Visual Abstract
Abstract
Endovenous stenting has emerged as the method of choice to treat iliofemoral venous outflow obstruction. It is used in patients with established postthrombotic syndrome (PTS) after previous deep vein thrombosis (DVT) to reduce symptoms of chronic pain and swelling and to aid ulcer healing in severe cases. Venous stenting is used to alleviate symptoms of obstruction in patients presenting with acute DVT, with the aim of preventing development of PTS. There is a low risk of morbidity and mortality associated with the use of endovenous stenting, and although significant advances have been made, particularly improvements in stent design for use in the venous circulation, data are lacking on beneficial long-term outcomes. Unmet research needs include optimal patient selection, anticoagulant choice and duration, best practice for postoperative surveillance, and use of validated assessment tools to measure outcomes. In this article, I address the potential benefits, as well as the challenges, of endovenous stenting.
Learning Objectives
Understand the role of venous stenting in patients who present with acute deep vein thrombosis or in patients with postthrombotic syndrome
Address the challenges associated with venous stenting
Introduction
The majority of patients presenting with venous thromboembolism (VTE) receive treatment with anticoagulation to prevent thrombus extension and embolization in order to reduce morbidity and mortality. However, up to 20% to 50% of patients develop PTS as a consequence of DVT despite anticoagulation.1
Symptoms of PTS include chronic limb swelling and pain, often leading to major disability and impaired QoL.2 In severe cases, development of leg ulceration leads to significant costs for health care services. Clinical scoring systems often used as outcome measures in studies of VTE have been developed to assess the severity of PTS, including the Villalta score3; Clinical, Etiology, Anatomic, Pathophysiology classification4; and Venous Clinical Severity Score.5
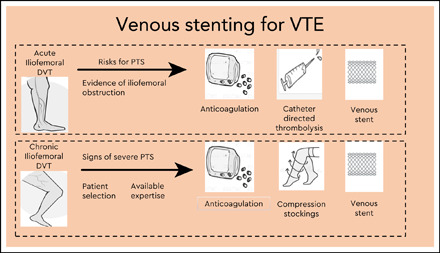
Mechanical methods such as thrombectomy and/or local thrombolysis to remove thrombus, potentially reducing the incidence or severity of PTS in patients with symptomatic iliofemoral DVT, have been in place since the 1990s. Endovenous stenting is increasingly used as a further treatment modality for management of underlying symptoms relating to venous outflow obstruction in patients with acute or chronic symptoms of VTE (Table 1). Although it appears to be a safe and promising treatment for prevention and management of PTS, data are lacking. This article aims to update the reader on the current use of venous stenting in VTE.
Table 1.
Patient selection criteria for endovenous stenting
| When to consider venous stenting |
|---|
| Acute DVT |
| Iliofemoral DVT |
| Symptoms present for <14 d and candidate for thrombolysis |
| Evidence of residual thrombus or obstruction following thrombolysis |
| Chronic DVT |
| Established PTS with significant moderate to severe symptoms using validated clinical assessment score (eg, Villalta or CEAP) |
| Iliofemoral stenosis or obstruction confirmed on imaging amenable to stent placement |
CEAP, Clinical, Etiology, Anatomic, Pathophysiology.
Case 1
Patient 1 is a 27-year-old woman who presented with a 3-day history of left lower limb pain and extensive leg swelling. She had experienced a postpartum left-sided deep vein thrombosis (DVT) 13 years previously and received short-term anticoagulation. She had been asymptomatic since.
A venous duplex scan demonstrated acute thrombus in the left external iliac, common femoral, superficial femoral, and popliteal veins, confirmed on the basis of a computed tomographic venogram (obtained while the patient was being considered for thrombolysis). She was anticoagulated with low-molecular-weight heparin (LMWH) and had catheter-directed thrombolysis (CDT) (without venous stenting) due to persistent symptoms. Her symptoms improved within 24 hours of CDT, and she was discharged to home with rivaroxaban.
She developed worsening symptoms within 2 to 3 weeks. Repeat imaging using magnetic resonance (MR) venography demonstrated an occluded left iliac vein. She can walk only short distances due to leg swelling and pain, uses crutches to mobilize, and is unable to work. She continues to take rivaroxaban (20 mg daily).
Case 2
Patient 2 is a 16-year-old boy who presented with gradual onset of right lower limb swelling and was diagnosed with iliofemoral DVT. Several small areas of ulceration were present on his shin, indicating chronic venous hypertension.
He was anticoagulated with LMWH followed by warfarin. In view of his age and presentation of acute-on-chronic DVT (leg ulceration caused by chronic venous insufficiency), it was recommended that he receive lifelong anticoagulation. His Villalta score was consistent with severe postthrombotic syndrome (PTS). His quality of life (QoL) had significantly deteriorated, and he had nonhealing leg ulcers despite treatment with compression bandaging.
Imaging demonstrated high-grade stenosis of the lower inferior vena cava and right common iliac vein. Venous stents were inserted in the inferior vena cava (IVC) and iliofemoral vein (one 14-mm × 90-mm and two 14-mm × 60-mm stents). Anticoagulation postoperatively was with warfarin and was later switched to rivaroxaban. His symptoms improved over 3 to 4 months, and his leg ulcers healed. He has intentionally lost 25 kg with the ability to exercise.
What is the history of endovascular stenting?
Endovascular stents have been in use since the 1960s. Traditionally, stents were designed for placement in the arterial system.
Endovenous recanalization of iliofemoral stenosis or occlusion with venoplasty and stent placement was first reported by Berger et al in 19956 and is now the treatment of choice to treat deep venous disease secondary to venous outflow tract obstruction.
Early attempts at endovenous stenting used arterial stents but were associated with high rates of reocclusion, since arterial stents are unsuitable for use in the venous system due to their small diameters and high radial force. Arterial and venous anatomies have significant differences. Venous stents need to have stent flexibility, radial strength and crush resistance (force required to compress the stent), and the ability to allow precise deployment (see Table 1).
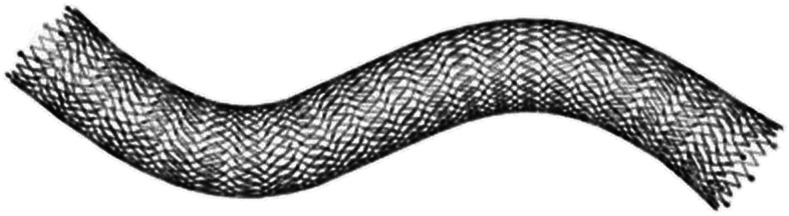
Most endovenous stents are “bare” (ie, uncovered), but expanded polytetrafluoroethylene-covered nitinol stents and drug-eluting stents or heparin-coated stents have been developed. The Vici (Boston Scientific [Figure 1] and Venovo (BD interventional) nitinol venous stents are currently the only US Food and Drug Administration–approved stents for use in iliofemoral venous occlusive disease, but studies of other devices are underway.
Figure 1.
The Vici venous stent system.
Table 2.
Ideal venous stent structure
| Stent structure | Material composition: steel/nickel/titanium/nitinol stent design, cell design (laser cut vs braided) |
|---|---|
| Mechanical properties | Radical strength, radical stiffness, acute recoil, foreshortening, crush resistance |
| Deployment method | Self-expandable vs balloon expandable |
| Stent covering | Bare metal vs coated vs drug eluting |
Table 3.
Venous stent devices
| Device | CE mark approval | FDA approved |
|---|---|---|
| Abre (Medtronic) | 2017 | — |
| blueflow (plus medica GmbH & Co. KG) | 2018 | — |
| sinus-Obliquus (optimed Medizinische Instrumente GmbH) | 2013 | — |
| sinus-Venous (optimed Medizinische Instrumente GmbH) | 2015 | — |
| WALLSTENT (Boston Scientific) | 2015 | — |
| Venovo (BD) | 2015 | 2019 |
| Vici (Boston Scientific) | 2013 | 2019 |
| Zilver Vena (Cook Medical) | 2010 | — |
CE mark, administrative marking that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area; FDA, US Food and Drug Administration.
When are venous stents used?
Venous stenting is used as an adjunctive treatment in patients presenting with acute iliofemoral DVT if there is a residual venous obstruction (RVOO) following thrombolysis and balloon angioplasty,7 with the aim of restoring vein patency and preventing PTS (Table 1). There appears to be a higher incidence of PTS and VTE recurrence if balloon angioplasty is used alone in patients with RVOO.8 Appropriate diagnostic methods to define the lesion using a combination of computed tomography or MR venogram and intravascular ultrasound (IVUS) should be used prior to treatment.9
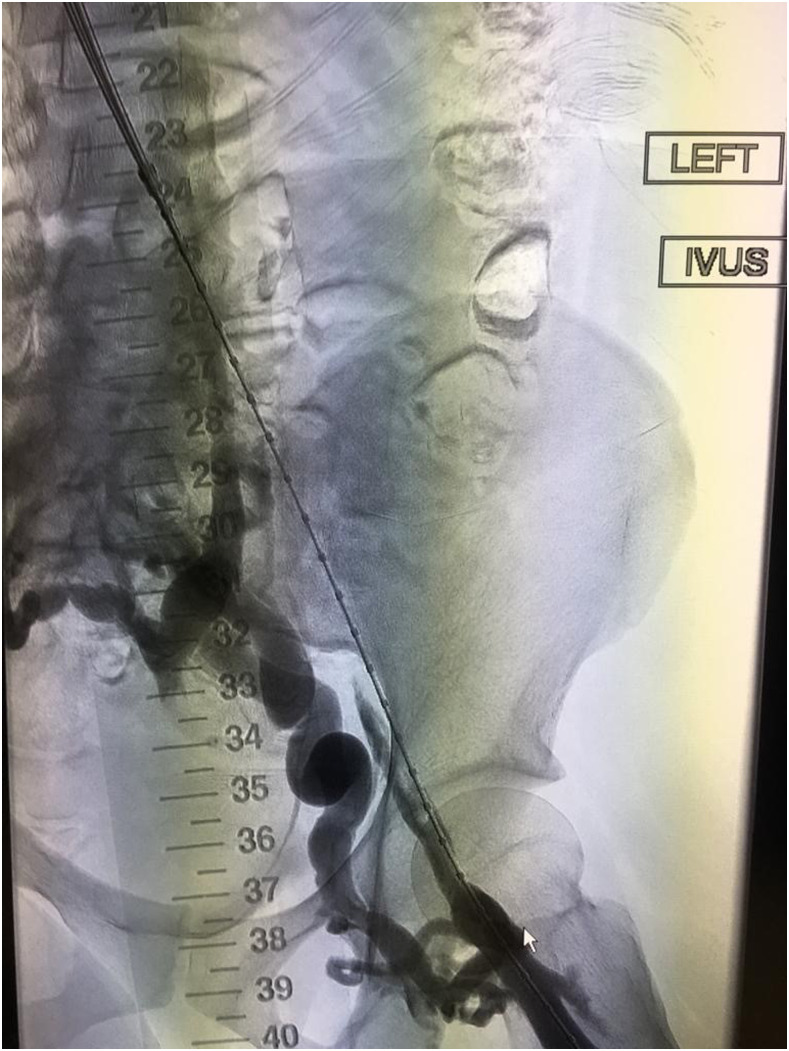
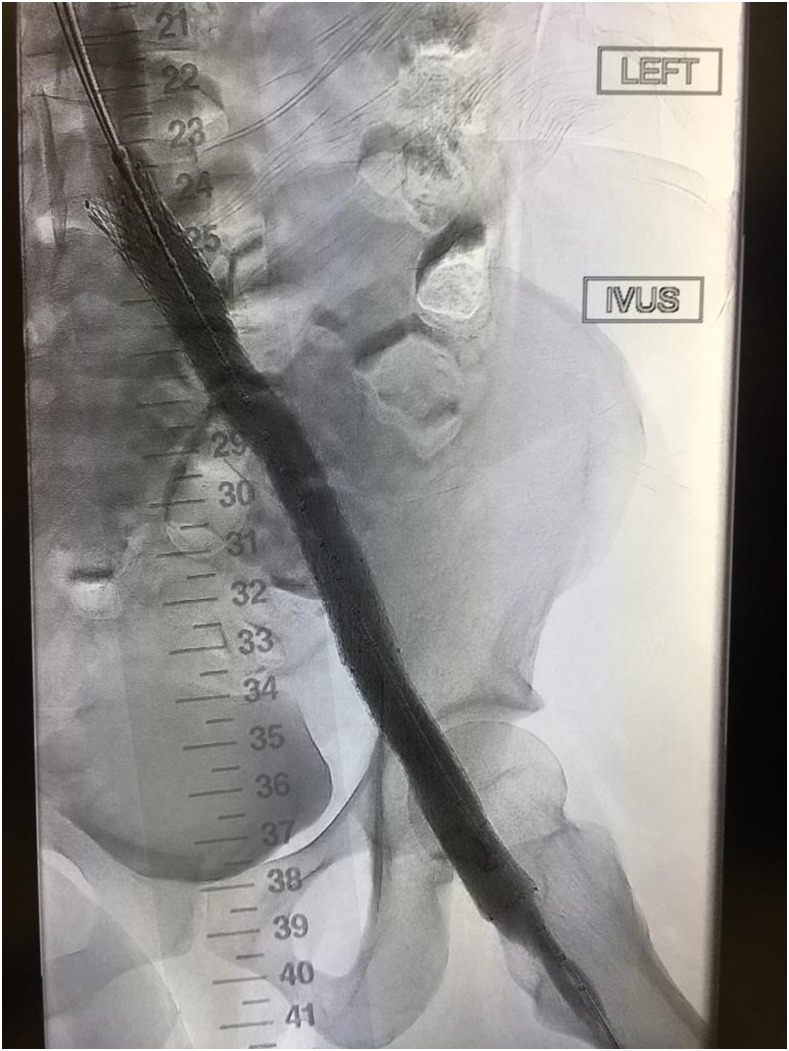
Severe PTS is often due to a chronic outflow obstruction, mainly the iliac vein, since there is usually poor collateralization of this vessel. Studies suggest when patients have severe symptoms; venous stenting is indicated when the obstruction is >50%, superficial collaterals form (Figures 2 and 3), and there is reflux in the deep and/or superficial veins.10 Femoropopliteal DVTs are best treated with anticoagulation only.
Figure 2.
Example of thrombosed iliofemoral lesion with collateralization before venous stenting.
Figure 3.
Improved vascular flow after venous stenting using the Abre stent (Medtronic).
Challenges of placing venous stents
Case selection is a key factor when patients are considered for endovascular treatment. Patients presenting with acute DVT require considerations different from patients with chronic symptoms. For acute iliofemoral DVT, strict selection criteria apply to use of thrombolysis with or without venous stenting, including bleeding risk, life expectancy, anatomy of the DVT, and severity of presenting symptoms; thrombolysis is usually reserved for those presenting with clinically severe thrombosis. There is a lack of convincing data, particularly medium- to long-term outcomes, to support use of venous stents in addition to CDT. Chronic venous obstruction can be postthrombotic or nonthrombotic, secondary to a number of causes, such as intrinsic, mural, and extrinsic pathology. External compression can be from surrounding tissues or localized compression from a pulsatile artery, as seen in May-Thurner configurations (compression of the left common iliac vein by the right common iliac artery). Chronically occluded veins are usually composed of collagen and often more difficult to treat. Outcomes vary according to whether a lesion is a postthrombotic or nonthrombotic iliac vein lesion (NIVL); therefore, the timing of stenting and the choice of stent design require careful consideration. The evidence for intervention is much less clear in NIVL, and it should be avoided until there are more convincing data.
The main complication of venous stents is in-stent restenosis or occlusion. Surveillance of stented limbs with duplex ultrasound is recommended on the day after stent placement, at 6 weeks, and yearly thereafter.11 It is our practice to perform duplex ultrasound the day after stent placement and at 6 weeks, 6 months, and yearly thereafter. If stents are >50% stenosed, reintervention, usually in the form of angioplasty to the in-stent stenosis, is required to maintain stent patency to prevent worsening of symptoms or occlusion.11
The risk of stent reocclusion is associated with a number of factors, including poor inflow to the obstructed vessel, external compression, or inappropriate stent design. Patient-related risk factors may include underlying thrombophilia.12 Other risks include stent misplacement or migration, stent fracture, and bleeding, although risk of major bleeding appears to be low (<1%).13
Patients presenting for surgical intervention usually have significantly limited function and a severe Villalta score before intervention. When venous stenting is offered, decision-making should be based on the patient's premorbid condition, anatomical extent of disease, and likelihood of symptomatic improvement. Patients need to be advised of the potential to reintervene and risks of bleeding and in- stent thrombosis.
What is the evidence for use of venous stenting in acute DVT?
Current American College of Chest Physicians, National Institute for Health and Care Excellence, and European Society of Cardiology guidelines do not provide recommendations for endovenous stenting after CDT or pharmacomechanical thrombectomy, likely due to lack of evidence. Most studies are retrospective, cohort series, or smaller trials with variable study design. Earlier studies used arterial stents rather than dedicated venous stents. Stent patency is the most frequently measured outcome, and few studies examined improvements in severity of PTS or QoL scores.
A systematic review of deep venous stenting in acute DVT identified 27 studies (542 patients). It included 3 randomized controlled trials (RCTs) and 21 cohort studies (8 prospective, 12 retrospective), and all patients included had undergone lysis, venoplasty, and stenting. The overall patency rates were 87.8% over a follow-up period of 12 to 19.7 months.14 PTS was assessed in 26 of 27 studies, with an observed rate of 14.6%, but PTS clinical scores and QoL were assessed in only 3 of 27 studies over a short period of 3 months. Chronic venous insufficiency questionnaires (CIVIQs) were used; only one compared stenting versus no stenting, but it did show a significant improvement in QoL using CIVIQ scores (22.67 ± 3.01 vs 39.34 ± 6.6; P < 0.01).15 Only 1 of the 3 randomized trials included15-17 was randomized between stenting and no stenting.15 In that study, the patency rate was 86% vs 54.8% in the stented vs nonstented group, and there was a significant reduction in CEAP score (1.61 ± 0.21 vs 0.69 ± 0.23).
The ATTRACT (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis)18 and CAVENT (Catheter-Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis)19 trials were large RCTs designed to address whether thrombolysis led to a reduction of PTS. The CAVENT trial19 showed a significant reduction in the incidence of PTS with CDT (49% vs 63%) but did not show any difference in QoL outcomes. The ATTRACT trial18 showed less reduction in the incidence of PTS, but it showed a reduction in severity of PTS at 6-, 12-, 18-, and 24-month follow-ups and, similar to CAVENT, showed no improvement in QoL. However, only a small number of patients included in the intervention arms of these trials had venous stenting after thrombolysis (28% in the ATTRACT trial and 18% in CAVENT), which may partly explain the poor outcomes of these trials.
Overall, although outcomes appear promising, there are few data to support use of stenting in patients with acute DVT, and it should be used only for highly selected patients.
Placement of a venous stent in patient 1 in addition to thrombolysis at the time of presentation of DVT might have prevented reocclusion and reduced her risk of developing significant PTS.
What is the evidence for the use of venous stenting in chronic DVT?
European Society for Vascular Surgery guidelines recommend angioplasty and stenting as first-line treatment for patients with clinically relevant chronic iliocaval or iliofemoral obstruction or those with symptomatic NIVL (class IIa, level B),20 while the American Heart Association guidelines recommend that it be considered in these situations (class IIb, level of evidence B).21
One of the first large single-center retrospective studies of endovenous stenting included 139 patients (78 patients with PTS secondary to previous DVT and 61 with NIVL). Patients with PTS showed a significant improvement in symptoms of pain and swelling (reduction of visual analog pain score from 4.2 to 2.2); 50% of 24 patients with leg ulcers showed complete healing.22 Stent reocclusion rates were 17%. While these initial results appeared promising, most later studies of venous stents were also retrospective, using stent patency as the main outcome measure. Few studies looked at reduction in symptom severity over the medium to long term.
A recent systematic review of venous stenting in chronic disease included 16 (mainly retrospective) studies of 2373 postthrombotic and 2586 nonthrombotic patients. Primary patency rates (open stent without need for reintervention) were 32% to 98%.23 Primary assisted patency rates (open stent but additional procedures required to prevent occlusion) were between 66% and 96%, and secondary patency rates (open stent required an additional procedure following occlusion) were between 68% and 96%. Twelve of the 16 studies reported on ulcer healing, which occurred in 56% to 100% of patients. Only 5 studies reported on severity of symptoms, but 4 of them showed a significant reduction in symptoms using validated scoring systems (CEAP and Villalta).
Similarly, only 3 studies reported on QoL outcomes (using CIVIQ and VEINESQOC scores) and showed general improvement in venous disease–related QoL scores.
Major complications were less than 2% across all studies (including bleeding, prolonged hospitalization, or need for further intervention), but reporting was variable between studies. No postoperative mortality was reported in 6 of the 16 studies, and no pulmonary emboli were reported.
It is accepted that chronic venous disease is more difficult to treat than NIVL and often leads to higher rates of stent reocclusion, particularly in patients with postthrombotic lesions.10,24,25 Significant drawbacks of most studies are that NIVLs are included with postthrombotic lesions, while control groups were rarely included, making it difficult to interpret whether stenting really improves outcomes.
Preliminary data from multicenter, multinational single-arm prospective studies (the VERNACULAR and VIRTUS trials) using newer dedicated venous stents have shown primary patency rates at 12 months to be 88.3%.and 84%, respectively.26,27 The VERNACULAR study showed a significant improvement in QoL scores (venous clinical severity score [VCSS] reduced at 12 months [4.0 ± 3.9]); further data from the VIRTUS trial are pending. The Arnsberg venous registry of 79 patients has shown a 6-month primary patency rate of 98% and significant decreases in revised VCSS scores, as well as ulcer healing in all 8 patients with ulcers.28 No major complications were seen.
Overall, evidence for use of venous stenting for treatment of chronic venous disease is weak, but potential particular benefits in improvement of QoL scores and ulcer healing have been shown. Randomized controlled trials using dedicated venous stents are needed to provide robust data on improvements in severity of PTS using clinical scores and QoL indicators, as well as complication rates over the long term.
Patient 2 underwent venous stenting and had significant reduction in the severity of his PTS, and his leg ulcers healed. Importantly, his QoL significantly improved, demonstrating that, in carefully selected patients, stenting can lead to improved outcomes.
How is anticoagulation managed in patients with venous stents, and how should they be followed up?
Few studies specifically address management of anticoagulation in patients with venous stents. The duration of anticoagulation is usually determined by underlying risks for VTE recurrence and not by the presence of a venous stent.
Most studies to date have involved the use of unfractionated heparin, LMWH, and vitamin K antagonists because many predate the use of direct oral anticoagulants. Eijgenraam et al concluded that anticoagulation regimens made no difference to outcomes in their systematic review of antithrombotic management.34 Taha et al could not draw any firm conclusions regarding anticoagulation, particularly extended use, in the setting of stenting in acute VTE.14 Use of antiplatelet agents did not seem to show any significant benefit.14
Pregnancy in women who have had previous VTE and stenting procedures may need careful consideration. Retrospective data suggest they are not at increased risk of stent occlusion or recurrent VTE during pregnancy or in the postpartum period,30 but further data are required.
Future research will need to focus on defining optimal peri- and postoperative management.
Conclusions
Endovenous stenting is a minimally invasive, relatively safe procedure with promising outcomes in reducing the severity of PTS and improving QoL. However, further research is needed to establish its usefulness in management of deep venous disease, particularly the long-term outcomes and cost-benefits.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SR, Solymoss S, Lamping DL, Abenhaim L. Long-term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15(6):425-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24. [Google Scholar]
- 4.Porter JM, Moneta GL; International Consensus Committee on Chronic Venous Disease. Reporting standards in venous disease: an update. J Vasc Surg. 1995;21(4):635-645. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31(6):1307-1312. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21(3):510-514. [DOI] [PubMed] [Google Scholar]
- 7.Black SA, Alvi A, Baker SJ, et al. Management of acute and chronic iliofemoral venous outflow obstruction: a multidisciplinary team consensus. Int Angiol. 2020;39(1):3-16. [DOI] [PubMed] [Google Scholar]
- 8.Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(3):768-773. [DOI] [PubMed] [Google Scholar]
- 9.Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord . 2018;6(1):48-56.e1. [DOI] [PubMed] [Google Scholar]
- 10.Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: A current comprehensive meta-analysis and systematic review. Phlebology. 2016;31(6):376-389. [DOI] [PubMed] [Google Scholar]
- 11.Raju S, Tackett P Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg. 2009;49(2):511-518. [DOI] [PubMed] [Google Scholar]
- 12.Neglen P. Chronic venous obstruction: diagnostic considerations and therapeutic role of percutaneous iliac stenting. Vascular. 2007;15(5):273-280. [DOI] [PubMed] [Google Scholar]
- 13.Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol. 2010;30(4):669-674. [DOI] [PubMed] [Google Scholar]
- 14.Taha MA, Busuttil A, Bootun R, Davies AH. A systematic review on the use of deep venous stenting for acute venous thrombosis of the lower limb. Phlebology. 2019;34(2):115-127. [DOI] [PubMed] [Google Scholar]
- 15.Meng QY, Li XQ, Jiang K, et al. Stenting of iliac vein obstruction following catheter-directed thrombolysis in lower extremity deep vein thrombosis. Chin Med J (Engl). 2013;126(18):3519-3522. [PubMed] [Google Scholar]
- 16.Sharifi M, Bay C, Mehdipour M, Sharifi J; Investigators TORPEDO. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Ther. 2012;19(2):273-280. [DOI] [PubMed] [Google Scholar]
- 17.Engelberger RP, Spirk D, Willenberg T, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(1):e002027. [DOI] [PubMed] [Google Scholar]
- 18.Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haig Y, Enden T, Grotta O, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(2):e64-e71. [DOI] [PubMed] [Google Scholar]
- 20.Wittens C, Davies AH, Baekgaard N, et al. Editor’s choice — management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49(6):678-737. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130(18):1636-1661. [DOI] [PubMed] [Google Scholar]
- 22.Neglen P, Berry MA, Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20(6):560-571. [DOI] [PubMed] [Google Scholar]
- 23.Seager MJ, Busuttil A, Dharmarajah B, Davies AH. Editor’s choice — a systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51(1):100-120. [DOI] [PubMed] [Google Scholar]
- 24.Raju S, Darcey R, Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51(2):401-408. [DOI] [PubMed] [Google Scholar]
- 25.Neglen P, Tackett TP Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48(5):1255-1261. [DOI] [PubMed] [Google Scholar]
- 26.Marston W, Razavi M. Improvement in quality of life after iliac vein stenting in a prospective clinical study of a nitinol venous stent [abstract]. J Vasc Surg Venous Lymphat Disord. 2019;7(2):P300. [Google Scholar]
- 27.Razavi MK. VIRTUS clinical trial: 12-month data. In: Leipzig Interventional Course. 22–25 January 2019. Leipzig, Germany. [Google Scholar]
- 28.Lichtenberg MKW, de Graaf R, Stahlhoff WF, Özkapi A, Rassaf T, Breuckmann F. Venovo venous stent in the treatment of non-thrombotic or post-thrombotic iliac vein lesions - short-term results from the Arnsberg venous registry. Vasa. 2019;48(2):175-180. [DOI] [PubMed] [Google Scholar]
- 29.Eijgenraam JW. Venous stenting after deep venous thrombosis and antithrombotic therapy: a systematic review. Rev Vasc Med. 2014;2(3):88-97. [Google Scholar]
- 30.Lestak K, Pouncey AL, Gwozdz A, et al. Midterm outcomes in postpartum women following endovenous treatment for acute iliofemoral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8(2):167-173. [DOI] [PubMed] [Google Scholar]