Abstract
Background:
Human reproduction is the most intricate event as ~ 20% of human pregnancies end in miscarriages for which chromosomal anomalies are a common factor. The chromosomal variations associated with reproductive failures include translocations, inversions, supernumerary marker chromosomes, heterochromatic polymorphisms, etc., Till date, the significance of heteromorphic variants in reproductive failures is unclear.
Aim:
The aim of this study is to investigate the role of chromosomal anomalies and polymorphic variations in reproductive failure.
Materials and Methods:
Chromosomal analysis using GTG banding was performed on 638 couples (1276 individuals).
Results:
In the present study, 138 of 1276 individuals showed chromosomal variations with respect to heterochromatic variants and Robertsonian translocations. The most common variants observed across the population studied were the pericentric inversion of the chromosome 9 [inv(9)(p11q13), 3.68%] followed by pstk + on the short arm of chromosome 15 (15pstk+, 1.95%) and Robertsonian translocation of chromosomes 13 and 14 [rob(13;14)(q10;q10), 1.25%]. The maximum percentage of heterochromatic variation was observed in females with recurrent pregnancy loss (Groups A, 4.78%) and males with wives having recurrent miscarriages (Group B, 3.68%) and the minimum was recorded in patients with in vitro fertilization (IVF) failures (Group C, 0.23%) and couples having a history of the malformed child (Group F, 0.23%).
Conclusions:
High level of chromosomal polymorphic variations in patients with reproductive failures warrants their in-depth analysis to nail down the causative factors. Hence, cytogenetic analysis coupled with genetic counseling becomes indispensable for patients suffering from infertility, reproductive failures and pregnancy losses before IVF treatment to rule out the carrier status.
KEYWORDS: Chromosomal anomalies, chromosomal polymorphic variations, reproductive failures
INTRODUCTION
The reproductive failures resulting from repeated miscarriages and infertility are quite often caused by constitutional chromosomal abnormalities. The anomalies associated with genetic and physiological oddities, infertility, and recurrent/spontaneous miscarriages may be transmitted across the generations with no certain clinical manifestation. Reproduction failure can be absolute (infertility, spontaneous abortions, and stillbirths) or relative (congenital malformation, genetic syndromes, and mental retardation). The genotypic and phenotypic aberrations present in either of the spouse or progeny probably result in a reproductive abnormality.[1] The term recurrent miscarriages refer to the loss of three or more consecutive gravidity before 20 weeks of gestation. The chromosomal anomalies in the fetus account for 60% of recurrent miscarriages.[2] The etiology of recurrent miscarriages in most of the cases is associated with multiple factors, including genetic, structural, hormonal, and environmental.[3,4] Meiotic errors during gametogenesis and embryonic development are the major cause of chromosomal anomalies resulting in miscarriages that are mostly de novo in nature.[5] Approximately, of the 20%–50% of the females affected with spontaneous miscarriages, only 15% are diagnosed clinically.[6,7] The fetal chromosomal abnormalities are major contributors for approximately half of the sporadic early abortions (12 weeks of gestation age) and to nearly one-third of the second-trimester miscarriages.[8] The chromosomal rearrangements occur in 3%–5% of the couples with miscarriages in either one of the affected partners as compared to 0.2% in the normal population.[9,10] A broad spectrum of chromosomal abnormalities in approximately 49% of sporadic miscarriages have been revealed by karyotyping, including numerical, structural, and miscellaneous (supernumerary, mosaicism, etc.) anomalies accounting for 86%, 6%, and 8%, respectively.[11] An Indian study reported 8.57% of the couples affected with miscarriages have numerical and structural chromosomal anomalies and polymorphic variants comprising of 0.95%, 2.87%, and 4.76%, respectively.[8,12]
In addition, infertility affects approximately 15% of couples during the age of reproduction. In both males and females, there seems to be an association between genetic abnormalities and infertility. Approximately, 40% of the infertility cases are caused by pathological conditions of both male and female, and 20% is related to the varying age group.[13] The majority of infertility in males is contributed by genetic factors, including chromosomal anomalies, gene mutation coupled with impaired spermatogenesis.[14,15] Chromosomal aberrations have also been encountered in males with spermatogenic failure, comprising of 5%–7% and 10%–15% in individuals with oligospermia and azoospermia, respectively. Besides, multiple etiological factors, chromosomal aberrations account for 10%–15% in male infertility. Of these, constitutional abnormalities with respect to numerical and structural, sex chromosomal anomalies and mosaicism associated with autosomal chromosome account for 5%, 80%–85%, and 2%, respectively.[16,17]
Polymorphic variations include the spectrum of heterochromatin regions, satellite or repeat sequences, and inversions.[18] Heteromorphism is defined as common cytogenetic polymorphisms detected by the GTG banding technique. These variations include, increase in the length of the heterochromatic regions on the long arms of chromosomes 1 (1qh+) and 9(9qh+), and an increase in the length of the short arm stalks of the acrocentric D and G group chromosomes (13, 14, 15, 21, and 22), these are designated, as 13pstk+, 14pstk+, 15pstk+, 21pstk+, and 22pstk.[19] The influence of chromosome heteromorphism has been reported earlier in infertility and recurrent miscarriages, however, the underlined mechanisms need to be clearly defined.[20] The present study provides a deeper insight into the chromosomal polymorphic and heterochromatic variations in recurrent/spontaneous abortion and other reproductive failures in humans. The detection of such variations becomes indispensable for the diagnosis of infertility, to take up follow-up treatment, and the assessment for risk in future pregnancy culminating in overall pregnancy management.
MATERIALS AND METHODS
Specimen collection
In the period January 2014 to January 2017, a total of 638 couples (1276 individuals) were studied. Written consent was obtained and clinical/medical condition was noted on a predesigned proforma to assess any history of disease and consanguinity in the patient. The mean age of the 1276 cases was 31 years (21–39 years) for females and 35 years (27–43 years) for males. Of these, 138 patients having reproductive failure were divided into six groups (Groups A-F): Group A; women with recurrent spontaneous miscarriages (women had two or more miscarriages); Group B; included males with wives having recurrent miscarriages; Group C; included patients with in vitro fertilization (IVF) failures; Group D; patients with primary and secondary infertility; Group E; encompassed patients with sterility and Group F; patients with a history of a malformed child (with genetic syndromes/abnormalities/mental retardation).
Metaphase culturing and harvesting
The peripheral blood leukocyte culture was performed as per the standard protocol.[21] Approximately 2–3 ml of venous blood was drawn into a sterile heparinized syringe, of which 0.5 ml was added to sterile culture vial containing 5 ml of RPMI-1640 culture medium, 1 ml (20%) of fetal bovine serum, and 0.5 ml of phytohemagglutinin (PHA). Then, the contents were mixed gently and incubated at 37°C for 72 h in the CO2 incubator, followed by addition of 50 μg/ml colchicine (Sigma Aldrich) to arrest mitosis 1 h before culture termination. Harvesting of the peripheral blood leukocytes was performed by treatment with hypotonic solution followed by fixation, and then G-banding was done.
Chromosomal analysis and photomicrography
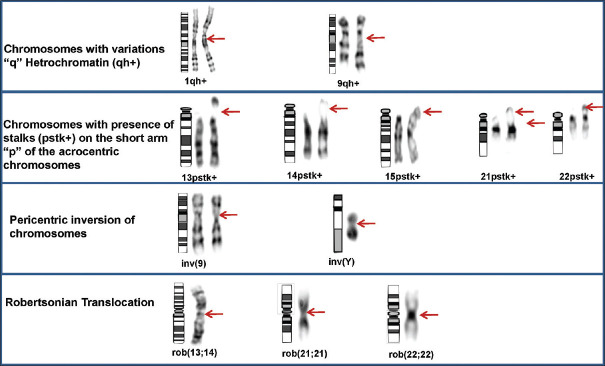
The metaphases were karyotyped using a GSL-120 automated microscope (Leica Biosystems) and automated Karyotyping software (Cytovision v7.2) at × 100, to rule out the presence of constitutional anomalies.[22] A minimum of twenty GTG banded metaphases (450–500 band level) were analyzed for each patient. Metaphases were karyotyped and interpreted according to the recommendations of the International System for Human Cytogenetic Nomenclature 2016.[22] Visualized polymorphic variations in the length of the centromeric heterochromatin on the long arms of chromosomes 1 and 9 (1qh+ and 9qh+) were documented. Distinct polymorphic variants of the length of stalks (pstk+) of the acrocentric chromosomes (13, 14, 15, 21, and 22) were also recorded. The pericentric inversion of chromosomes 9 and Y was considered as heteromorphisms [Figure 1]. For the classification of variants, at least a two-fold increase in the size of the corresponding region on the other homolog was considered, this works as an internal control during chromosomal analysis.[23] All karyotypes were examined independently, under a light microscope, by three laboratory technicians, at different time intervals to have consistent results.
Figure 1.
Partial karyotypes showing heterochromatic variations and structural chromosomal anomalies. The ideograms, normal and abnormal chromosomes are from left to right in each panel
RESULTS
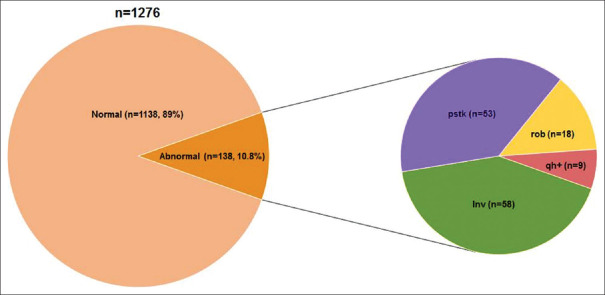
In this study, 1276 individuals were screened for cytogenetic analysis, of which 138 (10.8%) individuals were found to demonstrate chromosomal anomalies and polymorphic variations. Among these, 120 showed chromosomal polymorphism of heterochromatin and 18 showed Robertsonian translocations [Figure 2]. There was no significant difference in the frequency of chromosomal polymorphic variations in males (5.3%) and females (5.4%). The maximum occurrence of chromosomal variations was observed in Groups A and B, accounting for 4.78% and 3.68%, respectively. Alternatively, Groups C and F showed a minimum of chromosomal variations encompassing 0.23% each [Table 1]. The most common variants observed across the population studied were the pericentric inversion of the chromosome 9 [inv(9)(p11q13), 3.68%] followed by pstk+ on the short arm of chromosome 15 (15pstk+, 1.95%). In addition, the Robertsonian translocation of chromosomes 13 and 14 [rob(13;14)(q10;q10), 1.25%] was also frequently observed [Table 2 and Figure 3].
Figure 2.
An overview of the chromosomal variations and anomalies across the individuals analysed
Table 1.
Chromosomal anomalies and polymorphic variations across the group
| Type of variation/anomaly | Chromosome with variation/anomaly | Number of cases across the different groups | |||||
|---|---|---|---|---|---|---|---|
| Group A (females with RSA/2 or more abortions) | Group B (males with wife having history of abortion/BOH) | Group C (IVF failures) | Group D (primary and secondary infertility) | Group E (sterility) | Group F (history of malformed child) | ||
| Variation in “q” arm (qh+) | 1qh+ | 4 | 3 | 0 | 0 | 0 | 0 |
| 9qh+ | 0 | 1 | 0 | 1 (male) | 0 | 0 | |
| Presence of stalks (pstk+) on the short arm “p” of the acrocentric chromosome | 13pstk+ | 3 | 0 | 0 | 1 (male) | 0 | 0 |
| 14pstk+ | 2 | 0 | 0 | 0 | 0 | 0 | |
| 15pstk+ | 12 | 9 | 1 (female) | 1 (male) | 2 (1 male, 1 female) | 0 | |
| 21pstk+ | 5 | 2 | 0 | 0 | 0 | 0 | |
| 22pstk+ | 4 | 7 | 0 | 1 (male) | 2 | 1 (male) | |
| Pericentric inversion of chromosome | inv(9)(p11q13) | 20 | 14 | 2 (male) | 5 (4 female, 1 male) | 4 (3 male, 1 female) | 2 (female) |
| inv(Y)(p11q11) | 0 | 5 | 0 | 2 (male) | 4 (male) | 0 | |
| Robertsonian translocation | rob(13;14)(q10;q10) | 10 | 5 | 0 | 0 | 1 (male) | 0 |
| rob(21;21)(q10;q10) | 1 | 0 | 0 | 0 | 0 | 0 | |
| rob(22;22)(q10;q10) | 0 | 1 | 0 | 0 | 0 | 0 | |
RSA=Recurrent spontaneous abortion, BOH=Bad Obstetric history, IVF=In vitro fertilization
Table 2.
Frequency of chromosomal anomalies and polymorphic variations
| Type of variation | Chromosome with variation | Number of cases (n=1276) | Frequency of variation group (%) | |
|---|---|---|---|---|
| Variation in “q” arm (qh+) | 1qh+ | 7 | 0.54 | 0.69 |
| 9qh+ | 2 | 0.15 | ||
| Presence of long stalks (pstk+) on the short arm “p” of the acrocentric chromosome | 13pstk+ | 4 | 0.31 | 4.12 |
| 14pstk+ | 2 | 0.15 | ||
| 15pstk+ | 25 | 1.95 | ||
| 21pstk+ | 7 | 0.54 | ||
| 22pstk+ | 15 | 1.17 | ||
| Pericentric inversion of chromosome | inv(9)(p11q13) | 47 | 3.68 | 4.54 |
| inv(Y)(p11q11) | 11 | 0.86 | ||
| Robertsonian translocation | rob(13;14)(q10;q10) | 16 | 1.25 | 1.40 |
| rob(21;21)(q10;q10) | 1 | 0.07 | ||
| rob(22;22)(q10;q10) | 1 | 0.07 | ||
Figure 3.
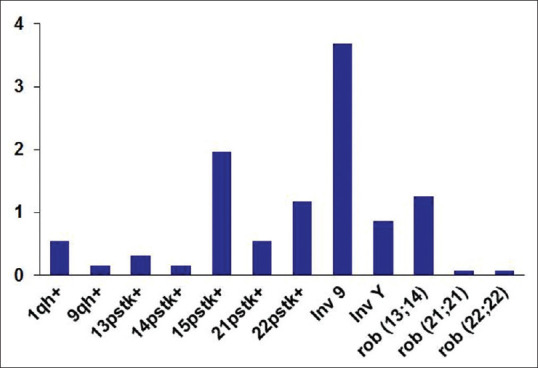
Distribution of chromosomal anomalies and polymorphic variations and anomalies across the population studied
Groups A and B demonstrated the highest incidence of pericentric inversion of chromosome 9 and the presence of elongated stalks of chromosome 15 with 1.56% and 0.94% and 1.09% and 0.7%, respectively. The most frequently affected chromosome with “pstk+” was 15, followed by 22pstk+ and 21pstk+, and the least affected ones were 13pstk+ and 14pstk+ of the heterochromatic variations [Table 1]. The maximum frequency was observed in cases with 3–5 abortions. The least affected variations were in chromosomes 1 (1qh+) and 9 (9qh+), with the variation in the q arm accounting for 0.31% with enlarged heterochromatin of both A and B groups. The maximum occurrence of Robertsonian translocations (rob[13;14][q10;q10]) in Group A was found to be 0.78% and in Group B was 0.39%. Exceptional cases corresponding to rob(21;21)(q10;q10) and rob(22;22)(q10;q10) were also observed in Groups A and B, respectively. Both Groups C and F had relatively lowest levels (~0.15%) of chromosomal anomalies and polymorphic variants showing inv(9)(p11q13) variation. Similarly, both males and females suffering from primary and secondary infertility belonging to Group D showed a significant increase in the number of inv(9)(p11q13), 0.39%. The sterile males of Group E showed the presence of most common variations of pericentric inversion of chromosomes 9 [inv(9)(p11q13)] and the distal heterochomatin of the Y [inv(Y)(p11q11)], both constituting about 0.31% each and a case depicting Robertsonian translocation indicates the crucial role of these variations correlating with infertility.
DISCUSSION
Constitutional chromosomal aberration may account for recurrent/spontaneous pregnancy loss, infertility, and congenital disabilities. Reproductive failures are an orchestration of potential genetic, hormonal, endocrine, immunologic, and environmental factors. Therefore, assigning proper etiological roles to each of these contributing factors is often uncertain. In the present study, 138 cases (70 females and 68 males) had chromosomal anomalies and polymorphic variations with no significant difference based on gender. Furthermore, we did not find any obvious correlation between the number of miscarriages and advanced maternal age, suggesting that the age is not the sole factor responsible for chromosomal anomalies. In our study, a total of 18 Robertsonian translocations were observed with most frequent being rob(13;14)(q10;q10) found in 16 of 18 cases and the other two being rob(21;21)(q10;q10) and rob(22;22)(q10;q10). There was a higher occurrence of Robertsonian translocations in females as compared to males. The reports suggest that the maternally derived Robertsonian translocation may pose an increased risk of fetus with unbalanced phenotypic manifestations. Studies indicate the genetic predisposition of miscarriage in couples with reciprocal translocations ranges from 25% to 50%, whereas with Robertsonian translocation, it is approximately 25%.[24] The synaptic alterations change the timing of the whole cell division, thereby disturbing or arresting meiosis, which eventually results in infertility. The study is an attempt to uncover the most frequent heterochromatic variations and structural chromosomal anomalies in the population suffering from reproductive failures. In this study, the pericentric inversion of chromosome 9 was most frequent in both male and female patients. Groups A and B showed the highest incidence of pericentric inversion of chromosome 9. Approximately, 1%–3% of the general population carries a pericentric inversion of chromosome 9.[25] Its most common form, inv(9)(p11q13) is reported to be associated with recurrent miscarriages, infertility, and congenital disabilities.[26,27,28]
The mechanisms of reproductive failure in couples with an inv(9) carrier suggest that the meiotic crossing over in an inversion loop leads to an unbalanced genetic makeup of the chromosome.[29,30] The inverted segment length has a detrimental role in ascertaining the effects of a pericentric inversion in the progeny. Therefore, large inversion in the meiotic loop may result in the production of genetically unbalanced gametes.[29] In a population of 6250 referred cases across four major ethnic groups, the antenatal cytogenetic analysis revealed the highest prevalence of inv(9) in the African population (3.57%), when compared to that in Hispanics (2.42%), and relatively low in Whites (0.73%) and Asians (0.26%).[30] In addition, we also observed a high occurrence of stalks in both Groups A and B. Although, to date, no specific functions have been reported to be associated with the stalk segments (pstk+). However, such variations in the couple may predispose the fetus to translocations, which may lead to fetal wastage.[20] The increased susceptibility of satellite association with nondisjunction results in a change in the number of chromosomes in gametes, posing a direct correlation between the occurrence of acrocentric variants and sterility.[31] The presence of nucleolar organizer regions on the short arms of all five acrocentric chromosomes predisposes them to nondisjunction.[32]
Besides these variations, Groups C and F encompassing the patients with IVF failures and with malformed child-bearing history, respectively, also showed a high occurrence of an increase in the centeromeric heterochromatin in chromosomes 1 (1qh+) and 9 (9qh+). These variants have been involved in mitotic instability and have an affinity towards a higher risk for aneuploidy.[33,34] Thus, the study was found to correlate with earlier work where variations in heterochromatin in chromosomes 1 and 9 have been associated with the loss of gestation product, recurrent/spontaneous miscarriages, embryonic development disorder, and abnormal phenotypes. The infertile males of Group E showed a higher incidence for both variations in terms of pericentric inversion of chromosomes 9 and Y.
The implication of chromosome 9, inversion, translocation, and other chromosomal anomalies in male infertility have been attributed by sperm abnormalities leading to abnormal clinical manifestations.[35] In addition, high heteromorphism of chromosome Y in infertile patients suggests the crucial role of these variations resulting in infertility. However, the influence of genetic variation of Y chromosome on the reproductive capacity is still unexplored. During meiosis, the pairing and synapsis of X and Y chromosomes are influenced by the DNA repeats at specific regions of the Y chromosome, which may decrease the reproductive capacity.[36,37] In the present study, although we have detected an increased ratio of chromosomal polymorphic variations and Robertsonian translocations they could not be regarded as the sole reason to govern reproductive failures. We believe molecular studies on similar lines would aid in establishing more certain roles of heterochromatin and chromosomal polymorphic variations that have not been realized yet.
CONCLUSIONS
The present study is an attempt to screen the chromosomal aberrations in reproductive failures in both males and females in the Indian population. In this work, pericentric inversion of chromosome 9, presence of satellite sequences on chromosome 15 coupled with Robertsonian translocations rob(13;14) were found to be the major factors governing reproductive failures. It suggests that these variations may have important cellular roles in different clinical conditions, including fertility. In the process, we have tried to create a baseline data which can assist the clinicians by increasing their awareness about the nature and frequency of chromosomal aberrations for take up proper prognostic assessment and genetic counseling for the affected couple. Hence, a thorough evaluation, evidence-based treatment, close monitoring, and supportive care, in couples with a history of reproductive failures in whom one and/or both the partners are a carrier of a chromosome polymorphism, could be associated with a marked improvement in the subsequent live birth rate.
Financial support and sponsorship
Dr. Lal PathLabs Limited.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors are grateful to the patient and their family to support the case study. We would also like to thank the management of Dr. Lal PathLabs Limited for providing excellent facilities.
REFERENCES
- 1.Lungeanu A, Stana A, Arghir A, Bari M, Budisteanu M. Cytogenetic abnormalities and reproductive failures. Med J Clin Med. 2007;2:11–20. [Google Scholar]
- 2.Carp H, Feldman B, Oelsner G, Schiff E. Parental karyotype and subsequent live births in recurrent miscarriage. Fertil Steril. 2004;81:1296–301. doi: 10.1016/j.fertnstert.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75:678–82. doi: 10.1016/s0015-0282(00)01801-x. [DOI] [PubMed] [Google Scholar]
- 4.Hogge WA, Byrnes AL, Lanasa MC, Surti U. The clinical use of karyotyping spontaneous abortions. Am J Obstet Gynecol. 2003;189:397–400. doi: 10.1067/s0002-9378(03)00700-2. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho B, Doria S, Ramalho C, Brandao O, Sousa M, Matias A, et al. Aneuploidies detection in miscarriages and fetal deaths using multiplex ligation-dependent probe amplification: An alternative for speeding up results? Eur J Obstet Gynecol Reprod Biol. 2010;153:151–5. doi: 10.1016/j.ejogrb.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 7.Warren JE, Silver RM. Genetics of pregnancy loss. Clin Obstet Gynecol. 2008;51:84–95. doi: 10.1097/GRF.0b013e318161719c. [DOI] [PubMed] [Google Scholar]
- 8.Goddijn M, Leschot NJ. Genetic aspects of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:855–65. doi: 10.1053/beog.2000.0124. [DOI] [PubMed] [Google Scholar]
- 9.Hirshfeld-Cytron J, Sugiura-Ogasawara M, Stephenson MD. Management of recurrent pregnancy loss associated with a parental carrier of a reciprocal translocation: A systematic review. Semin Reprod Med. 2011;29:470–81. doi: 10.1055/s-0031-1293201. [DOI] [PubMed] [Google Scholar]
- 10.Sugiura-Ogasawara M, Aoki K, Fujii T, Fujita T, Kawaguchi R, Maruyama T, et al. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J Hum Genet. 2008;53:622–8. doi: 10.1007/s10038-008-0290-2. [DOI] [PubMed] [Google Scholar]
- 11.Goddijn M, Leschot NJ. Geenetic aspects of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:855–65. doi: 10.1053/beog.2000.0124. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekhar M, Gopinath PM, Sreelakshmi K, Satyamoorthy K. A cytogenetic study of couples with miscarriages: An experience from Manipal Referral Centre. Int J Hum Genet. 2013;13:93–7. [Google Scholar]
- 13.Yoshida A, Miura K, Shirai M. Chromosome abnormalities and male infertility. Assist Reprod Rev. 1996;6:93–100. [Google Scholar]
- 14.Whitman-Elia GF, Baxley EG. A primary care approach to the infertile couple. J Am Board Fam Pract. 2001;14:33–45. [PubMed] [Google Scholar]
- 15.Quilter CR, Svennevik EC, Serhal P, Ralph D, Bahadur G, Stanhope R, et al. Cytogenetic and Y chromosome microdeletion screening of a random group of infertile males. Fertil Steril. 2003;79:301–7. doi: 10.1016/s0015-0282(02)04692-7. [DOI] [PubMed] [Google Scholar]
- 16.Siffroi JP, Le Bourhis C, Krausz C, Barbaux S, Quintana-Murci L, Kanafani S, et al. Sex chromosome mosaicism in males carrying Y chromosome long arm deletions. Hum Reprod. 2000;15:2559–62. doi: 10.1093/humrep/15.12.2559. [DOI] [PubMed] [Google Scholar]
- 17.Patsalis PC, Sismani C, Quintana-Murci L, Taleb-Bekkouche F, Krausz C, McElreavey K. Effects of transmission of Y chromosome AZFc deletions. Lancet. 2002;360:1222–4. doi: 10.1016/s0140-6736(02)11248-7. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer LG, Slovak ML, Campbel LJ. Basel, Switzerland: S Karger Publishing; 2009. ISCN 2009: An International System for Human Cytogenetic Nomenclature; pp. 53–4. [Google Scholar]
- 19.Borgaonkar DS. Chromosomal Variation in Man: A Catalog of Chromosomal Variants and Anomalies: Online NLM Version 1975. Bethesda (MD): National Centre for Biotechnology Information (US); 1975. [PubMed] [Google Scholar]
- 20.Purandare H, Fernandez NV, Deshmukh SV, Chavan S. Heterochromatic variations and pregnancy losses in humans. Int J Hum Genet. 2011;11:167–75. [Google Scholar]
- 21.Hungerford DA. Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCl. Stain Technol. 1965;40:333–8. doi: 10.3109/10520296509116440. [DOI] [PubMed] [Google Scholar]
- 22.McGowan-Jordan J, Simons A, Schmid M. ISCN 2016: An International System for Human Cytogenomic Nomenclature. Basel, Switzerland: S Karger Publishing; 2016. [Google Scholar]
- 23.Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod Biomed Online. 2005;11:726–32. doi: 10.1016/s1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- 24.Cortés-Gutiérrez EI, Cerda-Flores RM, Dávila-Rodríguez MI, Hernández-Herrera R, Vargas-Villarreal J, Leal-Garza CH. Chromosomal abnormalities and polymorphisms in Mexican infertile men. Arch Androl. 2004;50:261–5. doi: 10.1080/01485010490448750. [DOI] [PubMed] [Google Scholar]
- 25.Codina-Pascual M, Navarro J, Oliver-Bonet M, Kraus J, Speicher MR, Arango O, et al. Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum Reprod. 2006;21:1490–7. doi: 10.1093/humrep/del028. [DOI] [PubMed] [Google Scholar]
- 26.Teo SH, Tan M, Knight L, Yeo SH, Ng I. Pericentric inversion 9--incidence and clinical significance. Ann Acad Med Singapore. 1995;24:302–4. [PubMed] [Google Scholar]
- 27.Sahin FI, Yilmaz Z, Yuregir OO, Bulakbasi T, Ozer O, Zeyneloglu HB. Chromosome heteromorphisms: An impact on infertility. J Assist Reprod Genet. 2008;25:191–5. doi: 10.1007/s10815-008-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng R, Ma Y, Nie Y, Qiao X, Yang Z, Zeng R, et al. Chromosomal polymorphisms are associated with female infertility and adverse reproductive outcomes after infertility treatment: A 7-year retrospective study. Reprod Biomed Online. 2017;35:72–80. doi: 10.1016/j.rbmo.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Tempest HG, Simpson JL. Why are we still talking about chromosomal heteromorphisms? Reprod Biomed Online. 2017;35:1–2. doi: 10.1016/j.rbmo.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Fauth C, Bartels I, Haaf T, Speicher MR. Additional dark G-band in the p-arm of chromosome 19 due to a paracentric inversion with a breakpoint in the pericentromeric heterochromatin. Am J Med Genet. 2001;103:160–2. doi: 10.1002/ajmg.1520. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Jiang YT, Du RC, Zhang HG, Li LL, Liu RZ. Impact of chromosomal heteromorphisms on reproductive failure and analysis of 38 heteromorphic pedigrees in Northeast China. J Assist Reprod Genet. 2013;30:275–81. doi: 10.1007/s10815-012-9910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau N, Teyssier M. Variant D and G-chromosomes in male-related infertility. Arch Androl. 1982;9:307–10. doi: 10.3109/01485018208990256. [DOI] [PubMed] [Google Scholar]
- 33.García M, Dietrich A, Pujol R, Egozcue J. Nucleolar structures in chromosome and SC preparations from human oocytes at first meiotic prophase. Hum Genet. 1989;82:147–53. doi: 10.1007/BF00284048. [DOI] [PubMed] [Google Scholar]
- 34.Kalantari P, Sepehri H, Behjati F, Ashtiani ZO, Akbari MT. Chromosomal studies in infertile men. Tsitol Genet. 2001;35:50–4. [PubMed] [Google Scholar]
- 35.Minocherhomji S, Athalye AS, Madon PF, Kulkarni D, Uttamchandani SA, Parikh FR. A case-control study identifying chromosomal polymorphic variations as forms of epigenetic alterations associated with the infertility phenotype. Fertil Steril. 2009;92:88–95. doi: 10.1016/j.fertnstert.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 36.Nagvenkar P, Desai K, Hinduja I, Zaveri K. Chromosomal studies in infertile men with oligozoospermia & non-obstructive azoospermia. Indian J Med Res. 2005;122:34–42. [PubMed] [Google Scholar]
- 37.Antonelli A, Gandini L, Petrinelli P, Marcucci L, Elli R, Lombardo F, et al. Chromosomal alterations and male infertility. J Endocrinol Invest. 2000;23:677–83. doi: 10.1007/BF03343793. [DOI] [PubMed] [Google Scholar]