response.
Ongoing CD19 CAR T-cell trials in pediatric mature B-cell lymphoma
There are 31 actively recruiting trials evaluating CD19.CAR T cells for pediatric and young adult patients with r/r lymphomas. Commercial CAR T-cell products approved for adult B-cell lymphoma and pediatric B-ALL are now being tested in pediatric patients with r/r B-NHL. A phase 2 multicenter study is testing tisagenlecleucel in patients ≤25 years old with CD19+ r/r B-NHL in whom one or more therapies have failed, including HSCT (BIANCA, NCT03610724). As of November 2019, 8 patients were enrolled (4 with DLBCL, 3 with BL, 1 with gray zone lymphoma).13 CAR T cells were manufactured for all 8 patients, and all received bridging chemotherapy per investigator discretion. Five patients received tisagenlecleucel after Cy/Flu, and the safety profile was similar to that in B-ALL.
A phase 1/2 multicenter study is evaluating brexucabtagene autoleucel (KTE-X19, formerly KTE-C19) preceded by Cy/Flu for patients with r/r B-ALL or B-NHL aged ≤21 years in whom 2 or more lines of systemic therapy have failed, including HSCT (ZUMA-4, NCT02625480). JCAR017 (see above) after Cy/Flu is also being evaluated in a phase 1/2 study in patients with r/r B-NHL aged ≤25 years (NCT03743246). To date, no data have been reported regarding the B-NHL cohorts.
Although BL is aggressive, posing specific challenges for timely autologous CAR T-cell treatment, several cases of CD19.CAR T cells in BL have been reported. Avigdor et al14 described a 32-year-old patient with refractory BL and innumerable fluorodeoxyglucose-avid nodal, intestinal, and skeletal lesions visualized by PET-CT. The patient received Cy/Flu followed by infusion of CD3ζ-CD28.CD19 CAR T cells. He developed grade 2 CRS and neurotoxicity and achieved CR 1 month after infusion. He underwent haploidentical HSCT 3 weeks later, but he died 9 days after HSCT of sinusoidal obstruction syndrome and sepsis.
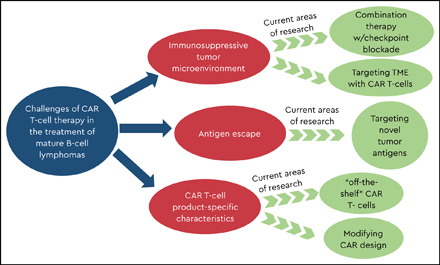
Deciphering the role of disease- and patient-specific characteristics
Tackling the immunosuppressive TME
In order to be effective, CAR T cells must expand in vitro to suitable numbers, engage the intended antigen, proliferate and kill tumor cells, and persist to provide durable tumor control. Although these predictors are relevant across malignancies, lymphomas present several unique challenges. Unlike ALL, lymphomas have a physical barrier that CAR T cells must penetrate.15 CAR T cells also must overcome immunosuppressive cells in the tumor microenvironment (TME) that protect lymphoma from immune attack.15 CAR T cells may also lack chemokines (eg, CXCR4 and CXCR5) that mediate entry into secondary lymphoid tissues, making them more likely to migrate into the circulation.16
The programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway in particular is an important component of the TME because it regulates immune responses by inhibiting T-cell cytokine production and cell-cycle progression.17 Thus, the expression of PD-L1 on tumor and immunosuppressive cells can prevent CAR T cells from infiltrating the tumor. Checkpoint blockade targeting the PD-1–PD-L1 axis can reverse T-cell exhaustion in peripheral and intratumoral T cells.18 Single-agent PD-1 inhibitors have been studied in the treatment of adult and pediatric r/r B-NHL, with select subtypes gaining more therapeutic benefit than others. On the whole, B-NHLs have been much less responsive to PD-1 inhibition than Hodgkin lymphoma.19 To address the heterogeneity of NHL, combination therapy has been attempted, with case studies reporting antitumor response when PD-1 inhibition is combined with CAR T cells for r/r DLBCL.20,21 One study treated 11 patients with r/r B-NHL with 4-1BB-CD3ζ CD19.CAR T cells followed by one 3 mg/kg nivolumab dose (anti–PD-1 antibody) 3 days later. Nine of 11 patients developed grade ≤3 CRS, and 1 developed transient neurotoxicity. With ORR and CR of 82% and 46%, respectively,21 combination therapy only slightly improved response rates compared with previous studies of CD19.CAR T cells alone. Larger trials will need to determine if combination therapy improves outcomes in this population.
In another trial, investigators combined PD-L1 inhibitor atezolizumab and axi-cel (ZUMA-6) in 12 patients with refractory DLBCL with an ORR of 92% (CR, 58%).22 Humanized PD-1/PD-L1 antibodies (eg, pembrolizumab and durvalumab) are also being studied with CD19.CAR T cells in B-NHL (NCT03310619, NCT03630159). As these strategies become more popular, in addition to determining whether PD-1/PD-L1 blockade enhances CAR T-cell efficacy, we must also consider the risk of T-cell “overactivation” and increased toxicity.
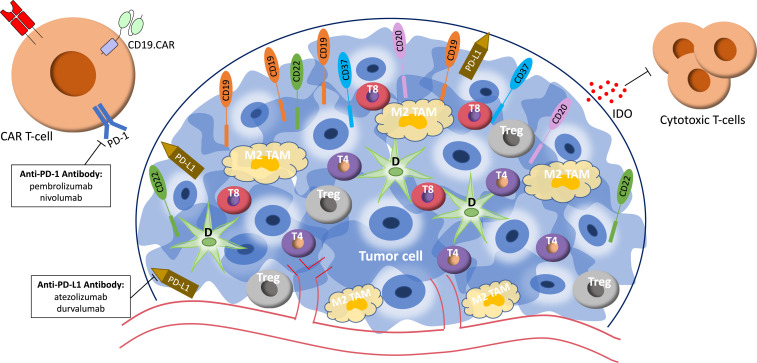
Other checkpoint receptors and inhibitory immune cells in the TME, including tumor-associated macrophages (TAMs) with an M2 phenotype, can decrease the efficacy of CAR T cells. TAMs minimize inflammation and immune surveillance, allowing tumor proliferation.23 Increased TAMs, which often express PD-L1/PD-L2, indicate poor prognosis in DLBCL.24 One proposed mechanism by which TAMs enable DLBCL to escape immune surveillance is by polarizing M1 TAMs to M2 TAMs, enabling them to engage PD-1 receptors on intratumoral T cells, further suppressing the antilymphoma response. Figure 1 illustrates proposed mechanisms to target the lymphoma TME to improve CAR T-cell efficacy. On the basis of these and similar studies, several groups, including ours, have generated immune effectors to simultaneously or separately target tumor antigens and the TME.25,26
Figure 1.
Schematic diagram of the lymphoma tumor microenvironment and treatment modalities currently being evaluated to target aspects of the tumor microenvironment. D, dendritic cell; IDO, indoleamine 2,3-dioxygenase; M2 TAM, M2 tumor-associated macrophage; T4, intratumoral CD4+ cell; T8, intratumoral CD8+ cell; Treg, regulatory T cell.
Targets beyond CD19
As in B-ALL, CD19-directed immunotherapies result in “antigen escape” and CD19-negative relapse in up to 20% of patients with B-NHL.1,27 On the basis of these risks and the success of the anti-CD20 monoclonal antibody rituximab in B-NHL, groups successfully developed CAR T cells targeting CD20.28,29 A phase 1 trial tested 4-1BB.CD20.CAR T cells in adult patients with r/r DLBCL. Four of 6 evaluable patients achieved CR by 1 month; however, 3 of 4 patients eventually relapsed, with only 1 achieving continued CR.28 Although promising, larger clinical trials need to be done to determine the efficacy of CD20 as a target for CAR T-cell therapy in B-NHL.
Other relevant targets, some of which have been studied in B-cell leukemias, include CD22, CD37, and the κ-light chain.30,31 Early results of a phase 1 trial investigating CD22.4-1BB/CD3ζ CAR T cells after Cy/Flu in children/young adults with r/r B-ALL showed 44% of patients attained minimal residual disease–negative remission. Although CR rates were lower than those achieved with similar CD19.CAR T cells, CD19.CAR T cells had previously failed in several patients and/or these patients experienced CD19 relapse.30 Though promising, there are no results yet from using CD22.CAR T cells in B-NHL. Table 2 shows clinical trials for CAR T cells targeting other antigens enrolling patients with r/r B-NHL.
Table 2.
Actively recruiting CAR T-cell trials for relapsed B-cell lymphomas using tumor antigen targets other than CD19
| Target | ClinTrials.gov identifier | Institution/sponsor | Age criteria (y) | Phase | Results reported (Y/N) |
|---|---|---|---|---|---|
| CD20 | NCT03277729 | Fred Hutchinson Cancer Research Center, Seattle, WA | ≥18 | 1/2 | N |
| NCT04316624 | Institute of Hematology and Blood Diseases Hospital, Tianjin, China | 18-75 | 1 | N | |
| NCT04169932 | First Affiliated Hospital with Nanjing Medical University, Jiangsu, China | 18-70 | 1 | N | |
| NCT04036019* | Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China | 14-70 | 1 | N | |
| CD22 | NCT04088890 | Stanford Medical Center, Stanford, CA | ≥18 | 1/1b | N |
| NCT04088864* | Stanford Medical Center, Stanford, CA | 1-30 | 1 | N | |
| NCT02650414* | Children’s Hospital of Philadelphia, Philadelphia, PA | 1-24 | 1 | Y (in ALL cohort) Ruella et al, 201725 | |
| NCT03262298 | Affiliated Hospital to Academic of Military Medical Sciences, Beijing, China | 18-65 | 1/2 | N | |
| NCT04007978* | Union Hospital, Tongji Medical College, Hubei, China | 14-70 | 1 | N | |
| Dual target: CD19/CD20 | NCT04186520 | Medical College of Wisconsin and Froedtert Hospital, Milwaukee, WI | ≥18 | 1/2 | N |
| NCT04007029 | UCLA/Jonsson Comprehensive Cancer Center, Los Angeles, CA | 18-70 | 1 | N | |
| NCT04215016 | Fujian Medical University, Fujian, China | ≥18 | 1 | N | |
| NCT03881761* | Cancer Hospital Affiliate to Zhengzhou University and Henan Cancer Hospital, Henan, China | 17-70 | 1 | N | |
| NCT03097770* | Chinese PLA General Hospital, Beijing, China | 16-70 | 1/2 | Y (74% CR) Zhang et al, 202032 | |
| Dual target: CD19/CD22 | NCT03233854 | Stanford University School of Medicine, Palo Alto, CA | ≥18 | 1 | N |
| NCT04204161* | Xiangya Hospital Central South University, Hunan, China | 1 mo to 18 y | 1 | N | |
| Dual target: CD19/CD22+ anti-PD1 Ab | NCT03287817 | Autolus Therapeutics | ≥18 | 1/2 | Y (55% CR) Osborne, 202046 |
| Dual target: CD19/CD20 or CD19/CD22 | NCT03398967* | Chinese PLA General Hospital, Beijing, China | 12-70 | 1/2 | N |
Ab, antibody; ALL, acute lymphoblastic leukemia; PLA, People’s Liberation Army; UCLA, University of California, Los Angeles.
Trials enrolling pediatric/adolescent patients.
Although patients with B-ALL whose disease progresses or relapses after receiving one CAR product are often enrolled in a trial targeting another antigen, this practice is rarely planned. Nevertheless, a small subcohort of pediatric patients with r/r BL was enrolled in a trial investigating sequential second-generation CD19, CD20, and CD22 4-1BB CAR T cells.32 Three of 5 patients with BL achieved CR by day 77 after infusion of CD19.CAR T cells following Cy/Flu. One patient showed a transient response at day 30 followed by tumor growth by day 60. This patient’s CAR T cells failed to expand, likely explaining the poor response. Subsequently, the patient received CD22.CAR T cells and 64 days later achieved a CR. Although promising, given small sample sizes and lack of correlative studies explaining differential responses, additional questions remain regarding this sequential approach.
Another report detailed an 8-year-old with relapsed BL who experienced disease progression 50 days after autologous 4-1BB/CD3ζ.CD19.CAR T cells and Cy/Flu. Seventy days later, he received lymphodepletion before 4-1BB/CD3ζ.CD22.CAR T cells manufactured from the initial cryopreserved apheresis product. Although a CT scan on day 35 indicated PR, biopsy revealed disease progression within 11 days with retained expression of CD19/CD20/CD22, eliminating antigen escape as the mechanism of failure. Finally, ∼70 days after receiving CD22.CAR T cells, he received Cy/Flu, then 2 weekly infusions of 4-1BB/CD3ζ.CD20.CAR T cells (manufactured from a new apheresis). PET-CT performed 64 days later showed CR.33 Although ultimately beneficial for this patient, this sequential treatment approach is labor intensive, cost prohibitive, and logistically challenging. More important, sequential CAR T-cell therapy may increase the risk of antigen escape, as demonstrated by a 12-year-old with refractory DLBCL who developed a largely CD19- and CD22-negative tumor following sequential treatment with CD19 and CD22.CAR T cells.27 Thus, many groups prefer to target multiple antigens simultaneously.
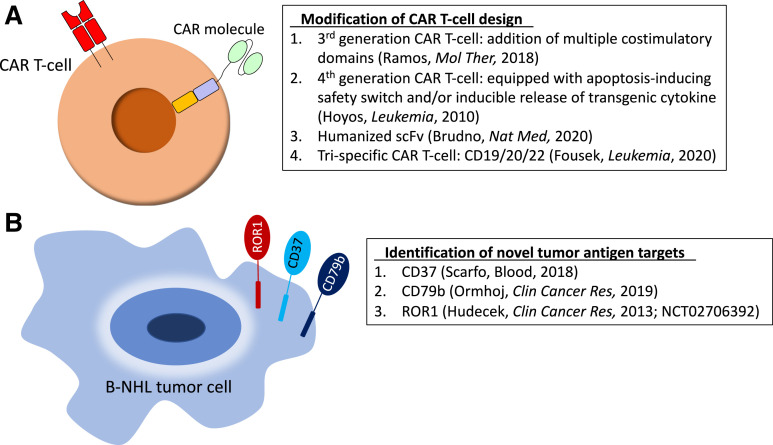
A phase 1/2 trial simultaneously targeting multiple antigens in a single product using tandem CD19/20.4-1BB/CD3ζ.CAR T cells after Cy/Flu enrolled 28 patients with r/r B-NHL and reported an ORR of 79% and a striking CR rate of 71%. Among patients with DLBCL, ORR was 75%. Rates of CRS and neurotoxicity were similar to single-antigen–targeting CAR T cells.34 In a preclinical report, Fousek et al35 manufactured CD19/20/22 CAR T cells that eliminated CD19-negative blasts from patients who experienced relapse after CD19.CAR T cells, as well as primary B-ALL in a CD19-knockout mouse model. Early evidence of CD22-targeting CARs and the success of CD20 monoclonal antibodies indicate this trispecific strategy holds promise for B-NHL. Identification of novel tumor antigen targets continues to be an active area of research, as shown in Figure 2, along with modifications to CAR design (as discussed in the CAR T cells for mature B-cell lymphomas section).
Figure 2.
Preclinical and early clinical strategies to optimize CAR T-cell therapy in B-NHL. (A) Modification of CAR T-cell design. (B) Identification of novel tumor antigen targets. ROR1, receptor tyrosine kinase-like orphan receptor 1; scFv, single-chain variable fragment.
Product-specific characteristics may impact responses
Patient age and T-cell quality both affect CAR T-cell function. Itzhaki et al36 analyzed differences in features and phenotypes of CD28-CD3ζ.CD19.CAR T cells manufactured from patients with r/r ALL or B-NHL. Although 100% and 94% of patients with ALL and B-NHL, respectively, reached the target dose of 1 × 106 CAR T cells per kilogram, products from patients with ALL had almost twice as many CAR T cells on expansion day 10 as those from patients with NHL. Furthermore, ALL products contained significantly more naive T cells and fewer central memory T cells than NHL products did. When expansion data were analyzed according to the age of patients with ALL (1-19 vs ≥20 years), CAR T cells from patients younger than 20 years old had significantly increased fold expansion compared with older patients. Aligning with previous studies, ORR (CR) was 84% (67%) in patients with ALL and 62% (31%) in patients with NHL. Although T-cell naivety from patients with ALL may contribute to superior in vitro expansion and cytokine secretion,37 the absence of other phenotypic differences (effector memory T-cell phenotype, expression of coinhibitory receptors) between the groups cannot fully explain differences in efficacy. Similarly, Singh et al38 reported pediatric patients with ALL with more naive T cells had in vitro expansion superior to that of the T cells from pediatric patients with NHL (few naive and central memory T cells). Thus, the quality of CAR T cells manufactured from patients with lymphoma may be inferior due to intrinsic T-cell features.
“Off-the-shelf” strategies
Though the percentage of adult patients with lymphoma in whom manufacture of autologous CAR T cells fails has decreased from its original 20%, manufacture failure remains a problem.6 Furthermore, patients with tumors such as BL often cannot wait the obligate 3 to 4 weeks for autologous CAR T-cell manufacture. Immediately available off-the-shelf options could overcome these limitations. HLA-mismatched allogeneic NK and NK-T cells, which are unlikely to cause graft-versus-host disease, have been used as off-the-shelf effectors with safe and promising early-phase results.39 In addition to eliminating manufacture failure/delays, off-the-shelf cord blood or healthy donor–derived, CAR-modified immune effectors minimize product heterogeneity, allowing selection of the product predicted to have the best in vivo activity. Our group is actively enrolling patients with r/r B-cell malignancies in a phase 1 clinical trial of CD19.CD28.CAR-NKT cells (NCT03774654). Other groups have used transcription activator–like effector nuclease–mediated gene editing of T-cell receptor α-chain and CD52 gene loci of non–HLA-matched donor cells transduced with a CD19.CAR to create a universal CAR T cell. These cells have been used successfully as a bridge to transplant in two pediatric patients.40 Phase 1 trials for CD19.CAR to create a universal CAR T-cell therapy are currently active and recruiting pediatric patients with r/r ALL (NCT02808442). Last, we and others are actively exploring alternative off-the-shelf CAR approaches using virus-specific T cells, which do not mediate graft-versus-host disease. Whether third-party CAR T cells can overcome the risk of allorejection to persist for the long term in relatively immunocompetent hosts is an open question.
Summary
Data for CAR T cells in pediatric mature B-cell lymphoma remain limited, lagging behind data in adult patients. Aside from accepted obstacles plaguing CD19.CAR T cells, lymphoma-specific characteristics may impact efficacy in pediatric B-NHL. Intrinsic differences in T cells collected from heavily pretreated patients with B-NHL, such as naivety and memory potential, affect CAR T-cell expansion, functionality, and persistence. Immunosuppressive cells, checkpoint molecules, and cytokines within the TME represent lymphoma-specific challenges. Ongoing trials combining checkpoint blockade or simultaneous targeting of inhibitory molecules as well as off-the-shelf strategies that may overcome two major obstacles of CAR therapy in lymphoma—manufacture failure and product heterogeneity—all hold promise. When revisiting the case above, we propose a stepwise approach: reimage in 3 to 4 weeks, given clinical improvement and the possibility of ongoing CAR T-cell response; maintain a low threshold for biopsy of an accessible PET-avid lesion to discriminate tumor flare from active disease; and consider addition of PD-1/PD-L1 inhibition in an effort to augment response.
Acknowledgment
We acknowledge Catherine Gillespie for scientific editing services.
References
- 1.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia [published correction appears in N Engl J Med. 2016;374(10):998]. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner RA, Finney O, Annesley C, et al. . Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Svoboda J, Chong EA, et al. . Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minard-Colin V, Brugières L, Reiter A, et al. . Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33(27):2963-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patte C, Auperin A, Gerrard M, et al. ; FAB/LMB96 International Study Committee. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109(7):2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minard-Colin V, Aupérin A, Pillon M, et al. ; Children’s Oncology Group. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med. 2020;382(23):2207-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anoop P, Sankpal S, Stiller C, et al. . Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53(10):1882-1888. [DOI] [PubMed] [Google Scholar]
- 11.Locke FL, Ghobadi A, Jacobson CA, et al. . Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramson JS, Palomba ML, Gordon LI, et al. . Pivotal safety and efficacy results from Transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed/refractory (R/R) large B cell lymphomas [abstract]. Blood. 2019;134(suppl 1):241. [Google Scholar]
- 13.Minard V, Maude SL, Buechner J, et al. . Bianca: phase II, single-arm, global trial to determine efficacy and safety of tisagenlecleucel in pediatric/young adult (YA) patients (Pts) with relapsed/refractory B-cell non-Hodgkin lymphoma (R/R B-NHL) [abstract]. J Clin Oncol. 2020;38(15 suppl):e22504. [Google Scholar]
- 14.Avigdor A, Shouval R, Jacoby E, et al. . CAR T cells induce a complete response in refractory Burkitt lymphoma. Bone Marrow Transplant. 2018;53(12):1583-1585. [DOI] [PubMed] [Google Scholar]
- 15.Enblad G, Karlsson H, Loskog ASI. CAR T-cell therapy: the role of physical barriers and immunosuppression in lymphoma. Hum Gene Ther. 2015;26(8):498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Giral S, Quintana NE, Cabrerizo M, et al. . Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76(2):462-471. [DOI] [PubMed] [Google Scholar]
- 17.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardeshna KM, Marzolini MAV, Norman J, et al. . Phase 1/2 study of AUTO3 the first bicistronic chimeric antigen receptor (CAR) targeting CD19 and CD22 followed by an anti-PD1 in patients with relapsed/refractory (r/r) diffuse large B cell lymphoma (DLBCL): results of cohort 1 and 2 of the Alexander study [abstract]. Blood. 2019;134(suppl_1):246. [Google Scholar]
- 19.Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1(26):2643-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill BT, Roberts ZJ, Rossi JM, Smith MR. Marked re-expansion of chimeric antigen receptor (CAR) T cells and tumor regression following nivolumab treatment in a patient treated with axicabtagene ciloleucel (axi-cel; KTE-C19) for refractory diffuse large B cell lymphoma (DLBCL) [abstract]. Blood. 2017;130(suppl 1):2825. [Google Scholar]
- 21.Cao Y, Lu W, Sun R, et al. . Anti-CD19 chimeric antigen receptor T cells in combination with nivolumab are safe and effective against relapsed/refractory B-cell non-Hodgkin lymphoma. Front Oncol. 2019;9:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke FL, Westin JR, Miklos DB, et al. . Zuma-6: phase 1-2 multicenter study evaluating safety and efficacy of axicabtagene ciloleucel (axi-cel; KTE-C19) in combination with atezolizumab in patients with refractory diffuse large b-cell lymphoma (DLBCL) [abstract]. J Clin Oncol. 2017;35(15 suppl):TPS7572. [Google Scholar]
- 23.Pham LV, Pogue E, Ford RJ. The role of macrophage/B-cell interactions in the pathophysiology of B-cell lymphomas. Front Oncol. 2018;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komohara Y, Niino D, Ohnishi K, Ohshima K, Takeya M. Role of tumor-associated macrophages in hematological malignancies. Pathol Int. 2015;65(4):170-176. [DOI] [PubMed] [Google Scholar]
- 25.Ruella M, Klichinsky M, Kenderian SS, et al. . Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7(10):1154-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parihar R, Rivas C, Huynh M, et al. . NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7(3):363-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalabi H, Kraft IL, Wang HW, et al. . Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103(5):e215-e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhang WY, Han QW, et al. . Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155(2):160-175. [DOI] [PubMed] [Google Scholar]
- 29.Till BG, Jensen MC, Wang J, et al. . CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah NN, Stetler-Stevenson M, Yuan CM, et al. . Minimal residual disease negative complete remissions following anti-CD22 chimeric antigen receptor (CAR) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) [abstract]. Blood. 2016;128(22):650.27281794 [Google Scholar]
- 31.Ramos CA, Savoldo B, Torrano V, et al. . Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126(7):2588-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Yang J, Zhou C, et al. . Early response observed in pediatric patients with relapsed/refractory Burkitt lymphoma treated with chimeric antigen receptor T cells. Blood. 2020;135(26):2425-2427. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Zhang Y. Sequential anti-CD19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: a case report and literature review [published correction appears in J Cancer Res Clin Oncol. 2020;146(8):2177]. J Cancer Res Clin Oncol. 2020;146(6):1575-1582. [DOI] [PubMed] [Google Scholar]
- 34.Tong C, Zhang Y, Liu Y, et al. . Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B cell lymphoma. Blood. 2020;136(14):1632-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fousek K, Watanabe J, Joseph SK, et al. . CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression [published online ahead of print 24 March 2020]. Leukemia. doi:10.1038/s41375-020-0792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itzhaki O, Jacoby E, Nissani A, et al. . Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients. J Immunother Cancer. 2020;8(1):e000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommermeyer D, Hudecek M, Kosasih PL, et al. . Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med. 2016;8(320):320ra3. [DOI] [PubMed] [Google Scholar]
- 39.Liu E, Marin D, Banerjee P, et al. . Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qasim W, Zhan H, Samarasinghe S, et al. . Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013. [DOI] [PubMed] [Google Scholar]
- 41.Maude SL, Laetsch TW, Buechner J, et al. . Tisagenlecleucel in children and young adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wierda WG, Bishop MR, Oluwole OO, et al. . Updated phase 1 results of Zuma-3: Kte-C19, an anti-CD19 chimeric antigen receptor T cell therapy, in adult patients with relapsed/refractory acute lymphoblastic leukemia [abstract]. Blood. 2018;132(suppl). Abstract 897. [Google Scholar]
- 43.Turtle CJ, Hanafi L-A, Berger C, et al. . CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turtle CJ, Hanafi L-A, Berger C, et al. . Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor–modified T cells. Sci Transl Med. 2016;8(355):355ra116-355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kochenderfer JN, Somerville RPT, Lu T, et al. . Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T Cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborne W, Marzolini M, Tholouli E, et al. . Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR T cell therapy, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. J Clin Oncol. 2020;38(15_suppl):8001. [Google Scholar]