Abstract
Background
Chronic lung allograft dysfunction (CLAD), the primary cause of poor outcome after lung transplantation, arises from fibrotic remodeling of the allograft and presents as diverse clinical phenotypes with variable courses. Here, we investigate if bronchoalveolar lavage (BAL) mesenchymal cell activity at CLAD onset can inform regarding disease phenotype, progression and survival.
Methods
Mesenchymal cell colony-forming units (CFU) were measured in BAL obtained at CLAD onset (n=77) and CLAD-free time post-transplant matched controls (n=77). CFU counts were compared using the Wilcoxon rank sum test. Cox proportional hazards and restricted means models were utilized to investigate post-CLAD survival.
Results
Higher mesenchymal CFU counts were noted in BAL at the time of CLAD onset than in CLAD-free controls. Patients with restrictive allograft syndrome (RAS) had higher BAL mesenchymal CFU count at CLAD onset compared to patients with bronchiolitis obliterans syndrome (BOS) (p=0.011). Patients with high mesenchymal CFU counts (≥10) at CLAD onset had worse outcomes compared to those with low (<10) CFU counts, with shorter average survival (2.64 years vs. 4.25 years, p=0.027) and shorter progression-free survival, defined as time to developing either CLAD stage 3 or death (0.97 years vs. 2.70 years, p<0.001). High CFU count remained predictive of decreased overall survival and progression-free survival after accounting for CLAD phenotype and other clinical factors in multivariable analysis.
Conclusion
Fulminant fibroproliferation with higher mesenchymal CFU counts in BAL is noted in RAS and is independently associated with poor survival after CLAD onset.
Introduction
Chronic lung allograft dysfunction (CLAD) is the primary cause of poor long-term outcomes after lung transplantation (1). CLAD can present as diverse clinical phenotypes (2–4). The predominant presentation, termed bronchiolitis obliterans syndrome (BOS), is identified by FEV1 decline with obstructive spirometric pattern and absence of radiographic opacities (4–6). Alternatively, restrictive allograft syndrome (RAS) presents with restriction on pulmonary function testing and persistent radiographic opacities (2, 4, 7–11). In addition to these, the recently updated guidelines classify patients with either restriction or CT scan opacities as undefined CLAD phenotype (U-CLAD), and yet others who meet criteria for both BOS and RAS as Mixed CLAD phenotype (4). Clinical, physiologic, and radiographic features at CLAD onset have been associated with survival after CLAD onset, with the RAS phenotype, early onset of disease, and higher extent of small airway disease correlating with poorer prognosis (8, 12–15). However, despite these advances, biological markers linking pathogenic mechanisms, CLAD phenotypes, and outcomes are lacking.
The histopathologic hallmark of CLAD, regardless of phenotype, is fibrotic infiltration of the transplanted lung. BOS is characterized by mesenchymal cell proliferation and collagen deposition in the submucosa or lumen of terminal airways leading to their partial or complete obstruction (5, 6). Patients with RAS have pleuroparenchymal fibroelastosis, with or without concomitant obliterative bronchiolitis on histology (16, 17). Fibrosis in the lung allograft is orchestrated primarily by lung-resident mesenchymal cells, (18–20) and we have previously identified the presence of donor-derived mesenchymal stromal cells (MSC) in the bronchoalveolar lavage (BAL) of lung transplant recipients via culture (21). Having increased mesenchymal cells in the BAL, defined as ≥10 mesenchymal colony forming units (CFU) per 2 ×106 cells plated, was associated with higher risk of subsequent development of CLAD (22). However, whether mesenchymal cell numbers in the BAL at the onset of lung dysfunction offer insight into CLAD phenotypes or prognosis remains to be elucidated.
In this study, by quantitating the mesenchymal cell component in BAL at CLAD onset, we provide evidence for active fibroproliferation in CLAD lungs and ascertain its association with specific CLAD phenotypes and disease progression. We show that mesenchymal CFU counts are higher in BAL collected at CLAD onset compared to CLAD-free control samples. Higher BAL mesenchymal CFU counts were more commonly found in RAS vs. BOS. Importantly, high BAL mesenchymal CFU counts at CLAD onset predicted poor outcomes with shorter time to disease progression and death, even after accounting for CLAD phenotype.
Materials and methods
Sample identification and consent
Lung transplant recipients undergoing bronchoscopy at the University of Michigan were enrolled in a prospective study permitting the collection of BAL fluid surplus to clinical requirements. All patients with a BAL sample collected near CLAD onset, defined as ≤60 days prior to or ≤90 days after diagnosis of CLAD, in which a mesenchymal CFU count was available were included in our study (n=77). For the 23 patients with more than one BAL collected near CLAD, the specimen with the highest mesenchymal count was analyzed (the median [IQR] of the intra-patient standard deviation of mesenchymal counts was 3.6 [1.3–18.2]). Control BAL samples were selected using a case-control matching algorithm from patients without CLAD at the time of bronchoscopy and who remained CLAD-free for at least 60 days following the BAL. The algorithm matched control samples to cases on the basis of similar time post-transplant and resolved multiple matches by selecting control samples with higher mesenchymal CFU counts. This study was approved by the University of Michigan Institutional Research Board and informed consent was obtained for participation in the study.
Bronchoalveolar Lavage & Mesenchymal Colony Forming Unit Assay
Bronchoscopy was performed per institutional protocol. Briefly, patients received conscious sedation and nebulized lidocaine. The bronchoscope was advanced via the mouth or nose and through the vocal cords and, after an airway exam, was wedged in the right middle lobe or lingula of the allograft unless another subsegment was clinically apparent. BAL was performed with instillation of between 120 and 300 ml of sterile isotonic saline. BAL samples were processed, and mesenchymal cells were cultured as previously described (18, 21–24). Briefly, BAL cells were pelleted by centrifugation and mononuclear cells were quantified and seeded in a density of 2 ×106 cells per 100 mm cell culture dish. Media consisted of high-glucose Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA); 100 U/ml penicillin/streptomycin (Invitrogen); and 0.5% fungizone (Invitrogen) and was changed every three days. The cells were incubated in 5% CO2 / 95% air at 37°C. CFU were identified under 25x magnification and counted between 14 and 21 days after initial plating. This CFU assay is a reproducible assessment of the mesenchymal cell component in BAL (22).
Patient demographics and clinical variables
Lung transplant patients were evaluated at least every 3 months and spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines (25). Baseline FEV1 and FVC were calculated as the average of the two highest values after transplant taken at least 21 days apart (8, 9, 26). The diagnosis of CLAD was established after 3 months of sustained decline in FEV1 to ≤80% of baseline despite evaluation and treatment of reversible causes (4). Patients whose lung function decline could potentially be ascribed to alternative etiologies (e.g. airway stenosis, persistent pleural effusions, neuromuscular disease, etc.) were not considered to have CLAD for this analysis. Per institutional protocol, at the time of initial lung function decline, patients received azithromycin (if not already taking) in the absence of contraindication. At the time of analysis, no patients have had subsequent recovery in pulmonary function. The date of CLAD onset was defined as the first date on which a patient had definitive FEV1 decline to ≤80% of baseline.
The CLAD phenotype was determined according to the International Society for Heart and Lung Transplantation (ISHLT) consensus report using clinical information, pulmonary function testing, and CT scan data available up to three months after the date of CLAD onset (Table 1) (4). Briefly, physiologic obstruction was defined as a ratio of FEV1:FVC of ≤70% for bilateral lung transplant recipients, (4) for single lung transplant recipients obstruction was defined as a decrease in FEV1:FVC ratio from the ratio of baseline FEV1 and FVC values. Restriction was defined as total lung capacity (TLC) ≤90% of post-transplant baseline; when TLC was not available, we defined physiologic restriction as a decline in FVC to ≤80% of baseline, similar to prior studies (8, 9, 11, 14, 15). Persistent radiographic opacities were defined as ground glass, consolidation, reticulation, septal and/or pleural thickening that were present on computed tomography (CT) at time of CLAD onset and which persisted for at least 3 months (4, 10).
Table 1:
CLAD Onset Cohort Clinical Characteristics & CLAD Phenotypes1
| Obstruction2 | Restriction3 | CT Opacities4 | n (%) | |
|---|---|---|---|---|
| BOS | + | − | − | 52 (67.5%) |
| RAS | − | + | + | 18 (23.4%) |
| Mixed | + | + | + | 3 (3.9%) |
| U-CLAD | + | − | + | 4 (5.2%) |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction, CT = computed tomography scan, BOS = bronchiolitis obliterans syndrome, RAS = restrictive allograft syndrome, U-CLAD = undefined CLAD phenotype
Assessed during CLAD onset period (i.e. first 3 months after CLAD onset)
Defined as FEV1/FVC ratio of ≤70%; for single lung transplant recipients obstruction was defined as a decline in FEV1/FVC ratio relative to the ratio of their baseline FEV1 and FVC values.
Defined as TLC ≤90% post-transplant baseline; when TLC information was not available, decline in FVC ≤80% of post-transplant baseline was used.
CT scan opacities were defined as ground glass, consolidation, reticulation, septal and/or pleural thickening not due to airways disease.
CLAD severity was graded according to ISHLT guidelines and defined as grades 1 (FEV1 66%−80% of baseline), 2 (FEV1 51%−65% of baseline) 3 (FEV1 50%−36% of baseline), and 4 (≤35% of baseline) (4, 6). Similar to prior work, patients were analyzed as low grade (CLAD grade 1) or high grade (CLAD grades 2–4) CLAD at time of BAL (12), and early CLAD onset was defined as development of CLAD <2 years after transplant (12). The indication for bronchoscopy was categorized as either surveillance (performed per protocol on asymptomatic patients with preserved lung function in the first year after transplant) or as diagnostic (to evaluate new respiratory symptoms, lung function decline, or imaging findings). BAL clinical results and serum donor specific antibody (DSA) presence was determined via medical record review.
Statistical Analysis
Statistical analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were summarized as percentages and compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous data were summarized as means ± standard deviations and compared using two-sample t-tests. Mesenchymal CFU counts were compared using the Wilcoxon rank-sum test. Time-to-event (survival) curves were presented using the Kaplan-Meier method (27). Univariate and multivariable time-to-event analyses were conducted using logrank (28, 29) and Cox proportional hazards (30) analyses, respectively. Unadjusted restricted mean estimates (31), with corresponding confidence intervals and tests for significance, are given pertaining to an approximate 7-year follow-up using methods appropriate for censored time-to-event data.
Results:
Increase in mesenchymal cells in BAL accompanies CLAD onset
To investigate the correlation of mesenchymal cells with CLAD onset, we first compared BAL samples obtained from lung transplant recipients at CLAD onset with time post-transplant matched CLAD-free control samples. The average time to CLAD onset in the CLAD group was 1013 ± 768 days. The demographic and clinical characteristics of the cases and controls are shown in Table 2. Preponderance of female sex was noted in the CLAD group and a higher proportion of CLAD onset samples demonstrated BAL neutrophilia as compared to the control group (66% vs. 43%). While there were over twice as many surveillance bronchoscopies included in the CLAD-free group, the majority of bronchoscopies in both groups were performed for clinical indications with no statistically significant difference between indication for bronchoscopy noted between the two groups (p=0.148).
Table 2.
Demographic and clinical characteristics of patients and samples included in analysis, stratified by CLAD status at time of BAL
| Variable | All (n=154) | CLAD-Free (n=77) | CLAD Onset (n=77) | p-value1 |
|---|---|---|---|---|
| Age in years, n (%) | 0.746 | |||
| Less than 30 | 27 (17.5) | 12 (15.6) | 15 (19.5) | |
| 30–40 | 12 (7.8) | 5 (6.5) | 7 (9.1) | |
| 40–50 | 16 (10.4) | 7 (9.1) | 9 (11.7) | |
| 50–60 | 64 (41.6) | 36 (46.8) | 28 (36.4) | |
| Older than 60 | 35 (22.7) | 17 (22.1) | 18 (23.4) | |
| Female sex, n (%) | 56 (36.4) | 20 (26.0) | 36 (46.8) | 0.007 |
| Pre-Transplant Diagnosis, n (%) | 0.281 | |||
| COPD/Emphysema | 39 (25.3) | 19 (24.7) | 20 (26.0) | |
| ILD | 66 (42.9) | 38 (49.4) | 28 (36.4) | |
| Cystic Fibrosis | 26 (16.9) | 12 (15.6) | 14 (18.2) | |
| Other | 23 (14.9) | 8 (10.4) | 15 (19.5) | |
| Bilateral Lung Transplant, n (%) | 106 (68.8) | 50 (64.9) | 56 (72.7) | 0.297 |
| BAL Performed for Surveillance, n (%) | 13 (8.4) | 9 (11.7) | 4 (5.2) | 0.147 |
| Days BAL after Lung Transplant, mean ± SD | 1016 ± 748 | 1002 ± 723 | 1030 ± 776 | 0.543 |
| Concurrent ACR biopsy grade ≥A12, n (%) | 20 (13.0) | 9 (11.7) | 11 (14.3) | 0.632 |
| Concurrent LB biopsy grade ≥B1R2, n (%) | 7 (4.6) | 3 (3.9) | 4 (5.2) | 0.699 |
| DSA Present3,4, n (%) | 31 (30.4) | 11 (23.9) | 20 (35.7) | 0.197 |
| BAL Neutrophils >15%2,5, n (%) | 79 (54.5) | 31 (43.1) | 48 (65.8) | 0.006 |
| Positive Bacterial Culture, n (%)2,6 | 21 (13.8) | 9 (11.8) | 12 (15.8) | 0.481 |
| Positive Respiratory Virus PCR, n (%)2,7 | 5 (4.6) | 3 (5.7) | 2 (3.5) | 0.588 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction, COPD = chronic obstructive pulmonary disease, ILD = interstitial lung disease, BAL = bronchioalveolar lavage, ACR = acute cellular rejection, LB = lymphocytic bronchiolitis, PCR = polymerase chain reaction qualitative test
Comparing CLAD-Free vs. CLAD Onset groups
Identified on bronchoscopy from which the mesenchymal CFU count was measured
DSA presence was defined as mean fluorescence intensity of ≥1000 on single bead antigen testing prior to bronchoscopy
n=102, 31 missing from CLAD Free group, 21 missing from CLAD onset group
n=145, 5 missing from CLAD free group, 4 missing from CLAD onset group
n=152, 1 missing from CLAD free group, 1 missing from CLAD onset group
n=110, 24 missing from CLAD free group, 20 missing from CLAD onset group
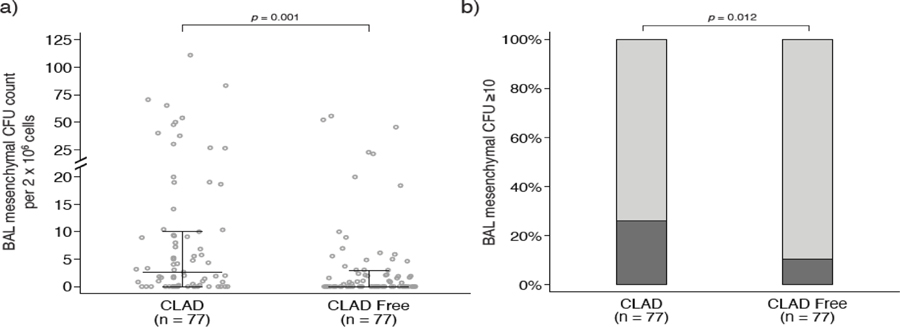
Mesenchymal cell numbers in the BAL samples, as quantitated by the CFU assay, was compared between the two groups. As shown in Figure 1, BAL samples obtained at CLAD onset demonstrated higher mesenchymal CFU counts compared with the control group (p=0.001, Figure 1a). The CLAD onset group also had a greater proportion of samples with high mesenchymal CFU counts, a cutoff defined as ≥10 CFU per 2 × 106 cultured mononuclear cells based on its clinical significance in prior research (22), than the CLAD-free group (26.0% vs 10.4%, p=0.012, Figure 1b). In a multivariate regression model, CLAD status at the time of BAL was the only variable associated with having a high mesenchymal CFU count (Supplementary Table S1).
Figure 1:
a) Scatterplot showing the distribution of mesenchymal cell colony forming units (CFU) in bronchioalveolar lavage (BAL) collected near chronic lung allograft dysfunction (CLAD) onset and from time-matched CLAD-free controls. Lines indicate medians and interquartile ranges. CLAD onset samples had higher mesenchymal CFU counts than samples collected while patients were CLAD-free. b) When mesenchymal CFU counts were stratified as low (<10) vs. high (≥10), the CLAD onset group had a greater proportion of BAL samples with high CFU counts vs. time-matched CLAD-free controls (26.0% vs 10.4%).
High Mesenchymal Cell Colony Forming Unit Counts Are More Common In Restrictive Allograft Syndrome
Next, we investigated whether BAL mesenchymal cell count at CLAD onset differed on the basis of CLAD phenotype at the time of CLAD onset (Table 1). Fifty-two (67.5%) patients had BOS, 18 (23.4%) had RAS, and 3 (3.9%) of patients had evidence of mixed CLAD. The remaining 4 (5.2%) had obstruction on spirometry with persistent opacities and, thus, were categorized as undefined CLAD (U-CLAD). The distribution of CLAD phenotypes and comparison of demographic and clinical variables is shown in Supplemental Table S2.
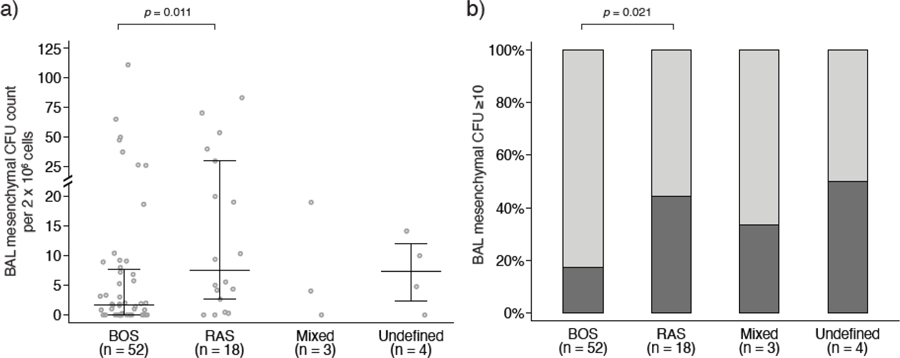
Comparison of BAL mesenchymal CFU counts at CLAD onset across various phenotypes demonstrated significantly higher mesenchymal cells in patients with RAS than in patients with BOS (RAS vs. BOS p=0.011, Figure 2a). Similarly, a higher proportion of RAS patients had high (≥10) CFU counts compared to those with BOS (44.4% vs. 17.3%, Figure 2b).
Figure 2:
a) Scatterplot showing the distribution of mesenchymal cell colony forming units (CFU) in bronchioalveolar lavage (BAL) collected near chronic lung allograft dysfunction (CLAD) onset for each CLAD phenotype. Lines indicate medians and interquartile ranges. Patients with restrictive allograft syndrome (RAS) had higher mesenchymal CFU counts than patients with bronchiolitis obliterans syndrome (BOS). b) When mesenchymal CFU counts were stratified as low (<10) vs. high (≥10), 9 (17.3%) BOS patients, 8 (44.4%) RAS patients, 1 (33.3%) mixed CLAD patient, and 2 (50%) U-CLAD patients had high CFU counts. Relative to BOS, a higher proportion of RAS patients had a high mesenchymal CFU count.
High Mesenchymal CFU Counts in BAL at CLAD Onset Portend a Poor Prognosis
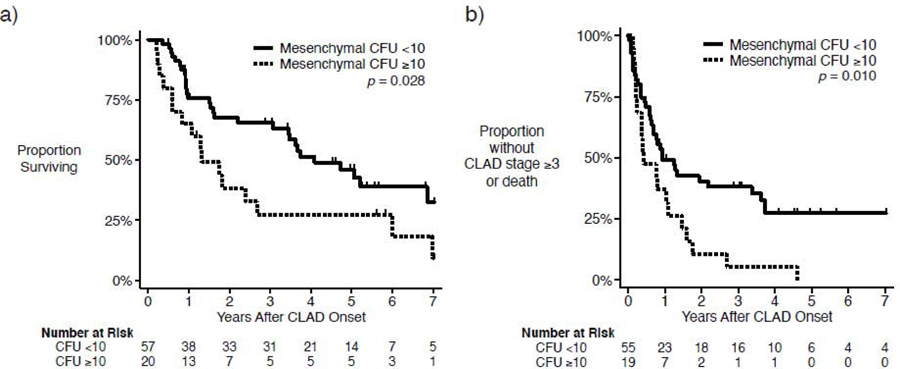
To investigate if higher mesenchymal cell activity in BAL predicts a more fulminant course and poor outcome, we first compared survival after CLAD onset between patients with high (≥10) vs. low (<10) mesenchymal CFU counts. Patients with high mesenchymal CFU counts had significantly decreased post-CLAD survival compared to patients with low mesenchymal CFU counts (Figure 3a, p=0.028). In restricted means analysis over 7.01 years of follow-up, we found that patients with low CFU counts survived an average of 60% longer after CLAD onset than those with high CFU counts (4.25 years [95% CI 3.54 – 4.96] vs. 2.64 years [95% CI 1.48–3.80], p=0.027).
Figure 3.
Kaplan-Meier curves, stratified by high (≥10) or low (<10) mesenchymal colony forming unit (CFU) count from bronchioalveolar lavage (BAL) fluid at time of chronic lung allograft dysfunction (CLAD) onset. a) Post-CLAD survival is decreased for patients with high CFU counts (p = 0.028). b) Likewise, patients with higher CFU counts develop either a FEV1 decline to ≤50% of the post-transplant baseline or death more quickly than those with low CFU counts (p = 0.010).
We also investigated whether high mesenchymal CFU counts were predictive of a more rapid decline in lung function. To determine this, we compared the progression-free survival, defined as the time until a patient developed Stage 3 CLAD or death, among patients with high and low mesenchymal CFU counts. Three patients had FEV1 values ≤50% of post-transplant baseline (i.e. consistent with CLAD stage 3) at the time of CLAD onset and were excluded from this analysis. As shown in Figure 3b, patients with high mesenchymal CFU counts had significantly shorter progression-free survival that shows with low CFU counts (p=0.010). The average time until CLAD stage 3 or death after CLAD onset for patients with a high CFU count was 0.97 years (95% CI, 0.25 – 1.46) vs. 2.70 years (95% CI, 1.91 – 3.50) for those with a low CFU count (p<0.001).
Importantly, BAL mesenchymal CFU count remained an independent predictor of survival (Table 3) and progression-free survival (Table 4) in multivariate models that accounted for CLAD phenotypes, the presence of donor specific antibodies, early vs. late CLAD onset, CLAD stage, BAL neutrophilia, age, sex, pre-transplant diagnosis, and bilateral vs. single lung transplant.
Table 3.
Multivariable Regression Model Evaluating Survival After CLAD Onset
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Age Category | ||
| Less than 30 | 1 | Referent |
| 30–39 | 1.08 (0.24 – 4.75) | 0.922 |
| 40–49 | 0.15 (0.02 – 1.04) | 0.055 |
| 50–59 | 0.24 (0.05 – 1.08) | 0.064 |
| 60 and older | 0.43 (0.08 – 2.49) | 0.348 |
| Female sex | 0.86 (0.40 – 1.82) | 0.686 |
| Pre-Transplant Diagnosis | ||
| COPD | 1 | Referent |
| ILD | 0.56 (0.22 – 1.46) | 0.236 |
| Cystic Fibrosis | 0.84 (0.15 – 4.85) | 0.847 |
| Other | 0.56 (0.12 – 2.70) | 0.469 |
| Bilateral Lung Transplant | 0.49 (0.18 – 1.31) | 0.154 |
| Mesenchymal CFU count ≥10 | 2.58 (1.01 – 6.57) | 0.047 |
| CLAD Phenotype | ||
| BOS | 1 | Referent |
| RAS | 3.06 (1.09 – 8.60) | 0.034 |
| Mixed CLAD | 6.60 (1.25 – 34.7) | 0.026 |
| U-CLAD | 2.55 (0.57 – 11.4) | 0.222 |
| CLAD Onset <2 years post-transplant | 1.43 (0.71 – 2.90) | 0.319 |
| CLAD Stage ≥2 at time of BAL | 1.57 (0.62 – 3.98) | 0.336 |
| DSA Present1 | 1.02 (0.44 – 2.38) | 0.959 |
| BAL Neutrophils >15%2 | 1.39 (0.64 – 2.98) | 0.403 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction, COPD = chronic obstructive pulmonary disease, ILD = interstitial lung disease, CFU = colony forming unit, BOS = bronchiolitis obliterans syndrome, RAS = restrictive allograft syndrome, U-CLAD = undefined CLAD phenotype, BAL = bronchoalveolar lavage, DSA = donor specific antibodies
DSA presence was defined as mean fluorescence intensity of ≥1000 on single bead antigen testing prior to bronchoscopy
Identified on bronchoscopy from which the mesenchymal CFU count was measured
Table 4.
Multivariable Regression Model Evaluating Progression-Free Survival After CLAD Onset
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Age Category | ||
| Less than 30 | 1 | Referent |
| 30–39 | 0.78 (0.18 – 3.38) | 0.744 |
| 40–49 | 0.26 (0.06 – 1.13) | 0.073 |
| 50–59 | 0.53 (0.15 – 1.81) | 0.308 |
| 60 and older | 0.36 (0.08 – 1.61) | 0.180 |
| Female sex | 0.49 (0.25 – 0.96) | 0.038 |
| Pre-Transplant Diagnosis | ||
| COPD | 1 | Referent |
| ILD | 0.64 (0.29 – 1.40) | 0.265 |
| Cystic Fibrosis | 0.93 (0.24 – 3.56) | 0.917 |
| Other | 0.52 (0.17 – 1.63) | 0.260 |
| Bilateral Lung Transplant | 1.06 (0.48 – 2.37) | 0.882 |
| Mesenchymal CFU count ≥10 | 3.39 (1.55 – 7.39) | 0.002 |
| CLAD Phenotype | ||
| BOS | 1 | Referent |
| RAS | 1.16 (0.53 – 2.54) | 0.707 |
| Mixed CLAD | 0.72 (0.16 – 3.38) | 0.682 |
| U-CLAD | 1.82 (0.54 – 6.14) | 0.333 |
| CLAD Onset <2 years post-transplant | 0.79 (0.43 – 1.46) | 0.452 |
| CLAD Stage ≥2 at time of BAL | 3.54 (1.40 – 8.92) | 0.007 |
| DSA Present1 | 1.03 (0.51 – 2.04) | 0.942 |
| BAL Neutrophils >15%2 | 1.10 (0.56 – 2.14) | 0.783 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction, COPD = chronic obstructive pulmonary disease, ILD = interstitial lung disease, CFU = colony forming unit, BOS = bronchiolitis obliterans syndrome, RAS = restrictive allograft syndrome, U-CLAD = undefined CLAD phenotype, BAL = bronchoalveolar lavage, DSA = donor specific antibodies
DSA presence was defined as mean fluorescence intensity of ≥1000 on single bead antigen testing prior to bronchoscopy
Identified on bronchoscopy from which the mesenchymal CFU count was measured
Discussion
CLAD arises from fibrotic remodeling of the lung allograft and is the leading cause of poor long-term outcomes after lung transplantation. Here we investigate if the previously understudied mesenchymal component of BAL can inform us regarding allograft fibroproliferation by studying the association of BAL mesenchymal cell numbers at CLAD onset with clinical phenotypes, disease progression, and survival. First, we confirm that mesenchymal CFU counts are higher in patients at CLAD onset compared to otherwise similar CLAD-free patients. Second, we show an association between higher mesenchymal CFU counts at CLAD onset and having RAS. Third, we demonstrate that having a high mesenchymal cell burden in BAL at CLAD onset is predictive of disease progression and death, even after controlling for CLAD phenotype and other relevant clinical variables. Together, these data shed novel light on mesenchymal cell responses in a chronically rejecting lung allograft and suggest that BAL mesenchymal cells can be a useful indicator of disease activity and progression.
Our present study is the first assessment of the mesenchymal cell component of BAL at the onset of spirometric decline. Higher mesenchymal CFU counts were noted in the BAL samples collected at CLAD onset vs. time post-transplant matched CLAD-free controls, offering insight into the fibroproliferative activity in the lung allograft which accompanies development of chronic graft failure. Unlike transbronchial biopsies, which have limited utility to diagnose CLAD because of the regional heterogeneity of this disease (3, 5), BAL allows for sampling of a larger allograft area. Prior studies of BAL specimens from lung transplant recipients have correlated immune cell counts (32–34), levels of cytokines and growth factors (35–39), and gene expression (40) with subsequent development of CLAD. We were first to demonstrate the presence of MSC in BAL samples obtained from human lung transplant recipients (21), and to show that a high (≥10) mesenchymal CFU count in BAL samples obtained from CLAD-free patients predicts future development of CLAD (22). However, whether assessment of mesenchymal cell activity in the BAL at the time of clinical deterioration can provide relevant information regarding ongoing fibroproliferation within an allograft had not been previously investigated. In this study, our cases (CLAD) and controls (CLAD free patients) were matched by time post-transplant, with a majority of the BAL in both cohorts obtained for clinical indications. Not only did we observe higher mesenchymal CFU counts in CLAD group, but we also note that four of the eight patients in the CLAD-free control group with a high mesenchymal CFU count developed CLAD within a year (Supplementary Table S3). Interestingly, three of the four CLAD-free patients with high mesenchymal CFU counts who recovered received high dose corticosteroids. Whether assessment of mesenchymal activity at the time of pulmonary function decline can identify patients more likely to respond to immunosuppression or anti-fibrotic treatment would require future prospective studies.
Among CLAD phenotypes, higher BAL mesenchymal CFU counts were noted among patients with RAS. Given the relatively recent recognition of the importance of CLAD phenotypes, there is a paucity of research into BAL features among CLAD phenotypes. Comparisons of immune cells counts between BOS and RAS have been inconclusive (42, 43); however, increased BAL immunoglobulins and complement have been linked with the development of RAS (42). In our study, we found that patients with RAS were more likely to have a high BAL mesenchymal CFU count than patients with BOS. This finding may suggest there is more active fibroproliferation in this phenotype, as might be suspected by the more rapid clinical decline seen in RAS (2, 4, 8–11). Alternatively, patients with RAS are known to have pleuroparenchymal fibrosis, organizing pneumonia, and intraluminal fibrotic plugs on histopathology (16, 17), and they have interstitial expansion and alveolar infiltration by fibrotic tissue on micro-CT (41). Therefore, it may be that that sampling the airspaces by BAL is more likely to detect mesenchymal cell activity in patients with RAS secondary to this parenchymal anatomic localization of fibrosis.
BAL mesenchymal cells also offer an opportunity to study the mechanisms of fibroproliferation in the allograft. Our group was the first to identify the mesenchymal cells cultured from the BAL of lung transplant recipients as representative of an allograft-derived lung-resident mesenchymal cell population via Affymetrix transcriptome analysis and sex chromosome karyotyping of sex-mismatched donors and recipients (18, 21). These donor-derived lung-resident mesenchymal cells have been shown to be the primary contributors to fibrosis in the allograft (18–20). Mesenchymal cells from patients with CLAD have a fibrotic phenotype, evidenced by increased expression of the matrix protein collagen 1 and the contractile filament α-smooth muscle actin, relative to mesenchymal cells from CLAD-free patients (18, 24, 45, 46). Secretome evaluation of BAL mesenchymal cells has found that their fibrotic differentiation can be regulated in an autocrine manner via secretion of anti-fibrotic lipid mediators, e.g. prostaglandin E2 (PGE2) (24), and pro-fibrotic mediators, e.g. lysophosphatidic acid (LPA) and endothelin-1 (ET-1) (45, 46). CLAD mesenchymal cells have been shown to have increased expression of LPA and ET-1 and lower expression of PGE2 and the PGE2 EP2 receptor (24, 45, 46). Further study into whether the migratory or fibrotic functions of mesenchymal cells differ on the basis of CLAD phenotype is warranted.
A key finding of our study was that having a high mesenchymal CFU count at CLAD onset predicts having a shorter time to disease progression and shorter overall survival. Importantly, having a high CFU count remained predictive of survival even after accounting for CLAD phenotypes and CLAD stage in multivariate analysis. To our knowledge, this is the first study to link a biological signal of fibrotic activity in the lung allograft with prognosis after CLAD onset. Previous studies have predominantly focused on physiologic and radiographic characteristics, noting decreased post-CLAD survival in patients with RAS (2, 8, 9, 11), increased functional small airways disease on CT scan (13), early onset CLAD (12), or larger FEV1 decline from baseline (12). Prognostic information in CLAD patients is important for consideration of treatment options such as re-transplantation. A biological marker of active fibroproliferation can also potentially allow for selection of patients likely to benefit from novel anti-fibrotic therapies and stratification of patient in clinical trials. Due to overall low numbers and lack of specific cell surface numbers, the standardized methodology for quantifying MSC in BAL fluid is the CFU assay, which requires plastic adherence and cell culture (21, 44). This method has been previously validated and correlated with other outcomes after lung transplant (18, 22). Further work is needed to develop culture-independent, less time-intensive methods to assess the mesenchymal cell population in the BAL fluid.
In summary, we demonstrate that higher mesenchymal CFU counts in the BAL of lung transplant recipients at CLAD onset are associated with RAS, and that high BAL mesenchymal CFU counts are predictive of decreased survival and more rapid disease progression, even after accounting for CLAD phenotype. Further research is required to determine the role of serial measures of mesenchymal cell activity in the follow-up of patients with CLAD, and whether this biological marker of fibroproliferation can help guide therapeutic interventions.
Supplementary Material
Acknowledgements
The funding agencies had no role in the design, conduct, and analysis of the study or in the decision to submit the manuscript for publication. None of the authors have any relevant disclosures to report.
Funded by National Institutes of Health Grants T32 HL007749 (MPC), R01 HL118017 and R01 HL094622 (to VNL), Cystic Fibrosis Foundation Grant LAMA16XX0 (to VNL) and the Brian and Mary Campbell and Elizabeth Campbell Carr research gift fund (to VNL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Chambers DC, Yusen RD, Cherikh WS, et al. : The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047–59. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Waddell TK, Wagnetz U, et al. : Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 2011;30:735–42. [DOI] [PubMed] [Google Scholar]
- 3.Meyer KC, Raghu G, Verleden GM, et al. : An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479–503. [DOI] [PubMed] [Google Scholar]
- 4.Verleden GM, Glanville AR, Lease ED, et al. : Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38:493–503. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Fishbein MC, Snell GI, et al. : Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–42. [DOI] [PubMed] [Google Scholar]
- 6.Estenne M, Hertz MI: Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med 2002;166:440–4. [DOI] [PubMed] [Google Scholar]
- 7.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P: A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant 2014;33:127–33. [DOI] [PubMed] [Google Scholar]
- 8.Belloli EA, Wang X, Murray S, et al. : Longitudinal Forced Vital Capacity Monitoring as a Prognostic Adjunct after Lung Transplantation. Am J Respir Crit Care Med 2015;192:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerHovanessian A, Todd JL, Zhang A, et al. : Validation and Refinement of Chronic Lung Allograft Dysfunction Phenotypes in Bilateral and Single Lung Recipients. Ann Am Thorac Soc 2016;13:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glanville AR, Verleden GM, Todd JL, et al. : Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38:483–92. [DOI] [PubMed] [Google Scholar]
- 11.Todd JL, Jain R, Pavlisko EN, et al. : Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med 2014;189:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM: Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med 2010;182:784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belloli EA, Degtiar I, Wang X, et al. : Parametric Response Mapping as an Imaging Biomarker in Lung Transplant Recipients. Am J Respir Crit Care Med 2017;195:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verleden SE, Ruttens D, Vandermeulen E, et al. : Predictors of survival in restrictive chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2016;35:1078–84. [DOI] [PubMed] [Google Scholar]
- 15.Verleden GM, Vos R, Verleden SE, et al. : Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation 2011;92:703–8. [DOI] [PubMed] [Google Scholar]
- 16.Ofek E, Sato M, Saito T, et al. : Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol 2013;26:350–6. [DOI] [PubMed] [Google Scholar]
- 17.von der Thusen JH, Vandermeulen E, Vos R, Weynand B, Verbeken EK, Verleden SE: The histomorphological spectrum of restrictive chronic lung allograft dysfunction and implications for prognosis. Mod Pathol 2018;31:780–90. [DOI] [PubMed] [Google Scholar]
- 18.Walker N, Badri L, Wettlaufer S, et al. : Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol 2011;178:2461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousem SA, Sherer C, Fuhrer K, Cieply K: Myofibroblasts of recipient origin are not the predominant mesenchymal cell in bronchiolitis obliterans in lung allografts. J Heart Lung Transplant 2013;32:266–8. [DOI] [PubMed] [Google Scholar]
- 20.Mimura T, Walker N, Aoki Y, et al. : Local origin of mesenchymal cells in a murine orthotopic lung transplantation model of bronchiolitis obliterans. Am J Pathol 2015;185:1564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lama VN, Smith L, Badri L, et al. : Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 2007;117:989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badri L, Murray S, Liu LX, et al. : Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2011;183:1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvinen L, Badri L, Wettlaufer S, et al. : Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, Lama VN: Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med 2012;185:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, et al. : Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JD, Billingham M, Egan T, et al. : A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 1993;12:713–6. [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P: Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53:457–81. [Google Scholar]
- 28.HARRINGTON DP, FLEMING TR: A class of rank test procedures for censored survival data. Biometrika 1982;69:553–66. [Google Scholar]
- 29.Savage IR: Contributions to the Theory of Rank Order Statistics-the Two-Sample Case. 1956;27:590–615. [Google Scholar]
- 30.Cox DR: Regression Models and Life-Tables. Journal of the Royal Statistical Society: Series B (Methodological) 1972;34:187–202. [Google Scholar]
- 31.Pepe MS, Fleming TR: Weighted Kaplan-Meier statistics: a class of distance tests for censored survival data. Biometrics 1989;45:497–507. [PubMed] [Google Scholar]
- 32.DiGiovine B, Lynch JP 3rd, Martinez FJ, et al. : Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–202. [PubMed] [Google Scholar]
- 33.Scholma J, Slebos DJ, Boezen HM, et al. : Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med 2000;162:2221–5. [DOI] [PubMed] [Google Scholar]
- 34.Neurohr C, Huppmann P, Samweber B, et al. : Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant 2009;28:468–74. [DOI] [PubMed] [Google Scholar]
- 35.Hertz MI, Henke CA, Nakhleh RE, et al. : Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet-derived growth factor. Proc Natl Acad Sci U S A 1992;89:10385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charpin JM, Stern M, Grenet D, Israel-Biet D: Insulinlike growth factor-1 in lung transplants with obliterative bronchiolitis. Am J Respir Crit Care Med 2000;161:1991–8. [DOI] [PubMed] [Google Scholar]
- 37.Elssner A, Jaumann F, Dobmann S, et al. : Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation 2000;70:362–7. [DOI] [PubMed] [Google Scholar]
- 38.Belperio JA, Keane MP, Burdick MD, et al. : Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest 2001;108:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynaud-Gaubert M, Marin V, Thirion X, et al. : Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant 2002;21:721–30. [DOI] [PubMed] [Google Scholar]
- 40.Weigt SS, Wang X, Palchevskiy V, et al. : Gene Expression Profiling of Bronchoalveolar Lavage Cells Preceding a Clinical Diagnosis of Chronic Lung Allograft Dysfunction. PLoS One 2017;12:e0169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verleden SE, Vasilescu DM, McDonough JE, et al. : Linking clinical phenotypes of chronic lung allograft dysfunction to changes in lung structure. Eur Respir J 2015;46:1430–9. [DOI] [PubMed] [Google Scholar]
- 42.Vandermeulen E, Verleden SE, Bellon H, et al. : Humoral immunity in phenotypes of chronic lung allograft dysfunction: A broncho-alveolar lavage fluid analysis. Transpl Immunol 2016;38:27–32. [DOI] [PubMed] [Google Scholar]
- 43.Dettmer S, Shin HO, Vogel-Claussen J, et al. : CT at onset of chronic lung allograft dysfunction in lung transplant patients predicts development of the restrictive phenotype and survival. Eur J Radiol 2017;94:78–84. [DOI] [PubMed] [Google Scholar]
- 44.Pittenger MF, Mackay AM, Beck SC, et al. : Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999;284:143. [DOI] [PubMed] [Google Scholar]
- 45.Cao P, Aoki Y, Badri L, et al. : Autocrine lysophosphatidic acid signaling activates beta-catenin and promotes lung allograft fibrosis. J Clin Invest 2017;127:1517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salama M, Andrukhova O, Jaksch P, et al. : Endothelin-1 governs proliferation and migration of bronchoalveolar lavage-derived lung mesenchymal stem cells in bronchiolitis obliterans syndrome. Transplantation 2011;92:155–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.