Abstract
Background:
The management of poor responders is still a challenge in modern-assisted reproductive technology. Several researches are showing encouraging results with autologous bone marrow-derived stem cells (ABMDSCs) and platelet-rich plasma (PRP) individually. Hence, we decided to study the synergistic effect of ABMDSCs with PRP.
Aims and Objective:
The aim of the study was to assess the safety and efficacy of intraovarian instillation of ABMDSCs combined with PRP in poor responders.
Design:
This was an interventional pilot study. Study Period: January 2017 to January 2019.
Materials and Methods:
We designed a pilot study using Patient-oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) Group 3 and 4 poor responder patients (n = 20). The study group underwent laparoscopic/transvaginal intraovarian instillation of ABMDSCs combined with PRP and the outcome was analyzed – primary outcome – antral follicular count (AFC) and mature MII oocytes and secondary outcome – Anti-Mullerian hormone (AMH) levels and number of Grade A and B embryos frozen on day 3. The Wilcoxon signed-rank test and Pearson correlation were used for the statistical analysis and P < 0.05 was considered statistically significant.
Results:
After 6 weeks of intraovarian instillation ABMDSCs mixed with PRP, patients were reassessed for AFC and AMH and their response to subsequent controlled ovarian stimulation (COS) cycle was observed. Statistically significant improvement was seen in AFC, MII oocytes, and Grade A and Grade B embryos. AMH was also increased in some patients, but the result was not statistically significant.
Conclusion:
Our results suggest that intraovarian instillation of ABMDSCs combined with PRP is safe and it optimized the recruitment of existing dormant primordial follicles to improve oocyte yield and hence the number and quality of embryos after COS in POSEIDON Group 3 and 4 poor responders.
KEYWORDS: Antral follicular count, autologous bone marrow-derived stems cells, platelet-rich plasma, serum anti-Mullerian hormone, poor responder
INTRODUCTION
There have been multiple advances in assisted reproductive technology (ART) since the time of its birth. However, one group remains the most challenging – poor responders that make up to 9% to 24% of patients who are seeking therapy at ART clinics.[1]
Attempts to overcome the poor response have been made by many controlled ovarian stimulation (COS) protocols and addition of adjuvants before or during the stimulation, but none have proved successful.[2] This is attributed to a low number of antral follicular count (AFC). A small pool of quiescent primordial follicles remains in the ovaries of these patients, which could potentially contribute to a higher yield of oocytes.[3,4,5]
Autologous bone marrow-derived stem cells in ovarian rejuvenation
Recently, with the emergence of regenerative medicine, many studies have successfully used stem cells or platelet-rich plasma (PRP) for ovarian rejuvenation.
Bone marrow-derived stem cells (BMDSCs) are a type of adult stem cell with low immunogenicity and they have the potential of renewing themselves and differentiating into different tissue cells.[6] The exact mechanism to explain how the BMDSCs can activate residual follicles in those who are poor responders remains unknown, but various theories have been proposed to achieve tissue regeneration.
One of the theories is the Homing phenomenon where BMDSCs directly and impulsively migrate to the defective microenvironment and repair under the stimulation of multiple factors, which facilitates ovarian recovery. BMDSCs differentiate into a variety of cells such as theca cells, granulosa cells, corona radiata cells, and vascular endothelial cells.[7]
Paracrine effects of stem cells have been evaluated for their ability to activate the preexisting quiescent follicles. BMDSCs secrete chemokines, growth factors, and hormones to influence adjacent cells (the paracrine effect). Paracrine signaling is important in anti-inflammation, immunoregulation, antiapoptosis, antifibrosis, and controlling oxidative stress, thus improving the microenvironment to promote the recovery of damaged tissues.[8]
Autologous bone marrow-derived stem cells (ABMDSCs) express genes relative to vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), and interleukin-6 (IL-6) and promote angiogenesis in vivo and in vitro, thus stimulating neovascularization and facilitating blood perfusion to quiescent primordial follicles.[9]
ABMDSCs for ovarian rejuvenation are still under medical research. All subjects were made aware that this intervention is still under research and informed audio–visual consent was obtained. To find the genomic and epigenomic alterations in ABMDSCs, long-term follow-up will be necessary. ABMDSCs, unlike embryonic stem cells, are differentiated cell populations. Hence, there are less or no chances of uncontrolled multiplication.
Platelet-rich plasma in ovarian rejuvenation
PRP constitutes an autologous and highly concentrated platelet-rich solution of plasma which is prepared from the patient's own blood and contains a concentrated source of growth factors, namely VEGF, insulin-like growth factor-1 and 2 (IGF-1 and IGF-2), epidermal growth factor, transforming growth factor-beta, hormones, and cytokines.[10] Thus, PRP treatment may assist in tissue regeneration,[11] enhancement of anabolic signaling pathways,[12] cell differentiation and proliferation,[13] and angiogenesis.[14] Considering the angiogenic composition of the ovary and the pivotal influence of platelet-derived growth factors on vascular activation and stabilization, autologous PRP may have a therapeutic effect on ovarian tissue regeneration.[15]
Autologous PRP also attracts stem cells to the site of injury and boosts their response. Based on these information and our own experience,[16,17] we designed a pilot study, with the aim of combining ABMDSCs with PRP to induce ovarian rejuvenation in patients who are poor responders of Patient-oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) Group 3 and 4 to optimize the recruitment of the existent dormant follicles to improve the oocyte yield and hence the number of embryos available. Pantos et al.[18] observed folliculogenesis along with menstrual cycle restoration and natural conception with complication-free pregnancies with PRP instillation. PRP containing growth factors enhances the regeneration of ovaries and also control proliferation and migration of other cells essential to tissue repair.[19]
Homing mechanism and niches
Homing of stem cells means that they can directly and impulsively migrate to the injured tissue and survive there under the stimulation of multiple factors that reconstructs the local microenvironment called “niche.” Homing is an active process that allows for the migration of hematopoietic stem cells through the blood and vascular endothelium to different organs and niches. Chemokines and growth factor receptors, such as receptors for IL8 and hepatocyte growth factor, located on the surface of BMSCs are involved in migration and homing of BMSCs. MicroRNA-21 (miR-21) facilitates BMSCs migration by upregulating matrix metalloproteinase (MMP-2), potentially via the phosphatidylinositol-3-OH-kinase pathway in vitro. Furthermore, selectins play an essential role in cell movement called “cell rolling” on microvessels. BMSCs localize and survive in the affected ovary after stem cell transplantation, thus promoting the ovarian recovery of histological structure and endocrine function. Stem cells migrate into the ovary and differentiate into a variety of cells, including theca cells, granulosa cells, corona radiata cells, and vascular endothelial cells. The BMSCs also contribute to ovarian regeneration by enhancing angiogenesis.[20,21]
MATERIALS AND METHODS
After approval from the Institutional Ethics Committee, 20 poor responder patients with audio–visual and written informed consent about the nature of the procedure to be undertaken and after explaining the pros and cons of this procedure were included in the study. We have been given permission by our ethical committee at SoloStem cells, a Stem Cell Research and application Centre, Pune India. This ethical committee is registered with ICMR & has given us the permission for this pilot study.
This prospective interventional pilot study was designed for intraovarian instillation of ABMDSCs along with PRP in 20 patients who are poor responders defined according to the POSEIDON criteria.
Inclusion criteria were as follows
Age group of 20–45 years
Poor responder POSEIDON[22] Group 3 and 4 (the expected poor responder group where AFC <5 and anti-Mullerian hormone (AMH) <1.1 ng/ml)
Normal karyotype.
Normal semen parameters.
Exclusion criteria were as follows
POSEIDON Group 1 and 2
Abnormal karyotyping.
Autoimmune diseases
POI due to chemotherapy or radiotherapy
Severe oligoasthenoteratozoospermia
Active viral infections.
The AFC by transvaginal ultrasound and serum AMH levels were considered markers of ovarian response. Both were recorded in all patients under basal conditions before and 6 weeks after the procedure.
Methodology
Preparation of autologous bone marrow-derived stem cells
Bone marrow aspiration was done from the posterior superior iliac spine under local anesthesia using the Jamshidi needle (13G) and 20 ml syringe prewashed with heparin maintaining strict asepsis. Around 150 ml of bone marrow was aspirated. Sixteen milliliter BMDSC was separated using the fully automated cell separator, which uses optical sensor technology and simultaneous application of centrifugation and sedimentation. Processing of bone marrow sample was carried out in a completely closed circuit centrifugation unit. Stem cell count (using flow cytometer and hemocytometer) varies from 05 million to 13 million cells per ml of the final stem cell concentrate. Count varies from patient to patient depending on their yield of stem cells obtained from BM sample aspirated.
Preparation of platelet-rich plasma
Around 20 ml of peripheral blood in the heparinized syringe was taken and 2 ml of PRP was prepared after double centrifugation. This was mixed with 16 ml of ABMDSCs.
Intraovarian instillation
Intraovarian instillation was performed either transvaginally USG guided or laproscopically.
We had eight patients where ovaries were accessible transvaginally. In those patients, we did transvaginal USG-guided intraovarian instillation under general anesthesia. Younger patients aged between 20 and 35 normally have a good volume of ovaries through its main stroma. It was possible to inject up to 06 ml of ABMDSC's per ovary at multiple sites along the long axis of the ovary. Injection started from caudal end and continued by withdrawing the specially designed needle up to the cranial end. In few patients where the volume of ovaries was not good enough, the injected volume was up to 04 ml per ovary. In 12 patients where ovaries were high up, small, atrophic, or difficult to fix, we preferred laparoscopic instillation.
Follow-up of the patients
Patients were followed up weekly for any adverse effect of instillation. In the 6th week, they were reassessed for change in AFC and serum AMH levels. The criteria for a positive response were an increase in AFC to three or more follicles from basal measurements and/or an increase of 2 standard deviation in AMH levels compared with basal levels.
Controlled ovarian stimulation and intracytoplasmic sperm injection
After 6 weeks of treatment, patients underwent COS using minilong agonist protocol, in which injection leuprolide acetate was started 5 days prior to the expected menstrual date in the dose of 0.15 mcg and stepped up to 0.7 mcg on day 6/7 of stimulation. Gonadotropin stimulation was started from day 6 of leuprolide acetate using human menopausal gonadotropins 300–450 IU. Final oocyte maturation was triggered with Human Chorionic Gonadotropin, hCG (Ovitrelle 250 mg) when the majority of the follicles were between 16 and 20 mm. Oocyte pickup was performed 35 h after hCG administration. Denudation was done 3½ h later, MII oocytes were confirmed, and intracytoplasmic sperm injection was then performed using fresh sperm. Embryos were grown till day 3 and then cryopreserved.
RESULTS
The procedure was safe, and no patient had instillation-related adverse effects.
The mean age of the patients in our study group was 32.95 ± 7.060 years [Table 1].
Table 1.
Distribution of age
| Age | Number of patients (%) |
|---|---|
| 20-30 | 8 (40) |
| 31-35 | 4 (20) |
| 36-40 | 5 (25) |
| >40 | 3 (15) |
The mean age of patients is 32.95±7.06 years
The Wilcoxon signed-rank test was used to compare the clustered-paired AFC and AMH before and after ABMDSCs combined with PRP instillation for each patient. In our study, thecvalues of P < 0.05 were considered statistically significant.
All the analyses were performed using SPSS 22.0 (IBM Corp, Armonk, New York, United States).
The total AFC increase was statistically significant (P = 0.0001) [Table 2, Figure 1].
Table 2.
AFC Statistics Pre- and Post Instillation
| Parameter | n | Mean±SD | Minimum number | Maximum number | P | Statistical significance |
|---|---|---|---|---|---|---|
| AFC preinstillation | 20 | 3.35±0.98 | 2 | 5 | 0.0001 | Significant |
| AFC postinstillation | 20 | 5.7±1.75 | 2 | 8 |
With P<0.05 significant at 95% CI, there was a statistically significant difference between preinstillation and postinstillation AFC (P=0.0001). AFC=Antral follicular count, SD=Standard deviation, CI=Confidence interval
Figure 1.
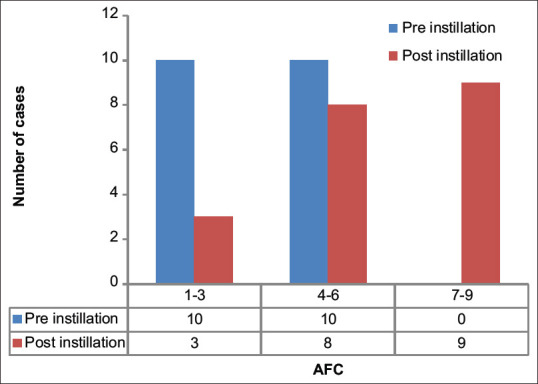
Comparison between preinstillation antral follicular count and postinstillation antral follicular count. AFC = Antral follicular count
We also observed an increase in AMH values in few patients, but it was not statistically significant (P = 0.584) [Table 3, Figure 2].
Table 3.
AMH Statistics Pre- and Post Instillation
| Variables | n | Mean±SD | P | Statistical significance |
|---|---|---|---|---|
| Preinstallation AMH | 20 | 0.6435±0.213 | 0.584 | Not significant |
| Postinstillation AMH | 20 | 0.632±0.205 |
With P<0.05 significant at 95% CI, there was no statistically significant difference between pre- and postinstillation AMH (P=0584). SD=Standard deviation, AMH=Antimullerian hormone, CI=Confidence interval
Figure 2.
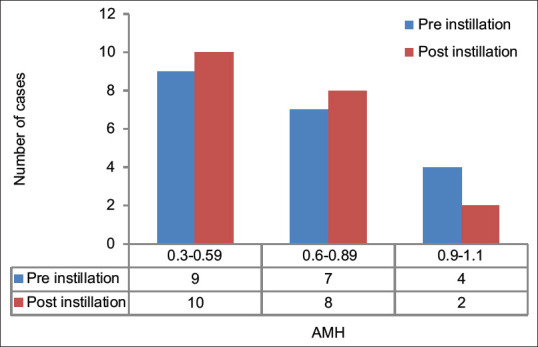
Comparison between preinstillation antimullerian hormone and postinstillation antimullerian hormone. AMH = Antimullerian hormone
After COS, the mean number of oocytes retrieved was 4 ± 1.654 [Table 4]. Using the Pearson correlation, we observed a positive correlation between MII oocytes with AFC [Figure 3] and MII oocytes with age [Figure 4]. The mean number of Grade A and B embryos frozen on day 3 was 2.5 ± 1.051 [Table 5].
Table 4.
Comparison of antral follicular count with the mean oocyte number
| AFC | Mean oocyte number±SD |
|---|---|
| 1-3 | 2.0±0.0 |
| 4-6 | 3.75±1.58 |
| 7-9 | 4.88±1.56 |
AFC=Antral follicular count, SD=Standard deviation
Figure 3.
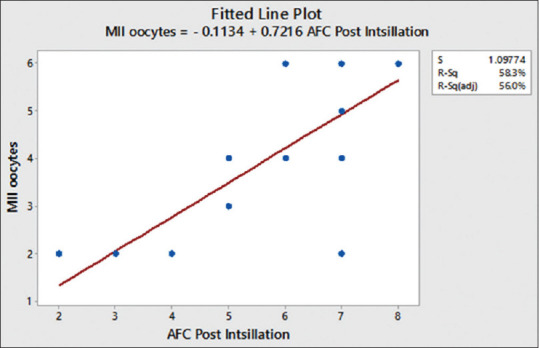
Correlation between antral follicular count with mean oocyte number. Pearson correlation of antral follicular count and MII oocyte = 0.763
Figure 4.
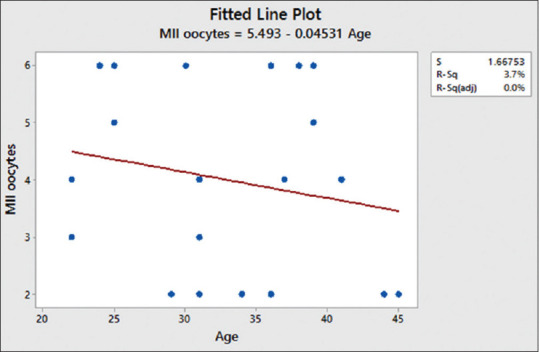
Correlation between age and MII oocytes. Pearson correlation of age and MII oocytes = −0.194
Table 5.
Comparison of antral follicular count with the mean oocyte number and embryo number
| AFC | Mean oocyte number±SD | Mean embryo number±SD |
|---|---|---|
| 1-3 | 2.0±0.0 | 1.33±0.57 |
| 4-6 | 3.75±1.58 | 2.12 ±0.64 |
| 7-9 | 4.88±1.56 | 3.33±0.86 |
AFC=Antral follicular count, SD=Standard deviation
With P < 0.05 significant at 95% confidence interval, there was a statistically significant difference between preinstillation and postinstillation AFC (P = 0.0001).
With P < 0.05 significant at 95% confidence interval, there was no statistically significant difference between preinstillation and postinstillation AMH (P = 0584).
DISCUSSION
In the present pilot study, an intraovarian instillation of ABMDSCs combined with PRP was used in 20 women, who are poor responders using POSEIDON Groups 3 and 4 criteria. Primary outcomes included clinical improvement in ovarian reserve measured by AFC and MII oocytes retrieved, and the secondary outcome included AMH levels and the number of Grade A and B embryos frozen on day 3.
Studies have shown that the BMDSCs secrete various soluble growth factors into plasma. This paracrine effect plays a crucial role in tissue regeneration by adult stem cells.[23] Herraiz et al. in their study observed an association between the presence of FGF-2 in plasma and the improvement in ovarian reserve biomarkers, BMSC infusion in chemotherapy-induced ovarian insufficient mice resulted in the production of higher numbers of preovulatory follicles, metaphase II oocytes, 2-cell embryos, and healthy pups.[24,25] The FGF-2 protein and its receptor are expressed in early follicles and play a key role in estrogen (E) production. Its overexpression is associated with improvement in follicular development and neoangiogenesis.[26,27,28] They also studied the positive effect of thrombospondin (THSP-1) on AFC and AMH enhancement. Cervello et al. also observed that the overexpression of THSP-1 has been associated with regenerative effects induced by CD133 cell therapy in an animal model in endometrial disease.[29]
Callejo et al. in their study employed PRP in an autologous ovarian transplantation to improve the quality and the vascularization potential of the implant.[30] Sfakianoudis et al. studied the role of intra-ovarian PRP in poor responders. In their study, there was a significant reduction in the patient's follicle-stimulating hormone levels 6 weeks following the autologous platelet-rich plasma treatment.[31] Based on these studies and with our own experience[32] of the beneficial effect of PRP on vascularization and rejuvenation of the endometrium, we decided to combine ABMDSCs with PRP so that we can have mutual beneficial effect of both in optimizing the results.
In our study, we observed positive ovarian response at 6 weeks, and AFC significantly increased following intraovarian instillation of ABMDSCs combined with PRP.
After COS, the mean number oocytes retrieved in our pilot study was 4 ± 1.654, and a positive correlation between AFC and oocytes retrieved was observed. Our study generated a mean of 2.5 ± 1.051 Grade A and B embryos frozen on day 3. We also observed the negative correlation between age and oocytes retrieved.
In our study, even AMH increased in some patients, but the increase was not statistically significant (P = 0.584). Herraiz et al.[24] reported a remarkable increase in AMH in few patients, but when all cases were analyzed together, the value of P was not significant (P = 0.14).
To our knowledge, it is the first study which reported the combined use of ABMDSCs and PRP in ovarian rejuvenation where young poor responders had a better outcome.
In a nutshell, we found that intraovarian instillation of ABMDSCs along with PRP mobilized and restored the pre-existing ovarian reserve in the poor responders.
CONCLUSION
In conclusion, intraovarian instillation of ABMDSCs combined with PRP is safe and efficacious. It could represent a paradigm shift for fertility treatment for poor responders. Further work is needed to validate results in a larger and homogeneous population, before considering ABMDSCs or PRP as a real alternative to managing poor responder patients.
Increased understanding of its mechanism will promote its wide clinical application and give a ray of hope to the infertile couples who do not wish to opt for donor-assisted reproductive techniques.
What is known?
Poor responders are known to give poor reproductive outcomes owing to poor quantity and quality of oocytes and hence embryos.
ABMDSCs and PRP have been individually attempted to improve reproductive outcomes/ovarian rejuvenation in poor responders.
What our study adds
To our knowledge for the first time, the combined use of ABMDSCs and PRP has been proposed to see the synergistic safety and efficacy to induce ovarian rejuvenation in poor responders.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
In the form, the patients have given their consent for their clinical information to be reported in the journal. The patients understand that their name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garcia-Velasco JA, Isaza V, Requena A, Martínez-Salazar FJ, Landazábal A, Remohí J, et al. High doses of gonadotrophins combined with stop versus non-stop protocol of GnRH analogue administration in low responder IVF patients: A prospective, randomized, controlled trial. Hum Reprod. 2000;15:2292–6. doi: 10.1093/humrep/15.11.2292. [DOI] [PubMed] [Google Scholar]
- 2.Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil Steril. 2009;91:749–66. doi: 10.1016/j.fertnstert.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 3.Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, et al. In vitro Activation of Follicles and Fresh Tissue Auto-transplantation in Primary Ovarian Insufficiency Patients. J Clin Endocrinol Metab. 2016;101:4405–12. doi: 10.1210/jc.2016-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–9. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–4. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raeth S, Sacchetti B, Siegel G, Mau-Holzmann UA, Hansmann J, Vacun G, et al. A mouse bone marrow stromal cell line with skeletal stem cell characteristics to study osteogenesis in vitro and in vivo. Stem Cells Dev. 2014;23:1097–108. doi: 10.1089/scd.2013.0367. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Chen D, Yang L, Hou Q, Ma H, Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9:263. doi: 10.1186/s13287-018-1008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilly JL, Johnson AL. Modulation of hen granulosa cell steroidogenesis and plasminogen activator activity hy transforming growth factor alpha. Growth Factors. 1990;3:247–55. doi: 10.3109/08977199009043909. [DOI] [PubMed] [Google Scholar]
- 9.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 10.Xia X, Yin T, Yan J, Yan L, Jin C, Lu C, et al. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant. 2015;24:1999–2010. doi: 10.3727/096368914X685267. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–56. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 12.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;4:73–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–40. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 14.Liao HT, James IB, Marra KG, Rubin JP. The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng Part A. 2015;21:2714–22. doi: 10.1089/ten.tea.2015.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bos-Mikich A, de Oliveira R, Frantz N. Platelet-rich plasma therapy and reproductive medicine.J. Assist Reprod Genet. 2018;35:753–6. doi: 10.1007/s10815-018-1159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Lodha P, Karthick MS, Tandulwadkar SR. Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: Review of literature and a case report of world's first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J Hum Reprod Sci. 2018;11:125–30. doi: 10.4103/jhrs.JHRS_57_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantos K, Simopoulou M, Pantou1 A, Rapani A, Tsioulou P, Nitsos N, et al. A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Trans. 2019;28:1333–40. doi: 10.1177/0963689719859539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott Sills E, Samue H. Wood Autologous activated platelet-rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep. 2019:39. doi: 10.1042/BSR20190805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Chen D, Yang LL, Hou Q, Ma H, Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9:263. doi: 10.1186/s13287-018-1008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truman AM, Tilly JL, Woods DC. Ovarian regeneration: The potential for stem cell contribution in the postnatal ovary to sustained endocrine function. Mol Cell Endocrinol. 2017;445:74–84. doi: 10.1016/j.mce.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol (Lausanne) 2018;9:461. doi: 10.3389/fendo.2018.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, Gómez-Seguí I. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110:496–505e1. doi: 10.1016/j.fertnstert.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Herriaz S, Pellicier A. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–17. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Abedini A, Zamberlam G, Lapointe E, Tourigny C, Boyer A, Paquet M, et al. WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 2016;30:1534–47. doi: 10.1096/fj.15-280313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price CA. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2016;228:R31–43. doi: 10.1530/JOE-15-0414. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Deutsch U, Jeong J, Lobe CG. Constitutive notch signaling in adult transgenic mice inhibitsbFGF-induced angiogenesis and blocks ovarian follicle development. Genesis. 2014;52:809–16. doi: 10.1002/dvg.22790. [DOI] [PubMed] [Google Scholar]
- 29.Cervello I, Gil-Sanchis C, Santamaria X, Cabanillas S, Diaz A, Faus A, et al. Human CD133(þ) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104:1552. doi: 10.1016/j.fertnstert.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Callejo J, Salvador C, González-Nuñez S, Almeida L, Rodriguez L, Marqués L, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6:33. doi: 10.1186/1757-2215-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pappas A, Pantou A, et al. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J Clin Med. 2018;8:1. doi: 10.3390/jcm8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tandulwadkar SR, Naralkar MV, Surana AD, Selvakarthick M, Kharat AH. Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: A pilot study. J Hum Reprod Sci. 2017;10:208–12. doi: 10.4103/jhrs.JHRS_28_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


