Visual Abstract
Abstract
Patients with hematologic malignancies are at increased risk of infection, with associated morbidity and mortality. Patients with acute myeloid leukemia (AML) have qualitative and quantitative deficits in granulocytes predisposing to bacterial and fungal infections. Acute lymphoblastic leukemia results in qualitative deficits in lymphocytes, resulting in hypogammaglobulinemia and reduced cell-mediated immunity predisposing to certain bacterial and viral as well as fungal infections. Chemotherapeutic regimens often compound these deficits, result in prolonged periods of severe neutropenia, and disrupt mucosal barriers, further elevating infection risk. Despite advances in antimicrobial therapies and prophylaxis, acute leukemia patients with disease- and treatment-related immunosuppression remain at risk for life-threatening infection, including with resistant organisms, antimicrobial-related adverse events, and higher treatment costs. Additionally, our knowledge of infection risk and drug-drug interactions with new immune-targeted cancer therapeutics is evolving. Here, we review 3 areas in which standard practice is evolving as challenges arise and new experience is gained, including antibiotic use in febrile neutropenia, fungal prophylaxis, and use of targeted therapies.
Learning Objectives
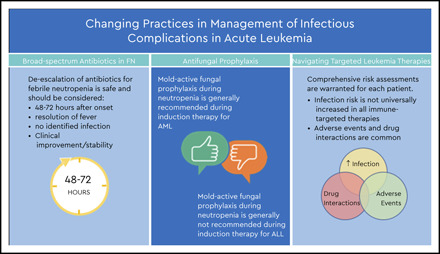
Review the management of febrile neutropenia including early de-escalation of broad-spectrum antibiotics
Review important issues in prophylaxis of fungal infections in patients with acute leukemia
Review infection risk and management considerations with some targeted therapies for treatment of acute leukemia
Introduction
Neutrophils are a critical component of the innate immune system. Qualitative deficits from the underlying malignancy compounded by periods of neutropenia from chemotherapeutic agents are major risk factors for development of bacterial and fungal infections in patients with acute leukemia. The degree and duration of neutropenia correlate with infection risk, particularly for invasive fungal infections. Antimicrobial prophylaxis is used to reduce the risk of life-threatening bacterial and fungal infections, particularly in patients with disruption of the gut mucosa. During treatment, most patients experience long-term antimicrobial exposure, which can lead to adverse effects, drug-drug interactions, added costs, altered gut microbiome, and increased risk for infection with multidrug-resistant organisms requiring shifts in management strategies. Additionally, the chemotherapeutic field is changing as well, with increasing use of immune-targeted therapies for treatment of acute leukemia. These therapeutics act on many different targets and with a theoretical consequent risk of infection, though it is often difficult to ascertain true infectious risk given confounding risk from underlying disease state and prior immunosuppressive therapies. As experience is gained with targeted therapies, there is growing evidence for an association with some agents and susceptibility to infection, whereas for others, clear correlation is lacking. Identifying the best practice for prevention and management of infectious complications during treatment of acute leukemia in this changing landscape creates a clinical challenge requiring the collaboration of specialists in infectious diseases, hematology, and pharmacy.
Clinical case
A 56-year-old man with no past medical history presented to his primary physician with 2 months of progressive fatigue. Laboratory tests revealed a “very high white count” and he was referred to the emergency department. His white blood cell count was 83.5 × 103/μL of blood with 65% blasts, his hemoglobin was 8.4 g/dL, and his platelet count was 35 × 109/L. A bone marrow biopsy revealed a hypercellular marrow (>95% cellularity) with 50% myeloid blasts with normal cytogenetics. He received 7+3 induction with cytarabine and daunorubicin, with levofloxacin, posaconazole, and acyclovir as antimicrobial prophylaxis. His course was complicated by febrile neutropenia (FN), treated with empiric cefepime. His fever resolved and no infectious agent was identified after 72 hours; cefepime was de-escalated to levofloxacin until neutropenia resolved. He achieved complete remission and underwent postremission chemotherapy.
Antibiotics for FN and their de-escalation
FN occurs in >80% of patients undergoing chemotherapy for acute leukemia. Despite improved diagnostic abilities in the last decade, an infectious etiology is identified in <50% of episodes.1,2 Empiric broad-spectrum antibiotic (BSA) therapy is universally recommended in patients with FN.3-5 Many studies have evaluated the best treatment regimens, and a number of consensus practice guidelines with stratified antibiotic recommendations are available. However, there is no consensus on duration of empiric treatment when patients clinically improve and no infectious etiology is identified.3,6-8 Based on prior guidance, practice has typically been to continue antibiotics until resolution of both symptoms and neutropenia4; we now recognize increasingly that in patients with prolonged neutropenia, this leads to long-term antibiotic exposure, which can be associated with antibiotic-related adverse events, selection for multidrug-resistant organisms, and alteration of the microbiome. The concept of earlier de-escalation or discontinuation of BSAs is not new. A number of small studies indicate that de-escalation after 72 hours is safe in clinically stable patients with no infection identified, regardless of ongoing neutropenia and in some cases ongoing fever.9-14 In recent years, early de-escalation has gained more traction. Guidelines from the European Conference on Infections in Leukemia advocate de-escalation after 48 hours if no infection is identified, regardless of anticipated duration of neutropenia.3 Since then, there have been additional attempts to assess the safety and feasibility of this approach. Studies have varied in methodology, but all have concluded that de-escalation to prophylaxis in patients with resolution of fever and no documented infection after 48 to 72 hours does not lead to a significant increase in subsequent bacterial infections, clinical decompensation, or in-hospital mortality.12,15-19 National Comprehensive Cancer Network (NCCN) guidelines now also suggest that clinically stable patients with persistent neutropenia without fever can be evaluated for discontinuation or de-escalation of BSAs to prophylaxis in some settings.5
Fungal prophylaxis
Invasive fungal infections (IFIs) are a major cause of morbidity and mortality in patients with acute leukemia. Patients with acute myeloid leukemia (AML) in particular are at increased risk of IFIs due to profound and prolonged duration of neutropenia, as well as the use of purine analogs in treatment.20 Azoles are the most common agents used for prevention and treatment of fungal infections during chemotherapy. Fluconazole, a first-generation triazole, is commonly used due to low cost and toxicity, but emergence of resistant Candida species and lack of activity against molds are limitations. Fluconazole is effective in decreasing Candida infection in transplantation and in patients with graft-versus-host disease, but studies have not shown benefit in preventing invasive mold infections.20-22 Voriconazole is a second-generation triazole that has activity against some opportunistic molds and is a first-line agent for treatment of invasive aspergillosis. Voriconazole has not been approved for use as primary prophylaxis, with studies showing a non–statistically significant trend toward decreased IFI incidence compared with fluconazole.23 Voriconazole has excellent bioavailability, although use is complicated by toxicities and drug-drug interactions.21
Posaconazole is another second-generation, extended-spectrum triazole with activity against Candida and Aspergillus spp, as well as other invasive molds including Fusarium and Mucorales. In several studies, including a multicenter randomized trial, prophylaxis with posaconazole in neutropenic patients with AML or myelodysplastic syndrome receiving induction chemotherapy significantly reduced the rate of IFIs (2% vs 8%; P < .001) and showed survival benefit (P = .04) when compared with fluconazole and itraconazole.24 Posaconazole is now recommended for primary fungal prophylaxis in patients with AML undergoing induction chemotherapy.5,25,26 The newer formulation of posaconazole with the extended-release tablet allows for better bioavailability compared with the oral suspension.27-29 Breakthrough IFIs have occurred, particularly at lower serum levels, thus therapeutic drug monitoring (TDM) may be warranted in obese patients or in those with concern for poor absorption.30 A trough level after 5 to 7 days of therapy with a goal concentration of >0.7 μg/mL is recommended. Although to a lesser degree than voriconazole, posaconazole is a potent cytochrome P450 3A (CYP3A) inhibitor that can complicate its use with other CYP3A substrates, resulting in increased bioavailability of many drugs leading to toxicity.31 Isavuconazole is a newer second-generation azole approved for treatment of both invasive aspergillosis and invasive mucormycosis. It is available in oral and IV formulations, has a broad spectrum of activity, and has a more favorable adverse effect profile and less significant drug-drug interactions compared with other triazoles. It is not routinely used for prophylaxis, although 1 study demonstrated safety and tolerability for use in high-risk patients.32 Unlike other triazoles, isavuconazole does not induce prolongation of the QTc interval, but rather a dose-dependent shortening of unclear clinical significance, and thus may be an alternative option in high-risk patients limited by toxicity or baseline QTc prolongation.33
Although mold-active antifungal prophylaxis has become standard of care during neutropenic periods of most AML treatment regimens, there is no similar standardized recommendation during acute lymphoblastic leukemia (ALL) treatment. Patients with ALL are at intermediate to high risk, with the rate of IFIs ranging between 3% and 12%,21 with higher rates in patients with longer duration of neutropenia, absence of antifungal prophylaxis, and relapsed disease. The strong inhibition of CYP3A4 by mold-active azoles can lead to significant toxicity when administered with several chemotherapy classes, particularly vinca alkaloids and alkylating agents, and targeted therapies such as tyrosine kinase (TK) inhibitors, which are mainstays in the chemotherapy regimens for ALL. In many instances, the azole would need to be discontinued prior to the chemotherapy initiation and not restarted until the chemotherapy agent has been discontinued and eliminated. In high-risk patients, the alternative use of an echinocandin or liposomal amphotericin may be warranted.3,5
Clinical case (continued)
At follow-up 9 months later, the patient’s laboratory tests showed pancytopenia. A bone marrow biopsy showed a 60% cellular marrow with 80% myeloid blasts. The next-generation sequencing hematologic malignancy panel showed NPM1-W290Sfs*10 (variant allele frequency, 25%) and IDH2-R140Q (variant allele frequency, 43%). He was started on enasidenib and Infectious Diseases was consulted to determine antimicrobial prophylaxis.
Infection risks in targeted therapies
Biological-targeted therapies are those designed to act on a therapeutic target considered important in the pathogenic process of the disease. In recent years, an increasing repertoire of agents has changed the landscape of therapeutics for acute leukemias. As more experience is gained with these targeted drugs, there is growing evidence for an increased association between some agents and susceptibility to infection, whereas for others, clear correlation with infectious risk is lacking. Complicating the picture, many agents are being used for a wide array of disease processes, often in combinations and in the setting of numerous prior chemotherapy regimens and relapse making it difficult to delineate association with infectious risk (Table 1).
Table 1.
Infection risk, drug interaction and prophylactic considerations associated with the use of therapeutic agents for acute leukemia
| Chemotherapy/Biological | Use | Infection risk | Interaction | Recommendations |
|---|---|---|---|---|
| Vinca alkaloids | ALL | Regimen related | Inhibits CYP3A4 | Avoid with azole |
| Alkylating agents | ALL | Regimen related | CYP3A4/2C | Avoid with azole |
| BCR-ABL TK inhibitors (imatinib, dasatinib, nilotinib, bosutinib, ponatinib) | Ph+ ALL | Modest risk: bacterial infections, CMV, PJP, HBV reactivation | CYP3A4 inhibitor | No clear benefit from routine prophylaxis Screen for HBV infection Avoid with azole Monitor QTc |
| Anti-CD19 bispecific T-cell engager (blinatumomab) | ALL | HSV, VZV, CMV, PJP, PML, fungal per NCCN | Consider ACV and PJP prophylaxis Screen for HBV |
|
| Anti-CD22 antibody drug conjugate (inotuzumab) | ALL | Risk similar to anti-CD20 | No clear benefit from routine prophylaxis Screen for HBV infection High risk for VOD |
|
| CD19 CAR-T (tisagenlecleucel) | ALL | Increased risk for IFI, PJP, prolonged IgG hypogammaglobulinemia in long-term; distinguish infection from CRS | Acyclovir viral prophylaxis PJP prophylaxis Screen for chronic HBV Consider levofloxacin and fluconazole prophylaxis Consider anti-mold azole if high-dose steroids or prolonged neutropenia |
|
| BCL-2 inhibitor (venetoclax) | AML | Possible increased risk of fungal infections in absence of antifungal prophylaxis | CYP3A4 | Avoid with azole If azole is indicated dose reduce venetoclax (>50%) |
| IDH1/2 inhibitor (ivosidenib, enasidenib) | AML | No clear increased risk of infection; distinguish infection from differentiation syndrome | Avoid with azole Monitor QTc |
|
| Hedgehog pathway inhibitor (glasdegib) | AML | No data | CYP3A4 | Avoid with azole Monitor QTc |
| Anti-CD33 antibody drug conjugate (gemtuzumab) | AML | Prolonged myelosuppression | Monitor QTc High risk for VOD |
|
| FLT3-TK inhibitor (midostaurin and gilteritinib) | AML | No significant increased risk of fungal infection | CYP3A4 | Monitor QTc Monitor for midostaurin toxicity and use posaconazole TDM |
ACV, acyclovir; BCL-2, B-cell lymphoma 2; CAR-T, chimeric antigen receptor T cell; CMV, cytomegalovirus; CRS, cytokine release syndrome; HBV, hepatitis B virus; HSV, herpes simplex virus; IDH, isocitrate dehydrogenase; IgG, immunoglobulin G; Ph+, Philadelphia chromosome–positive; PJP, Pneumocystis jirovecii pneumonia; PML, progressive multifocal leukoencephalopathy; TK, tyrosine kinase; VOD, veno-occlusive disease; VZV, varicella zoster virus.
Therapeutics for AML
Ivosidenib and enasidenib are small-molecule inhibitors of mutant isocitrate dehydrogenase 1 (IDH1) and IDH2, respectively, and may be used to treat relapsed or refractory AML. Thus far, small studies to assess efficacy and safety have not demonstrated a clear increased risk for infection. This class is a substrate of CYP3A4 and when used in combination with strong inhibitors such as posaconazole, serum concentrations of the drug may be increased. Current recommendation is to avoid use of azoles when possible, but when use is required to reduce the ivosidenib dose.
Glasdegib is a selective inhibitor of the Hedgehog signaling pathway with primary use in patients who are not candidates for intensive chemotherapy. There has been no additional infection risk associated with the use of this agent to date. When used in combination with strong CYP3A4 inhibitors, the serum concentration of glasdegib may be increased. Avoiding this combination if possible is recommended, but, if used, it should be monitored for prolongation of the QTc interval and other potential toxicities of glasdegib.
Gemtuzumab ozogamicin is an antibody drug conjugate targeting CD33 on the surface or normal and leukemic myeloid cells and blasts, which leads to profound and prolonged neutropenia and thrombocytopenia. To date, demonstrated rates of infection are comparable to other regimens causing neutropenia. Standard prophylactic strategies for patients with AML and neutropenia are recommended.34
FMS-like TK 3 (FLT3) inhibitors, including midostaurin and gilteritinib, have emerged as treatment options to improve survival in patients with FLT3 duplication mutations. Studies have not shown an increased risk of IFIs when used with induction or consolidation chemotherapy. These agents are metabolized by CYP3A4, and thus concomitant use with strong inhibitors can be challenging. Patients requiring azole therapy should be monitored closely for potential midostaurin-related toxicity, and TDM is recommended.35,36
Venetoclax is a B-cell lymphoma 2 inhibitor that can be used in combination for patients unsuitable for intensive chemotherapy. No clear increased risk of infection has been identified.37 However, venetoclax can induce severe and prolonged marrow suppression. When used in combination with CYP3A4 inhibitors, significant dose reduction of venetoclax is required (up to 75% dose reduction with strong CYP3A4 inhibitors such as posaconazole).38
Therapeutics for ALL
BCR-ABL TK inhibitors, including imatinib, dasatinib, nilotinib, bosutinib, and ponatinib, are used for Philadelphia chromosome–positive ALL. These may be associated with myelotoxicity, increasing risk for bacterial and fungal infection. There is some inhibition of CD4+ and CD8+ T-cell proliferation, which impairs cytomegalovirus (CMV)- and Epstein-Barr virus–specific CD8+ T-cell responses and proliferation. TK inhibitors significantly impair B-cell responses leading to less robust response to vaccinations. In studies evaluating the incidence of infectious complications, there appears to be a modest increase in the risk of infection, more so with dasatinib, notably CMV and hepatitis B virus (HBV) reactivation. Screening for chronic HBV infection is recommended prior to therapy. To date, there are no data to suggest a clear benefit in the routine use of anti-infective prophylaxis.5,37
CD-19 targeted agents, including blinatumomab, are designed to direct CD3-expressing cytotoxic T cells to CD19-expressing B cells resulting in B-cell depletion and reduction in immunoglobulin G levels and hypogammaglobulinemia. There may be some inhibition in B-cell–dependent T-cell activation similar to anti-CD20 agents. Thus far, CD19-targeted agents have not demonstrated a significant increased risk of infection compared with conventional chemotherapy for relapsed or refractory ALL, but the risk of herpesvirus reactivation (herpes simplex virus and varicella zoster virus) and Pneumocystis jirovecii pneumonia [PJP] warrants prophylactic acyclovir and PJP prophylaxis. Some centers also give antifungal prophy as well. Patients should be screened for HBV prior to initiation of therapy and monitored or treated accordingly.39
Inotuzumab is a CD22-directed antibody-drug conjugate that targets the CD22 antigen that is expressed on mature B cells and most B-cell blasts. Risk of infection is similar to those treated with anti-CD20 monoclonal antibodies such as rituximab. In early studies, there has been no increased incidence of infection demonstrated when compared with standard chemotherapy. There is no expected benefit from universal prophylaxis, although patients should be screened for HBV infection prior to initiation and managed accordingly.34
Chimeric antigen receptor (CAR)-engineered T cells are engineered to express a receptor recognizing a target protein on cancer cells for B-cell malignancies. Infections are common in CAR T-cell therapy but may be a result of the underlying malignancy, persisting depression in cell-mediated immunity, and prolonged myelosuppression from prior therapies, exacerbated by the need for corticosteroids and tocilizumab for management of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. Early infections after CAR T-cell therapy are more often bacterial, but risk for IFIs increases with prolonged neutropenia, and management would be per standard of care for these scenarios.40 Acyclovir for viral prophylaxis and fluconazole are recommended, with mold-active azoles to be considered depending on clinical scenario, duration of neutropenia, and need for high-dose steroids.5,40
As new antileukemic chemotherapy- or immune-based therapeutics are introduced into the armamentarium, vigilance in determining associated infection risk needs to be delineated so that appropriate caution and prophylaxis are considered. Many initial studies are performed in patients with relapsed or refractory disease, making it difficult to determine the additional infectious risk attributable to these agents vs associated with the underlying disease process and/or prior or concomitant immunosuppressive therapies. In addition, this list of agents is not exhaustive, and new agents enter the pipeline every year. Each patient should be managed based on a comprehensive risk assessment including disease status and prior and current therapies to ensure best management, and Infectious Diseases consultation and pharmacy involvement are strongly encouraged.
References
- 1.Nesher L, Rolston KV. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 2014;42(1):5-13. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer AJ, Freifeld AG. Optimal management of neutropenic fever in patients with cancer. J Oncol Pract. 2019;15(1):19-24. [DOI] [PubMed] [Google Scholar]
- 3.Averbuch D, Orasch C, Cordonnier C, et al. ; ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013;98(12):1826-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freifeld AG, Bow EJ, Sepkowitz KA, et al. ; Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-e93. [DOI] [PubMed] [Google Scholar]
- 5.Baden LR, Swaminathan S, Almyroudis NG, et al. . National Comprehensive Cancer Network. Prevention and Treatment of Cancer-Related Infections (Version 2.2020). Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf. [Google Scholar]
- 6.Cometta A, Kern WV, De Bock R, et al. ; International Antimicrobial Therapy Group of the European Organization for Research Treatment of Cancer. Vancomycin versus placebo for treating persistent fever in patients with neutropenic cancer receiving piperacillin-tazobactam monotherapy. Clin Infect Dis. 2003;37(3):382-389. [DOI] [PubMed] [Google Scholar]
- 7.Paul M, Dickstein Y, Borok S, Vidal L, Leibovici L. Empirical antibiotics targeting Gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev. 2014; (1):CD003914. [DOI] [PubMed] [Google Scholar]
- 8.Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev. 2013;(6):CD003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Marie S, van den Broek PJ, Willemze R, van Furth R. Strategy for antibiotic therapy in febrile neutropenic patients on selective antibiotic decontamination. Eur J Clin Microbiol Infect Dis. 1993;12(12):897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santolaya ME, Villarroel M, Avendaño LF, Cofré J. Discontinuation of antimicrobial therapy for febrile, neutropenic children with cancer: a prospective study. Clin Infect Dis. 1997;25(1):92-97. [DOI] [PubMed] [Google Scholar]
- 11.Slobbe L, Waal L, Jongman LR, Lugtenburg PJ, Rijnders BJ. Three-day treatment with imipenem for unexplained fever during prolonged neutropaenia in haematology patients receiving fluoroquinolone and fluconazole prophylaxis: a prospective observational safety study. Eur J Cancer. 2009;45(16):2810-2817. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Guisado M, Espigado I, Martín-Peña A, et al. . Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4(12):e573-e583. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen JJ, Rozenberg-Arska M, Dekker AW. Discontinuation of intravenous antibiotic therapy during persistent neutropenia in patients receiving prophylaxis with oral ciprofloxacin. Clin Infect Dis. 1995;21(5):1300-1302. [DOI] [PubMed] [Google Scholar]
- 14.Klaassen RJ, Allen U, Doyle JJ. Randomized placebo-controlled trial of oral antibiotics in pediatric oncology patients at low-risk with fever and neutropenia. J Pediatr Hematol Oncol. 2000;22(5):405-411. [DOI] [PubMed] [Google Scholar]
- 15.Gustinetti G, Raiola AM, Varaldo R, et al. . De-escalation and discontinuation of empirical antibiotic treatment in a cohort of allogeneic hematopoietic stem cell transplantation recipients during the pre-engraftment period. Biol Blood Marrow Transplant. 2018;24(8):1721-1726. [DOI] [PubMed] [Google Scholar]
- 16.Kroll AL, Corrigan PA, Patel S, Hawks KG. Evaluation of empiric antibiotic de-escalation in febrile neutropenia. J Oncol Pharm Pract. 2016;22(5):696-701. [DOI] [PubMed] [Google Scholar]
- 17.Le Clech L, Talarmin JP, Couturier MA, et al. . Early discontinuation of empirical antibacterial therapy in febrile neutropenia: the ANTIBIOSTOP study. Infect Dis (Lond). 2018;50(7):539-549. [DOI] [PubMed] [Google Scholar]
- 18.Rearigh L, Stohs E, Freifeld A, Zimmer A. De-escalation of empiric broad spectrum antibiotics in hematopoietic stem cell transplant recipients with febrile neutropenia. Ann Hematol. 2020;99(8):1917-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder M, Pasikhova Y, Baluch A. Early antimicrobial de-escalation and stewardship in adult hematopoietic stem cell transplantation recipients: retrospective review. Open Forum Infect Dis. 2017;4(4):ofx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpern AB, Lyman GH, Walsh TJ, Kontoyiannis DP, Walter RB. Primary antifungal prophylaxis during curative-intent therapy for acute myeloid leukemia. Blood. 2015;126(26):2790-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busca A, Pagano L. Prophylaxis for aspergillosis in patients with haematological malignancies: pros and cons. Expert Rev Anti Infect Ther. 2018;16(7):531-542. [DOI] [PubMed] [Google Scholar]
- 22.Hsu A, Matera R, Vieira K, Reagan JL, Farmakiotis D. Antifungal prophylaxis during 7 + 3 induction chemotherapy for acute myeloid leukemia is associated with improved survival, in a setting with low incidence of invasive mold infections [published online ahead of print 21 May 2020]. Support Care Cancer. doi:10.1007/s00520-020-05535-5. [DOI] [PubMed] [Google Scholar]
- 23.Wingard JR, Carter SL, Walsh TJ, et al. ; Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornely OA, Maertens J, Winston DJ, et al. . Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348-359. [DOI] [PubMed] [Google Scholar]
- 25.Maertens JA, Girmenia C, Brüggemann RJ, et al. ; European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and; European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221-3230. [DOI] [PubMed] [Google Scholar]
- 26.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. . Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(suppl 1):e1-e38. [DOI] [PubMed] [Google Scholar]
- 27.Cumpston A, Caddell R, Shillingburg A, et al. . Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother. 2015;59(8):4424-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58(7):432-436. [DOI] [PubMed] [Google Scholar]
- 29.Lenczuk D, Zinke-Cerwenka W, Greinix H, et al. . Antifungal prophylaxis with posaconazole delayed-release tablet and oral suspension in a real-life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother. 2018;62(6):e02655-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattaneo C, Panzali A, Passi A, et al. . Serum posaconazole levels during acute myeloid leukaemia induction therapy: correlations with breakthrough invasive fungal infections. Mycoses. 2015;58(6):362-367. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal SK, DiNardo CD, Potluri J, et al. . Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359-367. [DOI] [PubMed] [Google Scholar]
- 32.Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother. 2015;59(4):2078-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybak JM, Marx KR, Nishimoto AT, Rogers PD. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy. 2015;35(11):1037-1051. [DOI] [PubMed] [Google Scholar]
- 34.Drgona L, Gudiol C, Lanini S, Salzberger B, Ippolito G, Mikulska M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4). Clin Microbiol Infect. 2018;24(suppl 2):S83-S94. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay J, Teh BW, Micklethwaite K, Slavin M. Azole antifungals and new targeted therapies for hematological malignancy. Curr Opin Infect Dis. 2019;32(6):538-545. [DOI] [PubMed] [Google Scholar]
- 36.Stemler J, Koehler P, Maurer C, Müller C, Cornely OA. Antifungal prophylaxis and novel drugs in acute myeloid leukemia: the midostaurin and posaconazole dilemma. Ann Hematol. 2020;99(7):1429-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinwald M, Silva JT, Mueller NJ, et al. . ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70. [DOI] [PubMed] [Google Scholar]
- 38.DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. [DOI] [PubMed] [Google Scholar]
- 39.Mikulska M, Lanini S, Gudiol C, et al. . ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect. 2018;24(suppl 2):S71-S82. [DOI] [PubMed] [Google Scholar]
- 40.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]


