Abstract
Increased sympathoexcitation and renal sodium retention during high salt intake are hallmarks of the salt sensitivity of blood pressure (BP). The mechanism(s) by which excessive sympathetic nervous system release of norepinephrine (NE) influences renal sodium reabsorption is unclear. However, studies demonstrate that NE can stimulate the activity of the sodium chloride cotransporter (NCC) and promote the development of salt-sensitive hypertension (SSH). The adrenergic signaling pathways governing NCC activity remain a significant source of controversy with opposing studies suggesting a central role of upstream α1- and/or β-adrenoceptors in the canonical regulatory pathway involving with-no-lysine kinases (WNKs), STE20/SPS1-related proline alanine-rich kinase (SPAK), and oxidative stress response 1 (OxSR1). In our previous study, α1-adrenoceptor antagonism in NE-infused male Sprague-Dawley rats prevented the development of NE-evoked SSH in part by suppressing NCC activity and expression. In these studies, we used selective adrenoceptor antagonism in male Dahl Salt-Sensitive (DSS) rats to test the hypothesis that NE-mediated activation of the NCC in Dahl SSH occurs via an α1-adrenoceptor dependent pathway. A high salt diet evoked significant increases in NCC activity, expression, and phosphorylation in DSS rats that developed SSH. Increases were associated with a dysfunctional WNK1/4 dynamic and a failure to suppress SPAK/OxSR1 activity. α1-adrenoceptor antagonism initiated prior to high salt intake or following the establishment of SSH attenuated BP in part by suppressing NCC activity, expression, and phosphorylation. Collectively our findings support the existence of a NE-activated α1-adrenoceptor gated pathway that relies on WNK/SPAK/OxSR1 signaling to regulate NCC activity in SSH.
Keywords: adrenoceptors, blood pressure, norepinephrine, salt-sensitive hypertension, sodium chloride cotransporter, Dahl Salt-Sensitive
Graphical Abstract
INTRODUCTION
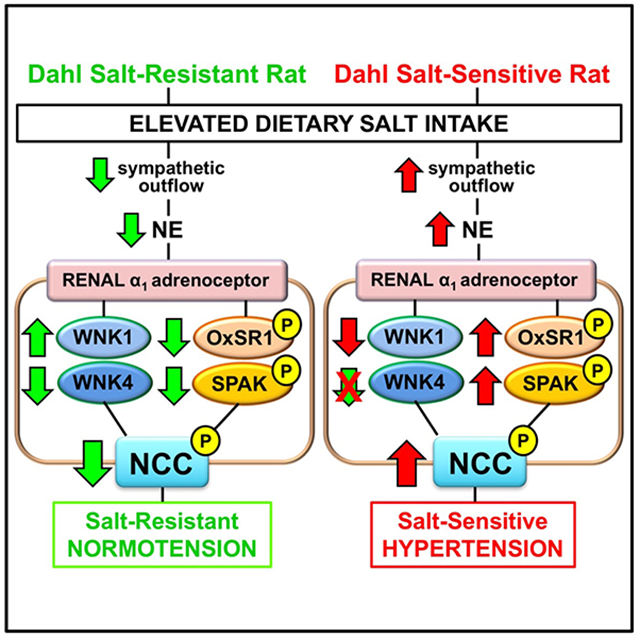
Dietary sodium intake has long been tied to the development of hypertension as increased sodium retention drives increases in blood pressure (BP).1–3 Roughly half of the hypertensive population and a quarter of the normotensive population demonstrate the salt sensitivity of BP.4, 5 The risk for developing hypertension is significantly increased in individuals with the salt sensitivity of BP, and given that U.S. adults consume approximately 3,400 mg of sodium per day, which is in excess of the daily recommended 1,500 mg of sodium per day by the American Heart Association.6, 7 salt-sensitive hypertension (SSH) represents a major public health risk. The Dahl Salt-Sensitive (DSS) rat has served as a model of SSH since its discovery in the 1960s.8 When fed a high salt diet, DSS rats exhibit a hallmark increase in BP that has been the subject of numerous investigations. Despite the plethora of existing studies, the mechanisms underlying Dahl SSH remain unclear. There is strong evidence for a role of the sympathetic nervous system (SNS) in SSH as increased SNS activity can contribute to renal sodium reabsorption and promote hypertension.9 Recent studies have reported that SNS-mediated release of norepinephrine (NE) elevates the activity of the sodium chloride cotransporter (NCC), resulting in increased sodium reabsorption at the level of the distal convoluted tubule (DCT).10, 11
The NCC is regulated by a complex network of kinases that include with-no-lysine kinases (WNK) 1 and WNK4, STE20/SPS1-related proline alanine rich kinase (SPAK), and oxidative stress response 1 (OxSR1).12–14 The regulatory pathway governing NCC activity is a source of continued controversy as opposing studies suggest that either WNK1 or WNK4 is the dominant regulatory kinase of the NCC.15–18 Moreover, there is also debate over which downstream kinase, SPAK or OxSR1, is responsible for phosphorylating and activating the NCC.14 Adding to the ambiguity, investigations into the adrenergic signaling pathways by which SNS release of NE exerts its effects on the NCC have resulted in multiple conflicting studies suggesting the existence of an α-adrenoceptor dependent,19 β-adrenoceptor dependent,16 or synergistic pathway that regulates NCC activity.20 In our previous study, NE-infused male Sprague-Dawley (SD) rats failed to downregulate NCC activity, expression, and phosphorylation and developed NE-evoked SSH.19 In this study, chronic administration of a low dose of the α1-antagonist terazosin, that did not lower BP compared to that observed in control animals, prevented the salt-sensitive component of NE-evoked hypertension and restored dietary sodium evoked suppression of NCC activity, expression, and phosphorylation.
We hypothesized that NE-mediated activation of the NCC in Dahl SSH occurs via an α1-adrenoceptor dependent pathway that is reliant upon WNK/SPAK/OxSR1 signaling. To test this hypothesis, we administered a low dose of either the α1-adrenoceptor antagonist terazosin, or a the β-adrenoceptor antagonist propranolol, in DSS rats and assessed their impact on BP, NCC activity, and NCC regulation in the development and maintenance of Dahl SSH. Collectively, this study offers new mechanistic insight into the SNS-activated adrenergic signal transduction pathways that regulate NCC activity, expression, and phosphorylation in Dahl SSH.
METHODS
A detailed description of experimental procedures is available in the online-only supplement. The authors declare that all supporting data are available within the article and its online supplementary files.
Animals
Groups of male Dahl Salt-Resistant (DSR) or DSS rats (9-12 weeks of age, Envigo, Indianapolis, IN, USA) were randomly assigned to a 21-day or 42-day normal salt (NS, 0.6% NaCl, Envigo Teklad, Teklad Global Diet #2918, 18% protein, 5% crude fat, 5% fiber, total potassium (K+) content 0.6%, total NaCl content 0.6% [174 mEq Na+/kg]) or high salt (HS, 4% NaCl, Envigo Teklad Diets, TD.03095, 19% protein, 5% crude fat, 3% fiber, total K+ content 0.8%, total NaCl content 4% [678 mEq Na+/kg]) diet and tap water ad libitum. All animals were randomly assigned to experimental treatment groups. All animal protocols were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee and performed using National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals.”
Statistical Analysis
Data are shown as mean ± SD. Comparisons were made between NS and HS dietary salt intake groups or HS and HS + adrenoceptor antagonist groups using a two-tailed Student’s t-test. One-way ANOVA was used to assess differences between groups, and the Tukey’s post-hoc test was used to evaluate variation among groups. Statistical analysis was carried out using GraphPad Prism version 7 (GraphPad). Statistical significance is defined as P <0.05.
RESULTS
Impact of high dietary sodium intake on blood pressure and NCC activity in male Dahl rats
When challenged with a high salt diet DSR rats increase the fractional excretion of sodium (FENa) and maintain normotension. Additionally, estimated blood volume (EBV), estimated plasma volume (EPV), and the vascular response to ganglionic blockade remain unchanged (Figure 1, Supplemental Table S1). Further, DSR rats exhibit dietary sodium evoked suppression of in vivo estimated NCC activity (expressed as the peak natriuretic response to hydrochlorothiazide (HCTZ)), estimated epithelial sodium channel (ENaC) activity (expressed as the peak natriuretic response to amiloride), plasma NE and renal NE content (Figure 1A). DSR rats did not exhibit HS-evoked alterations in baseline sodium or potassium excretion during the renal transporter assay (Supplemental Table S2). Reduced NCC activity in DSR rats fed a HS diet was paralleled by dietary-evoked suppression of both total NCC expression and NCC Thr53 phosphorylation (i.e., no change in the ratio of phosphorylated to total NCC) normalized to Coomassie blue (Figure 1B, Supplemental Figure S1). In contrast, in DSS rats, HS intake decreased FENa and increased BP, EPV, and the depressor response to ganglionic blockade (Figure 1). Critically, DSS rats fed a HS diet exhibit significant increases in in vivo NCC activity, total NCC expression and Thr53 phosphorylation (pNCC) compared to rats fed a NS diet (Figure 1, Supplemental Table S1). This increase in NCC activity was matched by a significant increase in plasma and renal NE content in DSS rats fed a HS diet (Figure 1A). As seen in DSR rats dietary sodium evoked suppression of ENaC activity was observed in DSS rats and no changes in baseline sodium and potassium excretion during the acute renal transporter assay were observed (Figure 1A, Supplemental Table S2).
Figure 1. Impact of high dietary sodium intake on blood pressure and NCC activity in Dahl rats.
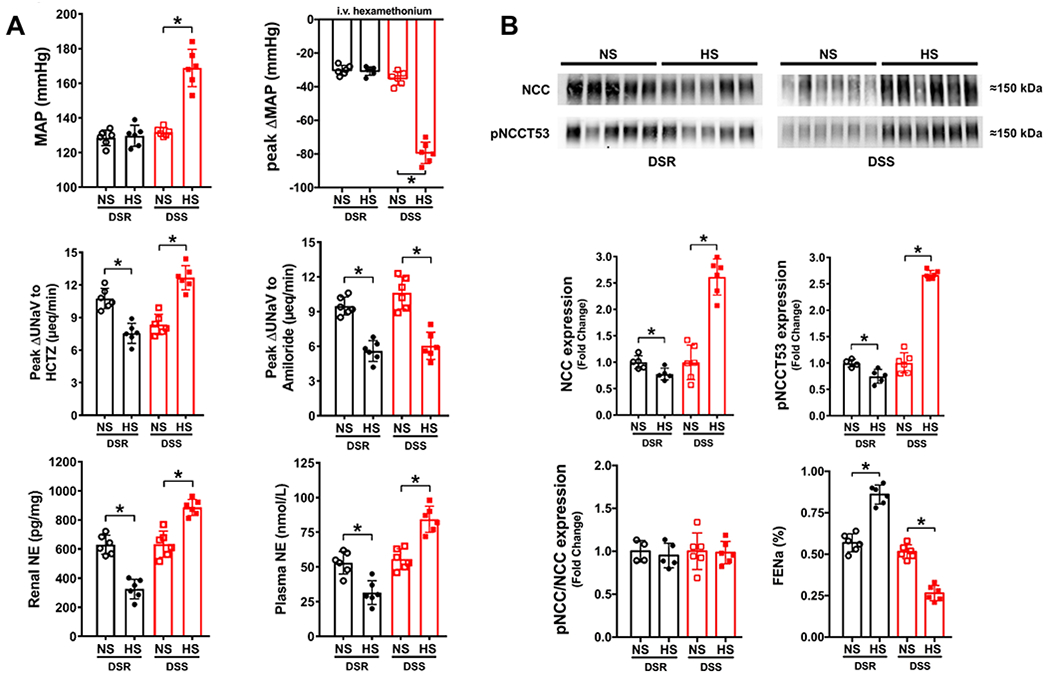
(A) Mean arterial pressure (MAP; mmHg), peak ΔMAP (mmHg) in response to i.v. hexamethonium (30 mg/kg), in-vivo NCC activity expressed as peak natriuretic response (ΔUNaV) to intravenous hydrochlorothiazide (HCTZ; 2 mg/kg bolus, 2 mg/kg hour infusion), in-vivo ENaC activity expressed as peak ΔUNaV to intravenous amiloride (2 mg/kg bolus, 2 mg/kg hour infusion), renal NE (pg/mg) and plasma norepinephrine (NE, nmol/L); (B) representative immunoblots for NCC, pNCCT53 and total NCC expression, pNCCT53 expression, pNCC/NCC ratio, and FENa (%) in 3-month old male Dahl Salt-Resistant (DSR) and Dahl Salt-Sensitive (DSS) rats fed a 21-day normal salt (NS; 0.6% NaCl) or high salt (HS; 4% NaCl) diet N=5/6 per group mean ± SD. Protein expression is shown as fold change with NS target protein expression set to 1 for each group. MAP = mean arterial pressure, UNaV = urinary sodium excretion, NE = norepinephrine, FENa = Fractional Excretion of Sodium. Differences in peak natriuretic responses, MAP, plasma and renal NE, and protein expression within each group between NS and HS diets were determined using a student’s t test. *P < 0.05 vs. respective NS group.
Impact of high dietary sodium intake on NCC regulation in male Dahl rats
DSR rats fed a HS diet show increased expression of the upstream regulatory kinase WNK1 and downregulation of WNK4, SPAK and OxSR1 compared to a NS diet normalized to Coomassie blue (Figures 2A&B, Supplemental Figure S1). Moreover, DSR rats downregulate the activity of SPAK and OxSR1, assessed as a decrease in the collective phosphorylation of these kinases at threonine sites in response to a HS diet (Figure 2C). Unlike DSR rats, DSS rats downregulated WNK1 abundance and maintained dietary sodium evoked suppression of WNK4 abundance (Figure 2A) in response to a HS diet. Further, DSS rats failed to suppress total SPAK and OxSR1 expression (Figure 2B) as seen in DSR rats. Moreover, in contrast to DSR rats, DSS rats exhibit significant increases in pSPAK(T222)/pOxSR1(T185) abundance in response to a HS diet (Figure 2C).
Figure 2. Impact of high dietary sodium intake on NCC regulation in Dahl rats.
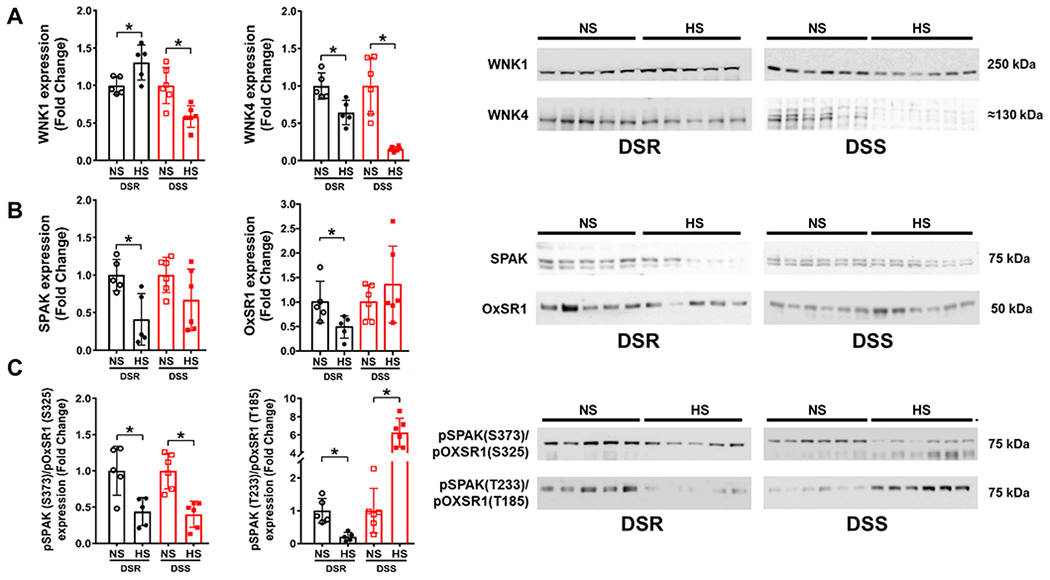
(A) total WNK1 and WNK4 expression and respective immunoblots, (B) total SPAK and OxSR1 expression, and respective immunoblots and, (C) total pSPAK (S373)/pOxSR1 (S325) expression and pSPAK (T233)/pOxSR1 (T185) expression and respective immunoblots in 3-month old male Dahl Salt-Resistant (DSR) and Dahl Salt-Sensitive (DSS) rats fed a 21-day normal salt (NS; 0.6% NaCl) or high salt (HS, 4% NaCl) diet. N=5/6 per group mean ± SD. Protein expression is shown as fold change with NS target protein expression set to 1 for each group. Differences in NS and HS target protein expression within each treatment group were determined using a student’s t test. *P < 0.05 vs. respective NS group.
Impact of chronic adrenoceptor antagonism on the development of male DSS rat hypertension
To study the involvement of adrenoceptors in the development of Dahl SSH, we performed chronic infusion of the α1-adrenoceptor antagonist terazosin or the β-adrenoceptor antagonist propranolol. During the 21-day experimental HS diet intake period α1-antagonism attenuated the development of Dahl SSH, prevented increases in EPV and FENa, and resulted in a significant decrease in NCC activity in DSS rats on a HS diet (Figure 3, Supplemental Table S1). α1-antagonism also suppressed NCC phosphorylation, without changing the total NCC abundance, resulting in a significantly decreased pNCC Thr53/total NCC ratio normalized to Coomassie blue (Figure 3B, Supplemental Figure S2). These changes occurred without impacting the enhanced depressor response to ganglionic blockade or suppression of estimated ENaC activity observed in saline control DSS rats (Figure 3A). Chronic infusion of the β-adrenoceptor antagonist propranolol in DSS rats did not alter the development of SSH, or reduce NCC activity, expression, or phosphorylation (Figure 3, Supplemental Table S1). Selective adrenoceptor blockade, confirmed pharmacologically (Supplemental Figure S1), had no impact on baseline sodium or potassium excretion during the renal transporter assay (Supplemental Table S2).
Figure 3. Impact of chronic adrenoceptor antagonism on the development of Dahl Salt-Sensitive (DSS) hypertension.
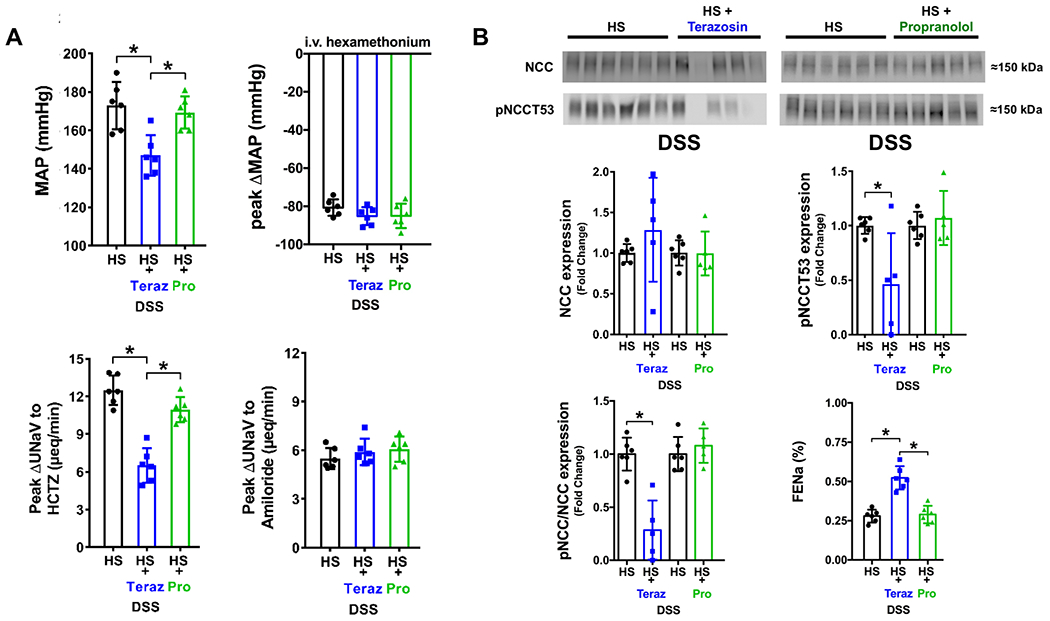
(A) Mean arterial pressure (MAP; mmHg), peak ΔMAP (mmHg) in response to i.v. hexamethonium (30 mg/kg), in vivo NCC activity expressed as peak natriuretic response (ΔUNaV) to intravenous hydrochlorothiazide (HCTZ; 2 mg/kg bolus, 2 mg/kg hour infusion), in vivo ENaC activity expressed as peak ΔUNaV to intravenous amiloride (2 mg/kg bolus, 2 mg/kg hour infusion); (B) representative immunoblots for NCC, pNCCT53 and total NCC expression, pNCCT53 expression, pNCC/NCC ratio and FENa (%) in groups of 3-month old male untreated DSS rats or groups of DSS rats that received a subcutaneous (s.c.) infusion of saline/DMSO vehicle terazosin (Teraz) or propranolol (Pro) dissolved in saline/DMSO during a 21-day HS diet. N=5/6 per group mean ± SD. Treatment group protein expression was compared to the same untreated HS group expression via individual blots to avoid comparing separate immunoblots. MAP = mean arterial pressure, UNaV = urinary sodium excretion, FENa = Fractional Excretion of Sodium. Protein expression is shown as fold change with untreated DSS HS target protein expression for each individual blot set to 1. Differences in peak natriuretic responses and MAP were determined using a one-way ANOVA to assess differences between groups, and a Tukey post-hoc test was used to evaluate variation among groups. Differences in untreated DSS HS and HS + adrenoceptor antagonist target protein expression were determined using a student’s t test. *P < 0.05 vs. respective DSS HS group.
Impact of chronic adrenoceptor antagonism on NCC regulation in male DSS rat hypertension
In contrast to vehicle treated animals DSS rats treated with the α1-adrenoceptor antagonist terazosin exhibited significant increases in WNK1 expression, with unchanged levels in the abundance of WNK4 and SPAK normalized to Coomassie blue (Figures 4A&B, Supplemental Figure S2). Critically, α1-antagonism resulted in a significant decrease in OxSR1 expression and a decrease in SPAK/OxSR1 phosphorylation (Figure 4). Although β-adrenoceptor antagonism increased WNK1 and WNK4 levels, it did not influence the expression of SPAK or OxSR1 (Figure 4A&B). Similar to the response observed during α1-antagonism, phosphorylation of SPAK/OxSR1 was decreased during β-adrenoceptor antagonism (Figure 4). It should be noted 1 data point in the DSS HS + Propranolol treated group for SPAK/OxSR1 phosphorylation was excluded from analysis as a GRUB statistical outlier.
Figure 4. Impact of chronic adrenoceptor antagonism on NCC regulation in Dahl Salt-Sensitive (DSS) hypertension.
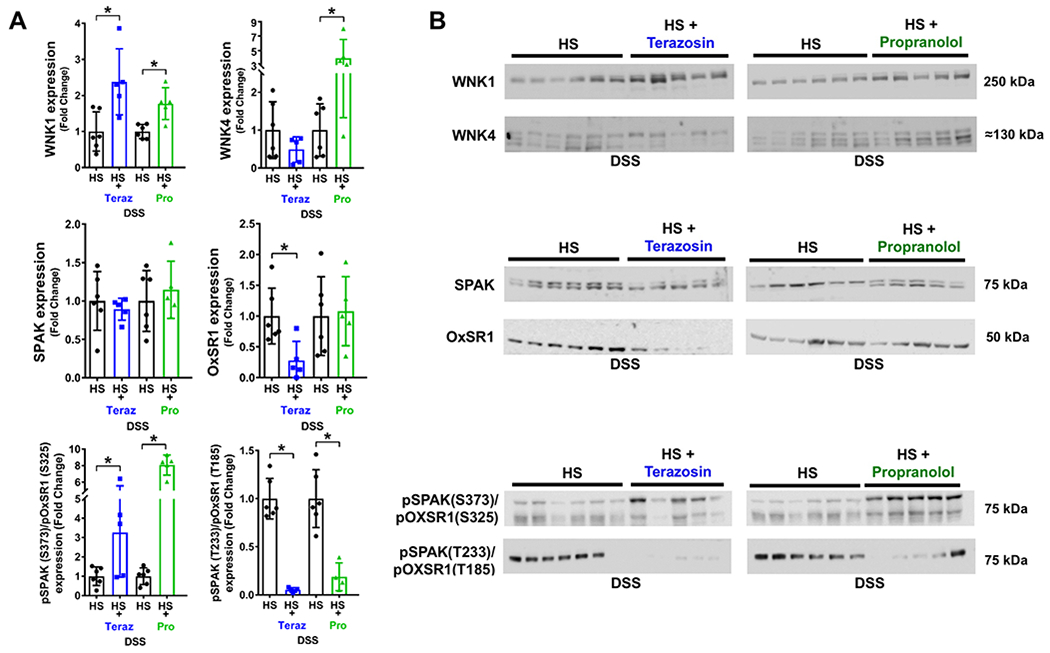
(A) total WNK1 and WNK4 expression and respective immunoblots, (B) total SPAK and OxSR1 expression and respective immunoblots and, (C) total pSPAK (S373)/pOxSR1 (S325) expression and pSPAK (T233)/pOxSR1 (T185) expression and respective immunoblots in groups of 3-month old male s.c. saline/DMSO vehicle treated DSS rats or groups of DSS rats that received a subcutaneous (s.c.) infusion of terazosin (Teraz) or propranolol (Pro) dissolved in saline/DMSO during a 21-day high salt (HS, 4% NaCl) diet. N=5/6 per group mean ± SD. Treatment group protein expression was compared to the same untreated HS group expression via individual blots to avoid comparing separate immunoblots. Protein expression is shown as fold change with untreated DSS HS target protein expression for each individual blot set to 1. Differences in untreated DSS HS and HS + adrenoceptor antagonist target protein expression were determined using a student’s t test. *P < 0.05 vs. respective DSS HS group.
Impact of chronic α1-adrenoceptor antagonism on established male DSS rat hypertension
DSS rats chronically infused with saline vehicle and fed a 42-day HS diet develop profound SSH of a greater magnitude than that observed after 21-day HS intake. Chronic α1-adrenoceptor blockade (confirmed pharmacologically, Supplemental Figure S5) during the final 21-days of a 42-day HS diet attenuated established Dahl SSH (Figure 5A). Moreover, decreases in BP with α1-adrenoceptor antagonism were coupled with reductions in NCC activity, expression, and phosphorylation. The dramatic reduction in pNCC Thr53 resulted in a significant reduction in the ratio of pNCC/total NCC normalized to Coomassie blue (Figure 5, Supplemental Figure S3). The expression of the upstream regulatory kinases WNK1 and WNK4 was also suppressed by α1-adrenoceptor antagonism. While total SPAK and OxSR1 expression remained unchanged, α1-adrenoceptor antagonism did result in the significant reduction of the phosphorylation of SPAK/OxSR1 (Figure 6). It should be noted that 1 data point in the DSS HS and terazosin group was excluded from analysis of pSPAK(T222)/pOxSR1(T185) as a GRUB statistical outlier.
Figure 5. Impact of chronic α1-adrenoceptor antagonism on established Dahl Salt-Sensitive (DSS) hypertension.
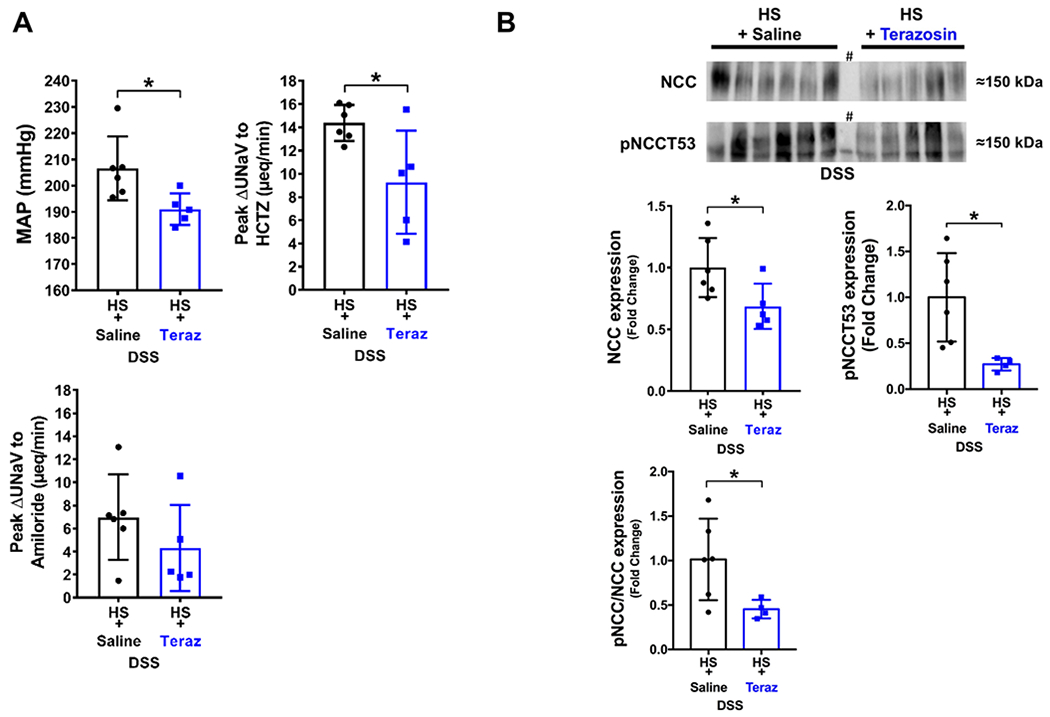
(A) Mean arterial pressure (MAP; mmHg), in vivo NCC activity expressed as peak natriuretic response (ΔUNaV) to intravenous hydrochlorothiazide (HCTZ; 2 mg/kg bolus, 2 mg/kg hour infusion), in vivo ENaC activity expressed as peak ΔUNaV to intravenous amiloride (2 mg/kg bolus, 2 mg/kg hour infusion); (B) representative immunoblots for NCC, pNCCT53 and total NCC expression, pNCCT53 expression and pNCC/total NCC ratio in groups of DSS rats fed a 42-day high salt (HS, 4% NaCl) diet that received a subcutaneous (s.c.) infusion of saline/DMSO or terazosin/DMSO during days 21-42 of the 42-day HS diet. N=5/6 per group mean ± SD. MAP = mean arterial pressure, UNaV = urinary sodium excretion. Protein expression is shown as fold change with HS + saline target protein expression set to 1. Differences between treatment groups were determined using a student’s t test, *P < 0.05 vs. saline group. # lane was not considered in quantification.
Figure 6. Impact of chronic α1-adrenoceptor antagonism on NCC regulation in established Dahl Salt-Sensitive (DSS) hypertension.
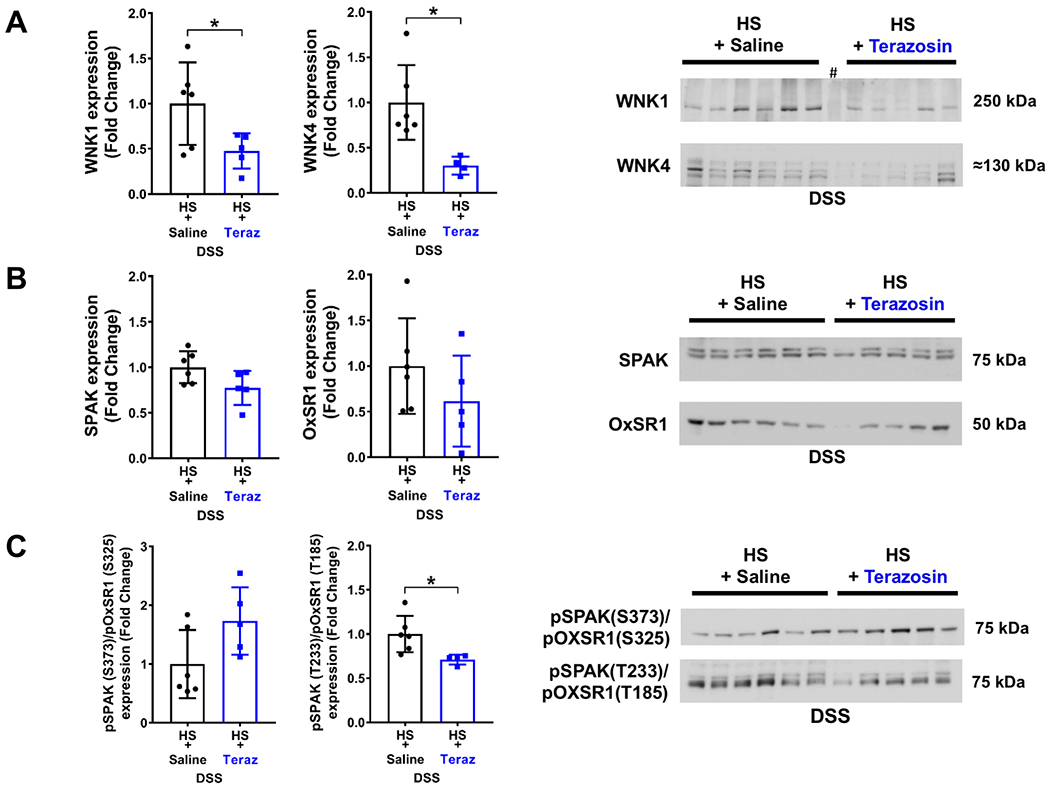
(A) total WNK1 and WNK4 expression and respective immunoblots, (B) total SPAK and OxSR1 expression, and respective immunoblots and, (C) total pSPAK (S373)/pOxSR1 (S325) expression and pSPAK (T233)/pOxSR1 (T185) expression and respective immunoblots in groups of DSS rats fed a 42-day HS diet that received a s.c. infusion of vehicle (saline/DMSO) or terazosin/DMSO during days 21-42 of the 42-day HS diet. N=5/6 per group mean ± SD. Protein expression is shown as fold change with HS + saline target protein expression set to 1. Differences between treatment groups were determined using a student’s t test, *P < 0.05 vs. saline group. # lane was not considered in quantification.
DISCUSSION
The major finding of this study is that α1-adrenoceptor antagonism attenuated the development and maintenance of SSH in male DSS rats in part by suppressing NCC activity and regulation. Critically, β-adrenoceptor antagonism failed to influence SSH, NCC activity, or regulation in male DSS rats.
Impact of dietary salt intake on BP and NCC activity in male DSR versus DSS rats
DSR rats exhibit dietary sodium evoked suppression of plasma and renal NE, estimated ENaC activity, NCC activity, expression, and phosphorylation, no changes in vascular tone, EBV or EPV, and increased FENa – all of which contribute to maintaining salt resistance and normotension. These findings are consistent with our previous studies in normotensive male SD rats that exhibit dietary sodium evoked suppression of sympathetic outflow, estimated ENaC activity, and NCC activity and expression.11, 19 Our findings are also supported by a recent study in DSR rats where a HS diet reduced renal NE turnover and blunted the diuretic and natriuretic responses to the NCC antagonist HCTZ.21 These data support our hypothesis of dietary sodium evoked suppression of sympathetically-driven NCC activation in salt-resistant rats.
In contrast, DSS rats fed a HS diet not only failed to suppress plasma and renal NE, NCC activity, expression, or phosphorylation, but exhibited significant increases in these parameters in addition to increased vascular tone, increased EPV and decreased FENa – factors which may promote the observed salt sensitivity of BP. The inability of DSS rats, which exhibit global and renal specific increases in sympathetic outflow, to downregulate NCC activity and expression is supported by our previous studies in male SD rats whereby NE-infusion prevented dietary sodium evoked suppression of NCC activity and expression and promoted the development of SSH.11, 19 Further support of our current data is provided by a recent study in DSS rats that reported no change in the diuretic and natriuretic responses to HCTZ between a NS and HS diet.21 Our finding that DSS rats maintain HS-evoked suppression of estimated ENaC activity suggests that ENaC activity does not drive Dahl SSH. This data is consistent with our prior finding in NE-infused male SD rats that suppressed ENaC activity but developed SSH.11, 19
Impact of dietary salt intake on NCC regulation in male DSR versus DSS rats
In response to a HS intake DSR rats exhibit increased expression of WNK1, downregulation of WNK4, and an altered WNK1 to WNK4 ratio. In contrast this pattern of altered dynamics of WNK1 and WNK4 abundance was not observed in DSS rats. In addition to altered WNK levels, DSR rats exhibited dietary sodium evoked suppression of SPAK and OxSR1 expression and phosphorylation – a response largely absent in DSS rats. Interestingly, DSS rats show a substantial 6-fold increase in SPAK and OxSR1 threonine phosphorylation that corresponded with a two-fold increase in NCC phosphorylation. The relative contributions of individual threonine phosphorylation on SPAK/OxSR1 activity is unclear. Prior studies have shown that threonine phosphorylation is required for kinase activity,14 suggesting that the observed increase in threonine phosphorylation may be driving NCC phosphorylation and activity in DSS rats.
Recent studies, conducted in vitro, demonstrate that WNKs are able to form heteromultimers12 – a phenomenon that may occur in vivo. The formation of WNK heteromultimers may result in an additive, sub-additive or synergistic effect on NCC depending on the ratio of WNK kinases versus SPAK/OxSR1.12 Given the dramatically altered WNK and SPAK/OXSR1 dynamics in response to HS intake in DSR versus DSS rats we speculate that WNK heterodimers may be mediating the observed effects on NCC activity and expression observed in DSR versus DSS rats.
Chronic α1-adrenoceptor antagonism suppresses NCC activity and attenuates the development of SSH in male DSS rats
To investigate the adrenergic signaling pathways influencing NCC activity and the development of Dahl SSH, the α1-adrenoceptor antagonist terazosin was administered, at a low dose that does not lower blood pressure in normotensive rats,19 throughout a 21-day HS diet. Terazosin preferentially blocks the α1B adrenoceptor,22 which makes up 50% of the rat distal tubule/collecting duct cells’ α-adrenoceptors. α1-adrenoceptor antagonism attenuated Dahl SSH by approximately 20 mmHg, without impacting the vascular response to ganglionic blockade. In contrast, α1-adrenoceptor antagonism increased the FENa, prevented an increase in EPV and decreased NCC activity and phosphorylation – data that strongly suggest a renal but not vascular action of α1-adrenoceptor antagonism. Our current observations in the DSS rat are in accordance with our previous work where α1-adrenoceptor antagonism abolished NE-evoked SSH and restored dietary-evoked suppression of NCC activity and phosphorylation in male SD rats.19 In terms of the mechanisms regulating NCC activity although α1-adrenoceptor antagonism did not promote the WNK1 and WNK4 expression dynamic observed in DSR rats, it did result in a decrease in OxSR1 expression suggesting a greater role for OxSR1 in NCC regulation as SPAK expression remained unchanged. This trend was also present in our prior study in NE-infused male SD rats treated with the α1-adrenoceptor antagonist terazosin, where OxSR1 downregulation corresponded with a decrease in NCC phosphorylation.19 In the current study there was also a significant decrease in SPAK/OxSR1 threonine phosphorylation with α1-adrenoceptor antagonism that correlated with a decrease in NCC phosphorylation, supporting the theorized requirement of threonine phosphorylation for kinase activity.14
Chronic β-adrenoceptor antagonism does not reduce NCC activity or counter Dahl SSH
The nonselective β-adrenoceptor antagonist, propranolol, was used to investigate the potential role of β-adrenoceptor signaling pathways on NCC activity. DCT cells express three times more β1- than β2- adrenoceptor subtypes, with no detectable expression of β3 adrenoceptors.23 Thus, we believe that the observed effects of propranolol are mediated by β1- or β2- adrenoceptors. As observed with α1-adrenoceptor antagonism β-adrenoceptor antagonism did not impact baseline sodium excretion recorded during the baseline period of the acute renal sodium transporter assay, and the resultant data obtained in this assay reflect the effects of diuretic. Chronic β-adrenoceptor antagonism in DSS rats fed a HS diet did not reduce NCC activity, NCC expression or NCC phosphorylation, and did not attenuate the magnitude of SSH. The inability of β-adrenoceptor antagonism to lower BP and NCC activity is in opposition to prior studies that suggested a sympathetically-mediated β-adrenoceptor gated pathway drives NCC activity in the pathophysiology of SSH.16 The difference between this study and our own observations may reflect species differences between mice and rats and differences in experimental approach may also have influenced the obtained results. In contrast to our studies in the DSS rat model prior studies that reported a role of β-adrenoceptors in NCC regulation infused C57BL/6 mice with NE and isoproterenol, a β-adrenoceptor agonist during a 5-day HS diet.16 A second study assessed the effect β-adrenoceptor antagonism, with propranolol, on the activity of the DCT potassium channel Kir4.1 in whole cell recording studies on DCTs prepared from C57BL/6 mice. These studies suggest that propranolol would reduce NCC activity given its suppression of Kir4.1 activity in response to NE stimulation.24 In strong support of our findings a recent study in DSS rats treated chronically with β-adrenoceptor antagonism during a 6-week HS diet period failed to detect a β-adrenoceptor antagonist-mediated BP lowering effect or any impact on NCC activity.21 Further, β-adrenoceptor antagonism did not restore the WNK expression dynamic observed in DSR rats, nor did it affect SPAK or OxSR1 expression despite reducing the phosphorylation of these kinases. However, there was no resultant decrease in phosphorylated NCC despite the activity of these intermediate kinases being suppressed. This may be explained by a failure to suppress OxSR1 expression as there is evidence that SPAK (and also most likely OxSR1) can directly activate the NCC in a WNK-independent manner.
Chronic α1-adrenoceptor antagonism suppresses NCC activity and attenuates established Dahl SSH
We observed an anti-hypertensive effect of α1-adrenoceptor antagonism in DSS rats with established SSH in concert with reduced NCC activity, reduced total and phosphorylated NCC abundance and a reduced phosphorylated NCC/total NCC ratio. Critically, these data suggest NCC activity is likely contributing not only to the development of Dahl SSH, but also the maintenance of Dahl SSH. Despite the WNK expression dynamic remaining perturbed during α1-adrenoceptor antagonism in established Dahl SSH, we observed significantly reduced phosphorylation of SPAK/OxSR1, a response that may underlie the observed decrease in phosphorylated NCC abundance. This finding suggests that the major factors regulating NCC activity may be different in the development versus maintenance phase of established Dahl SSH. Collectively, our findings with α1-adrenoceptor antagonism lend further support for the existence of a sympathetically mediated WNK4/SPAK/OxSR1 pathway that chronically modulates NCC activation.
Study limitations
Our studies are partially limited by the global administration of adrenoceptor antagonists which can influence BP via multiple mechanisms. We acknowledge that propranolol may act as an agonist of the β3-adrenoceptor in some systems. Owing to DCT cells lacking detectable expression of β3 adrenoceptors23 we believe this phenomenon has not adversely impacted our study. While our data suggest that the impact of terazosin on reducing BP is largely mediated by renal mechanisms future studies are needed to explore the renal specific effects of this antagonist. This study’s approach to direct BP measurement is a methodical limitation as animals are acutely instrumented. Nonetheless, this approach represents the only way in which the measurement of BP and in vivo physiological activity of the NCC can be assessed within the same animal.25, 26 This approach increases the reproducibility and rigor of our current and previously published data and supports a strong correlation between in vivo NCC activity and NCC phosphorylation.11, 19 At present the impact of phosphatases, that may respond to dietary manipulation27, 28 and can modulate NCC phosphorylation, on NCC activity remain unknown. Additionally, the potential roles of K+ channels and their modulation in response to adrenoceptor antagonism in DSS hypertension remain to be investigated.
Perspectives
The present study, in the DSS rat that exhibits endogenous sympathoexcitation and SSH, supports our previous mechanistic findings in the salt-resistant SD rat strain of NE-evoked SSH.19 These data provide convincing evidence for sympathetically-mediated α1-adrenoceptor mediated NCC activation, via a WNK/SPAK/OxSR1 pathway, in the development and maintenance of SSH. Significantly, these studies demonstrate the ability to selectively target and attenuate the development and maintenance of SSH using α1-adrenoceptor antagonism. The observed WNK1/WNK4 dynamic further promotes the significance of WNK signaling in the pathophysiology of SSH and NCC regulation. Ultimately, this work expands our understanding of adrenergic signaling that promotes NCC mediated sodium reabsorption and the pathophysiology of SSH to identify represent potential new therapeutic targets for SSH.
Supplementary Material
Novelty and Significance.
-
What is New?
NCC plays a critical role in Dahl Salt-Sensitive hypertension.
α1-adrenoceptor antagonism attenuates NCC activation and the salt sensitivity of blood pressure.
-
What is Relevant?
Exaggerated sympathetic activity trigged by increased dietary sodium intake can evoke salt-sensitive hypertension, which exists in ~50% of hypertensive patients.
Renal α1-adrenoceptors are implicated in the pathogenesis of NCC-mediated salt-sensitive hypertension.
-
Summary
In response to a high salt diet NCC activity increased in Dahl Salt-Sensitive rats and decreased in Dahl Salt-Resistant rats.
α1-adrenoceptor antagonism attenuates the development and maintenance of salt-sensitive hypertension in Dahl Salt-Sensitive rats via suppression of NCC activity and expression.
β-adrenoceptor antagonism fails to influence blood pressure or NCC activity in the Dahl Salt-Sensitive rat.
Acknowledgments
Sources of Funding
This work was supported by NIH R56 AG057687, R01 HL139867, R01 HL141406 and R01 AG062515 to R.D.W., NIH F31 DK116501 to A.A.F., the Boston University Undergraduate Research Opportunities Program to E.F., the ASN Foundation for Kidney Research Pre-Doctoral Fellowship award and the APS William Townsend Porter Physiology Development Fellowship award to F.P.
Footnotes
Disclosures
None
REFERENCES
- 1.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, Lopez-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The lancet commission on hypertension. Lancet. 2016;388:2665–2712 [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85:251–255 [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. A global brief on hypertension: Silent killer, global public health crisis: World health day 2013. 2013
- 4.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC Jr., Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the american heart association. Circulation. 2011;123:1138–1143 [DOI] [PubMed] [Google Scholar]
- 5.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737 [DOI] [PubMed] [Google Scholar]
- 6.Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the united states. JAMA. 2017;317:912–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agriculture USDoHaHSaUSDo. 2015-2020 dietary guidelines for americans. 2015;8th Edition [Google Scholar]
- 8.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–763 [DOI] [PubMed] [Google Scholar]
- 9.Yasuhiro Nishida MT-H, Takehito Kemurlyama, Kohsuke Hagisawa. Sympathetic activity in dahl salt-sensitive hypertension - why is a nitric oxide-induced inhibitory system up-regulated in the sympathetic center? J Nephrol Ther. 2013;3 [Google Scholar]
- 10.Sonalker PA, Tofovic SP, Bastacky SI, Jackson EK. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol. 2008;35:594–600 [DOI] [PubMed] [Google Scholar]
- 11.Walsh KR, Kuwabara JT, Shim JW, Wainford RD. Norepinephrine-evoked salt-sensitive hypertension requires impaired renal sodium chloride cotransporter activity in sprague-dawley rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CL, Cheng CJ. A unifying mechanism for WNK kinase regulation of sodium-chloride cotransporter. Pflugers Arch. 2015;467:2235–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 2017;25:285–299 [DOI] [PubMed] [Google Scholar]
- 14.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol. 2016;78:367–389 [DOI] [PubMed] [Google Scholar]
- 15.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol. 2006;290:F619–624 [DOI] [PubMed] [Google Scholar]
- 16.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–580 [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Feng X, Liu D, Chen J, Zhang Y, Niu B, Gu Y, Cai H. Dietary salt modulates the sodium chloride cotransporter expression likely through an aldosterone-mediated WNK4-ERK1/2 signaling pathway. Pflugers Arch. 2012;463:477–485 [DOI] [PubMed] [Google Scholar]
- 18.Argaiz ER, Chavez-Canales M, Ostrosky-Frid M, Rodriguez-Gama A, Vazquez N, Gonzalez-Rodriguez X, Garcia-Valdes J, Hadchouel J, Ellison D, Gamba G. Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of wnk4 and ncc. Am J Physiol Renal Physiol. 2018;315:F734–F745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frame AA, Puleo F, Kim K, Walsh KR, Faudoa E, Hoover RS, Wainford RD. Sympathetic regulation of the NCC in norepinephrine-evoked salt-sensitive hypertension in Sprague-Dawley rats. Am J Physiol Renal Physiol. 2019; 317:F1623–F1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terker AS, Yang CL, McCormick JA, Meermeier NP, Rogers SL, Grossmann S, Trompf K, Delpire E, Loffing J, Ellison DH. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension. 2014;64:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zicha J, Hojna S, Vanourkova Z, Kopkan L, Vaneckova I. Is renal beta-adrenergic-WNK4-NCC pathway important in salt hypertension of dahl rats? Physiol Res. 2019; 68: 873–882 [DOI] [PubMed] [Google Scholar]
- 22.Quaresma B, Pimenta AR, Santos da Silva AC, Pupo AS, Romeiro LAS, Silva CLM, Noel F. Revisiting the pharmacodynamic uroselectivity of alpha 1-adrenergic receptor antagonists. J Pharmacol Exp Ther. 2019;371:106–112 [DOI] [PubMed] [Google Scholar]
- 23.Gesek FA, White KE. Molecular and functional identification of beta-adrenergic receptors in distal convoluted tubule cells. Am J Physiol. 1997;272:F712–720 [DOI] [PubMed] [Google Scholar]
- 24.Duan XP, Gu L, Xiao Y, Gao ZX, Wu P, Zhang YH, Meng XX, Wang JL, Zhang DD, Lin DH, Wang WH, Gu R. Norepinephrine-induced stimulation of Kir4.1/Kir5.1 is required for the activation of nacl transporter in distal convoluted tubule. Hypertension. 2019;73:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashek A, Menzies RI, Mullins LJ, Bellamy CO, Harmar AJ, Kenyon CJ, Flatman PW, Mullins JJ, Bailey MA. Activation of thiazide-sensitive co-transport by angiotensin ii in the cyp1a1-Ren2 hypertensive rat. PLoS One. 2012;7:e36311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66–76 [DOI] [PubMed] [Google Scholar]
- 27.Ishizawa K, Wang Q, Li J, Yamazaki O, Tamura Y, Fujigaki Y, Uchida S, Lifton RP, Shibata S. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A. 2019;116:3155–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoda W, Nomura N, Ando F, Mori Y, Mori T, Sohara E, Rai T, Uchida S. Calcineurin inhibitors block sodium-chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int. 2017;91:402–411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


