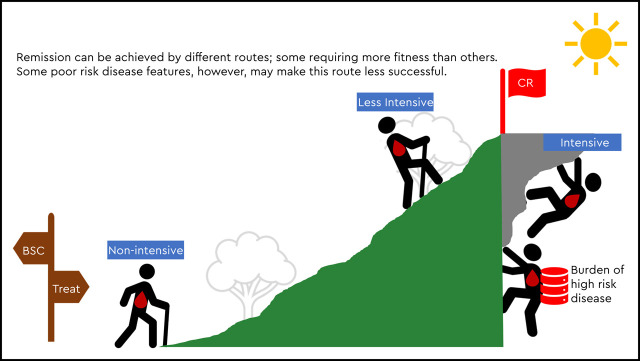
Visual Abstract
Abstract
The treatment choice for newly diagnosed patients with acute myeloid leukemia (AML) is no longer straightforward. Historically, patient fitness has been a major driver of the initial therapy decision based on the belief that intensive chemotherapy would be the optimal choice if a patient were “fit” enough to receive it. Tools based on chronological age, performance status, and comorbidities have been developed to help estimate patient fitness. With newer approved therapies that include nonintensive options such as IDH1 inhibition or less intensive options such as hypomethylating agent (HMA)- or low-dose cytarabine (LDAC)-based combinations with venetoclax, the choice of frontline AML therapy places more emphasis on disease-specific features, including cytogenetics and mutational profile. Moreover, newer treatments have higher response rates than what has been expected with older nonintensive options such as LDAC or HMA monotherapy. We present cases of three patients with AML with varying cytogenetic and molecular risks to demonstrate the important but changing role of patient fitness in the current era of expanding therapeutic options.
Learning Objectives
Learn how to incorporate fitness and disease features in frontline AML treatment selection
Know the tools available for estimating patient fitness and disease responsiveness
Introduction
The choice of therapy for newly diagnosed patients with acute myeloid leukemia (AML) is increasingly complicated. Until recently, therapeutic options have been limited to intensive induction chemotherapy with combination cytarabine and daunorubicin (eg, “7 + 3”) for any “fit” patient or nonintensive strategies with hypomethylating agent (HMA) or low-dose cytarabine (LDAC) monotherapy. Various algorithms incorporate prognostic factors such as chronological age and comorbidities to estimate patient fitness and predict the likelihood of treatment success and treatment-related mortality.1–4 The Ferrara et al consensus-based criteria (Table 1) also provide a practical framework for assessing fitness for intensive chemotherapy and have been used in recent phase 3 AML trials for this purpose.5,6 However, these tools are not routinely used in clinical practice, and patient fitness remains a largely subjective determination for many clinicians. With the approval of newer AML treatment options, the role of patient fitness in treatment selection has only grown in complexity. Now, clinicians must determine not only whether a newly diagnosed patient with AML is fit to withstand intensive induction chemotherapy but also whether induction chemotherapy is the optimal option, given the patient’s disease features and availability of newer, less intensive treatment options.
Table 1.
Consensus criteria proposed by Ferrara et al for defining patients unfit for intensive chemotherapy
| Fulfillment of at least one criterion suggests patient is unfit for intensive induction chemotherapy. |
| Advanced age (over 75 y) |
| Severe cardiac comorbidity |
| Severe pulmonary comorbidity |
| Severe renal comorbidity |
| Severe hepatic comorbidity |
| Active infection resistant to anti-infective therapy |
| Cognitive impairment |
| Low performance status (ECOG functional scale) |
| Any other comorbidity that the physician judges to be incompatible with chemotherapy |
Adapted from Ferrara et al.6
ECOG, Eastern Cooperative Oncology Group.
Frontline AML treatment landscape in the modern era
Current frontline intensive treatment options include cytarabine and daunorubicin-based induction chemotherapies such as conventional 7 + 3 and CPX-351. CPX-351 is a liposomal formulation of cytarabine and daunorubicin in a fixed molar ratio that is approved for older patients with newly diagnosed therapy-related AML or AML with myelodysplastic syndrome (MDS)-related changes. In the randomized phase 3 trial comparing CPX-351 to 7 + 3 for patients aged 60 to 75 years with newly diagnosed high-risk/secondary AML (defined as patients with a history of prior cytotoxic treatment, preceding MDS, or chronic myelomonocytic leukemia or having MDS-associated cytogenetic abnormalities), CPX-351 was associated with an improved overall remission rate (47.7% vs 33.3%; P = .016) and median overall survival (OS; 9.56 vs 5.95 months; hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.52 to 0.90; P = .003).7
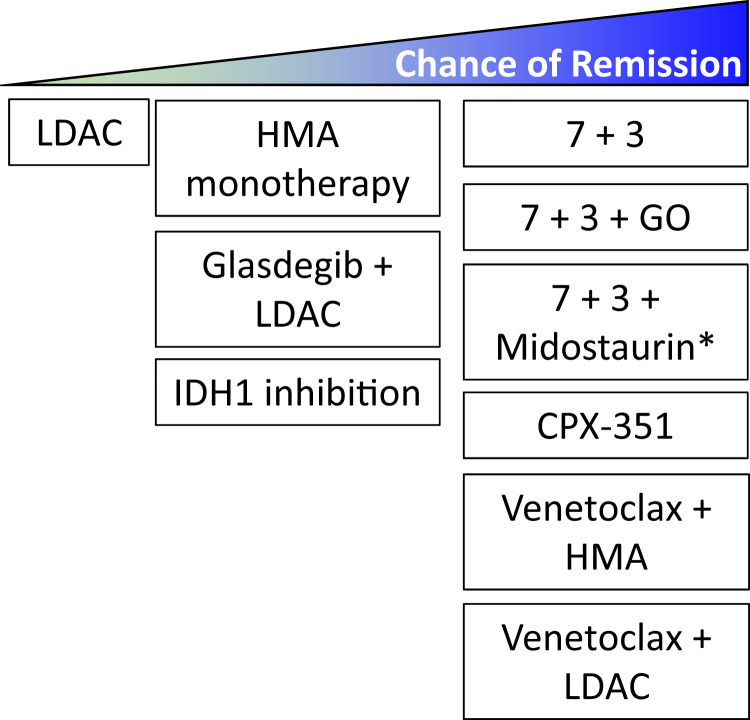
Current less intensive frontline options have expanded greatly and include HMA- and cytarabine-based combinations with venetoclax or glasdegib, and current frontline nonintensive options include the targeted IDH1 inhibitor ivosidenib (Figure 1 and Table 2). Venetoclax plus HMA (Ven/HMA) or LDAC (Ven/LDAC) and glasdegib plus LDAC combination therapies gained U.S. Food and Drug Administration (FDA) accelerated approval in 2018 for the treatment of patients with newly diagnosed AML age 75 years or older or of those who have serious cardiac, pulmonary, renal, or liver comorbidities that preclude intensive induction chemotherapy. These approvals were based on early-phase trials that demonstrated safety, tolerability, and robust composite response rates with each combination. Rates of complete remission (CR) and CR with incomplete count recovery (CRi) for Ven/HMA8 and Ven/LDAC9 were 67% and 62%, respectively. The subsequent phase 3 VIALE-A study confirmed that Ven plus azacitidine (Ven/Aza) compared with placebo plus azacitidine (Pbo/Aza) was associated with an improved CR + CRi rate (66.4% vs 28.3%, respectively) and median OS (14.7 vs 9.6 months; HR, 0.66; 95% CI, 0.52 to 0.85; P < .001).5 In the concurrent phase 3 VIALE-C study, the planned primary analysis confirmed the improved composite response rate with Ven/LDAC compared with Pbo/LDAC (48% vs 13%), but there was only a trend toward improved median survival with Ven/LDAC (HR, 0.75; 95% CI, 0.52 to 1.07; P = .11).10 A subsequent unplanned analysis with an additional 6 months of follow-up demonstrated a median OS of 8.4 months with Ven/LDAC compared with 4.1 months with Pbo/LDAC (HR, 0.70; 95% CI, 0.50 to 0.98; P = .04).10 Of note, in the phase 1b/2 study, prior HMA exposure compared with those without was associated with worse outcome with Ven/LDAC (4.1 months; 95% CI, 2.9 to 10.1 months).9 For the combination of LDAC + glasdegib, FDA approval was made after demonstration of an improved median OS of 8.8 months (80% CI, 6.9 to 9.9 months) compared with 4.9 months (80% CI, 3.5 to 6.0 months) with LDAC alone (HR, 0.51; 80% CI, 0.39 to 0.67, P = .0004).11
Figure 1.
Spectrum of frontline AML induction therapies. Though most frontline regimens have not been compared directly, the chance of remission varies among frontline options. *The 7+3+midostaurin regimen (RATIFY trial47) was assessed in patients aged 18 to 59 years old with FLT3-mutated AML. The RATIFY trial did not show a difference in complete remission (CR) rate between 7+3+midostaurin and 7+3 alone using the strict protocol criteria of remission achievement within 60 days. However, when expanding the response definition to include CRs during protocol treatment and within 30 days after treatment discontinuation, the CR rate was significantly higher in patients randomized to the midostaurin arm (68% vs 61%; P = .04).
Table 2.
Clinical outcomes, including response, survival, and notable toxicities in clinical trials of current frontline acute myeloid leukemia therapies
| Phase | Patient population | Therapy arms | Sample size | CR | Other clinical parameters: duration of response, PFS, OS | Notable toxicities | Reference | Take-Home | |
|---|---|---|---|---|---|---|---|---|---|
| Grade ≥3 AEs | Frequency | ||||||||
| 3 | Age 17-60 y Treatment-naïve AML |
Daunorubicin 45 mg/m2 × 3 d + cytarabine 100 mg/m2 × 7 d | 318 | 57.3%* | OS 15.7 mo* | Cardiac | 7.2% (ns) | Fernandez et al47 | Daunorubicin 90 mg/m2 improves upon 45 mg/m2 |
| F + N | 34.9% (ns) | ||||||||
| Death | 4.5% (ns) | ||||||||
| Daunorubicin 90 mg/m2 × 3 d + cytarabine 100 mg/m2 × 7 d | 315 | 70.6%* | OS 23.7 mo* | Cardiac | 7.9% (ns) | ||||
| F + N | 35.9% (ns) | ||||||||
| Death | 5.5% (ns) | ||||||||
| 3 | Age ≥60 y Treatment-naïve AML |
Daunorubicin 45 mg/m2 × 3 d + Cytarabine 200 mg/m2 × 7 d | 411 | 54%* | 2-y EFS (age 60-65 y) 14%* | Infection | 79%* | Löwenberg et al48 | Daunorubicin 90 mg/m2 improves upon 45 mg/m2 but up to age 65 y |
| 2-y OS (age 60-65 y) 23%* | 30-d mortality | 12% (ns) | |||||||
| Daunorubicin 90 mg/m2 × 3 d + cytarabine 200 mg/m2 × 7 d | 402 | 64%* | 2-y EFS (age 60-65 y) 29%* | Infection | 87%* | ||||
| 2-y OS (age 60-65 y) 38%* | 30-d mortality | 11% (ns) | |||||||
| 3 | Age 16-72 y Treatment-naïve AML |
Daunorubicin 60 mg/m2 × 3 d + cytarabine 100 mg/m2 × 7 d | 602 | 75% (ns) | 2-y OS 60% (ns) | 60-d mortality | 5%* | Burnett et al49 | Daunorubicin 90 mg/m2 is not superior to 60 mg/m2 |
| 2-y RFS 48% (ns) | |||||||||
| Daunorubicin 90 mg/m2 × 3 d + cytarabine 100 mg/m2 × 7 d | 604 | 73% (ns) | 2-y OS 59% (ns) | 60-d mortality | 10%* | ||||
| 2-y RFS 51% (ns) | |||||||||
| 3 | Age 18-59 y Treatment-naïve AML |
Placebo + daunorubicin 60 mg/m2 × 3 d + cytarabine 200 mg/m2 × 7 d | 354 | 53.5% (ns) | mOS 25.6 mo* | F + N | 82% (ns) | Stone et al50 | Midostaurin with induction chemotherapy is approved for newly diagnosed FLT3-mutant AML |
| 4-y OS 44.3% (ns) | |||||||||
| Midostaurin + daunorubicin 60 mg/m2 × 3 d + cytarabine 200 mg/m2 × 7 d | 335 | 58.8% (ns) | mOS 74.7 mo* | F + N | 82% (ns) | ||||
| 4-y OS 51.4% (ns) | |||||||||
| 3 | Age 50-70 y Treatment-naïve AML, CD33 expression not required |
Daunorubicin 60 mg/m2 × 3 d + cytarabine 200 mg/m2 × 7 d | 139 | 72% (ns) | 2-y EFS 17.1%* | TRM | 8% (P = .051) | Castaigne et al51 | GO + induction chemotherapy is approved for newly diagnosed CD33+ AML |
| 2-y OS 41.9% | |||||||||
| 2-y RFS 22.7%* | |||||||||
| Daunorubicin 60 mg/m2 × 3 d + cytarabine 200 mg/m2 × 7 d + gemtuzumab ozogamicin (3 mg/m2) | 139 | 73% (ns) | 2-y EFS 40.8%* | TRM | 2% (P = .051) | ||||
| 2-y OS 53.2%* | |||||||||
| 2-y RFS 50.3%* | |||||||||
| 3 | Age 60-75 y t-AML, s-AML, or de novo AML with MDS-related cytogenetic abnormalities | Daunorubicin 60 mg/m2 × 3 d + cytarabine 100 mg/m2 × 7 d | 156 | 25.6%* | mOS 5.95* | F + N | 70.9% (ns) | Lancet et al7 | CPX-351 is approved for t-AML, s-AML, or de novo AML with MDS-related changes |
| mEFS 1.31mo* | |||||||||
| CPX-351 100 U/m2 (44mg/m2 daunorubicin and 100mg/m2 cytarabine) | 153 | 37.3%* | mOS 9.56mo* | F + N | 68% (ns) | ||||
| mEFS 2.53mo* | |||||||||
| 3 | Primarily age >60 y | Hydroxyurea | 99 | 1%* | OS odds ratio 0.61* | Cardiac | 11% (ns) | Burnett et al52 | Single-agent LDAC is a low-intensity option |
| LDAC 20 mg bid | 103 | 18%* | Cardiac | 10% (ns) | |||||
| 3 | Age ≥65 y Treatment-naïve AML, >30% bone marrow blasts |
Conventional care regimens (induction, LDAC, BSC only) | 247 | 21.9% (ns) | mOS 6.5 mo* | F + N | 30% (ns) | Dombret et al53 | Single-agent azacitidine is a low-intensity option |
| 1-y OS 34.2%* | |||||||||
| mEFS 4.8 mo (ns) | |||||||||
| mRFS 10.5 mo (ns) | |||||||||
| Azacitidine 75 mg/m2 | 241 | 19.5% (ns) | mOS 10.4 mo* (benefit driven by comparison of azacitidine with BSC) | F + N | 28% (ns) | ||||
| 1-y OS 46.5%* | |||||||||
| mEFS 6.7 mo (ns) | |||||||||
| mRFS 9.3 mo (ns) | |||||||||
| 3 | Age ≥65 y Treatment-naïve AML with poor- or intermediate-risk cytogenetics |
Treatment choice (supportive care, LDAC 20 mg/m2) | 243 | CR + CRp: 7.8%* | mOS (ITT) 5.0 mo* | F + N | 22% | Kantarjian et al54 | Single-agent decitabine is a low-intensity option |
| Decitabine 20 mg/m2 | 242 | CR + CRp: 17.8%* | mOS (ITT) 7.7 mo* | F + N | 32% | ||||
| 3 | Age ≥75 y Treatment-naïve AML, ineligible for standard therapy |
Placebo + azacitidine 75/mg/m2 | 145 | 17.9%* | 9.6 mo* | F + N | 19% | DiNardo et al5 | Led to accelerated approval for venetoclax + HMA for patients ineligible for intensive therapy |
| Venetoclax 400mg + azacitidine 75 mg/m2 | 286 | 36.7%* | 14.7 mo* | F + N | 42% | ||||
| 3 | Age ≥ 18 y Treatment-naïve AML, ineligible for intensive chemotherapy |
Placebo + LDAC 20 mg bid | 68 | 7%* | mOS 4.1 mo* | F + N | 29% | Wei et al 10 | Led to accelerated approval for venetoclax + LDAC for patients ineligible for intensive therapy |
| Venetoclax 600 mg + LDAC 20 mg bid | 143 | 27%* | mOS 8.4 mo* | F + N | 32% | ||||
| 2 | Age ≥55 y Treatment-naïve AML, ineligible for standard therapy; high-risk MDS included |
LDAC 20mg bid | 44 | 2.3%* | mOS 4.9mo* | F + N | 24.4% | Cortes et al11 | Glasdegib + LDAC approved for patients ineligible for intensive therapy |
| Glasdegib + LDAC 20mg bid | 88 | 17.0%* | mOS 8.8mo* | F + N | 35.7% | ||||
| 1 | Age 64-87 y IDH1-mutated, treatment-naïve AML, ineligible for standard therapy |
Single-agent ivosidenib 500 mg | 34 | 30.3% | mOS 12.6 mo | F + N | 6% | Roboz et al33 | Led to approval of ivosidenib for adults age ≥75 y with IDH1-mutant AML |
| 12-mo OS 51.1% | Differentiation syndrome | 9% | |||||||
| 1/2 | Age 58-87 y IDH2-mutant, treatment-naïve AML, ineligible for standard therapy |
Single-agent enasidenib | 39 | 18% | ORR 30.8% | TLS | 8% | Pollyea et al55 | Enasidenib as frontline therapy for IDH2-mutant AML has some benefit but is not yet approved |
| mOS 11.3 mo | Differentiation syndrome | 10% | |||||||
| mEFS 5.7 mo | |||||||||
| 2 | Age >60 y Newly diagnosed AML, newly diagnosed sAML, treated sAML, relapsed/refractory AML, high-risk MDS |
Venetoclax 400 mg + 10 d of decitabine 20 mg/m2 | 184 | CR + CRi or marrow CR: 86% (newly-diagnosed AML) | mOS 18.1 mo (newly diagnosed AML) | F + N with infection | 46% | Maiti et al44 | Venetoclax with 10-d decitabine is an emerging treatment option |
| 67% (untreated sAML) | mOS 7.8 mo (untreated sAML) | F + N | 28% | ||||||
| 39% (treated sAML) | mOS 6.0 mo (treated sAML) | ||||||||
| 42% (R/R AML) | mOS 7.8 mo (R/R AML) | ||||||||
*Denotes statistical significance with p < 0.05. AE, adverse event; AML, acute myeloid leukemia; bid, twice daily; CR, complete remission; CRi, complete remission with incomplete count recovery; HMA, hypomethylating agent; ITT, intention to treat; LDAC, low-dose cytarabine; MDS, myelodysplastic syndrome; OS, overall survival; PFS, progression-free survival; t-AML, therapy-related acute myeloid leukemia. TLS, tumor lysis syndrome; sAML, secondary AML; R/R, relapsed/refractory; ns, not statistically significant with p > 0.05; F+N, fever and neutropenia; GO, gemtuzumab ozogamicin; mRFS, median relapse-free survival; mEFS, median event-free survival; mOS, median overall survival; EFS, event-free survival; CBF, core binding factor; BSC, best supportive care; CRp; CR without platelet recover.
Importantly, clinical benefit with these newer, less intensive combination strategies have been observed across historically difficult-to-treat secondary AML and poor-risk cytogenetic and molecular subgroups commonly enriched in the older AML population.12 In the phase 1b/2 trial evaluating Ven/LDAC in patients with previously untreated AML who were ineligible for intensive chemotherapy, CR + CRi rates of 35%, 63%, and 42% were seen in secondary AML and intermediate- and poor-risk cytogenetic groups, respectively.9 In the VIALE-A study comparing Ven/Aza with Pbo/Aza, the CR + CRi rates were 74% vs 32% and 53% vs 23% in the intermediate- and poor-risk cytogenetic groups, respectively.5 In addition, the CR + CRi rate for patients with TP53 mutations was 55% with Ven/Aza compared with 0% for those who received Pbo/Aza.5
Notably, data in support of newer treatments have relied on composite CR data that incorporate rates of CRi or CR with partial hematologic recovery (CRh). Although these variations in CR are clinically meaningful when contrasted with rates of stable or progressive disease, they can obscure whether treatment has led to deep remission achievement vs less desirable outcomes such as treatment-related cytopenias. The impact of these treatments on minimal residual disease (MRD), which is highly prognostic for outcome,13 is also just beginning to be understood. In the phase 1b study of Ven/HMA, 83 of 97 patients with CR/CRi had MRD data, and the median OS had not been reached for the 28 patients who achieved MRD <10−3 or for the 55 patients who did not, though the median duration of response appears to favor those with MRD <10−3 (not reached vs 11.3 months if MRD ≥10−3).8 The rate of MRD <10−3 is lower with Ven/LDAC. In the VIALE-A study, 6% of patients who received Ven/LDAC vs 1% of patients who received Pbo/LDAC had a flow cytometry–based MRD <10−3.10
Overall, treatment selection in the modern era is increasingly influenced by disease-specific features such as cytogenetics or mutation profile and not solely by patient fitness. We present three patient cases to examine the current, changing role of patient fitness relative to other features in frontline AML treatment decision making using today’s expanded therapeutic armamentarium (Figure 1).
Case descriptions
Case presentation 1
A 61-year-old man with obesity (body mass index, 33 kg/m2), well-controlled type 2 diabetes mellitus (non–insulin dependent), coronary artery disease (without anginal symptoms since coronary artery stenting 5 years ago), and a 40–pack-year smoking history presented to the emergency department with shortness of breath. He admitted to feeling unwell for the past 6 months. He had an Eastern Cooperative Oncology Group (ECOG) performance status of 1 and was living alone. He has an older sister who lives nearby. He was found to have a white blood cell (WBC) count of 10 000/µL, a platelet count of 7000/μL, and 80% peripheral blasts. A bone marrow biopsy confirmed a diagnosis of AML with MDS-related changes, del7q cytogenetics, and mutations in SRSF2 (P95H; variant allele fraction [VAF], 29.8%), ASXL1 (G642fs; VAF, 24.7%), and SH2B3 (E208Q; VAF, 38.5%). His baseline echocardiogram revealed an ejection fraction of 55% with no evidence of heart failure. He had a normal chest radiograph. He was interested in treatment and bone marrow transplant but worried that his comorbidities may be limiting. How does fitness play a role in deciding between intensive and less intensive treatment?
This patient was ≥60 years old with multiple comorbidities, raising concerns about his fitness for intensive chemotherapy. There are no universally accepted criteria for assessing fitness. Chronologic age and performance status per the Karnofsky performance status scale or ECOG criteria are commonly used estimates of patient fitness, and both older age and poorer performance status (eg, ECOG ≥2) have been correlated with worse outcomes after intensive treatment.14,15 More comprehensive tools include the Charlson Comorbidity Index and Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), which capture organ dysfunction and predict early death rates with induction chemotherapy or transplant.3,4 AML-specific calculators such as the AML composite model (which integrates HCT-CI, cytogenetic risk, and age)1 and a treatment-related mortality calculator (which integrates age, performance status, and select laboratory indices)2 are also able to predict 2-year mortality with intensive therapy.16 Geriatric assessment strategies may predict survival even more accurately by including dimensions such as cognitive impairment and objectively measured physical function.17-19 Gait speed, for instance, is a marker of frailty that is prevalent among older patients with hematologic malignancies and is associated with increased chemotherapy-related toxicity, poor response to treatment, and mortality.20,21 Despite these assorted tools, assessing a patient’s fitness can remain a complex and subjective task for many clinicians, particularly when evaluating patients aged 55 to 75.
To assess the fitness of the patient presented in this case, our preference is to use the consensus-based, structured criteria proposed by Ferrara et al5,6 (Table 1) that were recently adapted to determine patient eligibility in the phase 3 VIALE-A and VIALE-C trials. Using these criteria, we see that the patient has several comorbidities, but none clearly preclude his consideration for intensive induction therapy. Instead, we would rely more on his cytogenetics, mutational profile, and stated interests in a bone marrow transplant for our treatment decision. Given the patient’s MDS-related cytogenetics and age between 60 and 75, CPX-351 would be recommmended.7 In the phase 3 study comparing CPX-351 with 7 + 3 chemotherapy, the frequency of adverse events was comparable, although the duration of neutropenia and thrombocytopenia was longer with CPX-351 than with 7 + 3 (median time to absolute neutrophil count ≥500/μL, 35 vs 29 days; median time to platelet count ≥50 000/μL, 36.5 vs 29 days). Notably, prolonged neutropenia with CPX-351 was not associated with an increase in infection-related events or early mortality (5.9% and 10.6% at 30 days; P = .149; 13.7% and 21.2% at 60 days; P = .097).7 In addition, for patients whom we intend to consolidate with a bone marrow transplant, CPX-351 is more likely than 7 + 3 to improve survival (HR, 0.46; 95% CI, 0.24 to 0.89; P = .009).7
If the patient had more significant comorbidities and were unfit for intensive induction chemotherapy, we would recommend the combination of Ven/Aza. In comparison with CPX-351, Ven/Aza has a high response rate among those with secondary AML (CR + CRi, 67%) and lower rates of febrile neutropenia,5 though it should be noted that a head-to-head comparison with CPX-351 in a randomized trial has not been done. When compared with Pbo/Aza in the phase 3 VIALE-A study, Ven/Aza treatment resulted in increased rates of grade ≥3 thrombocytopenia (45% vs 38%, respectively), neutropenia (42% vs 29%, respectively), and febrile neutropenia (42% vs 19%, respectively), though 30-day mortality rates were similar between arms (7% vs 6%, respectively). Whether Ven/Aza compared with CPX-351 can improve survival for fit, elderly (age 60 to 75 years) patients who undergo consolidation with bone marrow transplant has not yet been assessed. In the VIALE-A trial, the OS benefit was significant in patients age ≥75 years receiving Ven/Aza compared with Pbo/Aza (HR, 0.54; 95% CI, 0.39 to 0.73). In contrast, a statistically significant OS benefit was not appreciated in patients <75 years of age (HR, 0.89; 95% CI, 0.59 to 1.33), though the study was not adequately powered to assess differences based on age.5
Treatment of older patients with AML will evolve further as we continue to tailor therapy on the basis of mutational profiles. The phase 3 trial comparing 7 + 3 with or without the e-selectin antagonist GMI-1271 (NCT03701308) is underway (Table 2), as is the Beat AML Master Trial that uses rapid genomic screening (<7 days) to assign therapy for patients 60 years of age and older.22 Early results from Beat AML suggest that patients enrolled in the trial had longer OS than those who received standard therapy, but increases in OS were also appreciated in those who went on to receive alternative investigational therapy. There are also efforts to incorporate functional assays into treatment selection. Using 672 specimens from the Beat AML trial, large-scale integration of whole-exome sequencing, RNA sequencing, and in vitro drug sensitivity analyses has revealed drug sensitivities specific to previously unrecognized mutational combinations.23 Incorporation of other functional tools, such as mitochondrial BH3 profiling assays,24 into trial or treatment selection in the era of venetoclax-based therapies is also awaited.
Case presentation 2
A 74-year-old retired fireman was referred for a WBC count of 1000/μL with 40% peripheral blasts associated and 1 month of dyspnea on exertion. He had a history of well-controlled hypertension. Studies yielded a diagnosis of normal karyotype AML with mutations in DNMT3A (R882H, VAF, 41.5%), NPM1 (W288fs; VAF, 44.6%), and IDH1 (R132H; VAF, 43.3%). He had an ECOG performance status of 1. He was living with his supportive wife and was independent in all activities. He was eager to undergo treatment with the goal of prolonging his life, but he was not interested in bone marrow transplant. He refused to undergo any further isolation after struggling with the stay-at-home order during the coronavirus disease 2019 pandemic. He wished to spend as much quality time as possible with his four young grandchildren. He did not meet the Ferrara et al criteria for unfitness6. Which frontline treatment option is recommended?
This patient’s case highlights treatment options for fit, elderly patients with newly diagnosed AML who are relatively chemosensitive. This patient had a favorable risk, non-core binding factor AML per European LeukemiaNet (ELN) criteria due to the normal karyotype, present NPM1 mutation, and absence of an FLT3 internal tandem duplication mutation with a high allelic ratio.25 The favorable prognosis of NPM1-mutated AML is age dependent (median OS, 10.5 years vs 1.7 years in younger vs older patients,26 respectively) and possibly diminished in the presence of select co-occurring poor-risk mutations.27
Treatment options of various intensities are available for patients with previously untreated NPM1-mutated AML. HMA monotherapy is a low-intensity option, but it confers a modest CR rate of 23% to 28% and a median OS ranging from 4.8 to 9.3 months.28,29 Newer venetoclax-based combination therapy offers a higher response rate and more durable remission for this population (composite CR + CRi, >80%; 2-year OS, 71.8%).30 Notably, our patient has a co-occurring IDH1 mutation, and this can increase the relative chemosensitivity of NPM1-mutated AML.31 In the phase 3 VIALE-A trial, patients with IDH1/2-mutated AML had a very high response rate when receiving Ven/Aza compared with Pbo/Aza (75% vs 11%, respectively), and those with IDH1 mutations also had improved OS (HR, 0.28; 95% CI, 0.12 to 0.65).5 Standard intensive induction chemotherapy with 7 + 3 is also reasonable for fit older patients with favorable-risk disease, but compared retrospectively with venetoclax-based combinations, the relative clinical benefit was less robust, with a CR rate of 56%, 1-year OS rate of 36%, and median OS of 10.8 months in patients older than 65.29 Intensive induction chemotherapy also confers toxicities and risk of treatment-related mortality for patients >60 years old that are lessened with less intensive but active combinations such as Ven/Aza.
It should be noted that this patient technically does not meet the FDA indications for Ven/Aza: his age is <75 years, and he would not have been deemed unfit for intensive chemotherapy based on the adapted Ferrara et al criteria used by the phase 3 Ven/Aza VIALE-A study.6 However, although the Ferrara et al criteria can be a useful tool, they lack prospective data assessing their role in identifying the optimal induction strategy (eg, 7 + 3 based vs Ven/Aza) in older patients with AML. Thus, our overall recommendation would be consideration of a clinical trial. In the absence of a trial, we would favor off-label Ven/Aza as his frontline therapy, given his previously discussed molecular profile and preference to reduce risk of prolonged hospitalization and to avoid bone marrow transplant. Grade 3 to 4 febrile neutropenia is seen in 43% of patients treated with Ven/Aza.8 To reduce treatment-related toxicities such as neutropenic fever and infection that are seen with Ven/Aza, we recommend reviewing the management guidelines on antimicrobial, antifungal, and tumor lysis syndrome prophylaxis to reduce complications.32 Notably, Ven/Aza shares a similar 30- and 60-day treatment-related mortality (TRM) rate with Pbo/Aza.
If this patient were less fit and were unlikely to tolerate myelosuppression or neutropenia-related infections, the small molecule inhibitor ivosidenib would be a reasonable option. Ivosidenib was originally approved by the FDA for relapsed or refractory AML and gained additional approval in 2019 for the treatment of patients with newly diagnosed IDH1-mutated AML who are ineligible for standard therapy based on age ≥75 or the presence of comorbidities. An expanded phase 1 trial in which 34 newly diagnosed IDH1-mutated patients with AML were treated with ivosidenib demonstrated a composite CR + CR with CRh rate of 42.4%.33 The median duration of CR + CRh has not yet been reached, though the lower bound of the 95% CI was 4.6 months, and the median OS was 12.6 months (95% CI, 4.5 to 25.7).33
Even more treatment options may become available in the near future. Ivosidenib at the approved dose of 500 mg per day given continuously has been combined with standard dose azacitidine for up-front treatment in patients with IDH1-mutated AML who are ineligible for intensive chemotherapy based on investigator assessment.34 Treatment has generally been tolerable, with common adverse events including thrombocytopenia, nausea, diarrhea, and anemia. Notably, combination ivosidenib plus azacitidine has an overall response rate of 78% (CR, 57%; CRi/CRp, 13%; morphologic leukemia-free state, 9%) with a median time to response of 1.8 months. There were also high rates of mutation clearance (79%; 11 of 14 patients with CR + CRh) and measurable residual disease negativity (83%; 10 of 12 patients with CR + CRh) with azacitidine plus ivosidenib.35 Data from the phase 3 AGILE study comparing azacitidine plus ivosidenib vs azacitidine plus placebo are eagerly awaited to confirm these benefits of combination therapy. Finally, a phase 1b/2 study for IDH1-mutated myeloid malignancies is underway to assess the combination of ivosidenib and venetoclax with or without azacitidine to further deepen responses.36
Case presentation 3
A 64-year-old woman with a history of breast adenocarcinoma (definitively treated with surgical excision, local radiation, and 5 years of hormone therapy) and depression was referred to an oncology clinic after presenting to her primary care physician with petechiae. She had a WBC count of 4000/µL, hemoglobin of 8 g/dL, platelet count of 40 000/µL, and 30% circulating blasts. A bone marrow biopsy revealed monosomal complex karyotype (46,XY,del[5][q15q35],-12,-Y,-3,-7,-16[8]/43 to 44,idem,-Y,-3,del[7][q11.2],-16,-18,+22[2]/46,XY,del[5],del[9][p22][5]) and mutations in TP53 (R248Q; VAF, 76.2%) and U2AF1 (S34F; VAF, 11.3%). She had an ECOG performance status of 1. She was a recently retired nurse who lived with her partner. She was interested in remission-inducing therapy and transplant. She did not meet any criteria for unfitness according to Ferrara et al criteria.6
This patient has adverse-risk disease on the basis of ELN criteria, given her complex karyotype and presence of TP53 mutation. These patients are not only less responsive to intensive chemotherapy but also frequently older, less fit with more comorbidities, and experience a higher risk of treatment-related adverse events and TRM. For patients ≥70 years of age, TRM can be 26% during the first month of conventional induction chemotherapy,37 although TRM has been declining in newly diagnosed patients with AML who receive intensive induction regimens and may have less impact on outcomes than rates of disease resistance.15,38 Still, CR + CRi rates are generally lower for adverse-risk disease after intensive chemotherapy than for more favorable risk groups, and the 10-year OS is ∼10%.39 Older patients are also more likely to have a complex karyotype, with 40% to 60% of these patients having a concurrent TP53 mutation, for whom the median OS is 4 months with a 3-year OS <5%.40 This patient also has therapy-related AML featuring TP53 and a secondary-type lesion in U2AF1. The presence of secondary-type AML lesions defines a distinct disease subset associated with worse clinical outcomes, higher reinduction rates, and decreased event-free survival.41
This patient is relatively fit for intensive therapy, but TP53-mutated AML is a notoriously chemoresistant disease subtype that makes intensive induction chemotherapy such as 7 + 3 less effective.41 Although CPX-351 is an approved therapy for therapy-related AML, those with unfavorable cytogenetics did not benefit compared with conventional 7 + 3 (median OS, 6.6 vs 5.16 months, respectively; HR 0.73; 95% CI, 0.51 to 1.06).7 Furthermore, no clear benefit was observed among those with mutations in TP53 in either treatment arm (4.5 vs 5.1 months for CPX-351 and 7 + 3, respectively; HR, 1.19; 95% CI, 0.70 to 2.05).42 Thus, we would not recommend CPX-351 for this patient.
Instead, we would prefer a clinical trial that is HMA-based (Table 3) or, in the absence of a clinical trial option, off-label use of Ven/HMA, with the caveat that in the phase 3 VIALE-A study, fit patients <75 years old, like the one in our vignette, would not have been included. Granted, for patients with TP53-mutated AML, Ven/HMA offers a median OS of 7.2 months,8 which is not much longer than what has been observed with intensive chemotherapy, as described above. Durability of response remains a common issue for this poor-risk subset. However, Ven/HMA, which has a high CR + CRi rate for TP53-mutated AML (47%) compared with HMA alone, is much less toxic and has reduced TRM compared with 7 + 3, making it a more attractive regimen and bridge to bone marrow transplant. Another option is 10-day decitabine monotherapy, which results in a composite CR rate of 46% (including CR + CRi + marrow CR), robust though incomplete mutation clearance, and a median OS similar to that observed in intermediate-risk patients with AML who received the same regimen (median OS, 11.6 months).43
Table 3.
Ongoing investigational therapies for frontline acute myeloid leukemia
| ClinicalTrials.gov identifier | Study | Phase | Target/agent | Key patient population |
|---|---|---|---|---|
| NCT03826992 | CPX-351 + venetoclax | 1 | Venetoclax is a BCL-2 inhibitor. | Age <40 y relapsed/refractory |
| NCT03471260 | Venetoclax + ivosidenib + azacitidine | 1/2 | Ivosidenib is an IDH1 inhibitor. | Age ≥18 y IDH1 mutant, relapsed/refractory |
| NCT03248479 | Magrolimab vs magrolimab + azacitidine | 1 | Magrolimab is an anti-CD47 mAb. | Age ≥18 y relapsed/refractory or treatment-naïve and unsuitable for intensive chemotherapy |
| NCT04086264 | IMGN632 vs | 1/2 | IMGN632 is an anti-CD123 ADC. | Age ≥18 y CD123-positive relapsed/refractory or treatment-naïve |
| IMGN632 + azacitidine vs | ||||
| IMGN632 + venetoclax vs | ||||
| IMGN632 + azacitidine + venetoclax | ||||
| NCT04150029 | MGB453 + azacitidine + venetoclax | 2 | MGB453 is an anti-TIM-3 mAb. | Age ≥18 y treatment-naïve, unsuitable for intensive chemotherapy |
| NCT03701308 | 7 + 3 vs 7 + 3 + uproleselan (GMI-1271) | 3 | Uproleselan is an E-selectin inhibitor. | Age ≥60 y, eligible for induction |
| NCT03258931 | Crenolanib + 7 + 3 vs midostaurin + 7 + 3 | 3 | Crenolanib is an FLT3 inhibitor. | Age 18-60 y FLT3-ITD and/or D835 mutations, de novo AML |
| NCT04140487 | Azacitidine, venetoclax, and gilteritinib | 1/2 | Gilteritinib is an FLT3 inhibitor. | Age ≥18 y relapsed/refractory or newly diagnosed FLT-3-mutated AML (for phase 2 portion) |
| NCT03709758 | Venetoclax plus 7 + 3 | 1 | Venetoclax is a BCL-2 inhibitor. | Age 18-60 y without FLT3 mutation or inv16 or t(8;21) |
| NCT03113643 | Azacitidine, venetoclax, and SL-401 | 1 | SL-401 is a recombinant IL-3 genetically fused to truncated diphtheria toxin payload. | Age ≥18 y with CD123/IL3RA expression on myeloblasts |
AML, acute myeloid leukemia; mAb, monoclonal antibody, ADC, antibody-drug conjugate; ITD, internal tandem duplication.
When considering Ven/HMA for the subset of patients with TP53-mutated AML, the choice of 5- vs 10-day decitabine warrants particular consideration. Early data from combination 10-day decitabine plus venetoclax show an impressive median OS of 18.1 months for those with newly diagnosed AML, but a median OS of only 7.8 months for patients with untreated secondary AML (n = 15).44 However, encouraging early results show that all treatment-naïve patients treated with 10-day decitabine plus venetoclax and subsequently consolidated with bone marrow transplant were still alive at 1 year. Additional data are required to understand the optimal HMA dose in Ven/HMA-based therapies for the therapy-related TP53-mutated cohort.
Finally, two novel agents are under investigation with favorable early results for TP53-mutated AML, although the data are derived from small patient numbers (Table 3). Azacitidine plus the anti-CD47 antibody magrolimab showed a CR + CRi rate of 56% for the overall cohort and a CR + CRi rate of 75% for the TP53-mutated cohort (n = 12) with a 6-month OS rate of 91%.45 Azacitidine plus the small molecule “P53-refolding” agent APR-246 resulted in CR in 9 of 16 evaluable patients. Among the patients with CR, 7 of 9 had cytogenetic CR and 100% had TP53 mutation clearance (VAF cutoff was 2%).46
In summary, for the patient in our case vignette with very poor–risk disease, we would prefer a clinical trial if the patient is eligible and amenable. However, we recognize that a clinical trial may not be feasible on the basis of patient preference and location and that there may be strict eligibility criteria for early-phase studies. If a clinical trial is not an option, we would recommend a remission-inducing option such as Ven/HMA, which has a lower risk of TRM and higher chance of remission than current intensive chemotherapies such as CPX-351. We would also make a referral to our bone marrow transplant colleagues. If the patient’s fitness level declines with disease progression, an early referral to our palliative care colleagues would be essential.
Conclusion
Frontline treatment options for AML have expanded substantially in the past few years. For several decades, treatment options have been dichotomous between intensive induction chemotherapy strategies and nonintensive options such as supportive care, and patient fitness primarily influenced the decision between these two options. Treatment options in the modern era now exist on a spectrum of treatment intensity and toxicity while having efficacy for more specific disease cytogenetics and mutational profiles (Figure 1). Outcomes are eagerly awaited from ongoing studies of novel targeted therapies such as CD47 and e-selectin inhibition and triplet therapy approaches combining the backbone of Ven/HMA with a targeted therapy (Table 3). Novel agents are also increasingly incorporated into up-front treatment strategies with the goal of deepening response. Crucially, prospective studies are still lacking that compare intensive regimens such as 7 + 3 or CPX-351 with Ven/Aza, which would help in deciding whether patients objectively fit for intensive chemotherapy might actually benefit more from less intensive therapeutic options that are now available. Tools such as the Ferrara et al6 consensus criteria can be useful for determining unfitness for intensive induction chemotherapy, but, moving forward, functional and genomic biomarkers should be increasingly integrated to guide treatment selection, given the growing armamentarium of effective treatment options.
Acknowledgments
We thank Richard Stone and Daniel DeAngelo for critical review of the manuscript. J.S.G. is a recipient of a career development award from Conquer Cancer Foundation, a translational research project award from the Leukemia and Lymphoma Society, and National Cancer Institute grant K08CA245209. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number K08CA245209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2015;21(8):1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etienne A, Esterni B, Charbonnier A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376-1383. [DOI] [PubMed] [Google Scholar]
- 4.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624-627. [DOI] [PubMed] [Google Scholar]
- 5.DiNardo CD, Jonas B, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020;383:617-629 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. [DOI] [PubMed] [Google Scholar]
- 7.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei AH, Strickland SA Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva P, Neumann M, Schroeder MP, et al. Acute myeloid leukemia in the elderly is characterized by a distinct genetic and epigenetic landscape. Leukemia. 2017;31(7):1640-1644. [DOI] [PubMed] [Google Scholar]
- 13.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. [DOI] [PubMed] [Google Scholar]
- 15.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer BE, Elsawy M, et al. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models [abstract]. Blood. 2016;128(22):216. [Google Scholar]
- 17.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59(10):1837-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. 2019;134(4):374-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15(7):894-902. [DOI] [PubMed] [Google Scholar]
- 22.Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in older AML: results of Beat AML Master Trial [abstract]. Blood . 2019;134(suppl 1):175. [Google Scholar]
- 23.Tyner JW, Tognon CE, Bottomly D, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaemmanuil E, Döhner H, Campbell PJ. Genomic classification in acute myeloid leukemia [letter]. N Engl J Med. 2016;375(9):900-901. [DOI] [PubMed] [Google Scholar]
- 26.Mrózek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015;33(10):1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prata PH, Bally C, Prebet T, et al. NPM1 mutation is not associated with prolonged complete remission in acute myeloid leukemia patients treated with hypomethylating agents. Haematologica. 2018;103(10):e455-e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lachowiez CA, Loghavi S, Kadia TM, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020;4(7):1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. 2019;33(12):2795-2804. [DOI] [PubMed] [Google Scholar]
- 33.Roboz GJ, DiNardo CD, Stein EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinardo CD, Stein AS, Stein EM, et al. Mutant IDH1 inhibitor ivosidenib (IVO; AG-120) in combination with azacitidine (AZA) for newly diagnosed acute myeloid leukemia (ND AML) [abstract]. J Clin Oncol. 2019;37(15 suppl):7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daigle SR, Choe S, Quek L, et al. High rate of IDH1 mutation clearance and measurable residual disease negativity in patients with IDH1-mutant newly diagnosed acute myeloid leukemia treated with ivosidenib (AG-120) and azacitidine [abstract]. Blood. 2019;134(suppl 1):2706. [Google Scholar]
- 36.Lachowiez CA, Borthakur G, Loghavi S, et al. Phase Ib/II study of the IDH1-mutant inhibitor ivosidenib with the BCL2 inhibitor venetoclax +/− azacitidine in IDH1-mutated hematologic malignancies [abstract]. J Clin Oncol. 2020;38(15 suppl):7500. [Google Scholar]
- 37.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2014;28(2):289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimwade D, Hills RK, Moorman AV, et al. ; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354-365. [DOI] [PubMed] [Google Scholar]
- 40.Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114-2121. [DOI] [PubMed] [Google Scholar]
- 41.Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML) [abstract]. Blood. 2019;134(supplement 1):15. [Google Scholar]
- 43.Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiti A, Dinardo CD, Pemmaraju N, et al. Ten-day decitabine with venetoclax (DEC10-VEN) in AML and high-risk (HR) MDS [abstract]. J Clin Oncol. 2020;38(15 suppl):7519. [Google Scholar]
- 45.Sallman DA, Malki MA, Asch AS, et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results [abstract]. J Clin Oncol. 2020;38(15 suppl):7507. [Google Scholar]
- 46.Cluzeau T, Sebert M, Rahmé R, et al. APR-246 combined with azacitidine (AZA) in TP53 mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). a phase 2 study by the Groupe Francophone des Myélodysplasies (GFM) [abstract]. Blood. 2019;134(supplement 1):677. [Google Scholar]
- 47.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia [published correction appears in N Engl J Med. 2010;362(12):1155]. N Engl J Med. 2009;361(13):1235-1248. [DOI] [PubMed] [Google Scholar]
- 49.Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125(25):3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study [published correction appears in Lancet. 2018;391(10123):838]. Lancet. 2012;379(9825):1508-1516. [DOI] [PubMed] [Google Scholar]
- 52.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. [DOI] [PubMed] [Google Scholar]
- 53.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollyea DA, Tallman MS, de Botton S, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]