Visual Abstract
Abstract
Recent developments in the management of chronic lymphocytic leukemia (CLL) have moved the standard of care away from chemoimmunotherapy to targeted agents such as oral kinase inhibitors or BCL-2 antagonists, alone or in combination with anti-CD20 antibodies. Two different treatment approaches have evolved: continuous, indefinite treatment and, more recently, fixed-duration combination treatment. With venetoclax-based treatment, there is a requirement to follow the established guidelines for close monitoring during initiation and ramp up, to reduce the risk of tumor lysis syndrome. The patient’s risk should be assessed before the initiation of venetoclax. Appropriate management strategies should be used, including uricosuric agents, hydration, and routine laboratory monitoring, per guidelines. With early identification, immediate management, and dose adjustments, we suggest that tumor lysis syndrome and other toxicities, such as neutropenia and infections, with venetoclax-based treatment can be dealt with successfully.
Learning Objectives
Understand the clinical advances, opportunities and challenges associated with venetoclax therapy for patients with CLL
Learn about the recommendations on how to prevent and monitor for tumor lysis syndrome and other toxicities of venetoclax
Introduction
Because of the availability of numerous therapies for patients with chronic lymphocytic leukemia (CLL), it is important to develop a tailored treatment strategy for the individual patient that considers balance of efficacy, toxicity, and the patient’s preference.1 Two different approaches can be considered: continuous treatment with Bruton’s tyrosine kinase (BTK) inhibitors until disease progression or fixed-duration combination treatment with venetoclax and obinutuzumab. Despite the remarkable progress that has been made with these novel targeted therapies, neither is considered curative.2,3,4 Moreover, it is important to note that each approach has a distinctive toxicity profile. In addition, hematological toxicities such as neutropenia and thrombocytopenia and also infections are often similar in frequency and severity when compared with chemoimmunotherapy.5-9 Although tumor lysis syndrome (TLS) has not been a frequent complication in the management of indolent lymphoma,10 early trials of venetoclax in patients with relapsed/refractory CLL reported a few cases of TLS, some of them fatal.11 Based on these early observations, subsequent trials have implemented various measures of monitoring and mitigation to control venetoclax-associated TLS. With the drug now approved and widely available for routine clinical use, various procedures have been recommended to avoid or treat TLS in patients with CLL.12-14 With venetoclax increasingly becoming the backbone of many different combination regimens for CLL, a solid understanding of the best ways to mitigate toxicities is increasingly important to the practicing hematologist. We summarize the current evidence with regard to preventing and monitoring TLS and other toxicities related to venetoclax. Ultimately, we propose specific recommendations for the management of venetoclax-based therapy, to tailor prophylaxis and mitigate risk for patients with CLL. Particular emphasis will be on the discussion of toxicity data and risk reduction strategies of venetoclax-based therapies of recently published clinical trials that have defined the prevalent standard of care and the ongoing trials that may influence the next generation of treatment options.
Clinical case
A 75-year-old female patient with a diagnosis of CLL was referred to our cancer center evaluation of her treatment. The patient had been diagnosed with stage Binet A/Rai I CLL 5 years ago with mild lymphocytosis of 12 × 109/L.
Initiating frontline therapy
To date, there is no evidence of a potential benefit of early intervention for asymptomatic CLL.15-17 Therapy initiation should be postponed until active disease, defined according to International Workshop on CLL (iwCLL) guidelines, is observed.18 Clinical trials evaluating the early use of novel inhibitors are currently ongoing, but so far, neither of these includes the BCL-2 inhibitor venetoclax or provides evidence that alters the current “watch and wait” standard of care.16
Clinical case (continued)
During the most recent watch-and-wait visits, an increasing lymphocyte count up to 80 × 109/L, hemoglobin of 8.5 × 103/L, and a platelet count of 70 × 109/L were observed. Moreover, the patient reported fatigue that impaired her mobility and well-being. Based on the symptom burden and cytopenias with stage Binet C/Rai IV disease, the need for leukemia treatment was discussed with patient.
Biologic and clinical factors guiding individualized treatment
At present, a tailored treatment approach requires knowledge of the patient’s condition including the following parameters19: (1) the clinical stage, (2) the presence of TP53 mutation and/or deletion, (3) the fitness (ie, coexisting conditions, such as cardiac conditions, or renal dysfunctions) of the patient, (4) the immunoglobulin heavy chain variable (IGHV) mutational status, and (5) the symptoms of CLL. The selection of the appropriate treatment paradigm (continuous indefinite vs fixed-duration treatment) follows these characteristics, because advanced age and poor performance status, among other factors, confer the highest risk of increased toxicity and intolerance.
Clinical case (continued)
A molecular and cytogenetic workup revealed unmutated IGHV gene status and TP53 wild-type and 13q deletions. The patient had several coexisting conditions, including hypertension (well controlled with ramipril and amlodipine), type 2 diabetes (treated with metformin and insulin replacement therapy), and chronic kidney disease (grade 2 with creatinine clearance of 75 mL/min).
How we choose a frontline therapy
On the basis of the factors described, we suggest the following algorithm for choosing a frontline therapy.20 We seek to simplify the increasing number of treatment options and to tailor an individualized therapy. Discussion of toxicities and duration of therapy with patients is important and may aid in the decision of whether to treat with venetoclax and obinutuzumab or BTK inhibitors. Currently, to our knowledge, no data are available on a direct comparison.
Clinical case (continued)
Two treatment options were evaluated for the patient: ibrutinib as a continuous, but highly effective option, with a good chance of disease control over several years, and a fixed-duration option with a combination of venetoclax and obinutuzumab for 12 cycles, which required 8 infusions of an antibody and oral intake of a tablet for 12 cycles. Possible drug-specific toxicities were taken into account, including worsening of existing arterial hypertension with ibrutinib and worsening of renal function if TLS occurred with venetoclax treatment. The patient was in favor of a limited-duration treatment, and after careful consideration and receiving formal consent from the patient, we initiated treatment with venetoclax and obinutuzumab, according to the CLL14 protocol.
Venetoclax therapy for CLL
Venetoclax is a BH3-mimetic compound that selectively antagonizes BCL-2 and induces apoptosis of CLL cells. Its efficacy as a monotherapy has been described in patients with relapsed/refractory CLL, including those with del(17p).11,21 Venetoclax received initial approval in 2016 on the basis of a phase 2 trial evaluating patients with relapsed/refractory disease with del(17p).22 Subsequently, venetoclax was approved in combination with rituximab: the phase 3 MURANO trial showed improved progression-free survival, compared with chemoimmunotherapy with bendamustine and rituximab in patients with relapsed/refractory disease.23,24 For first-line therapy, the phase 3 CLL14 trial evaluated fixed-duration venetoclax and obinutuzumab in patients with previously untreated CLL and coexisting medical conditions compared with chlorambucil and obinutuzumab. The results demonstrated the superiority of the fixed-duration treatment regimen of venetoclax and obinutuzumab over chlorambucil and obinutuzumab.2 On the basis of these results, the combination of venetoclax and obinutuzumab was approved for the first-line treatment of patients with previously untreated CLL. The results influenced the choice of first-line therapy by establishing fixed-duration treatment as an alternative option to continuous, indefinite treatment with the BTK inhibitor. Most recently, longer follow-up confirmed a sustained benefit of fixed-duration venetoclax and obinutuzumab.25
Combinations of venetoclax and BTK inhibitors in clinical trials
With the favorable outcome of BTK inhibitors and BCL-2 inhibitors in patients with CLL, current trials are evaluating the combination of the 2 oral agents. The first data reported support high efficacy rates and manageable toxicity, although longer follow-up is warranted.26-28 Ongoing clinical trials that are addressing the question of combination vs single-agent targeted strategies are outlined in Table 1.
Table 1.
Selection of ongoing/planned trials for venetoclax-based therapy in previously untreated CLL
| Active disease | Study ID | Experimental agent(s) and comparator | N | Status | Trial registration |
|---|---|---|---|---|---|
| FLAIR | Fludarabine-cyclophosphamide-rituximab vs ibrutinib vs ibrutinib-venetoclax | 1516 | Recruiting | ISRCTN01844152 | |
| CLL13 | Fludarabine-cyclophosphamide-rituximab/bendamustine-rituximab vs venetoclax-rituximab vs venetoclax-obinutuzumab vs obinutuzumab-ibrutinib-venetoclax | 920 | Recruitment completed | NCT02950051 | |
| National Cancer Institute (ECOG 9161) | Ibrutinib-obinutuzumab vs obinutuzumab-ibrutinib-venetoclax in untreated younger patients | 720 | Recruiting | NCT03701282 | |
| National Cancer Institute (Alliance 041702) | Ibrutinib-obinutuzumab vs obinutuzumab-ibrutinib-venetoclax in untreated older patients | 454 | Recruiting | NCT03737981 | |
| CLL17 | Ibrutinib vs venetoclax-obinutuzumab vs ibrutinib-venetoclax | 920 | In preparation | EudraCT 2019-003854-99 |
All are phase 3, multicenter, open-label trials. No results have been submitted.
Active disease, according to iwCLL criteria (ref. 19); ECOG, Eastern Cooperative Oncology Group.
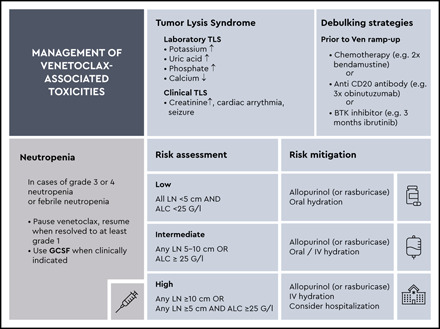
Tumor lysis syndrome
The Common Terminology Criteria for Adverse Events (version 5.0) define TLS as a disorder characterized by metabolic abnormalities that result from spontaneous or therapy-induced lysis of tumor cells. Diagnostic criteria by Howard et al have identified variables, such as hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia, to define laboratory indications of TLS without clinical symptoms.29 First laboratory signs usually occur 6 to 24 h after treatment is initiated.14,30 Clinical TLS is defined by clinical manifestations, most commonly renal, cardiac, or neuromuscular symptoms induced by worsening of the aforementioned metabolic and electrolyte in laboratory test results.31 The risk of TLS is a continuum based on multiple predisposing factors, including coexisting conditions. Patients with high tumor burden (eg, any lymph node with a diameter ≥5 cm or a high absolute lymphocyte count [ALC) of >25 × 109/L are at higher risk when initiating venetoclax.32 Reduced renal function (creatinine clearance, <80 mL/min) and concomitant medications such as CYP3A4 inhibitors further increase the risk.
Tumor lysis syndrome associated with venetoclax-based therapy
Venetoclax with its high antitumor activity, achieves deep remissions by potently inducing apoptosis and thereby increasing the risk of TLS. In early phase 1 trials with venetoclax 11,33 there were 2 fatal cases associated with TLS: one in a patient treated with a starting dose higher than the currently recommended 20 mg and the other in a patient whose dose was escalated to 1200 mg. Currently, a target daily dose of 400 mg is recommended.11,33 Consequently, initiation, escalation, and monitoring of venetoclax treatment were amended, and requirements for safe management were implemented. As a result of adherence to guidelines on the prevention of tumor lysis, the incidence of ∼1.1% to 3.8% for laboratory-confirmed TLS within clinical trials, with no cases of clinical manifestation after venetoclax initiation is considered low.2,24,26,27 Of note, monotherapy with obinutuzumab has been reported to be associated with an incidence of TLS of 4.8% in a phase 1/2 trial.34 Reports on patients treated with venetoclax outside of clinical trials are heterogenous, with 1 large retrospective analysis of 297 patients reporting an incidence of TLS of 5.7%,35 in contrast to a recent analysis of 48 patients with an incidence of 13%.36
Risk stratification of TLS associated with venetoclax-based therapy
The tumor mass burden varies from patient to patient, and several risk stratification and mitigation procedures have been implemented in the previously mentioned clinical trials. In general, the key parameters to estimate tumor mass are ALC and lymph node size (Table 2). Although physical examination and ultrasonography can provide a first impression of the lymph node mass, intraabdominal and intrathoracic lymph nodes cannot be safely assessed. Therefore, a CT or MRI scan of the neck, chest, abdomen, and pelvis is generally recommended. In addition, because most patients with CLL are >65 years of age and have coexisting conditions such as renal impairment, renal function must be taken into account. Patients with creatinine clearance <80 mL/min are at risk for developing TLS.37
Table 2.
TLS risk categories and prophylactic measures for venetoclax-based treatment in CLL
| Assessments before treatment | TLS risk category | Risk parameters | Mitigation measures | ||
|---|---|---|---|---|---|
| Prophylactic medication | Hydration | Hospitalization | |||
| Tumor burden assessment | Low | All lymph nodes <5 cm AND ALC <25 × 109/L | 2-3 d before venetoclax intake: allopurinol In cases of elevated uric acid: rasburicase | Oral hydration (1.5-2 L/d), starting 2 d before dose ramp up. | Outpatient, check TLS parameters and creatinine clearance at least 6 to 8 h and 24 h after each ramp up step |
| CT scan | |||||
| Lymphocyte count | |||||
| Blood chemistry | Medium | Any lymph node 5-10 cm OR ALC ≥ 25 × 109/L | Oral hydration or consider IV hydration | Outpatient, check TLS parameters and creatinine clearance at least 6 to 8 h and 24 h after each ramp up step OR inpatient, in case of preexisting abnormalities or relevant coexisting conditions (creatinine clearance <80 mL/min) | |
| Potassium | |||||
| Phosphate | |||||
| Calcium | |||||
| Uric acid | |||||
| Renal function | High | Any lymph node ≥10 cm OR Any lymph node ≥ 5 cm AND ALC ≥ 25 × 109/L | Oral hydration AND intravenous hydration | Admission to an inpatient or day hospital to ensure sufficient IV hydration and TLS monitoring | |
| Creatinine clearance | |||||
Clinical case (continued)
Before the patient started therapy, the risk for TLS was carefully assessed. Because of the lymphocyte count increasing to >25 × 109/L, the nonpalpable lymphadenopathy (which was confirmed via CT scan), and the mildly impaired renal function with a glomerular filtration rate <80 mL/min, an intermediate risk for development of TLS was assessed. The patient was treated in an outpatient setting, although admission for better monitoring generally can be discussed at the discretion of the treating physician in similar settings.
Debulking strategies for preventing TLS associated with venetoclax-based therapy
Because the risk of developing TLS is highest when treatment is initiated, when the overall tumor mass is highest, a further approach to mitigating TLS may be debulking. Pharmacological debulking strategies are commonly used in aggressive lymphoma to improve the tolerability and safety of first treatment cycles with chemoimmunotherapy.38 Similar approaches are being tested in CLL: chemotherapy,39-40 BTK inhibitors,26-28 and anti-CD20 antibodies2,41,42 have been shown to decrease the overall tumor burden and thereby reduce the individual risk for TLS.
Venetoclax in combination with chemotherapy
Chemoimmunotherapy can be an effective way to reduce the bulk of CLL before initiating venetoclax. Fludarabine-based and bendamustine-based regimens can be given as 1 to 3 cycles of standard-dose treatment before starting to increase the venetoclax dose. For instance, in the phase 2 CLL2-BAG trial, patients received sequential debulking treatment with 2 cycles of bendamustine followed by obinutuzumab and venetoclax.43 Although this strategy has indeed been shown to reduce TLS risk, the few chemotherapy cycles add toxicity,43 and therefore a careful risk-benefit evaluation should be made on an individual basis.
Venetoclax in combination with anti-CD20 antibodies
An early phase 1b/2 trial of venetoclax in combination with obinutuzumab evaluated a schedule with venetoclax followed by obinutuzumab and a schedule with obinutuzumab followed by venetoclax.41 Based on the overall risk profile in this trial and the phase 3 CLL14 trial,2 obinutuzumab was administered with 100 mg on day 1 and 900 mg on day 2 (or 1000 mg on day 1), 1000 mg on day 8, and 1000 mg on day 15 in the first treatment cycle before venetoclax ramp up. This treatment regimen allowed for an effective reduction of the ALC, which decreased the overall risk of TLS. The CLL14 trial reported that 3 patients developed laboratory-confirmed TLS, which was associated with obinutuzumab before exposure to venetoclax.44 It is therefore also recommended to watch out for laboratory signs of TLS after the first obinutuzumab infusions, particularly in patients with higher disease bulks.
Venetoclax in combination with BTK inhibitors
To establish an all oral venetoclax-based combination therapy, current clinical trials are evaluating the combination of BTK inhibitors with venetoclax. These trials initiated treatment with single-agent ibrutinib for 2 to 3 months before starting the venetoclax ramp up. This protocol allowed for an effective reduction of risk of TLS and led to an overall low incidence of TLS (1%-4%).27,28,45
Risk reassessment after debulking strategies
As the risk for TLS may change after debulking with anti-CD20 monoclonal antibodies or BTK inhibitor, a reassessment of risk could be considered based on lymphocyte count and physical examination. Further radiological examination could be performed when clinically indicated.
Clinical case (continued)
The patient had initial uric acid level of 12 mg/dL and was given allopurinol 5 days before the first infusion of obinutuzumab and then 1 infusion of 7.5 mg rasburicase on the day of obinutuzumab infusion. The first obinutuzumab dose was split into to 100 mg on the first day and 900 mg on the second day. She was advised to drink 1 to 2 L of fluids at home before the obinutuzumab infusion and received 1 L of crystalloid fluids together with the first obinutuzumab infusion. To avoid an infusion-related reaction, a triple combination of an H1 and H2 blocker, together with 100 mg prednisolone, together with 1000 mg oral acetaminophen were administered before the first obinutuzumab infusion. The obinutuzumab infusion was administered 3 times over 3 wk. Afterward, the ALC had dropped to 10 × 109/L. Venetoclax was initiated, with 20 mg given on day 21 of the first cycle, followed by a weekly dose ramp up of 50, 100, 200, and 400 mg. Around each dose escalation, electrolytes (potassium, calcium, and phosphate), uric acid, and creatinine clearance were checked before and 8 hours after dose administration to detect any signs of TLS. No electrolyte shifts or decline in creatinine clearance was observed.
Other toxicities associated with venetoclax
Venetoclax treatment is associated with common hematological toxicities, including grade 3 to 4 neutropenia in ∼40% of patients receiving single-agent venetoclax.22 This adverse event becomes more frequent in combination with anti-CD20 antibodies, where grade 3 to 4 neutropenia frequencies of up to 60% have been observed,2,24 or in combination with BTK inhibitors (up to 70%)26,27 The rates of febrile neutropenia are usually low (3%-5%).2,22,24,33 Specific guidance has been provided to react to a decrease in neutrophil count by use of granulocyte colony stimulating factor (GCSF), dose interruptions, or dose reduction.37 Apart from hematological toxicities, serious infections, including cases of sepsis with fatal outcome, have been reported.2 As the rate of opportunistic infections, such as pneumocystic jiroveci pneumonia, is very low with venetoclax, no specific antimicrobial prophylaxis is currently recommended.46 Hence, similar to any management of treatment of CLL, due diligence and timely action are necessary when patients develop signs of infection during treatment with venetoclax. In addition, gastrointestinal side effects have been reported with venetoclax monotherapy and venetoclax combination therapy, such as mild diarrhea and nausea in up to 40% of patients.22,24,25,33 After exclusion of possible infectious causes, these conditions are usually treated with supportive measures, such as loperamide or temporary dose reductions.37
Clinical case (continued)
At 200 mg of venetoclax, the patient developed grade 2 neutropenia without fever; GCSF was administered daily and the ramp up was continued to 400 mg after the neutrophil count had improved. GCSF was discontinued after 4 days, and the neutrophil count remained stable. The patient completed 6 cycles of obinutuzumab and 12 cycles overall of venetoclax and showed complete remission with undetectable minimal residual disease at final restaging.
Current challenges associated with venetoclax and outlook
The current challenge is to identify the best treatment strategy to achieve the long-term control of CLL with minimal toxicity and optimal quality of life. Within clinical trials, potential long-term toxicities including the incidence of second primary malignancies need to be continuously monitored. To investigate these questions, further clinical trials are currently being conducted or are about to open for recruitment (Table 1). The phase 3 FLAIR trial is investigating ibrutinib monotherapy vs the combination of ibrutinib plus rituximab vs ibrutinib and venetoclax vs fludarabine, cyclophosphamide, and rituximab and will show data of a randomized comparison with ibrutinib and venetoclax (Table 1). Although CLL14 enrolled older patients with coexisting conditions, one may be comfortable in extrapolating the results of the trial to younger, fit patients. In addition, for these patients, the CLL13 trial will determine whether a fixed-duration treatment of obinutuzumab and venetoclax or obinutuzumab and venetoclax and ibrutinib is superior to chemoimmunotherapy. In particular, the safety data from this trial will elucidate the extent of drug-related toxicity resulting from regimens with different combination partners of venetoclax (Table 1). The triple combination is also studied in 2 US trials compared with the combination of ibrutinib and obinutuzumab (Table 1). Moreover, in the near future, a direct comparison will be conducted within the CLL17 trial, to study the 2 different treatment approaches of continuous treatment with ibrutinib and fixed-duration combination treatment with venetoclax and obinutuzumab or venetoclax and ibrutinib (Table 1). Ultimately, such trials will improve the understanding of these regimens for treatment of CLL, including drug-related toxicities, discontinuations, and quality-of-life parameters.
References
- 1.Awan FT, Al-Sawaf O, Fischer K, Woyach JA. Current perspectives on therapy for chronic lymphocytic leukemia. Am Soc Clin Oncol Educ Book. 2020;40:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Barr PM, Robak T, et al. . Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kater AP, Wu JQ, Kipps T, et al. . Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study [published online ahead of print 28 September 2020]. J Clin Oncol. doi:10.1200/JCO.20.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Sawaf O, Zhang C, Tandon M, et al. . Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188-1200. [DOI] [PubMed] [Google Scholar]
- 5.Fischer K, Al-Sawaf O, Bahlo J, et al. . Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. [DOI] [PubMed] [Google Scholar]
- 6.Goede V, Fischer K, Busch R, et al. . Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101-1110. [DOI] [PubMed] [Google Scholar]
- 7.Moreno C, Greil R, Demirkan F, et al. . Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43-56. [DOI] [PubMed] [Google Scholar]
- 8.Shanafelt TD, Wang XV, Kay NE, et al. . Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woyach JA, Ruppert AS, Heerema NA, et al. . Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride A, Trifilio S, Baxter N, Gregory TK, Howard SC. Managing tumor lysis syndrome in the era of novel cancer therapies. J Adv Pract Oncol. 2017;8(7):705-720. [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts AW, Davids MS, Pagel JM, et al. . Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gribben JG. Practical management of tumour lysis syndrome in venetoclax-treated patients with chronic lymphocytic leukaemia. Br J Haematol. 2020;188(6):844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iskierka-Jażdżewska E, Robak T. Minimizing and managing treatment-associated complications in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2020;13(1):39-53. [DOI] [PubMed] [Google Scholar]
- 14.Tambaro FP, Wierda WG. Tumour lysis syndrome in patients with chronic lymphocytic leukaemia treated with BCL-2 inhibitors: risk factors, prophylaxis, and treatment recommendations. Lancet Haematol. 2020;7(2):e168-e176. [DOI] [PubMed] [Google Scholar]
- 15.Dighiero G, Maloum K, Desablens B, et al. ; French Cooperative Group on Chronic Lymphocytic Leukemia. Chlorambucil in indolent chronic lymphocytic leukemia. N Engl J Med. 1998;338(21):1506-1514. [DOI] [PubMed] [Google Scholar]
- 16.Herling CD, Cymbalista F, Groß-Ophoff-Müller C, et al. . Early treatment with FCR versus watch and wait in patients with stage binet a high-risk chronic lymphocytic leukemia (CLL): a randomized phase 3 trial. Leukemia. 2020;34(8):2038-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoechstetter MA, Busch R, Eichhorst B, et al. . Early, risk-adapted treatment with fludarabine in Binet stage A chronic lymphocytic leukemia patients: results of the CLL1 trial of the german CLL study group. Leukemia. 2017;31(12):2833-2837. [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, et al. . iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745-2760. [DOI] [PubMed] [Google Scholar]
- 19.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524-1537. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94(11):1266-1287. [DOI] [PubMed] [Google Scholar]
- 21.Souers AJ, Leverson JD, Boghaert ER, et al. . ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202-208. [DOI] [PubMed] [Google Scholar]
- 22.Stilgenbauer S, Eichhorst B, Schetelig J, et al. . Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768-778. [DOI] [PubMed] [Google Scholar]
- 23.Kater AP, Seymour JF, Hillmen P, et al. . Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III Study. J Clin Oncol. 2019;37(4):269-277. [DOI] [PubMed] [Google Scholar]
- 24.Seymour JF, Kipps TJ, Eichhorst B, et al. . Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sawaf O, Zhang C, Tandon M, et al. . Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020. Sep;21(9):1188-1200. [DOI] [PubMed] [Google Scholar]
- 26.Hillmen P, Rawstron AC, Brock K, et al. . Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study [published correction appears in J Clin Oncol. 2020;38(14):1644]. J Clin Oncol. 2019;37(30):2722-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain N, Keating M, Thompson P, et al. . Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095-2103. [DOI] [PubMed] [Google Scholar]
- 28.Tam CS, Siddiqi T, Allan JN, et al. . Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): results from the MRD cohort of the phase 2 CAPTIVATE study [abstract]. Blood. 2019;134(suppl 1). Abstract 35. [Google Scholar]
- 29.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mughal TI, Ejaz AA, Foringer JR, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36(2):164-176. [DOI] [PubMed] [Google Scholar]
- 31.Cairo MS, Coiffier B, Reiter A, Younes A; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578-586. [DOI] [PubMed] [Google Scholar]
- 32.Jones GL, Will A, Jackson GH, Webb NJ, Rule S; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169(5):661-671. [DOI] [PubMed] [Google Scholar]
- 33.Davids MS, Hallek M, Wierda W, et al. . Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24(18):4371-4379. [DOI] [PubMed] [Google Scholar]
- 34.Cartron G, de Guibert S, Dilhuydy MS, et al. . Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124(14):2196-2202. [DOI] [PubMed] [Google Scholar]
- 35.Roeker LE, Fox CP, Eyre TA, et al. . Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res. 2019;25(14):4264-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehler AB, Leung N, Call TG, et al. . Incidence and risk of tumor lysis syndrome in patients with relapsed chronic lymphocytic leukemia (CLL) treated with venetoclax in routine clinical practice. Leuk Lymphoma. 2020;61(10):2383-2388. [DOI] [PubMed] [Google Scholar]
- 37.Venclyxto: Summary or product characteristics. Maidenhead, UK: Abbvie; revised April; 2020. Available at: https://www.medicines.org.uk/emc/product/2267/smpc. [Google Scholar]
- 38.Lakshmaiah KC, Asati V, Babu K G, et al. . Role of prephase treatment prior to definitive chemotherapy in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2018;100(6):644-648. [DOI] [PubMed] [Google Scholar]
- 39.Cramer P, V Tresckow J, Robrecht S, et al. . Bendamustine, followed by ofatumumab and ibrutinib in chronic lymphocytic leukemia (CLL2-BIO): primary endpoint analysis of a multicentre, open-label phase-II trial. Haematologica. 2020;haematol.2019.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Tresckow J, Cramer P, Bahlo J, et al. . CLL2-BIG: sequential treatment with bendamustine, ibrutinib and obinutuzumab (GA101) in chronic lymphocytic leukemia. Leukemia. 2019;33(5):1161-1172. [DOI] [PubMed] [Google Scholar]
- 41.Flinn IW, Gribben JG, Dyer MJS, et al. . Phase 1b study of venetoclax-obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood. 2019;133(26):2765-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kater AP, Kersting S, van Norden Y, et al. ; HOVON CLL study group. Obinutuzumab pretreatment abrogates tumor lysis risk while maintaining undetectable MRD for venetoclax + obinutuzumab in CLL. Blood Adv. 2018;2(24):3566-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cramer P, von Tresckow J, Bahlo J, et al. . Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(9):1215-1228. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sawaf O, Fink A-M, Robrecht S, et al. . Prevention and management of tumor lysis syndrome in patients with CLL and coexisting conditions treated with venetoclax-obinutuzumab or chlorambucil-obinutuzumab: results from the randomized CLL14 trial [abstract]. Blood. 2019;134(suppl 1). Abstract 4315. [Google Scholar]
- 45.Hillmen P, Munir T, Rawstron A, et al. . Initial results of ibrutinib plus venetoclax in relapsed, refractory CLL (bloodwise TAP CLARITY study): high rates of overall response, complete remission and MRD eradication after 6 months of combination therapy [abstract]. Blood. 2017;130(suppl 1). Abstract 428. [Google Scholar]
- 46.Teh BW, Tam CS, Handunnetti S, Worth LJ, Slavin MA. Infections in patients with chronic lymphocytic leukaemia: Mitigating risk in the era of targeted therapies. Blood Rev. 2018;32(6):499-507. [DOI] [PubMed] [Google Scholar]


