Visual Abstract
Abstract
Although the majority of indolent lymphomas (focusing on follicular lymphoma [FL]) have a prolonged waxing and waning course, a portion of patients experience histologic transformation (HT) to either diffuse large B-cell lymphoma or a higher-grade morphology, often with acquisition of MYC and BCL2 and/or BCL6 rearrangements (high-grade B-cell lymphoma–double-hit lymphoma/triple-hit lymphoma). The overall incidence of HT and transformed follicular lymphoma (tFL) may be declining, but outcomes remain inferior to those in simple indolent lymphoma progression. Recent data suggest that the majority of HT cases occur in higher-risk patients with FL, and they occur early after initial chemoimmunotherapy, comprising the majority of patients with progression of disease within 24 months. This latter point emphasizes the need for a sufficient biopsy at relapse in FL. Treatment options depend on the prior therapy for the indolent component as well as the histology at relapse, but they generally follow several principles discussed in this article. Anthracycline-naïve patients have the best outcomes if there is HT, and responses to R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) are similar to those of patients with de novo diffuse large B-cell lymphoma. Patients with anthracycline exposure prior to transformation have the best outcomes with salvage chemotherapy and a consolidative autologous stem cell transplant. However, a major challenge is the management of patients with tFL who experience relapse early after bendamustine-based treatment, in whom the role of consolidative transplant after anthracycline-based treatment is unclear. In the past several years, cellular therapy has emerged as an important tool for some but not all patients with tFL. This review focuses on the nuances of managing tFL.
Learning Objectives
Develop a treatment strategy for patients with transformed follicular lymphoma
Describe the time to transformation for high-risk patients and the need for a biopsy for patients with early progressing follicular lymphoma
Clinical cases
Patient 1
Patient 1 was a 59-year-old man who initially presented with an acute abdomen while traveling. He was found to have appendicitis but also was noted to have multiple retroperitoneal lymph nodes. An inguinal lymph node biopsy revealed follicular lymphoma (FL) grade 1 to 2. Staging showed diffuse adenopathy (largest lymph node, 4.6 cm). He had mild anemia but a normal lactate dehydrogenase (LDH) level. He was treated with bendamustine and rituximab that required dose reduction due to cytopenias for cycles 5 and 6. Twenty-two months later, he had progressive adenopathy in the axillary region. A biopsy again showed FL, but it was now grade 3A. A bone marrow biopsy was performed, which showed a hypercellular marrow (70%) comprised largely of CD20+CD10+BCL2+ large cells consistent with transformed follicular lymphoma (tFL) (Figure 1).
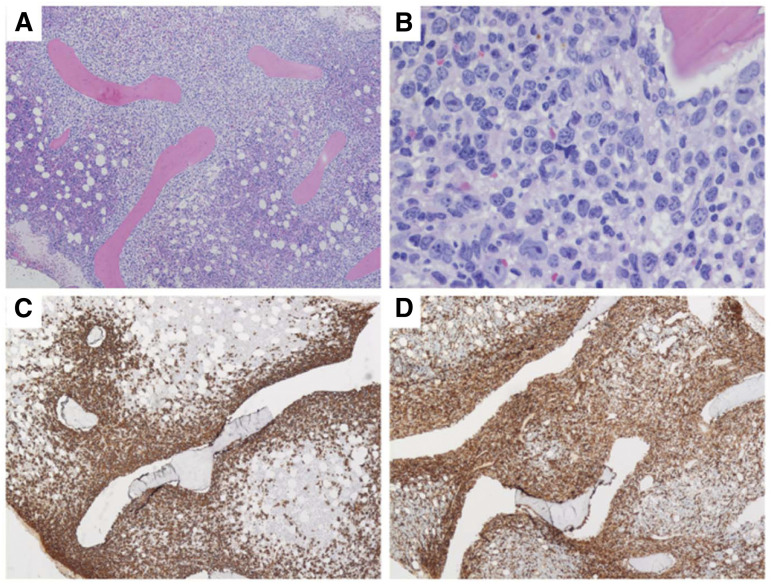
Figure 1.
Bone marrow biopsy pathologic images of patient 1. (A) Low power view showing extensive paratrabecular aggregates of lymphoma cells; (B) higher power view showing large polylobate centroblasts that are CD20 positive (C) and CD10 positive (D). (Images courtesy of Dr. Girish Venkataraman, University of Chicago.)
Patient 2
Patient 2 was a 61-year-old man who had presented with a right neck mass 8 years ago. A biopsy showed FL grade 1. He had undergone active observation until several months ago, when he noted right axillary swelling. He was evaluated in the clinic and was found to have an extensive mass in his right axilla and infiltrating the chest wall (Figure 2). A biopsy showed transformation to an aggressive lymphoma with a Ki67 of 95% and sheets of mitotically active intermediate to large cells with a high nucleus/cytoplasm ratio, CD20+CD10+MYC+BCL2+. Subsequent fluorescence in situ hybridization confirmed rearrangements of MYC and BCL2.
Figure 2.
Patient with FL transforming to HGBL-DHL/THL, showing massive infiltration of the right chest wall and axillary adenopathy.
Patient 3
Patient 3 was a 78-year-old man with a 15-year history of FL. He had previously been treated with rituximab monotherapy on 2 occasions, with the last one ∼10 years ago. He is feeling well but presents to the clinic with a new right axillary mass measuring 6.4 × 3.3 cm. He is not sure how quickly this might have grown. Biopsy shows diffuse large B-cell lymphoma (DLBCL) involving >90% of the lymph node with a residual area of FL. Fluorescence in situ hybridization testing reveals no abnormalities.
Introduction
Indolent lymphomas, including FL, marginal zone lymphoma, lymphoplasmacytic lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma, have the potential to undergo histologic transformation (HT) into an aggressive disease with a mandatory change in treatment approach. The historic outlook for transformed lymphomas was quite poor, but current treatment paradigms can lead to prolonged survival, particularly if patients are minimally pretreated before the transforming event. Very few prospective trials have been dedicated solely to transformed lymphomas, and most decision making derives from subset analysis of trials on aggressive lymphoma. Furthermore, it is unclear if the preceding indolent histology affects outcome or should impact selection of therapies. Richter’s transformation, the moniker for HT of chronic lymphocytic leukemia/small lymphocytic lymphoma to either an aggressive B-cell lymphoma or Hodgkin lymphoma, is the clearest example in which management of transformed disease may differ from that for aggressive lymphomas arising from a preceding FL or marginal zone lymphoma. Given the even more sparse data for nonfollicular HT, this review focuses on data impacting clinical decision making for tFL.
Incidence and diagnostic considerations in tFL
The incidence of transformation has been declining in the modern era, often attributed to better disease control of FL using rituximab and other anti-CD20 targeted agents.1 However, it is important to note that transformation rates in high-risk patients remain elevated, and outcome measures, although improved, still lag behind those used for patients without HT. A recent French analysis found that more than half of FL-related deaths are due to HT.2 In terms of incidence, the LymphoCare study, an observational FL dataset of >2600 patients, found that 14.3% of patients experience transformation with 6.8 years of median follow-up.3 This study identified poor performance status, more than one extranodal site, elevated LDH, and B symptoms as being associated with a higher risk of transformation. The PRIMA trial identified several predictors of HT, including high Follicular Lymphoma International Prognostic Index (FLIPI) score (and its components of anemia and increased LDH), poor performance status, and presence of B symptoms without impact of depth of response to chemoimmunotherapy induction or delivery of maintenance rituximab.4 Overall, 63% of patients with documented HT had a baseline FLIPI score of 3 to 5. Survival after transformation is inferior to that for progressive FL at 3.8 years (tFL) vs 6.4 years (FL) in the PRIMA trial. In addition to clinical factors, a number of genomic and biologic features may predict a higher risk of HT, including chromosome deletions and gains (del1q, del6q, +2, +3q, +5); loss of B2M; increased T-regulatory cells; or mutations in TP53, PIM1, B2M, and others (reviewed by Pasqualucci et al5). Whether newer FL prognostic indices such as the M7-FLIPI6 are also predictive of HT risk remains unknown.
Data from the prerituximab era estimated a continuous 3% risk of HT per year over 15 years, with a median survival of 1.7 years after transformation.7 In contrast, for patients with high tumor burden, many of whom also have high FLIPI scores, the time to transformation appears quite short. Furthermore, many patients with FL with progression of disease within 24 months (POD24)8 actually have HT rather than simple FL progression. In the PRIMA trial, for example, more than half of transformations occurred in the first year of follow-up, with the median time to transformation being only 9.6 months, underscoring the need to perform biopsies in patients with early progression. A Canadian population-based study found that 76% of patients with FL progressing within 2 years had HT, with a median time to transformation of 8.4 months.9 Two-year survival of patients with POD24 found to have HT was only 40%, emphasizing its dire prognosis. In these trials and others, it is highly possible that occult transformation was present at diagnosis.
Confirming HT requires a diagnostic biopsy of sufficient size. Functional imaging with 18-fluorodeoxyglucose–positron emission tomography can help guide the choice of biopsy site, based on the rationale that more highly proliferative lesions will have a higher standardized uptake value. However, this is somewhat controversial,10 and occasionally a biopsy is neither safe nor feasible. In this case, supportive evidence for HT includes rapid progression of adenopathy associated with a significantly elevated LDH and symptomatic presentation. Another key diagnostic consideration is that transformation is not always DLBCL histology; because the vast majority of patients with FL already harbor the t(14;18) rearrangement, acquisition of MYC rearrangements [usually t(8;14)] will lead to a diagnosis of high-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements (high-grade B-cell lymphoma/double-hit lymphoma/triple-hit lymphoma [HGBL-DHL/THL]). In this case, treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) is rarely sufficient, and more intensive regimens such as DA-EPOCH-R (etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride [hydroxydaunorubicin], and rituximab) should be considered. The possibility of HT to a higher-grade process such as HGBL-DHL/THL makes an adequate biopsy even more critical in order to deliver the optimal treatment.
What is the best initial treatment of tFL, and should patients receive a consolidative autologous stem cell transplant?
The best initial treatment depends on the prior therapy for the underlying indolent lymphoma and the histology at the time of transformation (Figure 3). There are several clinical scenarios to consider: tFL in treatment-naïve patients with FL (including simultaneous diagnosis of FL/tFL), tFL after prior anthracycline-based chemoimmunotherapy, and tFL developing after prior therapy that did not include cytotoxic chemotherapy. An example of a scenario with almost no data is when HT occurs after prior bendamustine-based chemoimmunotherapy and whether to treat these patients similarly to anthracycline-exposed patients is unclear.
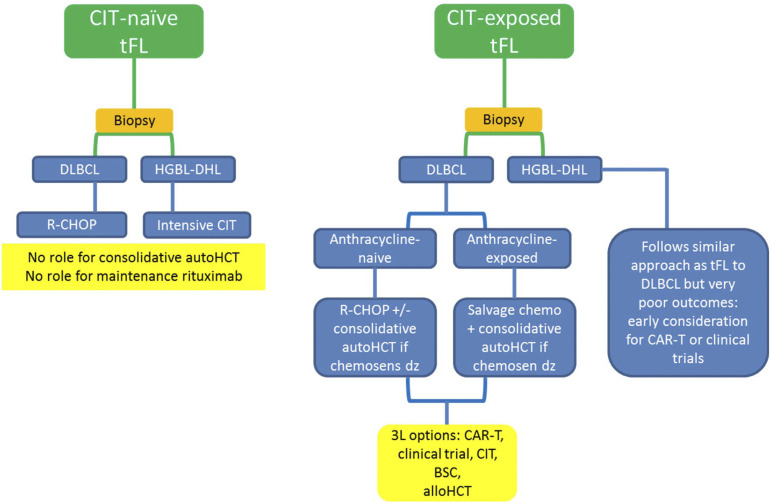
Figure 3.
Graphic summary of how I treat tFL. CIT, chemoimmunotherapy; BSC, best supportive care; HCT, hematopoietic stem cell transplant.
Initial approach for treatment-naïve or minimally pretreated patients with tFL
For patients who have never received treatment of FL or have only received nonchemotherapy approaches, management of tFL follows the same paradigm as de novo DLBCL or HGBL-DHL/THL, depending on the histology. A number of datasets show that tFL with no prior anthracycline exposure has a relatively good outcome similar to de novo DLBCL. A Mayo Clinic/Iowa study found equivalent event-free survival and overall survival (OS) among 109 patients with simultaneous presentation of FL and DLBCL when compared with patients with de novo DLBCL.11 An Molecular Epidemiology Resource analysis showed 5-year OS rates of 66%, similar to de novo DLBCL.1 A UK study of 87 patients with tFL reported a 5-year OS of 64% with R-CHOP–like therapy; importantly, the addition of autologous hematopoietic stem cell transplant (auto-HCT) for patients who were previously untreated for FL did not improve outcomes.12 A Danish National Lymphoma Registry analysis evaluated 85 patients with transformed indolent lymphomas, all of whom had biopsy confirmation of DLBCL in addition to the indolent component; the majority had tFL.13 The authors found that consolidative auto-HCT had the greatest benefit in patients with “sequential transformed indolent lymphoma,” where patients relapsed after prior treatment of their indolent lymphoma; in contrast, there was no significant benefit for patients presenting with simultaneous indolent and aggressive disease and no prior treatment. As might be expected, the magnitude of benefit was greatest in patients who were previously rituximab naïve. On the basis of these series, patients with tFL without prior chemotherapy for FL should be treated with anthracycline-based chemoimmunotherapy without a consolidative auto-HCT.
Maintenance rituximab in tFL
Given improved progression-free survival (PFS) and very prolonged response durations in FL, should maintenance rituximab be considered for patients with tFL? The preponderance of data suggests no benefit for tFL, similar to de novo DLBCL. For example, a Canadian registry analysis identified 107 patients with either discordant or composite lymphomas, of whom 55 received maintenance rituximab and 52 did not. With prolonged follow-up exceeding 7 years, there was no statistically significant difference in PFS, OS, or freedom from indolent progression.14 Similarly, an MD Anderson Cancer Center identified 311 patients with treatment-naïve tFL treated with R-CHOP–like chemoimmunotherapy, of whom 50 received maintenance rituximab.15 In a 1:2 propensity-based analysis, there were no PFS or OS advantages of maintenance rituximab. On the basis of these and other datasets, there is no indication for maintenance rituximab in tFL.
Initial approach for patients with tFL who have received prior chemoimmunotherapy
Patients who have received prior chemoimmunotherapy for FL and then experience a subsequent transformation are clinically challenging to manage and have poor outcomes. Prior anthracycline exposure appears to confer worse survival (21% vs 66%; P < .001) than in those without prior anthracycline exposure.1 The National Comprehensive Cancer Network found that the 2-year OS was substantially worse (35% vs 100%; P = .03) for patients treated with chemotherapy (mainly anthracycline based) before HT.16 A Spanish registry series reported that 5-year OS for patients treated with chemotherapy before HT was 55% (95% confidence interval, 38%-69%) vs 81% (95% confidence interval, 53%-93%; P = .009) for those who had not received prior chemotherapy.17 The poor prognosis for patients with prior chemoimmunotherapy may be independent of prior anthracycline exposure; for example, patients with HT after prior bendamustine, rituximab (BR) have a 2-year OS of only 40%.9 In this Canadian report, the authors reported that the main driver of POD24 was occult or early transformation.
There is sufficient evidence that patients with tFL previously treated with anthracycline-based chemoimmunotherapy for their underlying FL benefit from salvage chemotherapy and a consolidative autologous stem cell transplant if they have chemosensitive disease. A subset analysis of a Canadian intergroup study (NCIC CTG LY12) compared the outcomes of 87 patients with transformed lymphoma with outcomes of 429 patients with relapsed/refractory DLBCL; although many patient characteristics were similar (eg, time to relapse, percentage of refractory disease, median age), patients with transformed disease were more likely to have received more than one line of prior systemic therapy. Nevertheless, there was no difference in all outcome measures, including 4-year post-transplant event-free survival (45%), 4-year OS (∼40%), and transplant rates.18 The PRIMA trial described outcomes of 40 patients with documented HT at relapse. Seventeen (42%) of these patients underwent consolidative auto-HCT and had improved OS (not reached vs 1.7 years) compared with patients who either could not or did not undergo auto-HCT.4
A major unanswered question is whether patients who experience relapse after non–anthracycline-based chemoimmunotherapy such as bendamustine and rituximab should undergo consolidative auto-HCT. As mentioned above, the outcomes are poor, with 2-year OS only 40%.9 None of the above discussion addresses this specific, increasingly common clinical dilemma. The author’s personal perspective (acknowledging the paucity of data) is that tFL in this setting occurs early and confers a poor prognosis; therefore, anthracycline-based induction and auto-HCT is favored, particularly if the tFL occurred within 2 years of BR.
Allogeneic hematopoietic stem cell transplant (allo-HCT) is also an option for patients with relapsed/refractory tFL. Several factors limit its widespread use, including higher nonrelapse mortality and major questions regarding the optimal timing of allo-HCT. One of the few side-by-side analyses, albeit in a registry and not a prospective trial, found no difference in PFS or OS between those treated with allo-HCT and those who received auto-HCT, but a significantly higher nonrelapse mortality of 23% vs 5%, respectively, was reported.19 It should be noted that there are fundamental differences in patients selected for auto-HCT and those selected for allo-HCT, so these types of comparisons are difficult to interpret. According to the Center for International Blood and Marrow Transplant Research (https://www.cibmtr.org/ReferenceCenter), the number of allo-HCTs performed for lymphomas has declined in recent years as the introduction of cellular therapy has further challenged its role.
Is there a role for chimeric antigen receptor T-cell therapy in tFL?
The advent of cellular therapy has increased and improved options for patients with relapsed and refractory aggressive B-cell lymphomas, including tFL. To date, there are 2 US Food and Drug Administration–approved options, with a third agent close to approval. Anti-CD19 chimeric antigen receptor T-cell therapy (CAR-T) modifies autologous T cells to massively expand and become activated upon binding to target CD19 on the surface of B cells. All 3 compounds furthest along (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], and lisocabtagene maraleucel [liso-cel]) have included patients with tFL as part of development, but data separating outcomes of these patients from other patients with relapsed/refractory DLBCL are limited.
In the tisa-cel trial, 21 patients comprising 19% of the population proceeding to CAR-T had tFL.20 The trial overall showed a 40% complete remission (CR) rate, and remission at 3 months predicted a 12-month remission of >80%. The axi-cel trial lumped patients with primary mediastinal B-cell lymphoma and tFL, with 19 patients in this group.21 The overall outcomes showed an overall response rate of 82% with a CR rate of 58%; updated results with a median follow-up of 27 months showed a median duration of response of 11.1 months and a median OS >2 years.22 “Real-world” follow-up of almost 300 patients included 76 patients with tFL. A direct comparison of DLBCL vs primary mediastinal B-cell lymphoma vs tFL showed no difference in the incidence of grade ≥3 cytokine release syndrome or neurotoxicity, best CR at 12 months (62% for tFL), PFS at 12 months (51% for tFL), or OS at 12 months (70% for tFL).23
A heartening finding of this and other “real-world” analyses is that many patients treated with commercial products did not meet the strict criteria of the clinical trials leading to US Food and Drug Administration approval, but they still had meaningful benefit. In particular, patients were older and had more comorbidities.
Overall, the activity and outcomes are exciting, and CAR-T should be considered for patients with tFL who have either experience relapse after an autologous stem cell transplant or have chemoresistant disease precluding a hematopoietic stem cell transplant. Optimal patient selection is an ongoing dialogue, but the ability to achieve durable remission in a portion of patients with refractory tFL is highly encouraging.
Are there options for patients with tFL who are not transplant candidates?
Despite the promise of the approaches outlined above, many patients are either ineligible for transplant or cellular therapy or experience relapse despite these modalities. Again, there are no trials specifically dedicated to tFL or other patients with transformed indolent lymphoma, and nearly all data are derived by culling subsets from trials designed for relapsed/refractory DLBCL. An interesting observation is that there may be a role for lenalidomide in tFL despite this being a germinal center–derived disease. In a small prospective trial, lenalidomide monotherapy had an overall response rate of 57% with a median duration of response of 12.8 months in relapsed and refractory tFL.24 When combined with rituximab, a small prospective trial showed a response rate of ∼50%, but durability was limited.25 The addition of lenalidomide to tafasitamab appears active in transformed indolent lymphomas, with all patients responding; however, there were only 7 patients with transformed lymphomas included in this trial.26 Other regimens approved for DLBCL either had very limited patients with tFL or excluded them entirely. For example, the recently approved regimen of polatuzumab-BR excluded patients with transformed lymphomas.27 The median survival of patients with tFL in the relapsed/refractory setting is abysmal, and focused and biologically rational studies are needed.
Transformation to HGBL-DHL/THL
As discussed above, transformation of indolent lymphomas is not always to DLBCL, and acquisition of an MYC rearrangement in FL leads to DHL (or THL if BCL6 is also rearranged). There are almost no data separating tFL in this context. If there is transformation of FL to HGBL-DHL/THL, R-CHOP is insufficient, and an intensified regimen such as DA-EPOCH-R or another therapy should be considered.28,29 Extrapolating from retrospective series, there is no clear advantage to consolidative auto-HCT if patients have a metabolic CR after anthracycline-based treatment.29 Very few patients who relapse after intensive frontline regimens are able to proceed to transplant, emphasizing the dire prognosis. In one series, only 11 of 55 patients with HGBL-DHL/THL were able to undergo transplant after salvage chemotherapy.30 CAR-T trials have included patients with HGBL-DHL/THL but have not consistently described when disease has evolved from a lower-grade lymphoma; nevertheless, it is clear that CAR-T is effective in a portion of patients with HGBL-DHL/THL, and this would be an appropriate approach for patients with relapsed/refractory tFL and a high-grade histology.
Summary
Transformation of indolent lymphomas to an aggressive histology is a major event in patient management and forces a change in therapy. Although the overall incidence of transformation is declining, this remains a very high-risk disease and has a poor prognosis compared with the prognosis of patients without transformation. A biopsy at the time of suspected transformation is essential because there could be either a transformation to DLBCL or to a HGBL-DHL/THL. For patients without prior anthracycline exposure, R-CHOP or other anthracycline-based treatment is warranted. A major challenge is determining who benefits from consolidative auto-HCT, but the preponderance of data suggests this is best applied to patients who have HT after prior anthracycline treatment or early progression of disease with transformation after prior chemoimmunotherapy. CAR-T has provided a new option for patients with transformed lymphomas. Overall, the outcomes of patients with transformed lymphomas, particularly if hematopoietic stem cell transplant or cellular therapy approaches are unavailable or ineffective, remain highly unsatisfactory. For now, enrollment in a clinical trial should be the highest priority.
Back to the clinical cases
Patient 1
Patient 1 was treated with R-CHOP, entered complete metabolic remission, and had complete clearance of disease from the marrow. Given that he had both early progression of FL (POD24) based on the axillary biopsy and tFL (bone marrow), we proceeded with auto-HCT consolidation.
Patient 2
Patient 2 has HGBL-DHL transformation of his FL. He is being treated with DA-EPOCH-R and has had an excellent early clinical response. There are no plans for consolidation if he enters CR.
Patient 3
Patient 3 has tFL with DLBCL histology with no history of prior chemoimmunotherapy. He was treated with 6 cycles of R-CHOP and has entered complete metabolic remission. There are no plans for consolidation.
References
- 1.Link BK, Maurer MJ, Nowakowski GS, et al. . Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31(26):3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkozy C, Maurer MJ, Link BK, et al. . Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J Clin Oncol. 2019;37(2):144-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner-Johnston ND, Link BK, Byrtek M, et al. . Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood. 2015;126(7):851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkozy C, Trneny M, Xerri L, et al. . Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial [published correction appears in J Clin Oncol. 2016;34(26):3230.]. J Clin Oncol. 2016;34(22):2575-2582. [DOI] [PubMed] [Google Scholar]
- 5.Pasqualucci L, Khiabanian H, Fangazio M, et al. . Genetics of follicular lymphoma transformation. Cell Rep. 2014;6(1):130-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurinovic V, Kridel R, Staiger AM, et al. . Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood. 2016;128(8):1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. . Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5165-5169. [DOI] [PubMed] [Google Scholar]
- 8.Casulo C, Byrtek M, Dawson KL, et al. . Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study [published correction appears in J Clin Oncol. 2016;34(12):1430]. J Clin Oncol. 2015;33(23):2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman CL, Kridel R, Moccia AA, et al. . Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood. 2019;134(9):761-764. [DOI] [PubMed] [Google Scholar]
- 10.Mir F, Barrington SF, Brown H, et al. . Baseline SUVmax did not predict histological transformation in follicular lymphoma in the phase 3 GALLIUM study. Blood. 2020;135(15):1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Link BK, Witzig TE, et al. . Impact of concurrent indolent lymphoma on the clinical outcome of newly diagnosed diffuse large B-cell lymphoma. Blood. 2019;134(16):1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleeson M, Hawkes EA, Peckitt C, et al. . Outcomes for transformed follicular lymphoma in the rituximab era: the Royal Marsden experience 2003-2013. Leuk Lymphoma. 2017;58(8):1805-1813. [DOI] [PubMed] [Google Scholar]
- 13.Madsen C, Pedersen MB, Vase MO, et al. . Outcome determinants for transformed indolent lymphomas treated with or without autologous stem-cell transplantation. Ann Oncol. 2015;26(2):393-399. [DOI] [PubMed] [Google Scholar]
- 14.Kansara R, Connors JM, Savage KJ, et al. . Maintenance rituximab following induction R-CHOP chemotherapy in patients with composite or discordant, indolent and aggressive, B-cell non-Hodgkin lymphomas. Haematologica. 2016;101(10):e411-e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin CK, Rodriguez MA, Qing Y, et al. . Impact of maintenance rituximab in patients with de novo transformed indolent B cell lymphoma [published online ahead of print 6 July 2020]. Leuk Lymphoma. doi:10.1080/10428194.2020.1789631. [DOI] [PubMed] [Google Scholar]
- 16.Ban-Hoefen M, Vanderplas A, Crosby-Thompson AL, et al. . Transformed non-Hodgkin lymphoma in the rituximab era: analysis of the NCCN outcomes database. Br J Haematol. 2013;163(4):487-495. [DOI] [PubMed] [Google Scholar]
- 17.Méndez M, Torrente M, Sánchez-Beato M, et al. . Transformed follicular lymphoma in the rituximab era: a report from the Spanish Lymphoma Oncology Group. Hematol Oncol. 2019;37(2):143-150. [DOI] [PubMed] [Google Scholar]
- 18.Kuruvilla J, MacDonald DA, Kouroukis CT, et al. . Salvage chemotherapy and autologous stem cell transplantation for transformed indolent lymphoma: a subset analysis of NCIC CTG LY12. Blood. 2015;126(6):733-738. [DOI] [PubMed] [Google Scholar]
- 19.Wirk B, Fenske TS, Hamadani M, et al. . Outcomes of hematopoietic cell transplantation for diffuse large B cell lymphoma transformed from follicular lymphoma. Biol Blood Marrow Transplant. 2014;20(7):951-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster SJ, Bishop MR, Tam CS, et al. . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 21.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke FL, Ghobadi A, Jacobson CA, et al. . Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nastoupil LJ, Jain MD, Feng L, et al. . Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czuczman MS, Vose JM, Witzig TE, et al. . The differential effect of lenalidomide monotherapy in patients with relapsed or refractory transformed non-Hodgkin lymphoma of distinct histological origin. Br J Haematol. 2011;154(4):477-481. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Fowler N, Wagner-Bartak N, et al. . Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902-1909. [DOI] [PubMed] [Google Scholar]
- 26.Salles G, Duell J, Gonzalez Barca E, et al. . Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. [DOI] [PubMed] [Google Scholar]
- 27.Sehn LH, Herrera AF, Flowers CR, et al. . Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrich AM, Gandhi M, Jovanovic B, et al. . Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354-2361. [DOI] [PubMed] [Google Scholar]
- 29.Landsburg DJ, Falkiewicz MK, Maly J, et al. . Outcomes of patients with double-hit lymphoma who achieve first complete remission. J Clin Oncol. 2017;35(20):2260-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landsburg DJ, Ayers EC, Bond DA, et al. . Poor outcomes for double-hit lymphoma patients treated with curative-intent second-line immunochemotherapy following failure of intensive front-line immunochemotherapy. Br J Haematol. 2020;189(2):313-317. [DOI] [PubMed] [Google Scholar]