Abstract
Purpose:
To find the agreement and repeatability of Icare ic100 tonometer.
Methods:
We included 150 subjects above the age of 18 years for this cross-sectional, multicenter study with intraocular pressure (IOP) ≥7 mmHg. After the initial ophthalmic examination, two masked examiners took five IOP measurements using three different instruments; Icare ic100, Icare TA01i, and Goldmann applanation tonometer (GAT) in only one eye of the participants. Comparison of agreement of IOP using different instruments was quantified with intraclass correlation coefficient (ICC) using the two-way random effects models of absolute agreement and Cronbach's alpha. The test-retest variability of the instruments was assessed by deriving repeatability coefficient (RC) and coefficient of variation (CV).
Results:
Agreement between the tonometers across the different IOP groups had no statistically significant difference in their mean IOP. Icare ic100 was found to have good reliability across all IOP groups (ICC value >0.78) when compared with Icare TA01i. In comparison with GAT, Icare ic100 showed good reliability across all IOP groups (ICC >0.87) except >16 to <23 mmHg group where it showed moderate reliability (ICC = 0.52). Icare ic100 showed good repeatability with RC and CV of 2.67 and 4.89, respectively.
Conclusion:
Icare ic100 rebound tonometer can measure IOP with relatively small measurement error and can provide a reliable and repeatable reading in comparison with GAT across a wide pressure range without hampering corneal health.
Keywords: Agreement, Icare-tonometer, intraocular pressure, repeatability, tonometer
Elevated intraocular pressure (IOP) is one among the major risk factors for glaucoma. The widely used instrument to measure IOP is the gold standard Goldmann applanation tonometer (GAT).[1] Icare rebound tonometers, available in the market since 2005, are self-calibrated handheld devices which allow series of IOP measurements without topical anesthesia.[2] The new Icare ic100 tonometer is an upgraded version of the existing model of Icare TA01i. Readings of the Icare tonometer have shown a reasonable concordance with lOP measurements obtained by GAT.[3] However, in some cases, measurements of GAT and rebound tonometer (RBT) showed disagreement.[2,4] In this study, we estimate the agreement between Icare ic100 with rebound tonometer TA01i and GAT.
Methods
This cross-sectional study was conducted from May 2015 to March 2016. The study was approved by the Institutional Review Board and was performed according to the guidelines of the Declaration of Helsinki. Subjects were recruited from two study sites. A detailed explanation of the study procedures was given to the participants and written informed consent was obtained before the enrollment.
Inclusion exclusion criteria
Subjects with intraocular pressure (IOP) ≥7 mmHg were enrolled in the study. Age <18 years, uncorrected near visual acuity (UCNVA) of 20/200 (binocular) or less, poor or eccentric fixation in the study eye, corneal astigmatism >3D in the study eye(s), corneal scarring, history of prior incisional glaucoma surgery or corneal surgery (including corneal laser surgery) were not included in the study. Other exclusion criteria were microphthalmos, buphthalmos, contact lens use, symptoms of dry eye syndrome, signs of dry eye on examination of cornea, nystagmus, keratoconus, any other corneal or conjunctival pathology or infection, central corneal thickness greater than 0.60 mm or less than 0.50 mm, and cataract extraction within last 2 months for this study. Inclusion-exclusion criteria were confirmed after reviewing the patient file, performing auto-refraction, slit-lamp examination, and pachymetry. Pachymetry was performed either on the previous day of enrollment or on the same day after all IOP measurements.
Randomization of eye and masking of the examiner
Measurements were taken only in one eye of each subject. Where both eyes were eligible, the eye with higher pressure was selected. If the pressure were equal in both eyes, then a random allocation of the right or left eye was performed.
After the initial examination, IOP measurements were taken by two masked examiners using three different instruments; Icare ic100, Icare TA01i, and GAT. Five IOP measurements each was taken with each instrument in the study eye; first with Icare ic100 followed by Icare TA01i and GAT.
The test tonometer (Icare ic100) and one of the reference tonometers (Icare TA01i) did not require anesthetizing drop as they use rebound technology. Both the Icare devices were handled by examiner one, from whom the IOP readings displayed on the device were masked and were recorded by examiner two. Examiner two measured IOP using GAT and the measurements were recorded by examiner one in the study eye.
Assessment of corneal health
During slit-lamp examination, the Oxford Scheme (a scale from 0–5) of corneal grading was used to grade corneal epithelial defects before the IOP measurements as well as after. Cornea was examined for defect by examiner two after every sequence of IOP measurements as described above.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (SPSS Inc, Chicago, Illinois, USA) and STATA 14.0 (StataCorp, College Station, TX). IOP measurements with the Icare ic100, Icare TA01i, and GAT were compared. Comparison of agreement of IOP using different instruments was quantified with intraclass correlation coefficient (ICC) using the two-way random effects models and absolute agreement and Cronbach's alpha. The test-retest variability of the instruments was assessed by deriving repeatability coefficient (RC) and coefficient of variation (CV). P value less than 0.05 was considered statistically significant.
Results
Among 150 participants, 86 (57.3%) were males and 64 (42.7%) were females with mean ± SD age of 49.70 ± 18.25 years (range: 18–87 years). Based on the intraocular pressure values, the participants were divided into three groups as follows: 7–16 mmHg (n = 60), >16 to <23 mmHg (n = 42), and ≥23 mmHg (n = 48) for analysis.
Icare ic100, Icare TA01i, and GAT were compared across the different IOP groups (based on GAT). There was no statistically significant difference in the mean IOP between the three instruments [Table 1].
Table 1.
Comparison of IOP using the Icare ic100, Icare TA01i, and Goldmann Applanation Tonometer (GAT)
| IOP (mmHg) (n) | Mean±SD | P | ||
|---|---|---|---|---|
| Icare ic100 | Icare TA01i | GAT | ||
| 7-16 (60) | 12.83±2.74 | 13.17±2.38 | 13.00±2.22 | 0.7591 |
| >16-<23 (42) | 18.07±2.30 | 18.67±2.39 | 19.10±1.56 | 0.0876 |
| ≥23 (48) | 30.06±6.91 | 29.85±6.97 | 30.75±6.83 | 0.8018 |
| Overall (150) | 19.81±8.59 | 20.05±8.35 | 20.39±8.62 | 0.8423 |
Reliability of the Icare ic100 was assessed in comparison to the other tonometers. Icare ic100 was found to have good reliability across all IOP groups with ICC value >0.78 when compared with IcareTA01i. In comparison with GAT, it showed good reliability across all IOP groups (ICC >0.87) except >16 to <23 mmHg group where it showed moderate reliability (ICC = 0.52). Agreement in IOP measurement between the instruments across the IOP groups is given in Table 2.
Table 2.
Comparison of Agreement of IOP Using the Icare ic100, Icare TA01i, and Goldmann Applanation Tonometer (GAT)
| Group | ICC 95% CI | 95% LoA | Cronbach’s alpha | |
|---|---|---|---|---|
| Icare ic100 vs. | 7-16 | 0.872 (0.786-0.923) | −3.69, 3.03 | 0.874 |
| Icare TA01i | >16-<23 | 0.785 (0.600-0.885) | −4.37, 3.18 | 0.797 |
| ≥23 | 0.988 (0.978-0.993) | −2.79, 3.21 | 0.988 | |
| Overall | 0.989 (0.985-0.992) | −3.64, 3.18 | 0.989 | |
| Icare TA01i vs. GAT | 7-16 | 0.818 (0.695-0.891) | −3.39, 3.72 | 0.816 |
| >16-<23 | 0.713 (0.471-0.845) | −3.27, 4.13 | 0.718 | |
| ≥23 | 0.976 (0.948-0.988) | −2.95, 4.74 | 0.979 | |
| Overall | 0.987 (0.981-0.990) | −4.11, 3.43 | 0.987 | |
| GAT vs. Icare ic100 | 7-16 | 0.876 (0.793-0.926) | −3.08, 3.42 | 0.876 |
| >16-<23 | 0.528 (0.138-0.744) | −3.18, 5.23 | 0.574 | |
| ≥23 | 0.979 (0.960-0.989) | −2.95, 4.33 | 0.981 | |
| Overall | 0.987 (0.980-0.991) | −3.13, 4.28 | 0.988 |
Repeatability of Icare ic100, Icare TA01i, and the GAT in measuring IOP was found to be excellent. The ICC, RC, and CV were 0.997 (95% confidence interval [CI]: 0.997–0.998), 2.67 (95% CI: 2.37%–2.97%), and 4.89% (95% CI: 4.33–5.44), respectively, for Icare ic100 [Table 3].
Table 3.
Repeatability of IOP measurement using Icare ic100, Icare TA01i, and Goldmann Applanation Tonometer (GAT)
| Icare ic100 | Icare TA01i | GAT | |
|---|---|---|---|
| ICC | 0.997 | 0.998 | 0.998 |
| 95% CI Lower | 0.997 | 0.997 | 0.997 |
| 95% CI Upper | 0.998 | 0.998 | 0.998 |
| Sw | 0.96 | 0.88 | 0.92 |
| 95% CI Lower | 0.85 | 0.78 | 0.82 |
| 95% CI Upper | 1.07 | 0.98 | 1.03 |
| RC | 2.67 | 2.43 | 2.55 |
| 95% CI Lower | 2.37 | 2.15 | 2.26 |
| 95% CI Upper | 2.97 | 2.70 | 2.84 |
| CVw (%) | 4.89 | 4.36 | 4.54 |
| 95% CI Lower | 4.33 | 3.86 | 4.02 |
| 95% CI Upper | 5.44 | 4.85 | 5.05 |
Sw is within subject standard deviation and CVw is the within-subject coefficients of variation
Using GAT IOP as a reference standard, the difference between Icare ic100 and GAT measurements did not increase with increasing IOP (P = 0.122) [Fig. 1].
Figure 1.
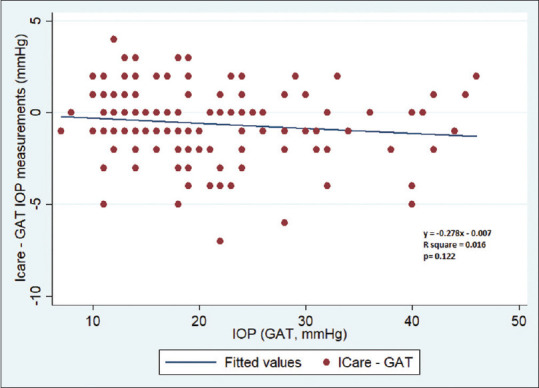
A scatter plot between IOP (GAT) and the difference between Icare ic100 and GAT measurements
IOP values measured by Icare ic100 and GAT showed a high correlation (r = 0.9556, P < 0.0001) [Fig. 2].
Figure 2.
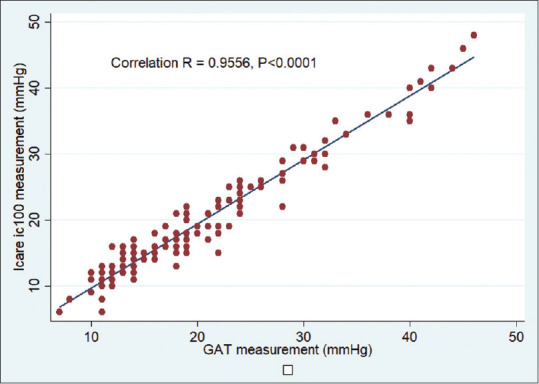
A scatter plot showing the correlation between GAT and the Icare ic100 measurements
Corneal health was assessed using the Oxford Scheme of corneal grading (a scale from 0–5) and was performed at baseline and after each attempt to measure IOP. Only 0.7% of eyes experienced an increase in staining after Icare ic100 measurements compared to baseline, whereas after the series of GAT measurements, 6.6% eyes experienced an increase in staining. And no eye experienced a clinically significant increase in corneal staining, i.e., two grades or more.
Discussion
This study showed that Icare ic100 is a reliable and repeatable instrument in measuring the intraocular pressure clinically over a range of 7–46 mmHg in both normal and glaucomatous eyes. We limited the eyes with central corneal thickness within 0.5–0.6 mm and corneal astigmatism less than 3D to reduce their effect on the IOP measurements.[5,6,7,8]
Although the GAT is considered as the gold standard in the measurement of IOP, GAT is a contact procedure which requires anesthetizing drop and a trained examiner. Studies have reported the variability of over 2 mmHg among two different GAT and this variability is further influenced by calibration challenges of GAT.[9,10,11] To take care of this calibration error, our GAT was calibrated daily in order to obtain accurate measurement.
Owing to its design and the working principle, Icare tonometers does not require any anesthesia during IOP measurements.[2] Studies have shown that Icare tonometers agree well with GAT and other applanation tonometers even though they overestimate and underestimate GAT readings in thicker and thinner corneas, respectively (530.5 ± 44.1 μm, range 420–636 μm).[3,12] Restricting the CCT of our study population from 500–600 μm and, thus, excluding thicker and thinner corneas in this study furthermore helped us to get accurate readings from individuals with normal CCT.
While prior studies have shown that the Icare TA01i RBT readings have less agreement with higher Goldmann tonometry intraocular pressures, in this study, in comparison with GAT, Icare TA01i showed good reliability across all IOP groups except for the group with IOP >16 to <23 (17–22) mmHg where it showed moderate reliability. This was probably the result of our inclusion criteria of corneal thickness and corneal astigmatism <3D.[13,14,15]
In this study, 5 sets of IOP Rebound Tonometer measurement were performed using 2 Icare tonometers followed by GAT measurement. This process helped in getting repeatable IOP measurements using all three tonometers, but multiple measurements in the same order could have an influence on IOP. When comparing the two RBT, Icare ic100 was found to have good reliability across all IOP groups when compared with IcareTA01i. Icare ic100 showed a moderate ICC compared to GAT for an IOP range of 17–22 (>16 to <23) mmHg.
Rebound tonometers are known to overestimate IOP compared to GAT in all range of central corneal thickness.[16,17] We found that the CCT of those 42 eyes with GAT IOP between 17 and 22 mmHg was 545.95 ± 25.9 μm (range: 503–597 μm). Although the mean IOP measured by Icare ic100 and GAT was similar (18.07 ± 2.30 vs. 19.10 ± 1.56, P = 0.0876), the individual difference in IOP does not get reflected in a mean value. To confirm this finding, we found our Icare ic100 IOP measurements to range between 13 and 23 mmHg, showing both underestimation and overestimation of IOP compared to the GAT range of 17–22 mmHg.
In a study, comparing repeatability of three different tonometers such as Icare pro, Tonopen, and GAT suggested that in a clinical setting, all three devices may be used interchangeably because of their good repeatability. But comparatively, GAT was found to be superior and our study similar to this study suggested that the other devices should only be used when it is not possible to use GAT.[18]
Similar to our study, Gao et al. also reported not a single eye with corneal epithelial defect after the rebound tonometer measurements.[19] In the present study, only 0.7% of eyes experienced an increase in staining after ic100 measurements compared to baseline.
The current study did not include factors such as high central corneal thickness, high astigmatism, dry eye, children with glaucoma or glaucoma suspect, and recent history of ocular surgery. This was done to avoid possible confounding effect on IOP measurements due to these factors, but it will be good to explore further clinical agreement and repeatability study in patients with these factors.
Goldmann applanation tonometry will remain the gold standard for the foreseeable future. Our study results show that both the Icare tonometers to have good repeatability. ICC values for GAT, Icare ic100, and Icare TA01i are very much similar suggesting that the devices are as repeatable as GAT.
Conclusion
The results of the study demonstrate that Icare ic100 rebound tonometer can measure Intraocular pressure with relatively small measurement error and can provide a reliable and repeatable reading in comparison with GAT across a wide pressure range without hampering corneal health.
Financial support and sponsorship
ICare Finland Oy provided the funding to conduct this work.
Conflicts of interest
There are no conflicts of interest.
References
- 1.De Moraes CG, Prata TS, Liebmann J, Ritch R. Modalities of tonometry and their accuracy with respect to corneal thickness and irregularities. J Optom. 2008;1:43–9. [Google Scholar]
- 2.Dahlmann-Noor AH, Puertas R, Tabasa-Lim S, EI-Karmouty A, Kadhim M, Wride NK, et al. Comparison of handheld rebound tonometry with Goldmann applanation tonometry in children with glaucoma: A cohort study. BMJ Open. 2013;3:e001788. doi: 10.1136/bmjopen-2012-001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L. Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2006;15:213–7. doi: 10.1097/01.ijg.0000212208.87523.66. [DOI] [PubMed] [Google Scholar]
- 4.Munkwitz S, Elkarmouty A, Hoffmann EM, Pfeiffer N, Thieme H. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes Arch Clin Exp Ophthalmol. 2008;246:875–9. doi: 10.1007/s00417-007-0758-3. [DOI] [PubMed] [Google Scholar]
- 5.Shimmyo M, Ross AJ, Mostafavi R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasian, Asians, Hispanics, and Hispanics, and Africans. Am J Ophthalmol. 2003;136:603–13. doi: 10.1016/s0002-9394(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 6.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–6. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 7.Bhan A, Browning AC, Shah S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol. 2002;43:1389–92. [PubMed] [Google Scholar]
- 8.Bron AM, Creuzot-Garcher C, Goudeau-Boutillon S, d'Athis P. Falsely elevated intraocular pressure due to increased central corneal thickness. Graefes Arch Clin Exp Ophthalmol. 1999;237:220–4. doi: 10.1007/s004170050222. [DOI] [PubMed] [Google Scholar]
- 9.George R, Arvind H, Baskaran M, Ramesh SV, Raju P, Vijaya L. Agreement between two Goldmann type applanation tonometers. Indian J Ophthalmol. 2008;56:516–7. doi: 10.4103/0301-4738.43381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhari NS, George R, Baskaran M, Vijaya L, Dudeja N. Measurement of Goldmann applanation tonometer calibration error. Ophthalmology. 2009;116:3–8. doi: 10.1016/j.ophtha.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Choudhari NS, Jadhav V, George R, Vijaya L. Variability in the calibration error of the goldmann applanation tonometer. J Glaucoma. 2011;20:492–6. doi: 10.1097/IJG.0b013e3181f464b8. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Darhad U, Tatsumi Y, Fujioka M, Kusuhara A, Maeda H, et al. Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006;142:332–4. doi: 10.1016/j.ajo.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L. Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2006;15:213–7. doi: 10.1097/01.ijg.0000212208.87523.66. [DOI] [PubMed] [Google Scholar]
- 14.Iliev ME, Goldblum D, Katsoulis K, Amstutz C, Frueh B. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br J Ophthalmol. 2006;90:833–5. doi: 10.1136/bjo.2005.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KN, Jeoung JW, Park KH, Yang MK, Kim DM. Comparison of the new rebound tonometer with Goldmann applanation tonometer in a clinical setting. Acta Ophthalmol (Copenh) 2013;91:e392–6. doi: 10.1111/aos.12109. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia-Sanchez J. Reproducibility and clinical evaluation of rebound tonometry. Invest ophthalmol. 2005;46:4578–80. doi: 10.1167/iovs.05-0586. [DOI] [PubMed] [Google Scholar]
- 17.Davies LN, Bartlett H, Mallen EA, Wolffsohn JS. Clinical evaluation of rebound tonometer. Acta Ophthalmol (Copenh) 2006;84:206–9. doi: 10.1111/j.1600-0420.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 18.Schweier CM, Toeteberg-Harms M, Funk J. Repeatability of intraocular pressure measurements in sitting and supine position with Icare Pro™. Invest Ophthalmol. 2012;53:5075. [Google Scholar]
- 19.Gao F, Liu X, Zhao Q, Pan Y. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer. Exp Ther Med. 2017;13:1912–6. doi: 10.3892/etm.2017.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]


