Abstract
A young 33 year old male presented with non-resolving corneal infiltrate for 2 month duration in the right eye. KOH/ Calcoflour wet mount revealed sparsely septate fungal hyphae. Post therapeutic penetrating keratoplasty 3 doses of intracameral voriconazole(100μg/0.1ml) was administered suspecting recurrence. Fungal culture revealed non sporulating mould on SDA. PCR based DNA sequencing targeting the ITS region identified the fungal isolate as Mortierella wolfii (M. wolfii) belonging to zygomycetes. To the best of our knowledge, this is the first report of human fungal keratitis caused by M. wolfii.
Keywords: DNA sequencing, fungal keratitis, Mortierella wolfii, non sporulating mould (NSM), therapeutic penetrating keratoplasty
Mortierella wolfii (M. wolfii) is a saprophytic fungus and belongs to member of the family Mortierellaceae with a worldwide distribution naturally found harboring the moldy grass in silage. M. wolfii is an important causative agent of bovine mycotic abortion, pneumonia, and systemic mycosis in New Zealand, Australia, Europe, and America.[1] M. wolfii has very rarely been reported to cause systemic infection in humans. The spores of M. wolfii may enter the body through the respiratory tract, alimentary tract, conjunctiva, or skin wounds. It may then cause local infection of the organ involved, or may undergo a hematogenous or lymphatic spread to a distant organ causing a disseminated systemic infection.[1]
However, to the best of our knowledge, ocular infection by this fungus has not been reported in literature. We hereby present the first case report of fungal keratitis caused by M. wolfii.
Case Report
A 33-year-old non-diabetic male presented with pain, redness and photophobia in the right eye since 2 months, following an injury with a flying insect. He was treated elsewhere with topical Amphotericin B (0.15%) QID, Natamycin eye drops (5%) QID and Atropine sulphate eye drops (1%) BID. His best corrected visual acuity (BCVA) in the right eye was hand movements (HM+) and in the left eye was 6/6 for distance and N6 for near. The anterior segment examination of the right eye showed lid edema, severe conjunctival congestion, a dry whitish anterior stromal infiltrate (5 × 6 mm) with an overlying corneal epithelial defect, superficial corneal vascularization in the superior and inferior quadrants, and an endothelial plaque with hypopyon of 4.0 mm in the anterior chamber [Fig. 1a]. Intraocular pressure was digitally normal in both eyes. Fundus examination in the right eye was not possible. The ocular examination in the left eye was normal.
Figure 1.
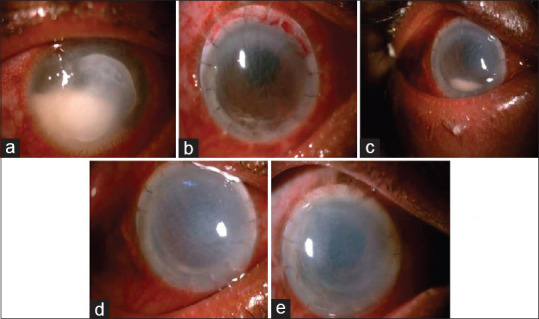
(a) Day of Presentation, 5 × 6 mm infiltrate with underlying endothelial plaque and 4.0 mm hypopyon. (b) PostOP Day 1 with intact graft host junction with minimal hypopyon. (c) 3rd post op week showing graft edema with resolving hypopyon. (d) 4 th post operative week showing resolving hypopyon. (e) 2 nd month post operative showing failed graft with resolved hypopyon
Topical Natamycin (5%) and Homatropine hydrobromide eye drops (2%) in the right eye along with oral Ketoconazole (200 mg) was initiated. The patient underwent a therapeutic penetrating keratoplasty (TPK), in the right eye under local anesthesia after 2 days worsening of the infiltrate. On the first post-operative day the visual acuity was hand movements (HM). Anterior segment examination showed lid edema, conjunctival congestion, and graft edema (2+) with intact graft-host junction, and well-formed anterior chamber with dispersed hyphema. Patient continued topical and oral anti-fungal therapy. Ultrasonography (USG) for the posterior segment in the right eye was normal. A streak hypopyon was noted on the second post-operative day 2 [Fig. 1b]. Topical Voriconazole (1%) 1 hourly and Timolol maleate (0.5%) BID was added. The next day, 0.1 ml of intracameral Voriconazole (100 μg/ml) was injected under local anesthesia with aseptic precautions. Persistent hypopyon was highly suggestive of recurrence. He further received two more doses of intracameral Voriconazole (100 μg/ml) within next 2 weeks. Resolving hyopyon was noted on the third post operative week [Fig. 1c]. Topical and oral anti-fungal treatments were continued for 8 weeks and subsequently topical prednisolone acetate (1%) was initiated in a tapering dose. The hypopyon gradually resolved and no subsequent recurrence was noted in the subsequent follow ups [Fig. 1d and e]. The corneal graft had failed with ectasia at the inferior graft-host junction, 10 months after the TPK. The patient then underwent an optical penetrating keratoplasty (PK) under local anesthesia. A paramedian temporary tarsorrhaphy was done for a persistent epithelial defect after a month. At the 3 month follow-up after the last PK, the BCVA in the right eye was 6/36 with + 0.00 Dsph/−6.00 × 40, 6/36 for distance and N16 for near with + 3.00 Dsph. The anterior segment examination in the right eye showed an inferior graft-host junction ectasia, deep anterior chamber, irregular pupil with broken posterior synechiae, and posterior sub capsular cataract. The intraocular pressure in the right eye was 22 mm Hg. The optic disc in the right eye showed a vertical cup-disc ratio of 0.75:1 with disc pallor and bipolar notching. Gonioscopy revealed 360° of synechial angle closure in the right eye. Humphrey visual field analysis (24-2) showed a biarcuate defect in the right eye. A fixed dose combination of Timolol maleate (0.5%) and Brimonidine tartrate (0.2%) was started twice daily. The intraocular pressure in the right eye was under control during the follow-up period. Another optical penetrating keratoplasty with extra capsular cataract extraction with posterior chamber intraocular lens implantation was advised for the right eye and at the last follow up the right eye BCVA was 6/24 with + 2.00 Dsph/−6.00 × 130 and near vision was N10 with + 3.00 Dsph.
The microbiological investigation of the corneal scraping revealed the presence of sparsely septate filaments in the direct smear by KOH–Calcoflour staining. The corneal scraping simultaneously inoculated on Sabouraud's dextrose agar showed the rapid growth of a whitish grey colonies covered with soft hairy structures (downy colonies) with a broadly zonated surface appearance with no reverse pigmentation after incubation for 2 days at 25°C [Fig. 2a]. The lactophenol cotton blue staining of the slide culture under a light microscope showed sparsely septate hyphae of variable diameter and non-parallel walls with bulbous dilations and non-dichotomous branching at right angle, consistent with a zygomycete fungi [Fig. 2b] and it remained as a non sporulating fungus and was reported as non sporulating mould (NSM). The same fungus was also isolated from the corneal button of the same patient. Subsequently, PCR based DNA sequencing targeting ITS region confirmed the final identification of the isolated NSM as M. wolfii with 97% homology of M. wolfii reference strain in Genbank. The nucleotide sequences of the M. wolfii isolated in this study was deposited in Genbank (Genbank accession number - MK630014.1).
Figure 2.
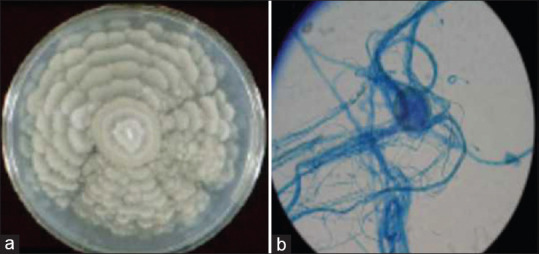
(a) Growth of M. wolfii on SDA. Growth of M. wolfii on SDA showing grayish white, downy, with a broad zonated rosette- like colony. (b) Morphology of M. wolfii in Lactophenol cotton blue staining. LPCB under × 40 magnification showing the sterile mycelia (Non Sporulating Mould (NSM))
Discussion
M. wolfii is associated invasive disease and the source of infection is oil, rotten silage, and hay. M. wolfii is predominantly known to cause infection in animals, especially in cattle. In cattle, it commonly causes abortion as a result of placentitis and endometritis during pregnancy, but may rarely cause pyogranulomatous pneumonia, nephritis and meningoencephalitis.[2] It is apparently more common in New Zealand, where M. wolfii causes up to 46% of cases of bovine mycotic abortions. There is also a report on microbiologically and cytologically proven M. wolfii keratomycosis in a horse[3] in literature.
The status of the M. wolfii as true human pathogen is refuted. Risk factors for infection in humans include immuno suppressive status with decreased production or function of macrophages and/or neutrophils, diabetes mellitus, ketoacidosis, malignancies, solid organ transplantation, bone marrow transplantation, intravenous drug use, and iron chelation therapy.[4] There are only a few reports of M. wolfii causing human infections found in literature–two cases of cutaneous sites infection reported in humans caused by M. wolfii based on their cultural and morphological structures. But, in both cases, the identification of M. wolfii was notdescribed sufficiently and was inconclusive.[5,6] Layios N et al. (2014) reported M. wolfii from liver biopsy specimen of a patient who underwent hematopoietic stem cell transplantation by both conventional culture and PCR based DNA sequencing targeting ITS 1-ITS 2 and D1–D2 regions of rRNA genes.[7] In the present study, though the isolated fungus showed the typical colony morphology on culture, the definite identification as M. wolfii was possible only by PCR based DNA sequencing targeting ITS region which exactly matched with the phenotypic characteristics (colony morphology).
Conclusion
To the best of our knowledge, this is the first report of human M. wolfii fungal keratitis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies JL, Ngeleka M, Wobeser GA. Systemic infection with Mortierella wolfii following abortion in a cow. Can Vet J. 2010;51:1391–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Wada S, Ode H, Hobo S, Niwa H, Katayama Y, Takatori K. Mortierella wolfii keratomycosis in a horse. Vet Ophthalmol. 2011;14:267–70. doi: 10.1111/j.1463-5224.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(Suppl 1):S8–15. doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciferri R, Ashford BK. New species of Mortierella isolated from human skin. P R J Pub Health Trop Med. 1929;5:134–43. [Google Scholar]
- 6.Nicolau E, Evolcenu R. Recherchesmycologiquesdans un cas de mycetome du pied a grains noirs.(Mortierellamycetomi) Annu Bull Dermatol. 1947;8:330–43. [PubMed] [Google Scholar]
- 7.Layios N, Canivet JL, Baron F, Moutschen M, Hayette MP. Mortierella wolfii-associated invasive disease. Emerg Infect Dis. 2014;20:1591–2. doi: 10.3201/eid2009.140469. [DOI] [PMC free article] [PubMed] [Google Scholar]


