Visual Abstract
Abstract
Despite the significant improvement in survival outcomes of multiple myeloma (MM) over the past decade, it remains an incurable disease. Patients with triple-class refractory MM have limited treatment options and a dismal prognosis. Chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen has transformed the treatment armamentarium of relapsed/refractory MM (RRMM), with unprecedented overall response rates in this difficult-to-treat patient population. However, a significant proportion of patients ultimately relapse despite achieving deep remission. Several innovative approaches, including alternative/dual-antigen–specific CAR T-cell constructs, genetically engineered “off-the-shelf” CAR T cells, and strategies to counteract an immunosuppressive microenvironment, may dramatically reshape the field of CAR T-cell therapy in the future. These strategies are being actively investigated in preclinical and early clinical trial settings with the hopes of enhancing the durability of responses and, thereby, improving the overall survival of RRMM patients after CAR T-cell therapy.
Learning Objectives
Summarize the current landmark clinical trials of CAR T cells for RRMM
Describe the underlying mechanism of failure in patients with RRMM treated with CAR T-cell therapy
Discuss the ongoing investigational strategies to overcome current barriers and enhance CAR T-cell efficacy in RRMM
Clinical case
A 65-year-old female was diagnosed with high-risk immunoglobulin G λ multiple myeloma (MM), International Staging System (ISS) stage III, in March of 2014. Bone marrow study at the time of diagnosis revealed extensive involvement by monoclonal plasma cells (90%) with fluorescence in situ hybridization cytogenetics analysis positive for +1q and −13q. She underwent induction therapy and autologous stem cell transplant in September of 2014 and achieved a partial response (PR), followed by lenalidomide maintenance. Her disease progressed in August of 2015. Since then, she relapsed after multiple lines of therapy, consistent with triple-class refractory myeloma. Ultimately, in September of 2017, she was evaluated for anti–B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T cells. Bone marrow pathology revealed clonal plasma cells of 30%. She was treated with anti-BCMA CAR T-cell therapy, MCARH171 (dose, 450 × 106 total CAR T cells) after receiving fludarabine-cyclophosphamide lymphodepletion (LD) chemotherapy. The end-of-treatment evaluation at day 30 postinfusion showed a 63% reduction in monoclonal protein (from 1.16 g/dL to 0.43 g/dL), an undetectable free light chain, and no evidence of abnormal plasma cells in bone marrow, consistent with PR.
Introduction
Over the past decades, the treatment landscape for patients with MM has evolved significantly. The incorporation of several novel therapies, including immunomodulatory agents, proteasome inhibitors, and, more recently, monoclonal antibodies, to the MM treatment paradigm has improved the response rate and survival of these patients. However, MM generally remains an incurable disease. Historically, patients who fail to respond or relapse early after these novel-based treatments carry a dismal prognosis and ultimately die of disease progression.1
CAR T-cell therapy for relapsed/refractory MM
Recently, clinical trials of CAR T-cell therapy against MM-associated antigens have demonstrated promising clinical activity, providing unprecedented response rates in these heavily pretreated patients. The target of most active CAR T-cell trials in MM is B-cell maturation antigen (BCMA). BCMA, a member of the tumor necrotic factor receptor superfamily, is highly specific to and expressed on the surface of plasmablasts, plasma cells, and activated B cells; thus, it is an attractive target for cellular immunotherapy of MM.2 In all studies, patients received LD chemotherapy with fludarabine and cyclophosphamide. In 1 such study, Raje et al investigated idecabtagene vicleucel (Ide-cel; previously bb2121), lentiviral vector–based 4-1BB-CD3ζ BCMA-targeted CAR T cells.3 The initial phase 1 report was of 33 patients with heavily treated relapsed/refractory MM (RRMM). The overall response rate (ORR) was 85%, with a complete response (CR) rate of 45%. Sixteen patients achieved minimal residual disease (MRD)-negative status at a sensitivity of ≤10−4 cells. Most patients attained a response early after infusion, with a median time to first PR or better of 1.0 month. The incidence of cytokine release syndrome (CRS) was high (25 patients, 76%), but severe (grade ≥ 3) CRS only occurred in 2 patients. Recently, Munshi et al reported initial results of the follow-up phase 2 open-label KarMMa trial of 128 RRMM patients treated with Ide-cel at a dose of 150 to 450 × 106 CAR T cells.4 The study confirmed the efficacy of Ide-cel with an ORR and CR rate of 73% and 33%, respectively. Among patients who attained CR, 33% achieved MRD negativity at a sensitivity of 10−5 nucleated cells. Several other groups have reported results for BCMA-directed CAR T cells. A handful of studies of BCMA CAR T cells in RRMM have demonstrated remarkable response rates and well-tolerated adverse event profiles (Table 1). In addition to Ide-cel, JNJ-68284528 (previously known as LCAR-B38M, ciltacabtagene autoleucel, lentiviral; CAR T-cell product containing 2 BCMA-targeting single domain nanobodies) and JCARH125 (orvacabtagene autoleucel, lentiviral; fully human 4-1BB-CD3ζ CAR) are among several BCMA CAR T-cell products that have advanced into later stages of clinical trials. It is worth noting that the difference in safety and efficacy profiles between trials could be attributed to several factors (eg, CAR T-cell constructs, LD chemotherapy, patient’s characteristics). Although the data from the original bb2121 study showed a low response rate (33%) in the 50 × 106 CAR T-cell cohort, and at least a very good partial remission (VGPR) was observed only in ≥150 × 106 cell cohorts, deep responses were seen in the 50 × 106 cell cohorts in the trials using JCARH125 and FCARH143 (lentiviral vector transduced fully human 4-1BB-CD3ζ CAR T cells with a defined ratio of CD4+/CD8+ lymphocytes in the final product) while still being able to safely dose escalate to similarly high doses; however, the clinical relevance of an optimal CAR T-cell dose remains unknown.5,6 Recently, the phase 1b/2 CARTITUDE-1 study investigating LCAR-B38M BCMA CAR T cells, the identical BCMA CAR T-cell product used in the LEGEND-2 study,6,7 reproduced an ORR of 100% in this heavily pretreated RRMM setting (CR rates of 74% and 86% in updated results from LEGEND-2 and CARTITUDE-1, respectively).7-9 The updated results from the EVOLVE study (JCARH125, 300 to 600 × 106 cell cohorts) demonstrated a high response rate (ORR 92%, CR 36%) and an excellent safety profile. Several phase 3 clinical trials comparing BCMA CAR T cells with standard-of-care treatment options in earlier disease settings (eg, Ide-Cel in KarMMa-3 [NCT03651128] and LCAR-B38M in CARTITUDE-4 [NCT04181827]) are enrolling patients.
Table 1.
Selected landmark clinical trials of BCMA-targeted CAR T cells in RRMM (with n > 10)
| Study | n | Phase | Vector | Product | Costimulatory domain | LD chemotherapy | CAR T-cell dose | Lines of prior treatment, median (range), n | Triple class refractory, % | Previous ASCT, % | CRS any grade, % | CRS grade ≥3, % | ICANS grade ≥3, % | Anti–IL-6 therapy, % | ORR, % | ≥VGPR, % | CR, % | MRD negative, %* | PFS, median | OS, median |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRB-4013 | 33 | 1 | Lenti | Ide-cel (bb2121) | 4-1BB | Flu/Cyc | 50/150/450/800 × 106 cells | 7 (3-23) | N/A | 97 | 76 | 6 | 3 | 21 | 85 | 72 | 45 | 94 (15/16; ≥PR patients) | 11.8 mo | N/A |
| KarMMA4 | 128 | 2 | Lenti | Ide-cel (bb2121) | 4-1BB | Flu/Cyc | 150/300/450 × 106 cells | 6 (3-16) | 84 | 94 | 84 | 6 | 3 | 2 | 73 | 53 | 33 | 33 (26/128; CR patients) | 8.8 mo | 19.4 mo |
| LEGEND-29 | 57/74 | 1 | Lenti | LCAR-B38M (JNJ68284528) | 4-1BB | Cyc | 0.5 × 106 (0.07-2.1) cells/kg | 3 (1-9) | N/A | 18 | 90 | 7 | 0 | 46 | 89 | 78 | 74 | 68.4 (39/57; CR patients) | 19.9 mo | 36.1 mo |
| CARTITUDE-17,50 | 29 | 1b/2 | Lenti | LCAR-B38M (JNJ68284528) | 4-1BB | Flu/Cyc | 0.75 × 106 (0.5-1.0) cells/kg | 5 (3-18) | 86 | 86 | 93 | 7 | 3 | 76 | 100 | 97 | 86 | 81 (13/16; CR patients) | 87% (9 mo) | N/A |
| EVOLVE5 | 44 | 1 | Lenti | Orvacabtagene autoleucel (JCARH125) | 4-1BB | Flu/Cyc | 50/150/450 × 106 cells | 7 (3-23) | N/A | 68 | 80 | 9 | 7 | 34 | 82 | 48 | 27 | 67 (6/9) at day 29 (≥PR patients) | N/A | N/A |
| EVOLVE47 | 62 | 1 | Lenti | Orvacabtagene autoleucel (JCARH125) | 4-1BB | Flu/Cyc | 300/450/600 × 106 cells | 6 (3-18) | 94 | 94 | 89 | 3 | 3 | 76 | 92 | 68 | 35 | 96 (21/25) at 3 mo (≥PR patients) | N/A | N/A |
| NCI13 | 16 | 1 | Retro | N/A | CD28 | Flu/Cyc | 9 × 106 cells/kg | 9.5 (3-19) | N/A | N/A | 94 | 38 | 19 | 31 | 81 | 63 | 13 | 100 (≥PR patients) | 31 wk | N/A |
| UPENN14 | 25 | 1 | Lenti | N/A | 4-1BB | None or Cyc | 10/50/100/500 × 106 cells | 7 (3-13) | 44 | 92 | 88 | 32 | 12 | 28 | 63 | 28 | 8 | 33 (≥PR patients) | 65-125 d | 502 d |
| P-BCMA-10145 | 23 | 1/2 | PiggyBac transposon | P-BCMA-101 | 4-1BB | Flu/Cy | 51/152/456/845/1143 × 106 cells | 6 (3-11) | N/A | 83 | 10 (2/21) | 0 | 5 (1/21) | 5 (1/21) | 63 (12/19) | 26 (5/19) | N/A | N/A | N/A | N/A |
| FHCRC6 | 11 | 1 | Lenti | FCARH143 | 4-1BB | Yes (not specified) | 50/150 × 106 cells | 11 (8-20) | 91 | 82 | 18 | 0 | 0 | 18 | 100 | 82 | 36 | N/A | N/A | N/A |
| CT05351 | 24 | 1 | Retro | CT053 | 4-1BB | Flu/Cyc | 150 × 106 cells | 4.5 (2-11) | N/A | 42 | 63 | 0 | 4 | 53 (8/15) | 88 | 83 | 79 | 85 (17/20) | N/A | N/A |
| CT103A52 | 18 | 1 | Lenti | CT103A | 4-1BB | Flu/Cyc | 1/3/6/8 × 106 cells/kg | 4 (3-6) | N/A | 39 | 94 | 22 | 0 | N/A | 100 (17/17) | 88 (15/17) | 71 (12/17) | 100 at 10−4 (≥PR patients) | N/A | N/A |
ASCT, autologous stem cell transplant; Cyc, cyclophosphamide; Flu, fludarabine; ICANS, immune effector cell associated neurotoxicity syndrome; IL-6, interleukin-6; Lenti, lentivirus; N/A, not available or not applicable; OS, overall survival; PFS, progression-free survival; Retro, retrovirus.
MRD negative at a sensitivity of 10−5 cells.
Clinical case (continued)
The patient continued to do well on posttreatment surveillance. Response assessment at 6 months after infusion showed M protein of 0.08 g/dL (93% reduction from pre–CAR T-cell treatment) and normal free light chain ratio, consistent with VGPR. There was no evidence of abnormal plasma cells in the bone marrow, including negative MRD by multiparametric flow cytometry (sensitivity 10−5 nucleated cells). At 1 year, serum protein electrophoresis showed undetectable monoclonal protein, but a persistent monoclonal band on immunofixation was present, consistent with a persistent MRD-negative VGPR.
Response kinetics and durability
Delayed clearance of monoclonal protein is commonly observed after BCMA CAR T-cell therapy, thus translating into a prolonged duration until maximal response is achieved. Treatment response in CAR T-cell clinical trials is based upon the reduction of monoclonal protein and resolution of extramedullary plasmacytoma, according to International Myeloma Working Group criteria. The depth of response by monoclonal protein in MM patients and its prognostic value depend on the time of assessment.10 In recent years, disease assessment using highly sensitive methods to detect MRD was integrated into the International Myeloma Working Group response criteria. A negative MRD status is strongly associated with superior outcomes in patients achieving at least a VGPR.11,12 The discordance between serum and bone marrow response could reflect a difference in test sensitivity, significance of the assessment time point, and/or potential sampling error. The updated long-term results of the CARTITUDE-1 trial showed that the median time to CR was 3 months, indicating that a more profound response can be achieved over time.
The median progression-free survival (PFS) after BCMA CAR T-cell therapy was ∼12 months (range, 6-15 months), depending upon the study. In the updated Ide-cel BCMA CAR T-cell study, the median PFS of all treated patients was 8.8 months (95% confidence interval, 5.5-11.6), with increased median PFS in higher-dose cohorts (5.8 months in the 300 × 106 cell cohort and 11.3 months in the 450 × 106 cell cohort; 8.6 months for the whole cohort).4 PFS outcomes for other CAR T-cell trials in RRMM are shown in Table 1.
Clinical case (continued)
The patient remained in remission for 18 months after BCMA-targeted CAR T-cell therapy. However, in May of 2019, laboratory results showed progressively increased serum free light chain. A bone marrow study showed 10% abnormal plasma cells, consistent with relapse.
Current limitations and potential strategies to overcome treatment failure
Despite an exceptional response rate observed across several BCMA-targeted CAR T cells, response durability has remained an ongoing clinical dilemma, because a significant proportion of patients eventually relapse.
Similar to CD19+ B lymphoid malignancies, the mechanisms of CAR T-cell therapy failure in MM are multifactorial, involving patient-, malignancy-, and immune-associated factors. Tumor with low or negative antigen that evades CAR T-cell eradication (antigen escape) is 1 underlying mechanism of relapse after cellular immunotherapy. Downregulation or loss of BCMA expression was observed in patients who relapsed after CAR T-cell therapy.6,13,14 However, unlike CD19+ lymphoid malignancy, mutations at the DNA level have not been reported. CAR T-cell–mediated trogocytosis, a process by which malignancy-associated surface proteins are extracted from the cell surface via lymphocyte-tumor engagement, is another mechanism that could result in decreased target antigen density.15 Lack of CAR T-cell persistence is likely another contributing factor to relapse in these patients. In addition, the immunosuppressive effects of the tumor microenvironment (TME) and malignant plasma cells on the function of CAR T cells potentially play a role in the resistance to immune-based therapy in patients with MM.
Overcoming antigen loss: beyond BCMA and polyspecific CAR T-cell constructs
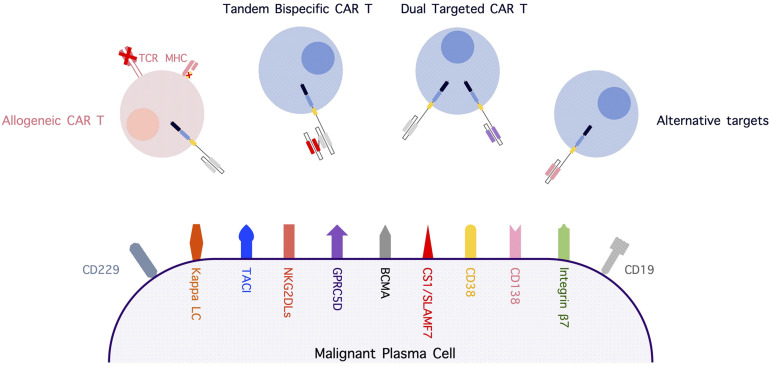
The potential strategy to overcome an antigenic loss in relapse after CAR T-cell therapy includes sequential/combined infusion with CAR T cells against targets other than BCMA, CAR T cells with novel dual-targeting vector design, and BCMA expression upregulation. In addition to BCMA, several antigens have been identified and explored as potential targets of immunotherapy, including adoptive cellular therapy for MM (Figure 1). These antigens include, but are not limited to, CD138, G-protein–coupled receptor class C group 5 member D (GPRC5D), transmembrane activator and calcium-modulator and cyclophilin ligand, signaling lymphocytic activation molecule family 7, natural killer group 2 member D (NKG2D) ligands, CD229, and integrin β7.16 Most of these non-BCMA–targeted CAR T cells are in early-stage clinical trials or preclinical phase studies. Our group demonstrated that GPRC5D is expressed on the surface of CD138+ multiple myeloma cells, independent of BCMA expression, but it is minimally expressed in other cell lines, with the exception of hair follicles; thus, it is a potential target of engineered immune effector cell–based therapy.17
Figure 1.
Alternative myeloma-associated targets for immune-based therapy and strategies involving novel CAR T-cell constructs. CS1, CD2 subset 1; LC, light chain; MHC, major histocompatibility complex; NKG2DLs, NKG2D ligands; SLAMF7, signaling lymphocytic activation molecule family 7; TACI, transmembrane activator and CAML interactor; TCR, T-cell receptor.
In addition to CAR T cells targeting antigens other than BCMA, engineering dual-targeted T cells is actively being investigated. Recently, our group reported preclinical data investigating dual-targeting approaches for CAR T-cell therapy, using BCMA and GPRC5D as a model. The study showed a superior antimyeloma response using a bicistronic construct encoding 2 independent 4-1BB CARs in preclinical models of MM with varying antigen expression compared with coinfusion of separate CAR T cells or a single-stalk tandem single-chain variable fragment (scFv) CAR design.18 In contrast to the results of this study, Zah and colleagues recently reported superior results using a tandem scFv “single-stalk” CAR design targeting BCMA and CS1.19 Transduction efficiency and gene expression were the limiting factors of the bicistronic approach; these challenges were not encountered in our study. Both studies revealed a trade-off between targeting 1 or the other antigens with a tandem single-stalk CAR design. There is reason to be hopeful that both approaches will enhance efficacy in patients, and it will be important to see how these strategies impact the durability of responses in the clinic.
One of the first clinical trials exploring such a dual-targeted approach was reported by Li et al, who evaluated a BCMA/CD38 tandem single-stalk CAR.20 Results from 22 patients with ≥2 prior lines of therapy included an ORR of 91% and a CR rate of 54.5%. Clinical trials using a split apheresis product transduced with unique vectors targeting distinct antigens, a so-called “CAR pool approach,” are also underway. Yan et al reported a phase 2 study of combined treatment with anti-CD19 and anti-BCMA CAR T cells in 21 RRMM patients.21 The median lines of prior therapies was 6 (range, 4-17), and 3 patients (14%) underwent autologous stem cell transplantation before CAR T-cell therapy. The investigators indicated that this strategy is a safe and active approach, with ORR, VGPR or better, CR or better, and MRD negativity rates of 95%, 81%, 57%, and 81%, respectively. The median PFS of patients who achieved a VGPR or better was 8 months (NCT04162353).
As discussed above, data from BCMA monotargeted CAR T-cell therapies have demonstrated decreased BCMA expression density after anti-BCMA CAR T-cell treatment.6,13,14 Preliminary data showed that γ-secretase inhibitor (GSI) inhibited the cleavage of BCMA and increased its expression on the plasma cell surface. It was hypothesized that this might improve the efficacy of BCMA CAR T cells in the future.22,23 Administration of an oral GSI with BCMA CAR T cells is being explored (NCT03502577).24 Cowan et al reported the preliminary results of this approach in patients with RRMM, with an ORR of 100% among 6 evaluable patients.24 All patients had increased BCMA expression on the plasma cell surface on serial bone marrow biopsies after receiving the GSI.
Impeding host immune response: decreasing CAR antigenicity and combating suppressive TME
An antimurine host immune response to CAR is a potential insult that can result in compromised in vivo CAR T-cell persistence. This was shown to be a clinically relevant concern for CD19-targeted CAR T-cell therapy in large cell lymphoma,25 and it was recently found to be a potential concern for BCMA-targeted CAR T-cell therapies incorporating the murine-derived 11D5-3 scFv.26 Engineering novel CAR T cells with humanized or a fully human CAR construct is an area of active research being explored by many groups and may ultimately be critical to providing long-term durability.21,27
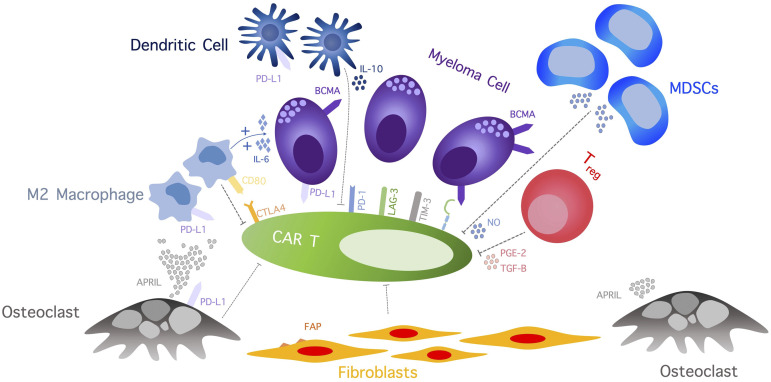
The immunosuppressive TME in bone marrow may also play an important role in resistance, immune escape, and progression of MM following CAR T-cell therapy. Preclinical and clinical data revealed a highly concentrated immune-resistant cytokine milieu and an increased number of immunosuppressive cells, including regulatory T cells, T helper 2 cells, myeloid-derived suppressor cells, cancer-associated fibroblasts (CAFs), tumor-associated macrophages, and osteoclasts (Figure 2).28 The immunosuppressive effect of myeloma cells and TME, along with an ongoing T-cell stimulation, contributes to T-cell dysfunction and activation-induced T-cell death. Therefore, targeting TME can alleviate some essential resistance pathways of MM to CAR T cells. Sakemura and colleagues conducted a preclinical study exploring CAR T-cell product targeting of fibroblast associated protein (FAP) and BCMA/CS1 in a CAF-enriched environment. Inhibition of CAFs by FAP CAR resulted in a superior myeloma killing effect of BCMA/CS1 target CAR T cells.29 An “armored” CAR T cell is among several approaches aiming to improve functions of engineered CAR T cells by preventing T-cell exhaustion, overcoming immunosuppressive TME, or enhancing killing function and T-cell persistence. Engineering CAR T cells to secrete programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1) antibody, which selectively binds to PD-L1 expressed by various cells in TME, thus preventing endogenous PD-1/PD-L1 axis activation, may alleviate TME-induced immune escape.30,31 Currently, an ongoing phase 1 clinical trial is exploring the safety of BCMA CAR T cells secreting a mutant PD-1 Fc fusion protein in RRMM (NCT04162119). The CAR T-cell construct inheriting an additional gene that leads to constitutive interleukin-12 secretion is another novel CAR T-cell model that was shown to have improved tumor-killing effect and overcome the immunosuppressive effect of TME in the preclinical models.32 Silencing immune checkpoint signaling using a genome-editing technique to enhance the anti-tumor killing effect and prevent T-cell exhaustion/activation-induced cell death was tested in a preclinical model.33,34 Recently, Stadtmauer et al presented the data from a phase 1 study of NY-ESO-1–targeted engineered T cells with a disrupted PDCD1 gene using a clustered regularly interspaced short palindromic repeats (CRISPR) gene-editing technique in 3 patients with refractory cancers, 2 of which were MM.35 The study showed a robust in vivo expansion with durable persistence and evidence of intratumoral infiltration of engineered T cells in all 3 patients. Although the treatment response observed in this study was modest, this finding proved the feasibility and safety of immune checkpoint disruption as a platform to improve the persistence of adoptive T cells. Rafiq et al demonstrated an enhanced CAR T-cell function and trafficking of CAR T cells to tumor sites in a preclinical model of PD-1 blocking scFv-secreting CAR T cells.30 Combining immunomodulatory agents (ie, lenalidomide) with CAR T-cell therapy was shown to enhance CAR T-cell function in the immunosuppressive TME. Works et al found that lenalidomide could potentiate cytokine production and cytolytic activities.36 In addition, lenalidomide prevented exhaustion of CAR T cells under low-antigen or immunosuppressive environments in a xenograft model.37
Figure 2.
The complicated immunosuppressive TME effect on CAR T cells includes a wide array of cellular network and cytokines that induce CAR T-cell exhaustion, inhibit CAR T-cell function, and promote CAR T-cell apoptosis. APRIL, a proliferation-inducing ligand; IL, interleukin; LAG-3, lymphocyte-activation gene-3, MDSCs, myeloid-derived stem cells; NO, nitric oxide; PGE-2, prostaglandin E2; TGF-B, transforming growth factor β; TIM-3, T-cell immunoglobulin mucin-3; Treg, regulatory T cell.
Universal adoptive engineered cellular therapy: allogeneic and iPSC-derived immune effector cells
In addition to data available from autologous CAR T cell trials, manufacturing CAR T cells using lymphocytes from allogeneic donors has long been investigated in several types of malignancy, including MM. Several novel bioengineering methods (ie, knocking out the T-cell receptor and major histocompatibility complex expression using various gene-editing techniques) have been implemented to moderate potential graft-versus-host toxicity and host rejection. The phase 1 UNIVERSAL (NCT04093596) and MELANI-01 (NCT04142619) trials are investigating the safety and feasibility of 2 allogeneic CAR T cells in RRMM patients (Table 2). In addition to donor-derived immune effector cells, induced pluripotent stem cell (iPSC)-derived immune cells are a promising platform for adoptive cellular therapy. In addition to their “off the shelf” availability, iPSC-derived lymphocytes offer a unique advantage via clonal selection: a highly selected, multiply gene-edited, and consistent tumor-specific immune cell product can be produced.38 Combining advanced techniques in developmental biology and novel genetic engineering, iPSC-derived T cells exhibit potent antitumor activity similar to conventional CAR T cells, but they tend to maintain the innate phenotype, which can translate into fewer concerns about graft-versus-host disease.39 Several iPSC-derived CAR immune cells are currently under investigation in hematologic malignancies. Recently, Bjordahl et al reported preclinical data using FT576 cells, a novel dual-target CAR iPSC-derived natural killer (NK) cell against BCMA and CD38 that shows high cytotoxic activity against myeloma cell lines.40 The additional hypothesized advantage of CAR NK cells is the absence of graft-versus-host disease development and a potentially lower risk for CRS compared with conventional CAR T cells. Several preclinical studies demonstrated cytotoxic activity and myeloma cell growth inhibition using CAR NK cells against various targets, including CS1, CD138, BCMA, and NKG2D ligands.41 A phase 1/2 study of BCMA CAR NK cells in RRMM is ongoing (NCT03940833).
Table 2.
Available allogeneic CAR-expressed immune effector cells in MM
| Product | Trial | ClinicalTrials.gov identifier | Phase | Type | Target | Vector | Gene editing event | Inclusion | n (estimated) | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| UCARTCS1 | MELANI-01 | NCT04142619 | 1 | CAR T | CS1 | Lentivirus | TALEN | RRMM | 18 | Recruiting |
| ALLO-715 | UNIVERSAL | NCT04093596 | 1 | CAR T | BCMA | Lentivirus | TALEN | RRMM | 90 | Recruiting |
| PBCAR269A | PBCAR269A-01 | NCT04171843 | 1/2a | CAR T | BCMA | Adenovirus | ARCUS endonuclease | RRMM | 48 | Recruiting |
| CTX120 | Unnamed | NCT04244656 | 1 | CAR T | BCMA | CRISPR/Cas9 | CRISPR/Cas9 | RRMM | 80 | Recruiting |
| BCMA-UCART | Unnamed | NCT03752541 | 1 | CAR T | BCMA | Unknown | Unknown | RRMM | 20 | Recruiting |
ClinicalTrials.gov access date was 30 May 2020.
CS1, CD2 subset 1; TALEN, transcription activator-like effector nucleases.
In addition to “off the shelf” availability, a major advantage of gene-modified allogeneic and iPSC-derived immune effector cells is the potential for superior fitness of healthy donor lymphocytes over autologous cells obtained from heavily treated patients, which can translate into better efficacy and survival outcomes. Garfall and colleagues demonstrated the influence of T-cell fitness on the function of CAR T cells as a clinically meaningful attribute of cellular therapies.42 Pheresis products collected from patients after initial induction therapy had a higher proportion of CD8+CD45RO−CD27+ memory T cells and CD4+/CD8+ ratio than from patients with heavily treated RRMM, which were predictors associated with clinical response in patients with RRMM treated with BCMA CAR T cells.14 The result of this study was similar to the finding in chronic lymphocytic leukemia (CLL) patients treated with CD19 CAR T cells.43
Strategies to overcome intrinsic T-cell defects
In concordance with data from B lymphoid malignancies, individual T-cell subsets have different replication potential and cytotoxic capacity, which play a critical role in the function of immune effector cells. Stem cell memory T cells and other less differentiated T cells carry a high potential for in vivo expansion, survival, and persistence and may be less susceptible to activation-induced exhaustion.44 The bb21217 anti-BCMA CAR T-cell product is generated by manufacturing T cells with phosphoinositide 3-kinase inhibitor, bb007, during the culture process to enrich the “memory-like” T-cell composition. This product induced an ORR of 83% and a toxicity profile comparable with other trials in 22 patients with RRMM.8 P-BCMA-101 is a nonviral-based BCMA targeted CAR T-cell product using the piggyBac transposon-based manufacturing system. The product contains a high proportion of CAR T cells with a stem cell memory T cell phenotype,45 which is hypothesized by the investigators to improve response rate and durability. Refining the ratio of CD4+/CD8+ in CAR T products is another approach that is being actively explored.46 Examples of CAR T-cell clinical studies focusing on this approach include FCARH143, a BCMA-targeted CAR T-cell product with separate CD4+ and CD8+ manufacturing and reinfusion at a fixed ratio of CD4+/CD8+ T lymphocytes, as well as JCARH125, a BCMA-targeting CAR T-cell product with a single-track manufacturing process and cytokine cocktail designed to result in a consistent CD4+/CD8+ ratio, with enrichment of CAR T cells with central memory phenotype in the final product. Both of these trials demonstrated high response rates (ORR > 90%) in heavily treated RRMM.6,47
Other actively investigated preclinical approaches to enhance CAR T-cell persistence and function include modifying the immunoreceptor tyrosine-based activation motifs (ITAMs) in the CD3ζ chain of the CAR endodomain and constructing T-cell receptor α constant–specific CAR using various genome-editing techniques.48,49 The typical construct of CD3ζ ITAMs in CAR T cells consists of 3 domains (ITAM1, ITAM2, ITAM3). Modulating activation potential by decreasing the expression of ITAMs affects T-cell signaling and function and controls T-cell fates. Feucht et al demonstrated improved CAR T-cell persistence in 1928ζ CAR T cells with a single ITAM domain.48 In a murine model, generating T-cell receptor α constant–specific CAR using CRISPR/Cas9 strengthened 1928ζ T-cell potency and elicited superior tumor-killing effect compared with conventional γ-retrovirus vector CAR T cells that result in multiple integration sites.49
Conclusions
In 2020, CAR T-cell therapy has reached a therapeutic milestone, offering great promise to patients with RRMM. However, despite an exceptional ORR, response durability remains a significant challenge. Further studies are needed to decipher current therapeutic dilemmas and to advance CAR T-cell therapy to additional disease settings for patients with MM.
Acknowledgments
K.W. receives salary support from the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center. S.M. acknowledges support from Memorial Sloan Kettering Core Grant P30 CA008748. E.L.S. acknowledges support from the National Institutes of Health National Cancer Institute (K08 CA241400), American Society of Hematology, Leukemia Lymphoma Society, Lymphoma Research Foundation, American Society of Clinical Oncology, Society for Immunotherapy of Cancer, Multiple Myeloma Research Foundation and Dana-Farber/Harvard Cancer Center Support Grant (P30 CA006516).
References
- 1.Gandhi UH, Cornell RF, Lakshman A, et al. . Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. . B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raje N, Berdeja J, Lin Y, et al. . Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi NC, Anderson LD Jr, Shah N, et al. . Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): initial KarMMa results. 2020;38(15 suppl):8503. [Google Scholar]
- 5.Mailankody S, Htut M, Lee KP, et al. . JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). Blood. 2018;132(suppl 1):957. [Google Scholar]
- 6.Green DJ, Pont M, Sather BD, et al. . Fully human Bcma targeted chimeric antigen receptor T cells administered in a defined composition demonstrate potency at low doses in advanced stage high risk multiple myeloma. Blood. 2018;132(suppl 1):1011. [Google Scholar]
- 7.Madduri D, Usmani SZ, Jagannath S, et al. . Results from CARTITUDE-1: a phase 1b/2 study of JNJ-4528, a CAR-T cell therapy directed against B-cell maturation antigen (BCMA), in patients with relapsed and/or refractory multiple myeloma (r/r mm). Blood. 2019;134(suppl 1):577.31416814 [Google Scholar]
- 8.Berdeja JG, Alsina M, Shah ND, et al. . Updated results from an ongoing phase 1 clinical study of bb21217 anti-Bcma CAR T cell therapy. Blood. 2019;134(suppl 1):927. [Google Scholar]
- 9.Wang B-Y, Zhao W-H, Liu J, et al. . Long-term follow-up of a phase 1, first-in-human open-label study of LCAR-B38M, a structurally differentiated chimeric antigen receptor T (CAR-T) cell therapy targeting B-cell maturation antigen (BCMA), in patients (pts) with relapsed/refractory multiple myeloma (RRMM). Blood. 2019;134(suppl 1):579.31076443 [Google Scholar]
- 10.Schinke C, Hoering A, Wang H, et al. . The prognostic value of the depth of response in multiple myeloma depends on the time of assessment, risk status and molecular subtype. Haematologica. 2017;102(8):e313-e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, et al. . International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 12.Munshi NC, Avet-Loiseau H, Rawstron AC, et al. . Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3(1):28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brudno JN, Maric I, Hartman SD, et al. . T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen AD, Garfall AL, Stadtmauer EA, et al. . B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamieh M, Dobrin A, Cabriolu A, et al. . CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmers M, Roex G, Wang Y, et al. . Chimeric antigen receptor-modified T cell therapy in multiple myeloma: beyond B cell maturation antigen. Front Immunol. 2019;10:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith EL, Harrington K, Staehr M, et al. . GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández de Larrea C, Staehr M, Lopez AV, et al. . Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple myeloma. Blood Cancer Disc. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zah E, Nam E, Bhuvan V, et al. . Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat Commun. 2020;11(1):2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Mei H, Hu Y, Guo T, Liu L, Jiang H, et al. . A bispecific CAR-T cell therapy targeting Bcma and CD38 for relapsed/refractory multiple myeloma: updated results from a phase 1 dose-climbing trial. Blood. 2019;134(suppl 1):930. [Google Scholar]
- 21.Yan Z, Cao J, Cheng H, et al. . A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6(10):e521-e529. [DOI] [PubMed] [Google Scholar]
- 22.Pont MJ, Hill T, Cole GO, et al. . γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent SA, Hoffmann FS, Kuhn PH, et al. . γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6(1):7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan AJ, Pont M, Sather BD, et al. . Efficacy and safety of fully human Bcma CAR T cells in combination with a gamma secretase inhibitor to increase Bcma surface expression in patients with relapsed or refractory multiple myeloma. Blood. 2019;134(suppl 1):204. [Google Scholar]
- 25.Turtle CJ, Hanafi LA, Berger C, et al. . Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N, Kochenderfer JN. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains [published correction appears in Nat Commun. 2020;11(1):1319]. Nat Commun. 2020;11(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EL, Staehr M, Masakayan R, et al. . Development and evaluation of an optimal human single-chain variable fragment-derived BCMA-targeted CAR T cell vector. Mol Ther. 2018;26(6):1447-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano Y, Moschetta M, Manier S, et al. . Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160-172. [DOI] [PubMed] [Google Scholar]
- 29.Sakemura R, Cox MJ, Hansen MJ, et al. . Targeting cancer associated fibroblasts in the bone marrow prevents resistance to chimeric antigen receptor T cell therapy in multiple myeloma. Blood. 2019;134(suppl 1):865. [Google Scholar]
- 30.Rafiq S, Yeku OO, Jackson HJ, et al. . Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez ER, Chang K, Sun J, et al. . Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341-34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7(1):10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Peng Y, Hublitz P, Zhang H, Dong T. Genetic abrogation of immune checkpoints in antigen-specific cytotoxic T-lymphocyte as a potential alternative to blockade immunotherapy. Sci Rep. 2018;8(1):5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupp LJ, Schumann K, Roybal KT, et al. . CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadtmauer EA, Fraietta JA, Davis MM, et al. . CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Works M, Soni N, Hauskins C, et al. . Anti-B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther. 2019;18(12):2246-2257. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Walter M, Urak R, et al. . Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin Cancer Res. 2018;24(1):106-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Themeli M, Kloss CC, Ciriello G, et al. . Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjordahl R, Gaidarova S, Goodridge JP, et al. . FT576: a novel multiplexed engineered off-the-shelf natural killer cell immunotherapy for the dual-targeting of CD38 and Bcma for the treatment of multiple myeloma. Blood. 2019;134(suppl 1):3214. [Google Scholar]
- 41.Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175-180. [DOI] [PubMed] [Google Scholar]
- 42.Garfall AL, Dancy EK, Cohen AD, et al. . T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3(19):2812-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraietta JA, Lacey SF, Orlando EJ, et al. . Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kravets VG, Zhang Y, Sun H. Chimeric-antigen-receptor (CAR) T cells and the factors influencing their therapeutic efficacy. J Immunol Res Ther. 2017;2(1):100-113. [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory T, Cohen AD, Costello CL, et al. . Efficacy and safety of P-Bcma-101 CAR-T cells in patients with relapsed/refractory (r/r) multiple myeloma (mm). Blood. 2018;132(suppl 1):1012. [Google Scholar]
- 46.Sommermeyer D, Hudecek M, Kosasih PL, et al. . Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mailankody S, Jakubowiak AJ, Htut M, et al. . Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011). J Clin Oncol. 2020;38(15 suppl):8504. [Google Scholar]
- 48.Feucht J, Sun J, Eyquem J, et al. . Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency [published correction appears in Nat Med. 2019;25(3):530]. Nat Med. 2019;25(1):82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyquem J, Mansilla-Soto J, Giavridis T, et al. . Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berdeja JG, Madduri D, Usmani SZ, et al. . Update of CARTITUDE-1: a phase Ib/II study of JNJ-4528, a B-cell maturation antigen (BCMA)-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. J Clin Oncol. 2020;38(15 suppl):8505. [Google Scholar]
- 51.Jie J, Hao S, Jiang S, et al. . Phase 1 trial of the safety and efficacy of fully human anti-Bcma CAR T cells in relapsed/refractory multiple myeloma. Blood. 2019;134(suppl 1):4435. [Google Scholar]
- 52.Li C, Wang J, Wang D, et al. . Efficacy and safety of fully human Bcma targeting CAR T cell therapy in relapsed/refractory multiple myeloma. Blood. 2019;134(suppl 1):929. [Google Scholar]