Abstract
Objectives:
The aim of this study is to evaluate HHV-6 and PVB19 infection using polymerase chain reaction (PCR) and immunofluorescent assay (IFA) in the myocardium of pediatric patients with dilated cardiomyopathy (DCM) and the impact of viral persistence in the cardiac allograft after heart transplantation (HT).
Methods:
Multiplex droplet digital PCR was used to analyze the prevalence of viral sequences in myocardial samples from 48 pediatric DCM patients and 10 control subjects. Of the 48 DCM patients, 44 underwent HT. After HT, consecutive endomyocardial biopsy (EMB) samples were analyzed for the presence of PVB19 and HHV-6 antigens using IFA and the patients were evaluated for rejections, coronary vasculopathy, and graft loss.
Results:
Of the 48 DCM patients, 14 had positive viral PCR results in explanted/autopsy hearts. Among them, PVB19 was found in 8/48, HHV6 in 4/48, both PVB19 and HHV6 in 1/48, and enterovirus in one, but no adenovirus was found. The EMB samples obtained after HT were positive for PVB19 and HHV-6 in 7/44 and 3/44 cases, respectively. Viral presence in both the explanted heart and the cardiac allograft was demonstrated in 4 patients, 3 of whom were positive for PVB19, and one of whom was positive for HHV-6 pretransplant. Coronary vasculopathy and graft loss were more common in patients with PVB19-positive myocardial tissues versus those who were PVB19-negative.
Conclusions:
There is an association between PVB19 and HHV-6 infection and DCM in children. The study suggests the persistence of PVB19 and HHV-6 in the host can lead to subsequent viral reactivation in the transplanted heart, even in those recipients who do not have active myocarditis. PVB19 in the cardiac allograft tended toward higher adverse post-HT events.
Keywords: Cardiotropic viruses, coronary vasculopathy, dilated cardiomyopathy, immunofluorescent assay, pediatric heart transplantation, polymerase chain reaction
INTRODUCTION
Viral infection is the most common cause of myocarditis and has been implicated in the development of dilated cardiomyopathy (DCM). The viruses that are frequently detectable in the myocardium of pediatric patients include parvovirus B19 (PVB19), human herpesvirus type 6 (HHV-6), enteroviruses, and adenovirus. Over the last two decades, the viral genomes most frequently detected in myocardium have changed from enterovirus and adenovirus to PVB19 and HHV-6 in children and adults.[1,2,3,4,5,6] A recent study by Pietra et al., which included both a pediatric cardiomyopathy registry and a pediatric heart transplant study database, concluded that myocarditis at presentation was associated with increased death after transplantation.[7] A number of other studies have correlated endomyocardial viral infection with pediatric heart transplant (HT) outcomes. Schowengerdt et al. reported the role of PVB19 endomyocardial viral infection in myocarditis and allograft rejection in pediatric transplant recipients.[5] Another study by Shirali et al. systemically evaluated over 500 biopsy specimens from transplanted children and showed an outcome difference based on the presence of the viral genome.[8] Similarly, Moulik et al. identified viral genomes, including PVB19, in 39% of myocardial biopsies from pediatric HT recipients and found an association between the viral presence and the development of cardiac allograft vasculopathy (CAV).[9]
The persistence of viral infection in the myocardium has been associated with disease progression in children,[10] and adults,[3] as well as relapse of inflammatory cardiomyopathy after transplantation.[10] One of the conundrums for clinicians is the role of the persistence of viruses in the myocardium of pediatric DCM patients and its functional significance. The aim of our study was to explore the presence of common cardiotropic viruses (PVB19, HHV-6, adenovirus, and enteroviruses) based on polymerase chain reaction (PCR) assays in the myocardium of pediatric DCM patients with endocardial fibroelastosis and without evidence of acute inflammation. The secondary aim was to evaluate the impact of the viral persistence on post-HT outcomes.
METHODS
Study population and data sources
Forty-eight consecutive DCM patients who were clinically followed at Children's Medical Center Dallas, Texas, USA, and 10 control subjects without any cardiac pathology from the same institution between 2010 and 2016 were included for this study. The explanted/autopsy heart tissues from both DCM cases and control subjects were sampled. All patients had a clinical diagnosis of DCM based on the echocardiographic finding of the dilated left ventricle with severely decreased systolic function and endocardial fibroelastosis confirmed by histopathology. There was no evidence of acute myocarditis in any of the 48 cases by Dallas criteria.[11] Control subjects, age < 18 years, included in this study from the same institution and same time period who died from non-cardiac causes such as head trauma (3), near-drowning (2), infants with bronchopulmonary dysplasia (3), acute asthma (1), and noncontrollable seizure (1) and the heart was included in the autopsy. None of the control subjects had any evidence of cardiomyopathy or myocarditis according to their final autopsy report.
Forty-four of the 48 DCM patients underwent HT at the same institution and were included for evaluation of the persistence of the virus in the cardiac allograft. Two hundred and fifty-eight endomyocardial biopsy (EMB) samples (formalin-fixed paraffin-embedded tissue) samples obtained within the 1st year of HT, median biopsies were 5 per each patient, ranged 1-10 biopsies per patient, and were evaluated for the presence of PVB19 and HHV-6 by immunofluorescent assay (IFA). The medical records were evaluated for demographic data and clinical variables including ventilator support, use of life support such as extracorporeal membrane oxygenation (ECMO) and ventricular assist device (VAD), prior sensitization (PRA > 10%), induction immunosuppression therapy (basiliximab versus anti-thymocyte globulin), positive serum PCR for viruses at the time of transplant, cytomegalovirus mismatch between organ donor and recipient, and posttransplant outcomes such as acute rejection, CAV, and graft loss (retransplantation or death) during a follow-up of 1–8 years. For the purpose of this study, all major adverse outcomes had prespecified definitions. The EMB protocol for rejection surveillance, and criteria for the diagnosis of acute rejections, and graft loss used in this study were per the institutional protocol and International Society for Heart and Lung Transplant (ISHLT) guidelines.[12] The diagnosis of CAV (any severity) was by angiography as per the ISHLT guidelines.[13] The details of institution protocol for the evaluation of pediatric transplant recipients at Children's Medical Center Dallas has been described previously.[14]
The PCR analysis for virus in all control subjects' autopsy heart tissues and DCM patients' explanted hearts at the time of transplant or autopsy was performed at the Molecular Virology Laboratory, University of Washington, Seattle, in a blinded fashion. The IFA analyses for the presence of PVB19 and HHV-6 of all EMB specimens from cardiac allografts were performed in the Virology Laboratory, University of Wuerzburg, Germany. This study was approved by the institutional review board of the Children's Medical Center and UTSW Medical Center Dallas.
Polymerase chain reaction and real-time polymerase chain reaction assay
Previously published real-time PCR (TaqMan) or RT-PCR assays were used employing primers and probes designed to amplify specific sequences of enteroviruses,[15] adenoviruses,[16] and HHV-6.[17] For the detection of PVB19 by real-time PCR, the forward and reverse primers were Parvo1860 (TGA AAA CTG GGC AAT AAA CTA CAC) and Parvo 1947 (CTG ATA CTG GTG TCT GT), respectively; the FAM-labeled TaqMan probe was Parvo 1917 (TGC CCT CCA CCC AGA CCT CCA AAC CA). Each 50 μl PVB19 PCR reaction contained 5 μl of ABI GeneAmp10x PCR buffer II, 4 μl glycerol, 10 μl MgCl2(25 mM), 2.2 μl forward and reverse primer mix (16.6 μM each oligonucleotide), 0.125 μl 5-carboxy-rhodamine-X, 1 μl deoxy nucleotide triphosphate mix (10 mM each nucleotide), 1 μl probe (10 μM), 0.5 μl AmpliTaq DNA polymerase, 0.25 μl TaqStart antibody, 0.05 μl uracil-DNA glycosylase, and 20 μl of extracted DNA. The thermocycling conditions were: 50°C for 2 min, 95°C for 2 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. A known amount of control molecule derived from jelly-fish DNA (EXO) and the specific PCR primers and VIC-labeled TaqMan probe were added to all reactions to monitor inhibition of PCR amplification. For the DNA viruses, the viral titers were normalized to per million myocytes, whereas Enterovirus was reported qualitatively as positive versus negative only.
Positive and negative controls for each viral detection assay
For this study, each DNA extraction run included one positive control consisting of a mixture of HHV-6 and PVB19, and each RNA extraction run had one enterovirus positive sample to serve as the positive control. Standards and two no template negative controls were included in every DNA PCR run. The EXO internal control was spiked into PCR mixtures to monitor for potential PCR inhibitors in the DNA. Negative results were accepted only if the EXO was amplified. The positivity cutoff was set as one copy per reaction. Enterovirus RT-PCR results were qualitative and accepted only if RPP-30 mRNA amplification proved that RNA was recovered from the samples, no template controls on the same run were negative and positive controls were positive. The positivity cutoff of enterovirus RT-PCR was ten copies per reaction.
Immunofluorescent assay
The IFA analysis was performed using specific antibodies against PVB19 and HHV-6 in 258 consecutive EMB samples from cardiac allografts of 44 transplant recipients with appropriate positive and negative controls. The protocol for this is previously described.[18] Notably, the IFA detected late viral antigens indicative of active replication at the time of biopsy. The IFA positive PVB19 and HHV-6 were confirmed using RNA in situ hybridization in the myocardial samples. There was no positive PVB19, HHV-6 in the blood sample of any of the patients before transplant. The IFA used for myocardial biopsy samples because for technical reasons. As the study spanned over 6 years, as per the virology team at WU Seattle, the sensitivity of the detection of the viral genome from FFPE tissue decreases after 5 years. The IFA using antibodies against late viral antigens indicate active viral infection in the EMB samples, which cannot be concluded from PCR assays. Although PCR is the most sensitive and it would have been ideal to perform only PCR, we acknowledged this in our limitation section.
Statistical analysis
Summary statistics are presented as mean ± standard deviation (SD) with medians, or described as numbers and percentages, as appropriate. Baseline characteristics between patients with and without virus in the myocardium were compared by an independent t-test (two-sided P values used) or Mann-Whitney U test for continuous variables, and the Chi-square test or Fisher exact test for categorical variables. Time from the transplant to adverse outcomes (CAV, rejections, and graft failure) was analyzed using the Kaplan–Meier (K-M) method and the over-all differences were evaluated by the log-rank test. A P < 0.05 was considered statistically significant.
RESULTS
Patients
A total of 48 pediatric DCM patients were included in the study, age range 0.1–17.6 (mean, 6.3 ± 6.2) years. Of these, 25 were male (52%), and 44 (91.6%) patients underwent successful HT. The characteristics of all 44 patients who underwent HT were divided into two groups based on the PCR positive viral status at the time of HT and described in Table 1. There were no significant differences in demographic or clinical characteristics, including induction immunosuppressive regimens between the two groups at the time of transplant. Patients were followed for 1–8 years after HT, and the average follow-up period was 4.5 ± 1.7 years. Our maintenance immunosuppression regimen includes tacrolimus and mycophenolate mofetil for all patients. Patients received appropriate cytomegalovirus prophylaxis as per institutional guidelines.[14] The 1-, 3-, and 5-year survival rates of the entire cohort were 100%, 98%, and 75%, respectively [Figure 1].
Table 1.
Characteristics of 44 dilated cardiomyopathy patients who underwent transplantation stratified by polymerase chain reaction viral status of explanted hearts
| Variables | With viral (n=14) | Without viral (n=30) | P |
|---|---|---|---|
| Age at HT, yrs | |||
| Range | 0.9-15.5 | 0.1-17.6 | 0.106 |
| Median | 10.4 | 2 | |
| Mean±SD | 9.0±6.3 | 5.6±6.2 | |
| Male, n (%) | 8 (57%) | 16 (53%) | 0.810 |
| Race, n (%) | |||
| Hispanic | 6 (43%) | 10 (33%) | 0.542 |
| African American | 4 (29%) | 8 (27%) | 0.879 |
| White | 4 (29%) | 12 (40%) | 0.465 |
| Ventilator use, n (%) | 2 (14%) | 7 (23%) | 0.490 |
| MCS, n (%) | 8 (57%) | 12 (40%) | 0.289 |
| PRA >10%, n (%) | 4 (29%) | 10 (33%) | 0.749 |
| CMV mismatch, n (%) | 3 (21%) | 12 (40%) | 0.226 |
| Induction therapy, n (%) | |||
| Basiliximab | 9 (64%) | 23 (77%) | 0.389 |
| ATG | 4 (29%) | 7 (23%) | 0.711 |
| DSA, n (%) | 6 (43%) | 11 (37%) | 0.697 |
| Adverse outcomes, n (%) | |||
| CAV | 9 (64%) | 13 (43%) | 0.197 |
| Rejection | 3 (21%) | 4 (13%) | 0.497 |
| Graft loss | 2 (14%) | 4 (13%) | 0.928 |
(HT=heart transplant; MCS=mechanical circulatory support, PRA=panel reactive antibody, CMV- cytomegalovirus; CAV=cardiac allograft vasculopathy, ATG=anti-thymocyte globulin)
Figure 1.
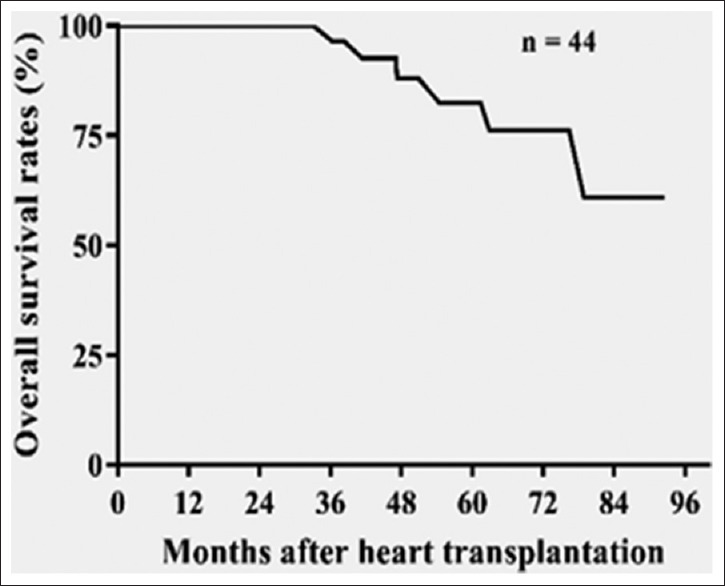
Kaplan–Meir survival after heart transplantation in pediatric dilated cardiomyopathy cohort
Results of viral testing
Among the viruses studied, PVB19 was positive in 8/48 (16.7%) of DCM patients before HT or death (viral load, range from 1.76E4 to 1.28E6 copies/10E6 cells), HHV-6 in 4/48 (8.3%) (viral load, range from 2.16E2 to 7.98E5 copies/10E6 cells), enterovirus in 1/37 (RPP-30 mRNA did not amplify in 11 cases, which could not be evaluated), and both HHV-6 and PVB19 in 1/48 (2.1%) (viral load, 1.86E3 and 1.28E6 copies/10 E6 cells, respectively). All of the HHV-6 cases detected by PCR in this study were due to HHV-6B type. Adenoviruses were not detected in any sample. Four patients died before HT and had negative PCR for any virus in their autopsy heart specimens. Among the 10 control subjects, one case (1/8 (12.5%), RPP-30 mRNA did not amplify in 2 cases) was positive for enterovirus only. Thus, using PCR, we found PVB19 DNA in the myocardium of 8 DCM patients and HHV-6 DNA in 4 patients, while neither virus was detected in the myocardium of the control group.
Parvovirus B19 and human herpesvirus type 6 before and after heart transplantation
Of the 48 DCM patients, 44 received HT and viral positivity was observed in the explanted myocardial tissue of 14 (31.8%) of them. PVB19 was found in 8 of the 44 patients and HHV-6 was detected in 5. We subsequently tested 258 serial biopsies from the cardiac allografts of 44 HT recipients within the 1st year of transplantation for the presence of PVB19 [Figure 2] and HHV-6 [Figure 3] by IFA. The cardiac allograft tissue was positive for PVB19 antigen in 7/44 cases and HHV-6 antigen in 3/44. All HHV-6 detected by IFA were also HHV-6B type. Among these, ten patients in whom active viral infection was observed after HT, 3 patients with PVB19-positive allografts had previously had PVB19-positive explanted myocardial tissue, and 1 patient with an HHV-6-positive allograft had also had HHV-6-positive explanted myocardial tissue.
Figure 2.
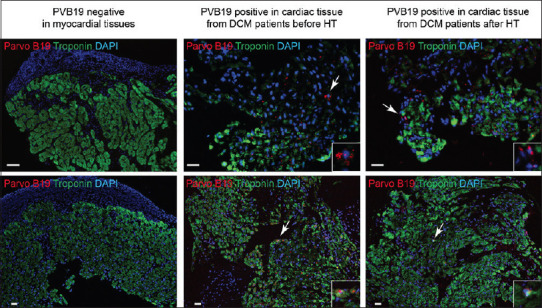
Representative images showing immunofluorescence analysis of PVB19 in PVB19 negative (control group), and positive PVB19 in cardiac tissue from dilated cardiomyopathy patient and myocardial biopsy samples from cardiac allograft after heart transplantation in the same patient. Troponin (green) staining was used as a control for cardiac tissue-specific staining. PVB19 positive areas (red) are indicated with white arrowhead. Expanded images of PVB19 positive staining are shown within white boxes wherever necessary. The scale bar represents 10 μm
Figure 3.
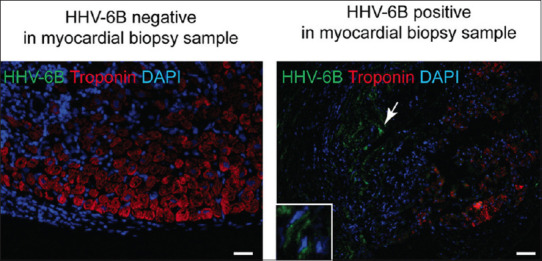
Representative images showing Immunofluorescence analysis of HHV-6B specific staining in cardiac allograft tissues using HHV-6B OHV-3 antibody. Troponin (red) staining was used as a control for cardiac tissue specific staining. HHV-6B positive areas (green) are indicated with white arrowhead. Expanded image of HHV-6B positive staining is shown within a white box. The scale bar represents 10 μm
Correlation between presence of parvovirus B19 and posttransplant outcomes
The K-M survival comparison between the PCR virus positive and negative groups starting at the time of HT showed no statistically significant difference for graft loss [log rank, P = 0.39, Figure 4]. However, when survival analysis was compared between patients who were PCR positive for PVB19 versus HHV-6, those with PVB19 had a trend toward a higher graft loss [log rank, P = 0.05, Figure 5]. After they received HT, we correlated the posttransplant adverse events: acute rejections, CAV, and graft loss with the viral status in their serial EMB samples. There were eight episodes of acute rejections in 7 PVB19 positive allografts compared to 38 episodes of acute rejections in 37 PVB19 negative allografts (Fisher's exact test, P = 0.45). Three of the 7 patients with PVB19-positive grafts (42.8%) had CAV and graft loss compared to 5 of the 37 patients with PVB19 negative allografts (13.5%) (relative risk = 3.17, odds ratio = 4.8). The 3 patients who had CAV and graft loss were the same and the numbers were too small to determine a direct relationship between the PBV19 and adverse outcomes.
Figure 4.
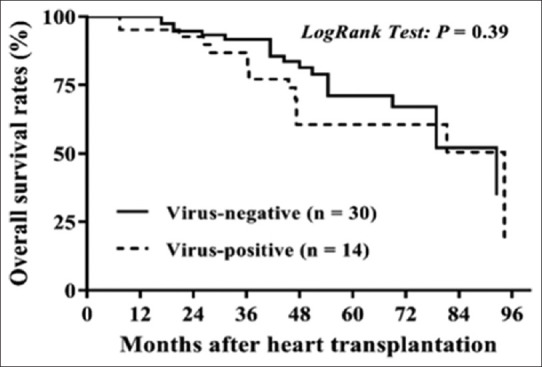
Kaplan–Meier analysis of overall graft survival rates after heart transplantation in pediatric dilated cardiomyopathy patients with virus-positive compared to virus-negative in the explanted heart
Figure 5.
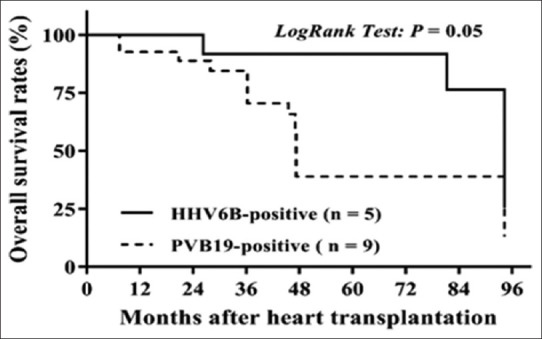
Kaplan–Meier analysis of overall graft survival rates after heart transplant in pediatric dilated cardiomyopathy patients with PVB19-positive compared to HHV-6-positive in explanted heart
DISCUSSION
We found an association between pediatric DCM and PVB19, and HHV-6, which were detected at higher frequency in patients than in the control group, suggesting that these viruses are causative or contributory to myocardial failure. Although enteroviruses have classically been identified as the prime viral agents in this condition,[6] new techniques to extract viral genomes from the myocardium in children has revealed that PVB19 and HHV-6 are an increasingly common cause of inflammatory cardiomyopathies.[2,3,4,5,19,20] The differential diagnosis between infectious and noninfectious DCM is of great clinical relevance not only because there is an impact of specific viruses on clinical outcomes in children who present with acute heart failure[10,21,22] but also because there is an association with poor survival outcomes after HT.[8,9,10] Despite the temporal relationship, there are still controversies on the functional importance of the persistence of viruses in DCM, partly due to the lack of standardization and reliable molecular assays for viral detection in the myocardium.[23] In inflammatory cardiomyopathy, as defined by AHA,[24] viruses can still be present despite normal-appearing EMB. Our findings on the presence of the virus in the absence of acute myocarditis is striking as it demonstrates the need for sensitive molecular tools such as PCR and RT-PCR capable of rapid and accurate viral detection and quantification in DCM patients hospitalized for unexplained heart failure.
Enterovirus was detected in only 1 patient and no adenoviruses were detected in the myocardium of explanted heart tissues in this study. Our results are similar to a previous study on children with inflammatory cardiomyopathy, in which 19 of 63 (30%) children were PCR positive for specific viruses and PVB19 was the most common virus.[21] One explanation for the predominance of PVB19 and HHV-6 in this study is similar to previous reports of the epidemiological shift in viral infection.[1,6] However, the absence of adenoviruses stands in contrast to other reports, in which they have been detected more often than other viruses.[25] This difference may be explained in part by variation in methods used in prior studies and also concur with recent studies suggesting an epidemiologic shift. An earlier study detected adenovirus in 12% (18/149) of DCM samples using qualitative PCR.[25] Another report described finding double the amount of adenovirus in biopsies from the right ventricular wall compared to those taken from the left ventricles.[26]
A high prevalence of HHV-6 (43.7%) in archival tissue of explanted hearts from pediatric patients with DCM has been reported previously.[19] The identification of PVB19 or adenovirus in the myocardium of pediatric transplant recipients was found to be predictive of adverse clinical events, including CAV, acute rejection, and graft loss.[1,5,8,9] However, latent viruses may also be found in the hearts of patients without any evidence of myocarditis or DCM, and a high degree of variability regarding the prevalence and viral load has been reported among different studies.[27,28]
Large studies determining the prognostic value of PVB19 viral load, co-infections, and virus activity are lacking. Nevertheless, EMB results can help provide a deeper understanding of the pathogenetic importance of PVB19 in the development of coronary vasculopathy. Graham et al. studied animal models and determined that natural killer (NK) cells play a direct role in the development of coronary vasculopathy and that an NK-cell-dependent pathway is triggered in the absence of T and B lymphocytes.[29] The PVB19 NS-1 upregulates the signal transducer and activator of transcription (STAT) pathway and may contribute to downregulation of T and B lymphocytes, allowing the virus to evade the immune response and establish persistent infection in human endothelial cells.[30] Persistent of the infection in the host, which could result in re-infection of the cardiac allograft tissue in the setting of immunosuppression. PVB19 in endothelial cells leads to the stimulation of inflammatory cytokines and could be accompanied by a disruption of endothelial cell barrier function, which could ultimately can lead to CAV.
We have shown that among DCM patients who had PVB19 positivity in their explanted hearts, there was a trend toward higher graft loss compared to HHV-6. The persistence of PVB19 infection in the cardiac allograft may be favored by the immunosuppression regimen the patients receive after transplant. Furthermore, we found that patients who have positive PVB19 in their cardiac allografts have a higher incidence of CAV and graft loss compared to those with PVB19-negative cardiac allografts. Our results are similar to the previous study of PVB19, causing coronary vasculopathy in pediatric transplant recipients.[1,9] In addition, 3 of our DCM patients who had PVB19 detected in the myocardium of their cardiac allografts were also positive for PVB19 by PCR in their myocardium pretransplant, which suggests the possibility of the persistence of previous PVB19 infection in the host, resulting in re-infection of the cardiac allograft in the setting of immunosuppression. The persistence of PVB19 supports the theory of chronic stimulation of inflammatory cytokines and damage to the endothelial barrier, which can predispose to CAV.
Limitations
The sample size for the present study is small, and it is a retrospective single-center study. However, the patients followed a uniform protocol for the evaluation and management of post-HT care and received the same immunosuppression regimen throughout. The control sample size is also small and includes mostly accidental death cases. Unfortunately, the invasive nature of EMB presents a challenge to compare DCM cases against healthy controls. The myocardial biopsy samples were obtained within the 1st year after HT; therefore, viral myocardial infection after the 1st year of transplant could not be ruled out. The posttransplant surveillance EMB samples were tested using IFA but not PCR, and both modalities differ in the sensitivity for the detection of viruses. However, the IFA using antibodies against late viral antigens indicates active viral infection in the EMB samples, which cannot be concluded from PCR assays. Furthermore, we hypothesized that the persistence of PVB19 supports the theory of chronic stimulation of inflammatory cytokines and damage to the endothelial barrier, which can predispose to CAV, we have not analyzed other risk factors for predisposition to CAV in this study.
CONCLUSIONS
In our study, there is an association between PVB19, and HHV-6 and pediatric DCM, as a higher frequency of these viruses are found in patients compared to the control group. Our study evaluated the effect of persistence viral genome in the allograft, and there is a trend toward higher graft loss if the myocardium was positive for PVB19 compared to HHV-6 in pediatric DCM patients, which needs to be studied further in large samples with multi-institutional studies. The viruses investigated in the explanted hearts showed that there was only one case of enterovirus and none associated with Adenovirus, which would suggest a shift in epidemiology. PVB19 may contribute to the downregulation of T and B lymphocytes, leading to persistent viral infection and subsequent NK-mediated CAV, which is a potential topic of investigation in future mechanistic studies.
Financial support and sponsorship
This project was funded by an HHV-6 Foundation Grant (Santa Barbara, CA, USA).
Conflicts of interest
There are no conflict of interest.
Acknowledgment
We are thankful to Ms. Angela Galipeau, Pathology Supervisor at Children's Medical Center Dallas, Texas, for her help in obtaining all myocardial tissue samples.
REFERENCES
- 1.Breinholt JP, Moulik M, Dreyer WJ, Denfield SW, Kim JJ, Jefferies JL, et al. Viral epidemiologic shift in inflammatory heart disease: The increasing involvement of parvovirus B19 in the myocardium of pediatric cardiac transplant patients. J Heart Lung Transplant. 2010;29:739–46. doi: 10.1016/j.healun.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen Y, Renois F, Leveque N, Giusti D, Picard-Maureau M, Bruneval P, et al. Virus detection and semiquantitation in explanted heart tissues of idiopathic dilated cardiomyopathy adult patients by use of PCR coupled with mass spectrometry analysis. J Clin Microbiol. 2013;51:2288–94. doi: 10.1128/JCM.00820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 4.Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N Engl J Med. 2010;362:1248–9. doi: 10.1056/NEJMc0911362. [DOI] [PubMed] [Google Scholar]
- 5.Schowengerdt KO, Ni J, Denfield SW, Gajarski RJ, Bowles NE, Rosenthal G, et al. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: Diagnosis using the polymerase chain reaction. Circulation. 1997;96:3549–54. doi: 10.1161/01.cir.96.10.3549. [DOI] [PubMed] [Google Scholar]
- 6.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 7.Pietra BA, Kantor PF, Bartlett HL, Chin C, Canter CE, Larsen RL, et al. Early predictors of survival to and after heart transplantation in children with dilated cardiomyopathy. Circulation. 2012;126:1079–86. doi: 10.1161/CIRCULATIONAHA.110.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirali GS, Ni J, Chinnock RE, Johnston JK, Rosenthal GL, Bowles NE, et al. Association of viral genome with graft loss in children after cardiac transplantation. N Engl J Med. 2001;344:1498–503. doi: 10.1056/NEJM200105173442002. [DOI] [PubMed] [Google Scholar]
- 9.Moulik M, Breinholt JP, Dreyer WJ, Kearney DL, Price JF, Clunie SK, et al. Viral endomyocardial infection is an independent predictor and potentially treatable risk factor for graft loss and coronary vasculopathy in pediatric cardiac transplant recipients. J Am Coll Cardiol. 2010;56:582–92. doi: 10.1016/j.jacc.2010.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese F, Rigo E, Milanesi O, Boffa GM, Angelini A, Valente M, Thiene G. Molecular diagnosis of myocarditis and dilated cardiomyopathy in children: Clinicopathological features and prognostic implications. Diagnostic Molecul Pathol. 2002;11:212–21. doi: 10.1097/00019606-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Aretz HT. Myocarditis: The Dallas criteria. Hum Pathol. 1987;18:619–24. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International society for heart and lung transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Das BB, Lacelle C, Zhang S, Gao A, Fixler D. Complement (C1q) binding de novo donor-specific antibodies and cardiac-allograft vasculopathy in pediatric heart transplant recipients. Transplantation. 2018;102:502–9. doi: 10.1097/TP.0000000000001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renaud C, Kuypers J, Ficken E, Cent A, Corey L, Englund JA. Introduction of a novel parechovirus RT-PCR clinical test in a regional medical center. J Clin Virol. 2011;51:50–3. doi: 10.1016/j.jcv.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Huang ML, Nguy L, Ferrenberg J, Boeckh M, Cent A, Corey L. Development of multiplexed real-time quantitative polymerase chain reaction assay for detecting human adenoviruses. Diagn Microbiol Infect Dis. 2008;62:263–71. doi: 10.1016/j.diagmicrobio.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerr DM, Yeung LC, Obrigewitch RM, Huang ML, Frenkel LM, Corey L. Case report: Primary human herpesvirus-6 associated with an afebrile seizure in a 3-week-old infant. J Med Virol. 2002;66:384–7. doi: 10.1002/jmv.2156. [DOI] [PubMed] [Google Scholar]
- 18.Das BB, Rakheja D, Lacelle C, Sedlak RH, Gulve N, Chowdhury SR, et al. Possible progesterone-induced gestational activation of chromosomally integrated human herpesvirus 6B and transplacental transmission of activated human herpesvirus 6B. J Heart Lung Transplant. 2016;35:1373–6. doi: 10.1016/j.healun.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Comar M, D’Agaro P, Campello C, Poli A, Breinholt JP, 3rd, Towbin JA, et al. Human herpes virus 6 in archival cardiac tissues from children with idiopathic dilated cardiomyopathy or congenital heart disease. J Clin Pathol. 2009;62:80–3. doi: 10.1136/jcp.2008.059568. [DOI] [PubMed] [Google Scholar]
- 20.Molina KM, Garcia X, Denfield SW, Fan Y, Morrow WR, Towbin JA, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390–7. doi: 10.1007/s00246-012-0468-4. [DOI] [PubMed] [Google Scholar]
- 21.Gagliardi MG, Fierabracci A, Pilati M, Chinali M, Bassano C, Saura F, et al. The impact of specific viruses on clinical outcome in children presenting with acute heart failure. Int J Mol Sci. 2016;17:486. doi: 10.3390/ijms17040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schranz D, Michel-Behnke I. Myocardial biopsies differentiate between myocyte- and endothelial-targeted myocarditis. Cardiol Young. 2012;22:485. doi: 10.1017/S1047951112000674. [DOI] [PubMed] [Google Scholar]
- 23.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–38. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 25.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–72. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 26.Tátrai E, Hartyánszky I, Jr, Lászik A, Acsády G, Sótonyi P, Hubay M. The role of viral infections in the development of dilated cardiomyopathy. Pathol Oncol Res. 2011;17:229–35. doi: 10.1007/s12253-010-9302-6. [DOI] [PubMed] [Google Scholar]
- 27.Kuethe F, Lindner J, Matschke K, Wenzel JJ, Norja P, Ploetze K, et al. Prevalence of parvovirus B19 and human bocavirus DNA in the heart of patients with no evidence of dilated cardiomyopathy or myocarditis. Clin Infect Dis. 2009;49:1660–6. doi: 10.1086/648074. [DOI] [PubMed] [Google Scholar]
- 28.Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca HP, et al. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: Review of the literature. Eur J Heart Fail. 2016;18:1430–41. doi: 10.1002/ejhf.665. [DOI] [PubMed] [Google Scholar]
- 29.Graham JA, Wilinson RA, Hirohashi T, Chase CM, Colvin RB, Madsen JC, et al. Viral infection induce de novo lesions of coronary allograft vasculopathy through a natural killer cell-dependent pathway. Am J Transplant. 2009;9:2479–84. doi: 10.1111/j.1600-6143.2009.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duechting A, Tschope C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, et al. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PAS3 in human endothelial cells. J Virol. 2008;82:7942–52. doi: 10.1128/JVI.00891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


