Abstract
We report the case of a 16-year-old girl diagnosed with myocarditis, although initial echocardiographic imaging was consistent with hypertrophic cardiomyopathy (HCM). The diagnosis of myocarditis was made with the findings of troponin elevation, presence of influenza A, and a more characteristic electrocardiogram. She eventually made a full recovery. Clinicians must be vigilant for such rare presentations of myocarditis masquerading as HCM.
Keywords: Hypertrophic cardiomyopathy, influenza A, myocarditis
INTRODUCTION
Myocarditis is a disease characterized by acute inflammation of the myocardium associated sometimes with the onset of cardiac dysfunction, arrhythmia, and even, sudden death. Although there are a variety of causes of myocarditis, a viral etiology is the most common, and it is postulated that the viruses trigger the immune system to attack cardiac myocytes, leading to lymphocytic infiltration of the tissue.[1,2] Given the broad ranges of presentation of these patients, myocarditis must be at least suspected in any patient presenting with new dysfunction, troponin elevation, and/or characteristic electrocardiogram (ECG) changes.[3,4] While advanced studies such as cardiac magnetic resonance imaging (cMRI) are of utility in recognizing this disease, serial re-evaluation is paramount to recognizing the changing characteristic of these hearts with regard to function and inflammation, and in supporting patients through acute changes in their function.[5] Herein, we expound upon a case of myocarditis in a 16-year-old girl presenting with syncope and severe cardiac hypertrophy in the setting of influenza A infection.
CASE REPORT
A 16-year-old, previously healthy female initially presented to her pediatrician with a chief complaint of syncope. The onset of illness started 6 days prior with the symptoms of malaise, fever, and fatigue, later progressing to include chest pain and shortness of breath. She had a presyncopal episode with dizziness, vision changes, and sweating, and later had a true syncopal episode, chipping her tooth upon collapse, but awakening within seconds. After an immediate visit with her primary care physician, she was transferred urgently to our emergency department (ED).
On presentation to the ED, she was afebrile with a blood pressure of 93/72 mmHg, pulse rate of 90s–100 beats per minute, respiratory rate of 16, and oxygen saturation of 100% on room air. Her physical examination showed a well-developed, well-nourished female in no distress aside from mild pain from her fall, with no apparent head/facial bruising. She was tachycardic with normal heart sounds and no murmurs, with lungs that were clear to auscultation bilaterally and an abdominal examination demonstrating no hepatosplenomegaly. An ECG showed normal sinus rhythm with low-voltage QRS complexes and T-wave inversions in inferior and anterior leads [Figure 1]. Laboratory evaluation revealed normal electrolytes, complete blood counts, and inflammatory markers, including her erythrocyte sedimentation rate and C-reactive protein. Transthoracic echocardiogram (TTE) showed mildly depressed left ventricular (LV) function with an ejection fraction (EF) of approximately 50%, a small pericardial effusion, and striking moderate-to-severe concentric LV hypertrophy (LVH) with a LV diastolic septal and posterior wall thickness of 1.4 cm [Figure 2a] without outflow tract obstruction. Differential diagnosis at the time included hypertrophic cardiomyopathy (HCM) with dysfunction, HCM with arrhythmia, and myocarditis, and she was admitted for close monitoring, including telemetry, and to follow serial troponins. After admission, her initial troponin I resulted at 0.977 ng/mL (laboratory index for upper limits of normal <0.029 ng/mL).
Figure 1.
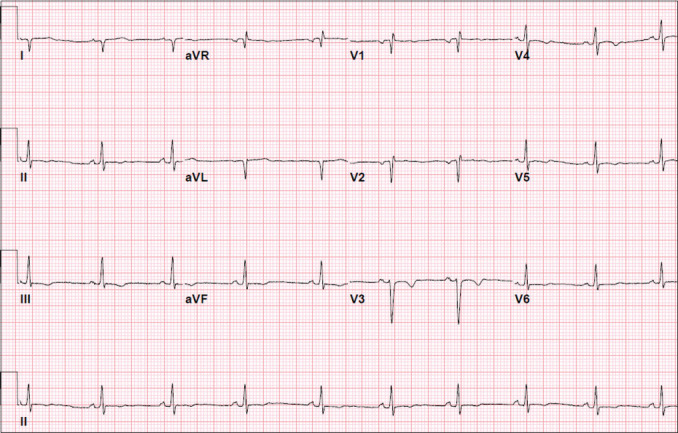
Admission electrocardiogram with normal sinus rhythm, low-voltage QRS complexes, and anteroinferior T-wave inversions
Figure 2.
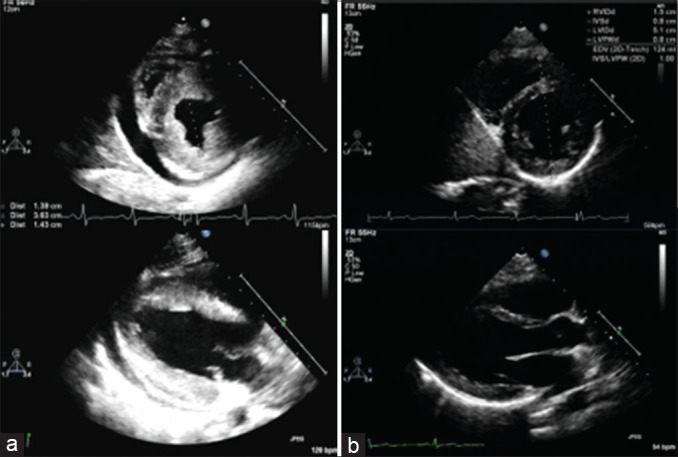
(a) Initial short- and long-axis views demonstrating echo bright and hypertrophied heart, with a septal thickness of 14 mm (b) 4 months after presentation, the same views demonstrating complete resolution of hypertrophy, with septal thickness normalized to 8 mm
On hospital day 1, she developed fluid refractory hypotension, sinus tachycardia, and tachypnea. She was transferred to the cardiac intensive care unit for vasopressor support including dopamine and milrinone as we had progressive concern for both myocarditis and likely viral sepsis. A variety of viral polymerase chain reactions were obtained including human herpesvirus-6, Epstein–Barr virus, enterovirus, parvovirus B19, adenovirus, and influenza. She was found to be influenza A positive and was subsequently started on peramivir. On hospital day 2, a repeat echocardiogram showed an increased pericardial effusion, and there was concern for tamponade physiology; pericardiocentesis was performed with pericardial drain placement. She also started having increased respiratory effort with hypoxemia and eventually was started on biphasic positive airway pressure for respiratory support. She was given a dose of 2 g/kg of intravenous immunoglobulin. From this point, she gradually improved and by hospital day 5, was weaned from intravenous infusions and supplemental oxygen. A repeat TTE demonstrated increased EF of >70% and persistent concentric LVH. On hospital day 7, all of her symptoms had resolved, and TTE revealed resolving LVH (LV diastolic septal and posterior wall dimensions of 1.2 cm) upon discharge. At her 4-month follow-up appointment, she had a cMRI which showed normal biventricular chamber sizes and biventricular systolic function, normal LV mass with no LVH, nor evidence of late gadolinium enhancement, corresponding to her echocardiogram at the same time demonstrating resolution of LVH [Figure 2b].
DISCUSSION
Transient LVH should be recognized as a complication and possible presentation of acute myocarditis. This case of influenza A myocarditis presented with profound myocardial edema, resulting in transient myocardial thickening, or hypertrophy, that may be easily confused with HCM. Influenza, though not a common cause of myocarditis, has been shown to cause myocarditis in all three of the most recent pandemics.[6] Without a high index of suspicion, cardiac involvement can go undiagnosed, especially in otherwise healthy children. In many patients with influenza who did undergo endomyocardial biopsy, interstitial edema was found in cardiac tissue,[7,8] which in progressive form is likely what contributes to transient hypertrophy in viral myocarditis. Prior case series have also documented some gross hypertrophy.
As shown in Table 1,[9,10,11,12] the reported pediatric cases in the literature involve both infantile and adolescent patients. While the etiology varies, all of the patients had an increase in LV septal wall thickness that normalized after a short period, as in our patient. This bimodal distribution in the reported literature could lead providers to have a higher index of suspicion in these age groups when presented with LVH in the setting of myocarditis, though further research is needed to identify the true incidence of this condition.
Table 1.
Case reports of transient pediatric left ventricular hypertrophy in acute myocarditis
| Reference | Age | Max septal thickness (cm) | Baseline septal thickness (cm) | Ratio (max/baseline) | Lowest ejection fraction (%) | Recovery? | Virus |
|---|---|---|---|---|---|---|---|
| Current | 16 years | 1.4 | 0.8 | 1.8 | 50 | Yes | Influenza A |
| [9] | 14 years | 1.6 | 1.3 | 1.2 | 29 | Yes | Influenza A |
| [10] | 17 days | NR | NR | NR | NR | Yes | Unknown |
| [11] | 4 months | 0.11 | 0.07 | 1.6 | NR | Yes | Coxsackie B |
| [12] | 15 years | 1.3 | 0.7 | 1.9 | 27 | Yes | Unknown |
NR: Not Reported
Transient ventricular wall thickening can lead to a diagnostic challenge for physicians in that it is difficult to distinguish from HCM on initial presentation. It is important to understand that this phenomenon exists, as myocarditis and HCM have very different treatment strategies and prognoses. Complete assessment of this patient, including an ECG that is more in fitting with myocarditis, history of a viral prodrome, presence of troponin elevation and pericardial effusion, and finding a virus known to cause myocarditis, all helped steer this diagnosis toward myocarditis and away from HCM. This, in turn, allowed us to involve our infectious disease colleagues early on and provide the best care for our patient. A high index of suspicion for myocarditis and awareness of the broad range of presentations is needed to make the right diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shauer A, Gotsman I, Keren A, Zwas DR, Hellman Y, Durst R, et al. Acute viral myocarditis: Current concepts in diagnosis and treatment. Isr Med Assoc J. 2013;15:180–5. [PubMed] [Google Scholar]
- 2.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 3.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–98. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 4.Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–28. doi: 10.1161/CIRCULATIONAHA.113.001372. [DOI] [PubMed] [Google Scholar]
- 5.Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J Cardiovasc Magn Reson. 2015;17:96. doi: 10.1186/s12968-015-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezkalla SH, Kloner RA. Influenza-related viral myocarditis. WMJ. 2010;109:209–13. [PubMed] [Google Scholar]
- 7.Hiramitsu S, Morimoto S, Kato S, Uemura A, Kubo N, Kimura K, et al. Transient ventricular wall thickening in acute myocarditis: A serial echocardiographic and histopathologic study. Jpn Circ J. 2001;65:863–6. doi: 10.1253/jcj.65.863. [DOI] [PubMed] [Google Scholar]
- 8.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, et al. Myocarditis A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 9.Wittlieb-Weber CA, Harris MA, Rossano JW. Transient left ventricular wall thickening in a 14-year-old girl with influenza A myocarditis. Cardiol Young. 2015;25:187–90. doi: 10.1017/S1047951114000018. [DOI] [PubMed] [Google Scholar]
- 10.Liao PK, Seward JB, Hagler DJ, Driscoll DJ. Acute myocarditis associated with transient marked myocardial thickening and complete atrioventricular block. Clin Cardiol. 1984;7:356–62. doi: 10.1002/clc.4960070607. [DOI] [PubMed] [Google Scholar]
- 11.Kosutic J. Severe transient left ventricular hypertrophy in an infant with acute myocarditis and heart failure. Pediatr Cardiol. 2004;25:677–80. doi: 10.1007/s00246-003-0617-x. [DOI] [PubMed] [Google Scholar]
- 12.Sagrera MR, Sala MF, Bárcena JP, Garcia JB, Cañellas CF, Juvé JI. Acute myocarditis and left ventricular “hypertrophy. Echocardiography. 2000;17(6 Pt 1):567–70. doi: 10.1046/j.1540-8175.2000.00567.x. [DOI] [PubMed] [Google Scholar]


