Abstract
Tuberculosis remains one of the most common infectious diseases. Miliary presentation is a rare and possibly lethal form, resulting from massive lymphohaematogenous dissemination of Mycobacterium tuberculosis bacilli. The authors describe the case of a 47-year-old immunocompetent woman, diagnosed with miliary tuberculosis, with both lung and central nervous system involvement, who showed total recovery after starting anti-tuberculous drugs. The atypical neutrophilic-predominant pleocytosis and negative cerebrospinal fluid microbiological results made the diagnosis even more challenging. Since prognosis largely depends on timely treatment, recognition and prompt diagnosis is important. Thus, clinicians should be aware and treatment should be initiated as soon as the diagnosis is suspected.
LEARNING POINTS
Cerebrospinal fluid (CSF) characteristics in central nervous system tuberculosis (CNS TB) are variable and may even be normal. Typical CSF findings include lymphocytic-predominant pleocytosis, although neutrophilic predominance may occur. CSF microbiological testing for Mycobacterium tuberculosis has low sensitivity, so a negative test does not eliminate the diagnosis.
Cerebral magnetic resonance imaging is usually the test of choice, given its superiority in CNS TB diagnosis over computed tomography (CT), which can be normal.
Chest x-ray may appear normal and miss miliary TB, which however a CT scan can identify.
Keywords: Miliary tuberculosis, meningitis, brain tuberculomas
CASE DESCRIPTION
A 47-year-old woman presented to the emergency department with a 24-hour history of behavioural changes, consisting of disorientation and psychomotor agitation. She also referred one vomiting episode and asthenia and weight loss of 10 kg in 2 months. There was no relevant medical history, nor did she smoke or use illicit drugs. No epidemiological context was known. On examination, the patient was agitated and uncooperative, with meningeal signs, without focal neurological deficits. Laboratory results were relevant for lymphopenia (800 μl), C-reactive protein elevation (64.2 mg/l) and a raised erythrocyte sedimentation rate (97 mm), anaemia of inflammation (haemoglobin 11.2 g/dl) and hyposmolar hyponatremia (131 mmol/l). Cranial computed tomography (CT) scanning did not show any acute findings. A lumbar puncture was performed and cerebrospinal fluid (CSF) analysis revealed neutrophilic-predominant pleocytosis (120 cells/μl, 42% neutrophils, 36% lymphocytes), proteinorrhachia (128 mg/dl) and hypoglycorrhachia (37 mg/dl, CSF/serum ratio 0.37). Acute bacterial meningitis was assumed, and empirical ceftriaxone, vancomycin and ampicillin were started.
However, there were no signs of improvement, so the lumbar puncture was repeated, revealing worsening of the previous CSF parameters (132 cells/μl, proteins 156 mg/dl, glucose <20 mg/dl) and elevated adenosine deaminase (14.3 U/l). Cerebral magnetic resonance imaging (MRI) demonstrated: numerous contrast-enhanced small intra-axial lesions in the supratentorial and infratentorial compartments, with a miliary pattern; leptomeningeal enhancement of basal cisterns, surrounding the bifurcation of the internal carotid arteries and, on the right, extending to the opercular region; and signs of moderate hydrocephalus (Fig. 1).
Figure 1.
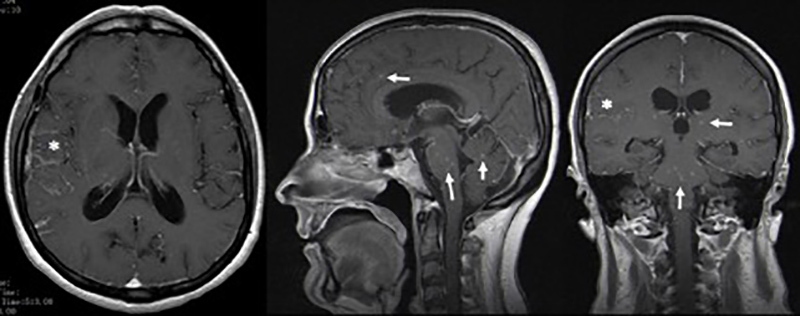
Cerebral magnetic resonance imaging demonstrating contrast enhanced small intra-axial lesions in the supratentorial and infratentorial compartments with a miliary pattern (-->), and leptomeningeal enhancement of basal cisterns, extending to the opercular region on the right (*).
As bacillary meningitis was suspected, ongoing antibiotic therapy was suspended and targeted therapy was started. However, CSF analysis (two samples) failed microbiological confirmation, with a negative acid-fast-bacilli (AFB) smear, culture, and polymerase chain reaction (PCR) for Mycobacterium tuberculosis (MT) DNA detection. A CSF cytological study showed no evidence of malignant cells and immunophenotyping testing was normal. Further study revealed: interferon gamma release assay (IGRA) positive; HIV, HCV and HBV serologies negative; VDRL test negative; no vitamin B12 or folic acid deficiency; normal thyroid function; and a normal immunological study. Chest x-ray (CXR) showed no evident alterations (Fig. 2). Thoracoabdominopelvic CT scanning demonstrated multiple millimetric pulmonary micronodules (Fig. 3). Bronchoscopy with bronchoalveolar lavage (BAL) was performed and MT was identified.
Figure 2.
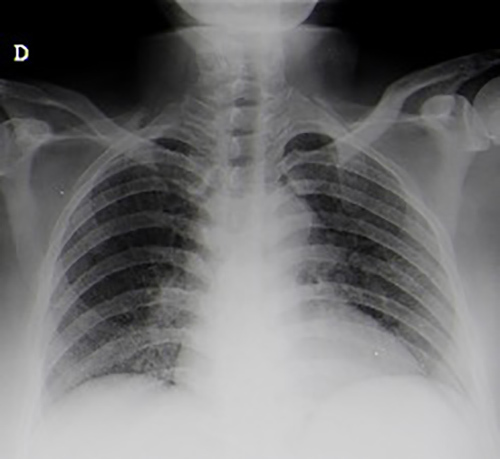
Chest x-ray without obvious alterations.
Figure 3.
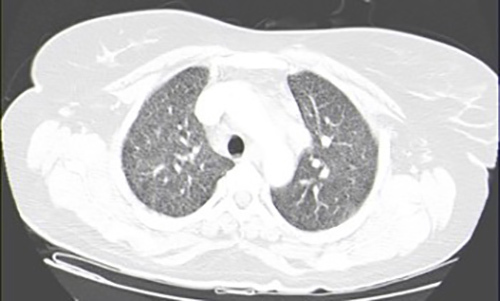
Thoracoabdominopelvic computed tomography scan showing multiple millimetric pulmonary micronodules.
Therefore, a diagnosis of miliary tuberculosis (MTB) was made, with lung and central nervous system (CNS) involvement, and presenting with meningitis and multiple tuberculomas. The patient completed treatment for 2 months with daily isoniazid, rifampin, pyrazinamide and ethambutol, along with 3 weeks of dexamethasone, followed by 10 months of isoniazid and rifampin. She showed a favourable evolution with total recovery. A follow-up MRI revealed complete resolution of the lesions.
DISCUSSION
MTB is a rare presentation of TB, accounting for 1–2% of all TB cases and 8% of extrapulmonary cases [1]. It results from massive lymphohaematogenous dissemination of MT, during primary infection or reactivation of latent disease [1]. TB aetiology should be included in the differential diagnosis of miliary pattern lesions, especially in endemic regions. The patient lives in a region of Portugal with a high TB incidence rate (31/100,000 persons).
Although only 1% of all TB cases present with CNS involvement, in MTB it can reach 10–30%, meningitis being the predominant form[1–3]. The spectrum of CNS involvement also includes tuberculomas, a conglomerate granulomatous parenchymal focus developed during disseminated bacillaemia, often clinically silent. When subependymal tubercles rupture into the subarachnoid space, meningitis occurs [4,5]. In this case, the patient presented with meningitis and miliary brain tuberculomas, the least frequently reported pattern of brain tuberculomas and a rare form of CNS involvement. The widespread and dense distribution of infectious foci seen in MT greatly increases the chance of juxta-ependymal tubercle establishment, and thus meningitis.
Individuals with an increased risk for miliary and extrapulmonary TB include patients with immunodeficiency caused by aging, alcoholism, malnutrition, malignancy, HIV infection, or drugs, such as tumour necrosis factor-alpha inhibitors [2,6,7]. None of these factors was identified in this patient.
The diagnosis of CNS TB is challenging and frequently delayed due to a variable and often non-specific presentation of the disease. The clinical course is usually subacute or chronic [8]. Although cognitive/behavioural changes had appeared in the previous 24 hours, our patient had already been experiencing constitutional symptoms.
CSF characteristics in CNS TB are variable and may even be normal. Typical CSF findings of TB meningitis include lymphocytic-predominant pleocytosis. In this case, the CSF sample showed an atypical neutrophilic predominance, although this can occur at an early stage of the disease [6, 8]. Additionally, in this patient, the AFB smear, culture and PCR testing were negative for MT in CSF samples. Extrapulmonary lesions are paucibacillary, so the efficacy of microbiological confirmation of CNS TB is unsatisfactory compared with that of pulmonary lesions. For a single CSF sample, the sensitivity of an AFB smear is about 20–40%, for culture about 40–80% and for PCR it can be as low as 30% [6, 8]. Consequently, a negative test does not exclude the diagnosis of CNS TB, nor does it obviate the need for empiric therapy if the clinical suspicion is high. The diagnosis is often presumptive [1,6,7]. Multiple lumbar punctures (up to four) and large-volume CSF samples (10–15 ml) can increase test sensitivity [6,8]. Adenosine deaminase activity (ADA) quantification in CSF was useful, since its elevation has been reported as suggestive of tuberculous aetiology [6, 9].
Neuroimaging supported the diagnosis, since typical findings were present. Classic MRI features of meningitis include basal meningeal enhancement and hydrocephalus, while brain tuberculomas appear as nodular lesions, better demonstrated in gadolinium-enhanced images[1,3,6]. MRI is usually the imaging test of choice, given its superiority over CT in the diagnosis of CNS TB, as demonstrated in this case.
Patient pulmonary TB diagnosis was essential to support bacillary aetiology of CNS infection. Although CXR remains the first imaging test in pulmonary TB evaluation, it may appear normal and miss MTB diagnosis, which CT can identify, as in this case.
Therefore, in this case, the CNS TB diagnosis was based on specific CSF and brain MRI findings, along with a simultaneous pulmonary TB diagnosis.
Regarding treatment, antimicrobial therapy is the same as for pulmonary forms. However, longer duration and adjunctive glucocorticoid therapy should be warranted in CNS disease [1, 6]. Another essential aspect is timely treatment, since prognosis largely depends on neurological status at presentation and time-to-treatment initiation. Thus, empiric treatment should be started as soon as the diagnosis is suspected [1,6]. This patient completed 12 months of an anti-tuberculous regimen, showing total recovery. Despite evidence of hydrocephalus, she did not require surgical intervention.
In conclusion, MTB is a rare and possibly lethal form of TB. Diagnosis is challenging and often delayed or even missed, so clinicians should be aware.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
REFERENCES
- 1.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5(7):415–430. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 2.Phypers M, Harris T, Power C. CNS tuberculosis: a longitudinal analysis of epidemiological and clinical features. Int J Tuberc Lung Dis. 2006;10(1):99–103. [PubMed] [Google Scholar]
- 3.Garg RK, Sharma R, Kar AM, Kushwaha RAS, Singh MK, Shukla R, et al. Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg. 2010;112(3):188–192. doi: 10.1016/j.clineuro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Rich AR. The pathogenesis of tuberculous meningitis. Bull John Hopkins Hosp. 1933;52:5. [Google Scholar]
- 5.Davis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ. The pathogenesis of tuberculous meningitis. J Leukoc Biol. 2019;105(2):267–280. doi: 10.1002/JLB.MR0318-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011 doi: 10.1155/2011/798764. 798764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farer LS, Lowell AM, Meador MP. Extrapulmonary tuberculosis in the United States. Am J Epidemiol. 1979;109(2):205–217. doi: 10.1093/oxfordjournals.aje.a112675. [DOI] [PubMed] [Google Scholar]
- 8.Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17(6):987–994. doi: 10.1093/clinids/17.6.987. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal AK, Bansal S, Nand V. A hospital based study on estimation of adenosine deaminase activity (ADA) in cerebrospinal fluid (CSF) in various types of meningitis. J Clin Diagn Res. 2014;8(2):73–76. doi: 10.7860/JCDR/2014/7154.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]


