Abstract
Pathological neovascularization in the eye is a leading cause of blindness in all age groups from retinopathy of prematurity (ROP) in children to age-related macular degeneration (AMD) in the elderly. Inhibiting neovascularization via antivascular endothelial growth factor (VEGF) drugs has been used for the effective treatment. However, anti-VEGF therapies may cause development of chorioretinal atrophy as they affect a physiological amount of VEGF essential for retinal homeostasis. Furthermore, anti-VEGF therapies are still ineffective in some cases, especially in patients with AMD. Hypoxia-inducible factor (HIF) is a strong regulator of VEGF induction under hypoxic and other stress conditions. Our previous reports have indicated that HIF is associated with pathological retinal neovascularization in murine models of ROP and AMD, and HIF inhibition suppresses neovascularization by reducing an abnormal increase in VEGF expression. Along with this, we attempted to find novel effective HIF inhibitors from natural foods of our daily lives. Food ingredients were screened for prospective HIF inhibitors in ocular cell lines of 661W and ARPE-19, and a murine AMD model was utilized for examining suppressive effects of the ingredients on retinal neovascularization. As a result, rice bran and its component, vitamin B6 showed inhibitory effects on HIF activation and suppressed VEGF mRNA induction under a CoCl2-induced pseudo-hypoxic condition. Dietary supplement of these significantly suppressed retinal neovascularization in the AMD model. These data suggest that rice bran could have promising therapeutic values in the management of pathological ocular neovascularization.
Keywords: hypoxia-inducible factor, age-related macular degeneration, vascular endothelial growth factor, food ingredients, rice bran, vitamin B6, retinal pigment epithelium
1. Introduction
Ocular pathological neovascularization is a leading cause of blindness worldwide [1,2]. It affects our lives in all age groups from children to elderly with various names of diseases such as retinopathy of prematurity (ROP), diabetic retinopathy (DR), retinal vein occlusion and age-related macular degeneration (AMD) [3]. To date, a blockade of vascular endothelial growth factor (VEGF) using anti-VEGF antibodies has been applied for the treatment of these diseases [4], as VEGF plays a crucial pathological role in the development of these diseases [5,6]. Even though small concentrations for the treatment to a local target site may not affect systemic side effects of the drugs, repetitive administrations of anti-VEGF drugs for chronic therapies could alter a systemic or local VEGF amount which may be required for normal vascular and neuronal maintenance [4]. Moreover, anti-VEGF drugs are still ineffective in some cases, especially in patients with AMD [7].
Hypoxia-inducible factor (HIF) plays a strong transcription regulator of VEGF induction under hypoxic and other stress conditions [8]. Under hypoxic conditions, HIF translocates to the nucleus and binds the hypoxia response element (HRE), inducing hypoxia responsive gene expressions including VEGF as well as B-cell lymphoma 2 interacting protein 3 (BNIP3) and phosphoinositide-dependent kinase 1 (PDK1) [9,10]. As the HIF/VEGF axis is a strong pathological pathway for neovascularization [11,12], inhibiting HIF activation could be an attractive target for antineovascularization therapies. Moreover, HIF expression was observed in human choroidal neovascular membranes in patients with AMD [13,14], and retinal pigment epithelial (RPE) cells resided in those membranes were localized with the presence of HIF and VEGF [14].
Previously, we demonstrated HIF inhibition-suppressed retinal neovascularization genetically and pharmacologically in murine models of oxygen-induced retinopathy (OIR), one of the ROP models, and laser-induced choroidal neovascularization (CNV), one of the AMD models [15,16,17,18,19]. Moreover, another HIF inhibitor, halofuginone, a synthetic derivative of febrifugine isolated from hydrangea, exerted retinal protection in a murine ischemia–reperfusion model [20].
In our daily lives, we consume extensive amounts of food. Even though food has been classically perceived as a simple means for energy production and body construction, their potentials have expanded to drug discovery and development for various diseases [21,22,23]. Diet that contains omega-3 fatty acids could enhance cognitive functions in humans [24]. A suggestive association of a vegetable-rich and low carbohydrate diet and a lower risk of early paracentral visual loss in primary open-angle glaucoma was recently reported in data from three United States cohorts [25]. There are still lots of food ingredients that could be unraveled in terms of potent therapeutic effects that they possess on various diseases including retinal neovascularization.
Along with this, we obtained various types of food ingredients and attempted to find novel effects of them through drug screenings: inhibitors of HIF activation. After screenings, we investigated therapeutic effects of positively selected ingredients on retinal neovascularization in a murine model of laser-induced CNV.
2. Materials and Methods
2.1. Animal
All experimental procedures were approved by the Ethics Committee on Animal Research of the Keio University School of Medicine (approved number #16017-2 on 12 October 2018) and followed with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the international standards of animal care and use in ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines (http://www.nc3rs.org.uk/arrive-guidelines). C57BL/6 and BALB/c male mice were obtained from CLEA Japan (Tokyo, Japan) and raised in a standardized temperature-controlled environment under a 12 h light-dark cycle with free access to water and food.
2.2. Cell Culture
A mouse cone photoreceptor 661W cell line was maintained in DMEM (Cat #08456-36, Nacalai Tesque, Kyoto, Japan) media with 10% FBS and 1% streptomycin-penicillin under an atmospheric condition containing 5% CO2 at 37 °C. A human cell line for retinal epithelial ARPE-19 was maintained in DMEM/F-12 (Cat #C11330500BT, Gibco, Waltham, MA, USA) media with the same supplements above in the same atmospheric condition.
2.3. Food Ingredient Preparation and Luciferase Assay
Food ingredients for the screening were prepared as listed in Table A1. Then, a luciferase assay for drug screenings on inhibition of HIF activation was performed as previously described in our papers [15,16,17,20]. Briefly, 661W and ARPE-19 cells, transfected with a HIF-luciferase reporter gene construct (Cignal Lenti HIF Reporter, Qiagen, Venlo, Netherlands) encoding a firefly luciferase gene under a control of the HRE, were seeded and treated with a well-known HIF inducer CoCl2 (200 µM, cobalt (II) chloride hexahydrate, Wako, Osaka, Japan). To evaluate inhibitory effects of food ingredients on HIF activation, cells were cotreated with 1 mg/mL of each ingredient and CoCl2. 24 h after incubation, luminescence was measured using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
2.4. MTT Assay
For determination of cytotoxicity of food ingredients under a CoCl2-induced hypoxic condition, ARPE-19 cells were seeded in 96-well plates and the ingredients were treated to the cells for 12 h. A total of 10 µL of MTT solution (Cat #M2128, Sigma, St. Louis, MO, USA) was added to each well and incubated for 2 h at 37 °C. After labeling the cells with MTT, media were removed from the wells and 100 µL of DMSO was added to each well. Then, absorbance of colored solution in the wells was measured at 540 nm (Synergy HT Multi-Mode Microplate Reader, Winooski, VT, USA).
2.5. Quantitative PCR and Western Blotting
RNA extraction, cDNA synthesis and real-time quantitative PCR were performed as same as previously described in our papers [15,16,17,20]. Primers used are listed in Table 1. Fold changes between levels of different transcripts were calculated by the ΔΔCT method.
Table 1.
Primer list.
| Name | Direction | Sequence (5′ → 3′) |
|---|---|---|
| GAPDH | Forward | TCCCTGAGCTGAACGGGAAG |
| Reverse | GGAGGAGTGGGTGTCGCTGT | |
| HIF-1α | Forward | TTCACCTGAGCCTAATAGTCC |
| Reverse | CAAGTCTAAATCTGTGTCCTG | |
| VEGF | Forward | TCTACCTCCACCATGCCAAGT |
| Reverse | GATGATTCTGCCCTCCTCCTT | |
| BNIP3 | Forward | GGACAGAGTAGTTCCAGAGGCAGTTC |
| Reverse | GGTGTGCATTTCCACATCAAACAT | |
| PDK1 | Forward | ACAAGGAGAGCTTCGGGGTGGATC |
| Reverse | CCACGTCGCAGTTTGGATTTATGC |
Protein extraction, electrophoresis and visualization of protein bands were performed as the same as previously described in our papers [15,16,17,20]. Antibodies used were anti-HIF-1α (1:1000, Cat #36169, Cell Signaling Technology, Danvers, MA, USA), anti-HIF-2α (1:1000, Cat #NB100-122, Novus Biologicals, Centennial, CO, USA) and anti-β-Actin (1:5000, #3700, Cell Signaling Technology, Danvers, MA, USA). For visualization, HRP-conjugated secondary antibodies (1:5000, GE Healthcare, Chicago, IL, USA) were used. Blotting was quantified using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.6. A Laser-Induced CNV Model and Measurement of CNV Volumes
A murine laser-induced CNV model was produced as previously described in our papers [15,16]. Briefly, the eyes of C57BL/6 mice were dilated by a combination of 0.5% tropicamide and 0.5% phenylephrine (Santen Pharmaceutical, Osaka, Japan) and the mice were anesthetized by a combination of midazolam (Sandoz, Tokyo, Japan), medetomidine (Orion, Espoo, Finland) and butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan), termed ‘MMB’. After anesthesia, the eyes of mice were covered with a contact lens (Haag-Streit Diagnostics, Koniz, Switzerland) to see the retinas clearly. A total of 5 laser spots (532 nm argon laser, 200 mw, 100 ms, 75 mm) were placed between the retinal vessels, located 2-disc diameters from the optic nerve head. During irradiation by laser, an air bubble was used as an index of Bruch’s membrane disruption. Laser lesions that lack the air bubble or have occurrence of hemorrhage were excluded from data analyses [15,16]. At day 7 after irradiation, the mice were anesthetized by MMB, followed by euthanasia, and the eyes were enucleated by forceps. The retinas from the eyes were flat-mounted and stained with isolectin B4 (Invitrogen, Carlsbad, CA, USA) [15,16]. We observed CNV with a laser microscope (Zeiss, Oberkochen, Germany) and measured a volume of CNV using Imaris (Bitplane, Zurich, Switzerland) as previously described in our papers [15,16].
2.7. A Light-Induced Retinopathy (LIR) Model and Measurement of Retinal Function by Electroretinography (ERG)
Development of a murine light-induced retinopathy (LIR) model was modified from our previous study [26], the exposure of 3000 lux white light to the eyes for 1 h. After 1 week following the light exposure, the eyes of BALB/c mice were dilated by a combination of 0.5% tropicamide and 0.5% phenylephrine (Santen Pharmaceutical, Osaka, Japan) and the mice were anesthetized by MMB under a dark room. After anesthesia, retinal function was evaluated by ERG, as previously described in our paper [18]. Briefly, active electrodes were recorded in the contact lens and a reference electrode was placed in the mouth. ERG responses were obtained from both eyes of each animal. Scotopic responses were recorded with various stimuli. The amplitudes of a-wave were measured from the baseline to the lowest point of a-wave. The amplitudes of b-wave were measured from the lowest peak of a-wave to the highest peak of b-wave. During the procedure, all mice were kept in warm conditions using heat pads.
2.8. Statistical Analysis
Data analyses were performed using Prism 5 (GraphPad, San Diego, CA, USA). Statistically significant differences were calculated using a two-tailed Student’s t-test or one-way ANOVA followed by a Bonferroni post hoc test. p-value of less than 0.05 was regarded as statistically significant.
3. Results
3.1. Rice Bran or Vitamin B6 Shows Inhibitory Effects on HIF Activation in ARPE-19 Cells under a CoCl2-Induced Hypoxic Condition
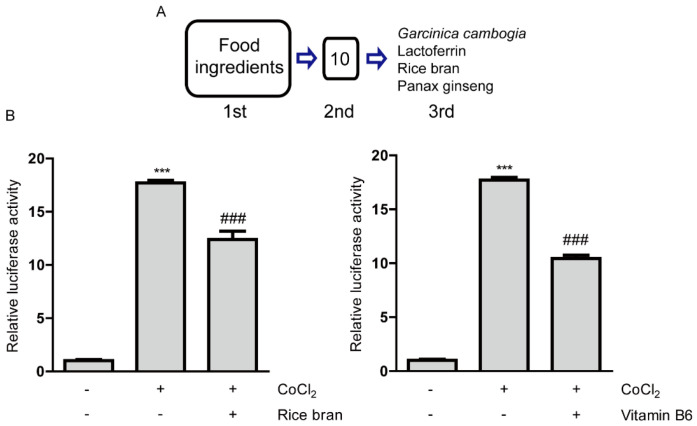
Food ingredients (with their expected components) were screened for inhibition of HIF activity (Figure 1A and Table A1). For the first gross screening, 661W cells (mouse immortalized cone photoreceptor cells with some features of retinal ganglion precursor-like cells) were utilized, as this cell line has been widely used as one of the in vitro cell models for ophthalmic drug development [27,28] and a HIF-luciferase reporter stable cell line has already been established [15,16]. Screened samples were in order of strong inhibitory tendencies on HIF activation (Table A1). At the first line, the top 10 samples with strong inhibitory tendencies were selected among 202 samples on HIF activation under a CoCl2-induced hypoxic condition (number 1–10 in Table A1). After collecting the 10 samples (garcinia fruit extract with or without water solubility and lactoferrin and lactoferrin from milk were considered as one sample after the gross first screening), we carefully evaluated inhibitory effects of them again at the second screening with the same system. We found that only six samples consistently showed statistically significant inhibitory effects on HIF activation under a CoCl2-induced hypoxic condition (Table A2).
Figure 1.
Inhibitory effects of rice bran and vitamin B6 on hypoxia-inducible factor (HIF) activity. (A) A process of drug screenings for HIF inhibitors. After the first screening, 10 samples were shown to be positive. After the second screening, four food ingredients (lactoferrin, rice bran, panax ginseng and Garcinia cambogia) with their expected 2 component compounds (hydroxycitric acid and vitamin B6) were selected as HIF inhibitors. (B) Quantitative analyses of HIF-reporter luciferase assay using ARPE-19 cells (n = 3 per group) showed that rice bran (1 mg/mL) and vitamin B6 (1 mg/mL) inhibited HIF activity induced by 200 µM CoCl2. *** p < 0.001, ### p < 0.001, compared with no treatment and 200 µM of CoCl2 treatment, respectively. Bar graphs were presented as mean with the ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
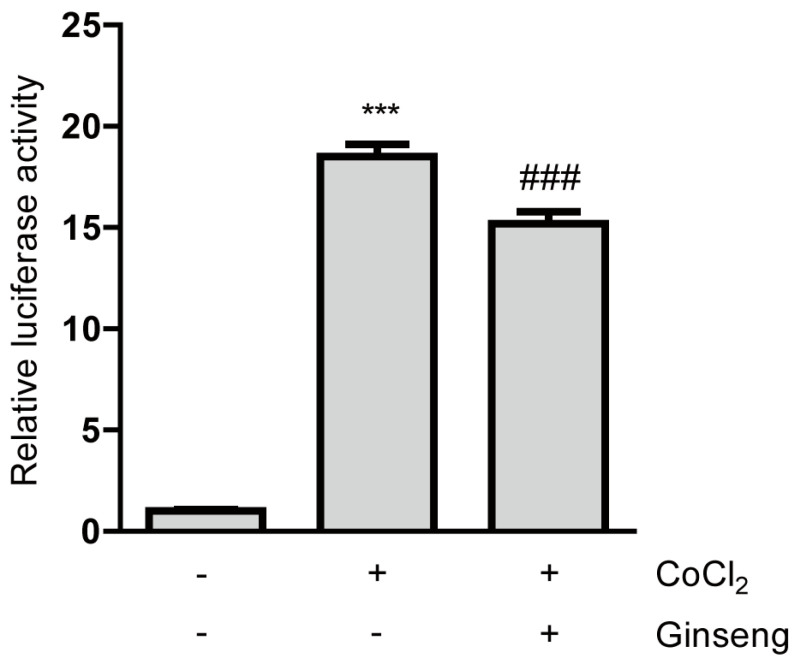
We proceeded to the next final screening with these six samples. For the third final screening, we used ARPE-19 cells (human retinal pigmented epithelium cells) as this cell line also has been widely used for ophthalmic drug development [27,29]. In addition, this cell type, RPE cells, has been suggested as one of main pathological reasons for the development of CNV, finally leading to AMD [30,31,32]. Through the final screening, we found that six samples showed statistically significant HIF inhibitory effects (Figure 1B and Figure A1 and [15,16]). Taken together, four food ingredients (with their expected two component compounds, hydroxycitric acid and vitamin B6) were positively selected as inhibitors of HIF activation, as listed ‘Garcinia cambogia’, ‘lactoferrin’, ‘rice bran (Oryza sativa Linne, Gramineae, defatted)’ and ‘ginseng’ (Figure 1A). Based on the screening results, we could demonstrate therapeutic effects of lactoferrin and Garcinia cambogia (and its abundant component hydroxycitric acid) via inhibition of HIF activation in murine models of CNV [15,16].
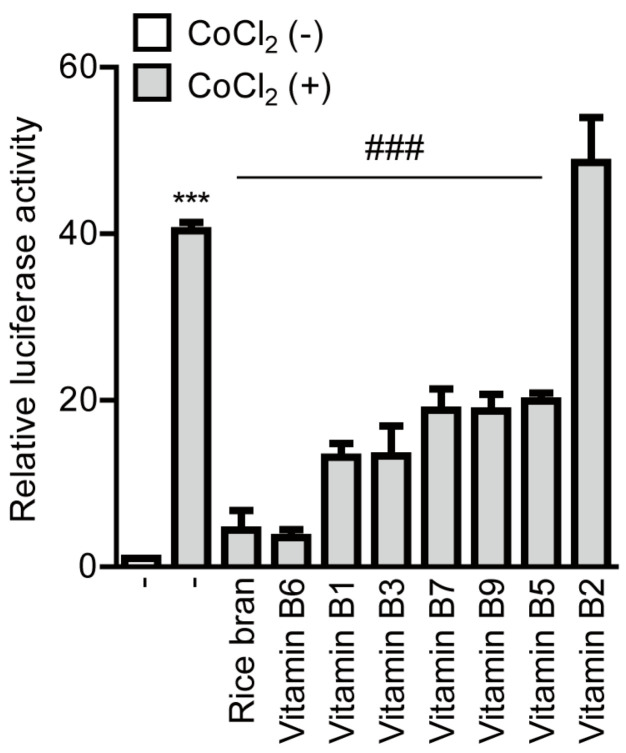
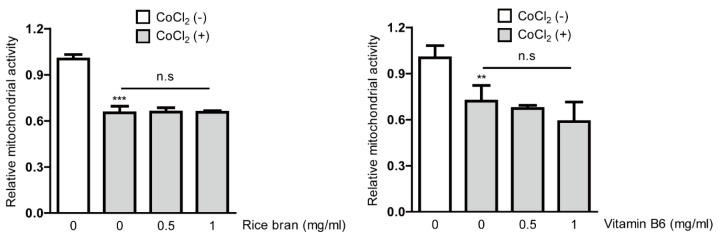
Next, with the rest of the positively selected food ingredients (rice bran or ginseng) from the screenings, we further attempted to examine which components inside defatted rice bran or ginseng could help it to exert HIF inhibitory effects. While we could not find which components inside ginseng could help it to have HIF inhibitory effects, among the components contained in rice bran (Table A3), we have found that vitamin B6 showed a significant and the most robust HIF inhibitory effect (Figure 1B and Figure A2). Taken together, in this current study, we mainly focused on unraveling potent effects of rice bran and vitamin B6 as novel HIF inhibitors. For further experiments under a CoCl2-induced hypoxic condition, we examined whether rice bran or vitamin B6 has cellular toxicity using MTT assay (Figure A3). Basically, cytotoxicity of them was not significantly detected although there was a decreasing tendency in mitochondrial activity in high-dose vitamin B6 (1 mg/mL)-treated group.
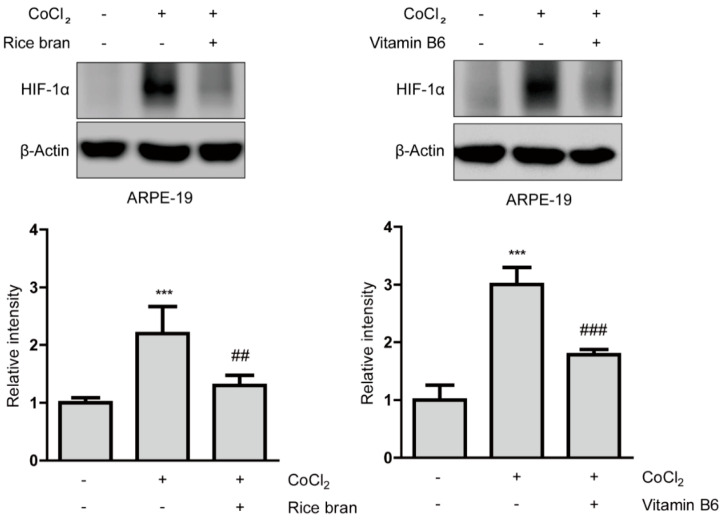
3.2. Rice Bran or Vitamin B6 Has Suppressive Effects on HIF Stabilization in ARPE-19 Cells under a CoCl2-Induced Hypoxic Condition
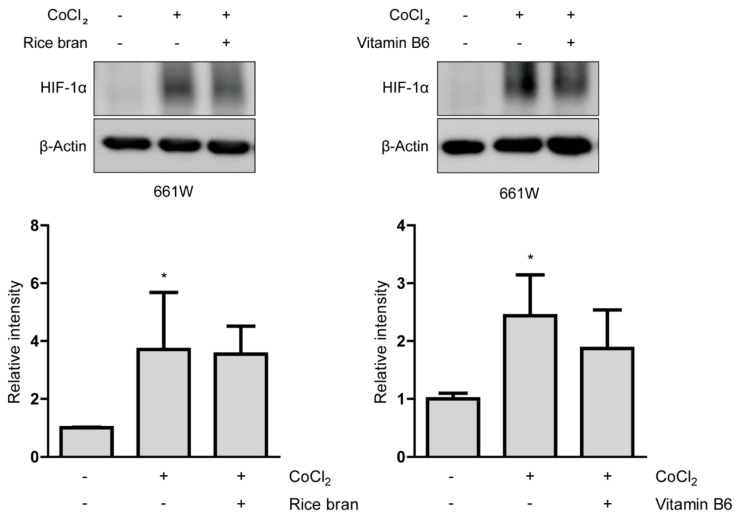
Suppressive effects of rice bran and vitamin B6 on HIF stabilization in protein levels were examined (Figure 2). HIF-1α expression was stabilized in ARPE-19 cells 6 h after incubation of 200 µM of CoCl2. Then, stabilized HIF-1α expression was significantly reduced by rice bran and vitamin B6 treatments, respectively. On the other hand, in 661W cells, there was no statistical difference by rice bran or vitamin B6 treatment in stabilized HIF-1α expression 6 h after incubation of 200 µM of CoCl2, (Figure A4). These results indicate that rice bran and vitamin B6 could have suppressive effects on HIF-1α stabilization in RPE cells more than neuronal cells.
Figure 2.
Suppressive effects of rice bran and vitamin B6 on HIF-1α stabilization. Representative immunoblot images and quantitative analyses (n = 4 per group) for HIF-1α and β-Actin showed that HIF-1α was stabilized in ARPE-19 cells under a CoCl2-induced pseudo-hypoxic condition. Rice bran (1 mg/mL) and vitamin B6 (1 mg/mL) significantly decreased stabilized HIF-1α expression. *** p < 0.001, compared with no treatment, ## p < 0.01, ### p < 0.001, compared with CoCl2 treatment. Bar graphs were presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
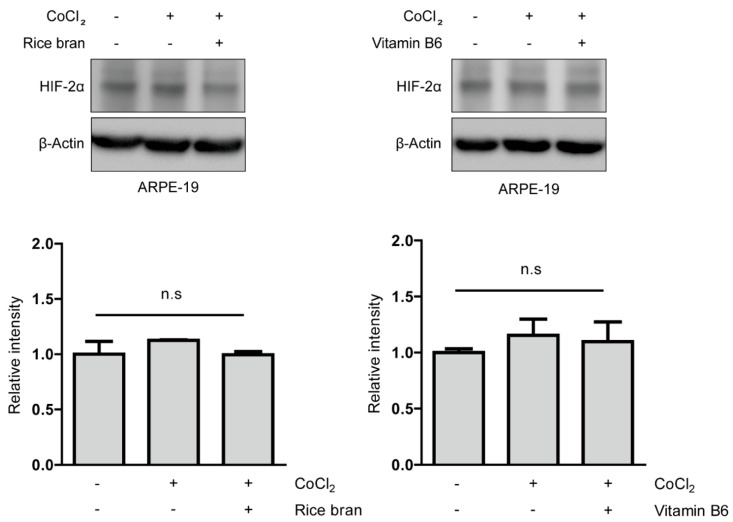
Next, we examined whether rice bran and vitamin B6 could act on another HIF expression (HIF-2α) in ARPE-19 cells under the same condition (Figure A5). We could not see a significant increase in HIF-2α expression under a CoCl2-induced hypoxic condition, and rice bran and vitamin B6 did not change its expression. Taken together, it indicates that HIF-1α (rather than HIF-2α) might be the major regulator in ARPE-19 cells under this hypoxic condition, which is in agreement with several previous reports using ARPE-19 cells [33,34,35].
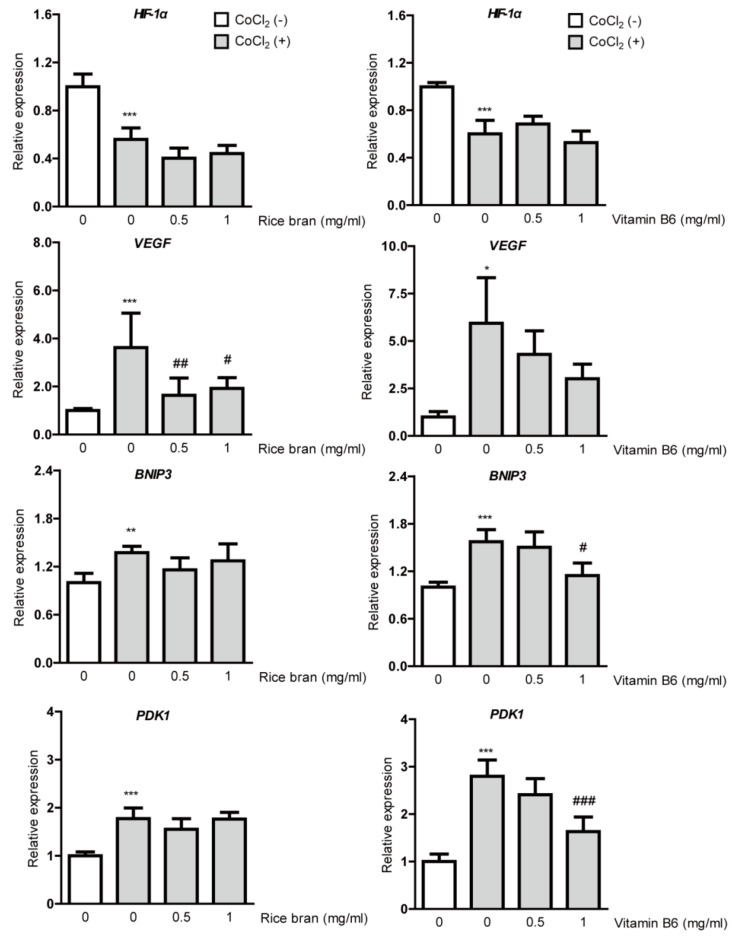
3.3. Rice Bran or Vitamin B6 Inhibits the HIF/VEGF Axis in ARPE-19 Cells under a CoCl2-Induced Hypoxic Condition
Suppression on HIF-1α stabilization in RPE cells could be followed by inhibition of DNA binding of stabilized HIF-1α, finally reducing induction of HIF target gene expressions, especially VEGF. Thus, we examined whether rice bran or vitamin B6 suppresses HIF downstream-target hypoxia-responsive gene expressions under a CoCl2-induced hypoxic condition (Figure 3). A total of 8 h after 200 µM of CoCl2 incubation in ARPE-19 cells, downregulation of HIF-1α mRNA expression was detected due to a negative feedback from the post translational HIF-1α protein modification, which has been consistently seen in several previous papers [15,16,19,36]. CoCl2 induced upregulation of VEGF, BNIP3 and PDK1 mRNA expressions as results of HIF activation. Upregulated VEGF mRNA expression was significantly reduced by rice bran treatment. Although upregulated VEGF mRNA expression was not significantly reduced by vitamin B6 treatment, its expression showed a decreasing tendency. On the other hand, upregulated BNIP3 and PDK1 mRNA expressions were not significantly reduced by rice bran treatment although these expressions were significantly reduced by vitamin B6 treatment.
Figure 3.
Suppression of hypoxia-responsive gene expressions by rice bran and vitamin B6. Quantitative analyses (n = 4–6 per group) showed significant changes in HIF-1α, VEGF, BNIP3 and PDK1 mRNA expressions 8 h after incubation of CoCl2 in ARPE-19 cells. Upregulated VEGF mRNA expression was significantly reduced by rice bran treatment. There was a decreasing tendency of upregulated VEGF mRNA expression by vitamin B6 treatment. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with no treatment, # p < 0.05, ## p < 0.01, ### p < 0.001, compared with CoCl2 treatment. Bar graphs were presented as mean with ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
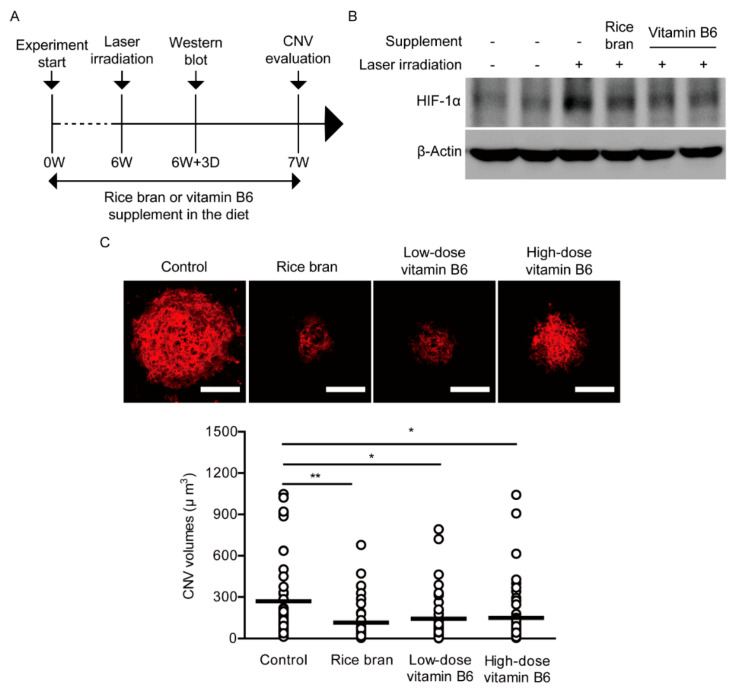
3.4. Rice Bran or Vitamin B6 Administration Suppresses Retinal Neovascularization in a Murine Model of CNV
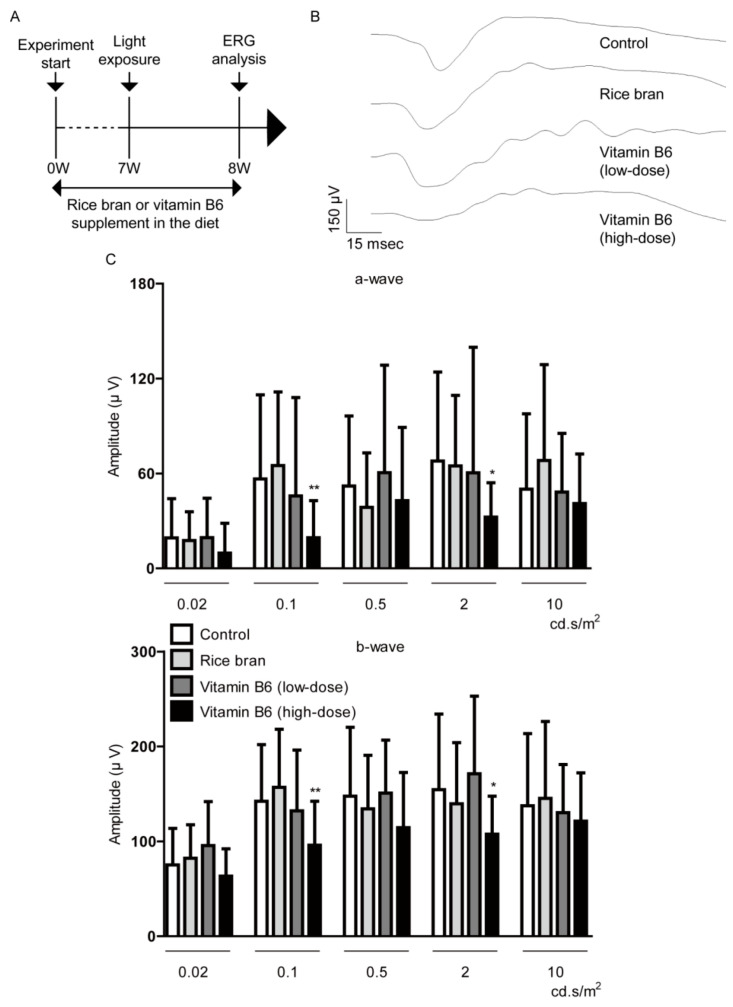
To assess therapeutic effects of rice bran and vitamin B6 on pathological neovascularization, a vitamin B6-deficient diet (AIN-93G, Oriental Yeast Co. Ltd., Tokyo, Japan) supplemented with vitamin B6 (9 or 35 mg/kg diet weight, DSM Nutritional Products Ltd., Kaiseraugst, Switzerland) or rice bran (defatted, 587.5 mg/kg diet weight, Oryza Oil & Fat Chemical Co., Ltd., Ichinomiya, Japan) with vitamin B6 (1 mg/kg diet weight) was given to 4-weeks-old male mice for total 7 weeks (Figure 4A). The control group was provided with the basic diet (AIN-93G) which only contains 1 mg of vitamin B6 in 1 kg of the diet. This amount of vitamin B6 is lower than that of normal diet commonly used for mouse studies; about 5–8 mg of vitamin B6 in 1 kg of diet, of which value could be considered as the roughly recommended amount for a daily life consumption.
Figure 4.
Suppression of neovascularization by rice bran and vitamin B6. (A) A schematic illustration demonstrates the murine choroidal neovascularization (CNV) model procedure and administration of rice bran or vitamin B6 to mice. (B) An immunoblot image for HIF-1α and β-Actin in the retina with or without the supplement of rice bran or vitamin B6, 3 days after the laser irradiation. (C) Representative images of CNV in the whole mount staining with isolectin B4 and quantitative analyses (n = 5–6 per group, n = 42–49 laser spots in the eyes per group) showed that the volume of CNV was significantly reduced by administration of rice bran (587.5 mg/kg diet weight) and vitamin B6 (9 or 35 mg/kg diet weight), respectively. Scale bars, 100 μm. * p < 0.05, ** p < 0.01. Dot plot graphs were presented as mean. The data were analyzed using Student’s t-test.
After 3 days of irradiation by laser which was performed on week 6 after administration of diet supplemented with rice bran or vitamin B6, we could see stabilized HIF-1α expression in the laser-irradiated retina and its expression was reduced by the rice bran- or vitamin B6-administered retina (Figure 4B). After 1 week of irradiation by laser, we quantified CNV volume as previously described [15,16]. A decreased volume of CNV was significantly observed in the rice bran- or vitamin B6-administered mice in comparison with that in the control mice, respectively (Figure 4C).
3.5. Rice Bran or Vitamin B6 Administration Did Not Directly Affect Neuronal Dysfunction in a Murine Model of LIR
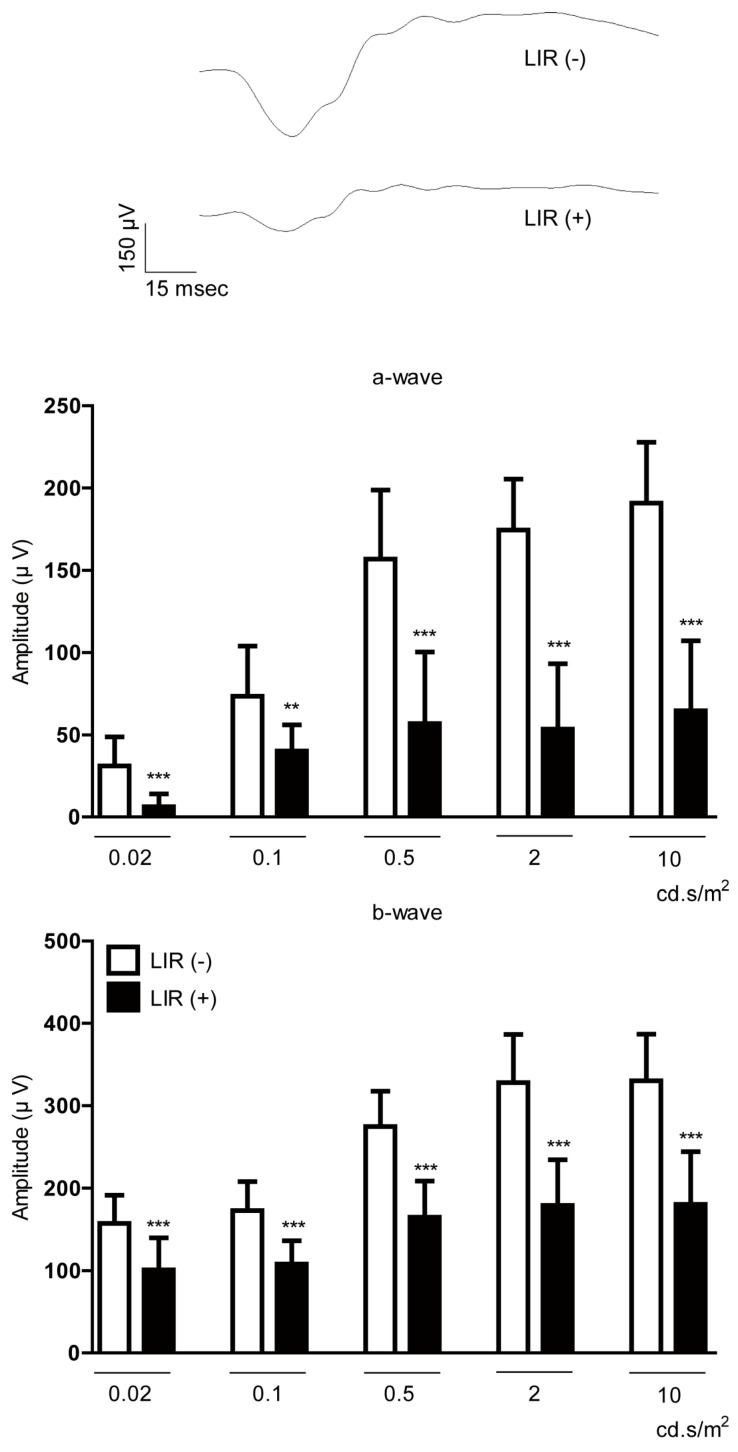
To assess the therapeutic effects of the dietary supplement on protection in retinal function, we employed a murine LIR model of retinal degeneration. In this model, we could observe retinal dysfunction 1 week after the light exposure analyzed by ERG (Figure A6), which is in the basic agreement with previous observations [26,37,38].
Next, we attempted to examine therapeutic effects of rice bran and vitamin B6 on direct retinal protection. A diet supplemented with rice bran or vitamin B6 was given to 5-weeks-old male mice for a total 8 weeks (Figure 5A). The control group was provided with a normal diet (which basically contains 1 mg/kg diet weight of vitamin B6) without the additional supplement. A total of 1 week after the light exposure which was performed on week 7 after administration of the diet supplemented with rice bran or vitamin B6, we found no significant change in the amplitudes of a- and b-waves among all of the groups except for the high-dose vitamin B6-administered group (Figure 5B,C).
Figure 5.
Direct retinal protection by rice bran and vitamin B6. (A) A schematic illustration demonstrates the murine light-induced retinopathy (LIR) model procedure and administration of rice bran or vitamin B6 to mice. (B,C) Representative waveforms of a- and b-waves (2 cd.s/m2) and quantitative analyses showed that rice bran (587.5 mg/kg diet weight) or vitamin B6 (9 mg/kg diet weight) did not change the amplitudes of a-wave and b-wave in LIR mice (n = 9–10 per group, 18–20 eyeballs per group). There was a significant decrease in the amplitudes of a-wave and b-wave in high-dose vitamin B6 (35 mg/kg diet weight)-administered LIR mice. * p < 0.05, ** p < 0.01, compared with control. The data were analyzed using Student’s t-test.
4. Discussion
Rice bran is a hard-outer covering of rice grains and it is produced as a byproduct of milling for the production of refined rice [39]. In East Asia, people simply use rice bran to enrich some dishes and wish to increase their intake of dietary fiber as it contains vitamins, minerals, fatty acids, dietary fiber, and other sterols [39]. Rice bran has been traditionally thought to be a normal fiber material and considered enough for making cooking oil or feeding their livestock such as cattle or pigs. However, its high nutritional values received considerable attention after their therapeutic potentials were reported in various diseases [40]. In the current study, we found a new aspect of uses of rice bran as an HIF inhibitor and suggested vitamin B6 would be a working compound for this role among the components.
HIF plays a critical role in the maintenance of cellular homeostasis responded by alteration of oxygen status [8,9,10]. However, under pathological hypoxic conditions, HIF activation can cause devastating outcomes, in this case, retinal neovascularization [11]. Previously, we demonstrated that pharmacological intervention via administration of doxorubicin or topotecan [18], marine products [17], and a mushroom product [19] showed therapeutic effects on retinal neovascularization through inhibition of HIF activation and suppression of its downstream VEGF expression. In addition, we also demonstrated a significant reduction of CNV volume in RPE-specific Hif-1α-conditional knockout mice in comparison with that in control mice [15,41], which implies that genetic intervention of HIF is also a beneficial target against pathological hypoxic conditions in the eyes. In our current study, oral administration of rice bran or vitamin B6 as a diet supplement also showed similar therapeutic effects on retinal neovascularization. Taken together, we think that inhibition of HIF activation could have benefits for managing pathological ocular neovascularization.
The development of AMD involves complex pathological mechanisms. Above all, increasing evidence suggests that dysfunction of RPE is crucially involved in neovascular and atrophic forms of AMD [42]. RPE is a monolayer of polarized cells and lies underneath the retina intimately interacting with photoreceptor cells, of which communication is important for retinal homeostasis [43]. RPE controls the outer blood-retinal barrier via regulation of nutrient and oxygen delivery to the retina and clearance of metabolic waste from photoreceptor cells. RPE physiologically produces growth factors such as VEGF to support the retina and choriocapillaris [42,44,45]. Damages could occur to RPE over many years with ageing and increasing pathological stresses, which causes dysfunction of RPE and following pathological release of growth factors from RPE, especially VEGF [30,31]. This may be one of main pathological reasons for the development of CNV that finally leads to AMD [32]. In our study, upregulated VEGF mRNA expression in APRE-19 cells under a CoCl2-induced hypoxic condition was reduced by rice bran treatment. However, more data are required to unravel the in vivo mode of action regarding the reduction in VEGF levels in the eye by administration of rice bran or vitamin B6. This will be further studied. Moreover, CNV is one of the complex tissues in the eye composed of vascular components including endothelial cells, vascular smooth muscle cells, and pericytes, and extravascular cells such as inflammatory cells, fibroblasts, glial cells, and RPE cells [46,47,48]. Recently, endothelial cells, vascular smooth muscle cells, macrophages, and RPE cells have been suggested to be main cell types of CNV formation [46,47,48]. It is still hard to tell which cells exactly give the most contribution of CNV formation. Therefore, we think that more comprehensive studies regarding the relationship of VEGF-producing cell types with contribution of CNV formation may be needed for a better understanding of pathological mechanisms for ocular neovascularization. Nonetheless, based on what we have found so far, we suggest that a strategy to control RPE using rice bran or vitamin B6 might be applicable for AMD therapy.
Secondly, we examined a direct therapeutic role of rice bran (along with vitamin B6) in the retina. However, we could not see the positive effect. Instead, high-dose vitamin B6 administration caused retinal damages, which implies an excessive amount of vitamin B6 may not be suitable for retinal function. Furthermore, stabilized HIF-1α expression in neuronal cells under a CoCl2-induced hypoxic condition was less affected by rice bran and vitamin B6. Although more studies are required, rice bran may directly work on RPE cells under pathological conditions in the eye rather than to work on neuronal cells.
Hypoxia-responsive genes other than VEGF were also examined in our study. Interestingly, BNIP3 and PDK1 expressions were not dramatically reduced by rice bran treatment while VEGF expression showed the expected result. Furthermore, BNIP3 and PDK1 expressions were significantly reduced by vitamin B6 treatment. This might be explained by the fact that rice bran contains several components other than vitamin B6 [39], which could induce other unknown signaling pathways to hinder inhibition of hypoxia-responsive gene induction under a CoCl2-induced hypoxic condition. More comprehensive studies regarding unknown effects of each component in rice bran may be further needed for a better understanding of pathological mechanisms for ocular neovascularization.
To date, effective treatments for pathological retinal neovascularization contain intravitreal injection of anti-VEGF drugs as well as laser photocoagulation, topical injection of corticosteroids, and vitreoretinal surgery [4,49].Chronic anti-VEGF therapies may induce photoreceptor cell atrophy [50] as it could abrupt normal physiological roles of VEGF for retinal function [51]. In addition, these treatments are very of high cost and even invasive to the eye, which is not patient-friendly [52,53]. In our current study, we targeted pathological HIF activation using rice bran to reduce HIF-induced pathological VEGF expression. In addition, edible rice bran is cost-effective and noninvasive to patients as it is always produced as a byproduct of milling for the production of rice [39] and possesses its beneficial features of good patient compliance and fear or pain avoidance [54].
5. Conclusions
In conclusion, we screened various types of food ingredients as prospective HIF inhibitors and demonstrated that rice bran and its component vitamin B6 possess HIF inhibitory effects. Furthermore, they showed their suppressive effects on pathological retinal neovascularization in a murine CNV model. Rice bran or vitamin B6 dietary supplement could be useful as stand-alone therapies or as adjuvants to anti-VEGF therapies for neovascularization in ocular diseases.
6. Patents
The data of the current research have applied for a patent (application number: 2019-038043).
Acknowledgments
We thank ROHTO Pharmaceutical Co. Ltd. for sharing food ingredients and their critical discussions. We also thank our lab members, S. Ikeda, Y. Katada, H. Jeong, K. Kurosaki, and A. Kawabata for their critical discussions.
Appendix A
Table A1.
First screening of HIF inhibitors.
| Number | Name | Fold Change |
|---|---|---|
| 1 | Hydroxycitric acid | 0.24 |
| 2 | Garcinia fruit extract | 0.30 |
| (hydroxycitric acid ≥ 50%, calcium ≥ 18%) | ||
| 3 | Garcinia fruit extract, water soluble | 0.40 |
| (hydroxycitric acid ≥ 60%) | ||
| 4 | Ginkgo biloba extract A | 0.46 |
| (flavonoid ≥ 24%, terpene lactones ≥ 6%) | ||
| 5 | Panax ginseng | 0.48 |
| 6 | Lactoferrin from milk | 0.49 |
| 7 | Rice bran, defatted | 0.51 |
| 8 | Lactoferrin | 0.57 |
| 9 | Vitamin B6 (pyridoxine hydrochloride) | 0.58 |
| 10 | Thiamine mononitrate | 0.63 |
| 11 | Tilia cordata flower extract A | 0.64 |
| 12 | Garcinia peel extract | 0.64 |
| (hydroxy citric acid ≥ 60%) | ||
| 13 | Enterococcus faecalis B | 0.65 |
| 14 | Summer pumpkin seed extract | 0.66 |
| (fatty acid ≥ 85%, total sterol ≥ 0.3%) | ||
| 15 | Grape pomace extract A | 0.68 |
| (oleanolic acid ≥ 2.0%) | ||
| 16 | Maqui berry fruit extract | 0.68 |
| (anthocyanins ≥ 35%, delphinidins ≥ 20%) | ||
| 17 | Dextrin | 0.69 |
| 18 | Strawberry seed extract | 0.71 |
| (polyphenol ≥ 2.0%, tiliroside ≥ 0.5%) | ||
| 19 | Ginsenoside Rf | 0.73 |
| 20 | Petasites japonicus extract | 0.74 |
| 21 | Vitamin A palmitate | 0.74 |
| 22 | Tomato extract A | 0.75 |
| (lycopene ≥ 15%, tocopherol ≥ 1.5%, | ||
| phytoene phytofluene ≥ 1.0%, β-carotene ≥ 0.2%) | ||
| 23 | Panax notoginseng root extract | 0.76 |
| 24 | Sasa veitchii leaf extract | 0.77 |
| 25 | Cockscomb extract | 0.80 |
| (hyaluronic acid ≥ 5%, hydroxyproline ≥ 8%) | ||
| 26 | Red dragon fruit extract | 0.80 |
| (betacyanin ≥ 0.05%) | ||
| 27 | Tilia cordata flower extract B | 0.85 |
| 28 | Seaberry fruit extract | 0.85 |
| (triterpenes ≥ 0.2%, isorhamnetin rhamnoside ≥ 0.2%) | ||
| 29 | Cyanocobalamin | 0.88 |
| 30 | Hovenia dulcis extract | 0.90 |
| 31 | Polyphenol | 0.91 |
| 32 | Siraitia grosvenorii extract | 0.91 |
| 33 | Peptide formulation derived from dairy protein | 0.92 |
| 34 | Phosphoryl oligosaccharides of calcium | 0.94 |
| 35 | Tilia cordata flower extract C | 0.95 |
| 36 | L-Carnitine | 0.96 |
| 37 | Myrciaria dubia fruit extract | 0.97 |
| (citric acid ≥ 1%) | ||
| 38 | Monostroma nitidum extract | 0.97 |
| 39 | Aspalathus linearis extract B | 1.00 |
| 40 | Parsley extract | 1.02 |
| 41 | Honey | 1.03 |
| 42 | Panax ginseng root extract H | 1.03 |
| 43 | Niacinamide | 1.04 |
| 44 | Maca extract | 1.04 |
| (benzyl glucosinolate ≥ 2.4%) | ||
| 45 | Broccoli sprout extract B | 1.05 |
| (glucoraphanin ≥ 3%) | ||
| 46 | Peptide formulation derived from milk protein A | 1.05 |
| 47 | Hydrolyzed rice bran extract | 1.05 |
| (peptide ≥ 60%) | ||
| 48 | Polysaccharide from yeast | 1.06 |
| 49 | Branched chain amino acids | 1.06 |
| 50 | Chlorogenic acid | 1.06 |
| 51 | Ginsenoside Rb1 | 1.07 |
| 52 | Panax ginseng root extract C | 1.08 |
| 53 | Chamomile flower extract | 1.09 |
| 54 | Salmon milt extract | 1.09 |
| 55 | Kidney beans extract B | 1.10 |
| 56 | Acerola fruit extract A | 1.12 |
| (vitamin C ≥ 30%) | ||
| 57 | Acerola fruit extract B | 1.12 |
| (vitamin C ≥ 20%) | ||
| 58 | Ginkgo biloba extract B | 1.14 |
| 59 | Eleutherococcus senticosus extract | 1.14 |
| (saponin ≥ 2%) | ||
| 60 | Vitamin K2 | 1.16 |
| 61 | Astragalus complanatus extract | 1.17 |
| (flavonoid ≥ 5%) | ||
| 62 | Peptide formulation derived from casein A | 1.20 |
| 63 | Selenium | 1.22 |
| 64 | Rosa canina fruit extract | 1.23 |
| 65 | Perilla leaf extract B | 1.26 |
| 66 | Mugwort leaf extract | 1.26 |
| 67 | Milk protein | 1.27 |
| 68 | Evening primrose oil | 1.27 |
| (cis-gamma-linolenic acid and linoleic acid 76%) | ||
| 69 | Glucosyl hesperidin A (hesperidin ≥ 70%) | 1.27 |
| 70 | Japanese hawthorn fruit extract | 1.28 |
| 71 | Chinese chive extract (S-allyl-l-cysteine ≥ 0.1%) | 1.29 |
| 72 | Enterococcus faecalis A | 1.30 |
| 73 | Coenzyme Q10 | 1.30 |
| 74 | Peptide formulation derived from casein B | 1.32 |
| 75 | β-Carotene | 1.35 |
| 76 | Peptide formulation derived from milk protein B | 1.37 |
| 77 | Moringa leaf extract | 1.38 |
| 78 | Ganoderma lucidum extract | 1.39 |
| 79 | Rice germ extract A (polyamine ≥ 0.2%) | 1.41 |
| 80 | Boswellic acid | 1.41 |
| 81 | Tocopherol | 1.43 |
| 82 | Kiwi fruit seed extract | 1.44 |
| (polyphenol ≥ 2%, quercitrin ≥ 0.05 mg/1 g) | ||
| 83 | Glucosyl hesperidin B | 1.45 |
| 84 | Calcium | 1.46 |
| 85 | Paprika extract B | 1.47 |
| (xanthophyll ≥ 27 mg/g, capsanthin ≥ 15 mg/g, | ||
| β-cryptoxanthin ≥1.5 mg/g) | ||
| 86 | Licorice extract A | 1.47 |
| 87 | Coprinus comatus extract | 1.49 |
| 88 | Alpinia speciosa leaf extract | 1.49 |
| 89 | Pomegranate extract (ellagic acid 80%) | 1.54 |
| 90 | Grifola frondosa mushroom extract | 1.54 |
| 91 | Olive fruit extract B (maslinic acid ≥ 10%) | 1.55 |
| 92 | Pearl barley seed extract | 1.55 |
| 93 | Saffron extract | 1.57 |
| 94 | Perilla frutescens leaf powder | 1.57 |
| 95 | Soybean protein | 1.57 |
| 96 | Cherry blossom flower extract | 1.58 |
| (caffeoyl glucose ≥ 2.0%, quercetin glucoside ≥ 0.05%) | ||
| 97 | Royal jelly (decenoic acid 1.6–1.8%) | 1.71 |
| 98 | Soybean extract | 1.77 |
| 99 | Seaweed mineral | 1.78 |
| 100 | Ginsenoside, compound K | 1.78 |
| 101 | Lactulos | 1.78 |
| 102 | Brewers’ yeast extract | 1.78 |
| 103 | Panax ginseng root extract D | 1.81 |
| 104 | Golden oyster mushroom extract | 1.88 |
| 105 | Barley powder (β-glucan 15%) | 1.88 |
| 106 | Tamarind extract | 1.95 |
| 107 | Isatis tinctoria extract | 2.05 |
| 108 | Indian long pepper fruit extract | 2.12 |
| 109 | Gardenia fruit extract B | 2.15 |
| 110 | Amla fruit extract (gallotannnin ≥ 15%) | 2.17 |
| 111 | Arctium lappa ferment extract | 2.19 |
| 112 | Arctium lappa root extract | 2.24 |
| 113 | Broccoli sprout extract A (sulforaphane ≥ 2%) | 2.24 |
| 114 | Euphrasia rostkoviana extract | 2.27 |
| 115 | Phellinus linteus extract | 2.27 |
| 116 | Chinese wolfberry fruit extract | 2.30 |
| 117 | Rosemary leaf extract | 2.31 |
| 118 | Black rice seed extract | 2.31 |
| (polyphenol ≥ 15%, anthocyanidin ≥ 5%) | ||
| 119 | Plant oil | 2.34 |
| 120 | Zingiber purpureum extract | 2.39 |
| 121 | Sweet clover extract | 2.40 |
| 122 | Grape bud extract | 2.41 |
| (resveratrol ≥ 20%, trans-resveratorol ≥ 5%, ε-viniferin ≥ 5%) | ||
| 123 | Cocoa seed extract | 2.43 |
| (theobromine ≥ 10%, polyphenol ≥ 20%) | ||
| 124 | Bilberry fruit extract A | 2.44 |
| (anthocyanosides ≥ 85%) | ||
| 125 | Linseed extract | 2.80 |
| (secoisolariciresinol diglucoside ≥ 40%) | ||
| 126 | Calcium ascorbate | 3.02 |
| 127 | Mulberry leaf extract (1-deoxynojirimycin ≥ 1%) | 3.10 |
| 128 | Siberian ginseng root extract | 3.11 |
| (eleutherosides B+E ≥ 0.9%) | ||
| 129 | Ginger extract B | 3.30 |
| 130 | Siberian larch extract (dihydroquercetin ≥ 88%) | 3.40 |
| 131 | Coconut oil | 3.43 |
| 132 | Marigold flower extract A (lutein ≥ 20%, zeaxanthin 1–2%) | 3.48 |
| 133 | Lemon verbena extract (acteoside and isoacteoside ≥ 9–11%) | 3.71 |
| 134 | Cyanidin 3-glucoside | 3.76 |
| 135 | Sichuan pepper peel extract | 3.84 |
| 136 | Pyrroloquinoline quinone | 3.87 |
| 137 | Olive leaf extract A (oleanolic acid ≥ 55%) | 4.05 |
| 138 | Gymnema sylvestre extract B (gymnemic acid ≥ 25%) | 4.09 |
| 139 | Psidium guajava leaf extract (tannin ≥ 18%) | 4.11 |
| 140 | Geranylgeraniol | 4.24 |
| 141 | Lemon balm leaf extract | 4.46 |
| 142 | Olive leaf extract B | 4.48 |
| 143 | Riboflavin | 4.57 |
| 144 | Kaempferia parviflora extract | 4.65 |
| (5,7-dimethoxyflavone ≥ 4%, polymethoxyflavonoid ≥ 15%) | ||
| 145 | Curcuma extract B | 4.69 |
| (curcuminoid 19–22%, curcumin ≥ 13%) | ||
| 146 | Coffee seed extract | 4.97 |
| (chlorogenic acid ≥ 24.0%) | ||
| 147 | Oleanolic acid | 5.00 |
| 148 | Cranberry extract C (proanthocyanidins ≥ 50%) | 5.20 |
| 149 | Panax ginseng root extract E (compound K ≥ 5 mg/g) | 5.21 |
| 150 | Ginger extract A | 5.25 |
| (gingerols ≥ 15%, 6,8,10-gingerols ≥ 12%, shogaol ≥ 3%) | ||
| 151 | Japanese horseradish extract | 5.29 |
| 152 | Curcuma extract A | 5.37 |
| (curcuminoid complex ≥ 95%, curcumin ≥ 65%) | ||
| 153 | Melinjo seed extract (resveratrol ≥ 20%) | 5.40 |
| 154 | Grape marc extract | 5.42 |
| (polyphenol ≥ 92%, proanthocyanidin ≥ 15%, | ||
| anthocyanin ≥ 2%, t-resveratrol ≥ 2500 ppm) | ||
| 155 | Ginsenoside C-K (compound K ≥ 98%) | 5.51 |
| 156 | Ginkgo biloba extract C | 5.53 |
| 157 | Ginsenoside Rg3 | 5.64 |
| 158 | Laurel leaf extract (deacetyl laurenobiolide ≥ 1%) | 5.88 |
| 159 | Aspalathus linearis extract A (aspalathin ≥ 20%) | 5.88 |
| 160 | Bacopa monniera extract | 5.95 |
| 161 | Perilla seed extract | 6.24 |
| 162 | Panax ginseng root extract K | 6.30 |
| 163 | Gymnema sylvestre extract A | 6.31 |
| 164 | Cat’s claw extract | 6.37 |
| 165 | Panax ginseng root extract F | 6.45 |
| 166 | Black chokeberry fruit extract | 6.49 |
| 167 | American panax quinquefolius root extract A | 6.91 |
| 168 | Coleus forskohlii extract | 6.96 |
| 169 | Panax ginseng root extract L | 7.12 |
| 170 | Mangosteen peel extract (maclurin glycosides ≥ 0.03%) | 7.22 |
| 171 | Docosahexaenoic acid | 7.22 |
| 172 | Mallotus japonicus peel extract (bergenin ≥ 12%) | 7.61 |
| 173 | Fucoxanthin B | 7.77 |
| 174 | Grape seed extract (polyphenol ≥ 95%, proanthocyanidin ≥ 40%) | 7.83 |
| 175 | Evening primrose seed extract | 7.94 |
| 176 | Citrus extract | 7.96 |
| 177 | Olive fruit extract A | 7.96 |
| 178 | Andrographis paniculata extract | 8.14 |
| 179 | Cranberry extract B | 8.43 |
| 180 | Banaba leaf extract | 8.67 |
| 181 | Peanut seed coat extract | 8.97 |
| 182 | Glucosylceramide | 9.16 |
| 183 | Pomegranate fruit extract | 9.86 |
| 184 | Artichoke leaf extract | 10.09 |
| 185 | Eicosapentaenoic acid | 10.23 |
| 186 | Propolis extract A | 10.44 |
| 187 | Black soybean extract | 10.64 |
| 188 | Licorice extract B (glycyrrhizinic acid ≥ 20%) | 10.71 |
| 189 | Propolis extract B | 10.82 |
| 190 | Bilberry fruit extract B | 11.44 |
| (anthocyanidin ≥ 25%, anthocyanin ≥ 36%) | ||
| 191 | Paprika extract A | 11.71 |
| (xanthophyll ≥ 9 mg/g, capsanthin ≥ 5 mg/g, | ||
| β-cryptoxanthin ≥ 0.5 mg/g) | ||
| 192 | Quercus salicina leaf extract (tannins ≥ 18%) | 12.00 |
| 193 | Hesperetin | 12.33 |
| 194 | Pterocarpus marsupium extract | 12.65 |
| (pterostibene ≥ 5%) | ||
| 195 | Tomato extract B (lycopene ≥ 10%) | 13.15 |
| 196 | Cranberry extract D (proanthocyanidins 27–33%) | 13.37 |
| 197 | Fucoxanthin A | 13.94 |
| 198 | Grape stem extract | 14.74 |
| (resveratrol ≥ 3.5%, oligo-stilbenes ≥ 1%, ε-viniferin ≥ 0.8%) | ||
| 199 | Salacia reticulata extract (mangiferin ≥ 1%, triterpenoids ≥ 20%) | 15.58 |
| 200 | Apocynum venetum extract (hyperoside and isoquercitin ≥ 4%) | 19.67 |
| 201 | Green tea extract | 20.65 |
| 202 | Gardenia fruit extract A (crocetin ≥ 75%) | 27.55 |
Fold changes of HIF activity by the ingredients were compared with the value of CoCl2-induced HIF activity in 661W cells (n = 1 per sample).
Appendix B
Table A2.
Second screening of HIF inhibitors.
| Number | Name | Fold Change + SD | p-Value |
|---|---|---|---|
| 1 | Garcinia fruit extract | 0.38 ± 0.12 | 0.003 ** |
| 2 | Hydroxycitric acid | 0.44 ± 0.20 | 0.035 * |
| 3 | Rice bran, defatted | 0.58 ± 0.05 | 0.001 ** |
| 4 | Lactoferrin | 0.61 ± 0.08 | 0.002 ** |
| 5 | Panax ginseng | 0.71 ± 0.04 | 0.040 * |
| 6 | Vitamin B6 | 0.74 ± 0.02 | 0.003 ** |
| 7 | Ginkgo biloba extract A | 0.99 ± 0.14 | 0.952 |
| 8 | Thiamine mononitrate | 1.26 ± 0.16 | 0.050 |
Fold changes of HIF activity by the samples were compared with the value of CoCl2-induced HIF activity in 661W cells (n = 3 per sample, * p < 0.05, ** p < 0.01).
Appendix C
Figure A1.
An inhibitory effect of panax ginseng on HIF activity. Quantitative analysis of HIF-reporter luciferase assay using ARPE-19 cells (n = 3 per group) showed that ginseng inhibited CoCl2-induced HIF activity. *** p < 0.001, ### p < 0.001, compared with no treatment and 200 µM of CoCl2 treatment, respectively. A bar graph was presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvent, ginseng: water.
Appendix D
Table A3.
Ingredient information (www.mext.go.jp/a_menu/syokuhinseibun).
| Components of vitamin B (in 100 g of rice bran) | B1 (thiamine): 3.12 mg |
| B2 (riboflavin): 0.21 mg | |
| B3 (niacinamide): 34.6 mg | |
| B5 (pantothenic acid): 4.43 mg | |
| B6 (pyridoxine hydrochloride): 3.27 mg | |
| B7 (biotin): 0.04 mg | |
| B9 (folic acid): 0.18 mg |
Appendix E
Figure A2.
Inhibitory effects of components of vitamin B in rice bran on HIF activity. Quantitative analysis of HIF-reporter luciferase assay using ARPE-19 cells (n = 3 per group) showed that vitamin B6 dramatically inhibited CoCl2-induced HIF activity more than any other components in rice bran. *** p < 0.001, ### p < 0.001, compared with no treatment and 200 µM of CoCl2 treatment, respectively. A bar graph was presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B: water.
Appendix F
Figure A3.
No cytotoxicity of rice bran and vitamin B6. Quantitative analyses (n = 4 per group) showed that a significant change in mitochondrial activity was not seen in ARPE-19 cells 12 h after rice bran or vitamin B6 treatment under a CoCl2-induced pseudo-hypoxic condition. However, high-dose vitamin B6 (1 mg/mL) tended to damage mitochondrial activity. ** p < 0.01, *** p < 0.001, compared with no treatment. Bar graphs were presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
Appendix G
Figure A4.
No effect of rice bran and vitamin B6 on HIF-1α stabilization. Representative immunoblot images and quantitative analyses (n = 4 per group) for HIF-1α and β-Actin showed that HIF-1α was stabilized in 661W cells under a CoCl2-induced pseudo-hypoxic condition. 1 mg/mL of rice bran and vitamin B6 did not significantly decrease stabilized HIF-1α expression. * p < 0.05, compared with no treatment. Bar graphs were presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
Appendix H
Figure A5.
No effect of rice bran and vitamin B6 on HIF-2α stabilization. Representative immunoblot images and quantitative analyses (n = 4 per group) for HIF-2α and β-Actin showed that HIF-2α was not significantly stabilized in ARPE-19 cells under a CoCl2-induced pseudo-hypoxic condition. 1 mg/mL of rice bran and vitamin B6 did not significantly change its expression. Bar graphs were presented as mean ± standard deviation. The data were analyzed using one-way ANOVA followed by a Bonferroni post hoc test. Solvents, rice bran: DMSO; vitamin B6: water.
Appendix I
Figure A6.
Evaluation of a mouse model of LIR. Representative waveforms of a- and b-waves (2 cd.s/m2) and quantitative analyses showed that the light exposure (3000 lux) significantly decreased the amplitudes of a-wave and b-wave in the retina (n = 6 per group, 12 eyeballs per group). ** p < 0.01, *** p < 0.001. Bar graphs were presented as mean ± standard deviation. The data were analyzed using Student’s t-test.
Author Contributions
T.K. provided technical and funding assistance. M.I. and T.K. designed the study. M.I., D.L., A.S. and Y.M. performed the experiments. M.I., D.L. and A.S. analyzed the results. D.L. summarized all of the results and wrote the manuscript. T.K. and K.T. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (18K09424 to Toshihide Kurihara) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Conflicts of Interest
The authors declare no conflict of interest except for the patent issue.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ding J., Wong T.Y. Current Epidemiology of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diabetes Rep. 2012;12:346–354. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S., Pascolini D., Etya’ale D., Kocur I., Pararajasegaram R., Pokharel G.P., Mariotti S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman L.G., Lilienfeld A.M., Ferris F.L., 3rd, Fine S.L. Senile macular degeneration: A case-control study. Am. J. Epidemiol. 1983;118:213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- 4.Fogli S., Del Re M., Rofi E., Posarelli C., Figus M., Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye. 2018;32:1010–1020. doi: 10.1038/s41433-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozaki H., Seo M.S., Ozaki K., Yamada H., Yamada E., Okamoto N., Hofmann F., Wood J.M., Campochiaro P.A. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am. J. Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabral T., Mello L.G.M., Lima L.H., Polido J., Regatieri C.V., Belfort R., Mahajan V.B. Retinal and choroidal angiogenesis: A review of new targets. Int. J. Retin. Vitr. 2017;3:31. doi: 10.1186/s40942-017-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Zhao J., Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016;10:1857–1867. doi: 10.2147/DDDT.S97653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaelin W.G., Ratcliffe P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Mole D.R., Blancher C., Copley R.R., Pollard P.J., Gleadle J.M., Ragoussis J., Ratcliffe P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majmundar A.J., Wong W.J., Simon M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krock B.L., Skuli N., Simon M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno H., Shirato K., Sakurai T., Ogasawara J., Sumitani Y., Sato S., Imaizumi K., Ishida H., Kizaki T. Effect of exercise on HIF-1 and VEGF signaling. J. Phys. Fit. Sports Med. 2012;1:5–16. doi: 10.7600/jpfsm.1.5. [DOI] [Google Scholar]
- 13.Inoue Y., Yanagi Y., Matsuura K., Takahashi H., Tamaki Y., Araie M. Expression of hypoxia-inducible factor 1alpha and 2alpha in choroidal neovascular membranes associated with age-related macular degeneration. Br. J. Ophthalmol. 2007;91:1720–1721. doi: 10.1136/bjo.2006.111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan C.M., Pate S., Hiscott P., Wong D., Pattwell D.M., Kent D. Expression of hypoxia-inducible factor−1α and −2α in human choroidal neovascular membranes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009;247:1361–1367. doi: 10.1007/s00417-009-1133-3. [DOI] [PubMed] [Google Scholar]
- 15.Ibuki M., Shoda C., Miwa Y., Ishida A., Tsubota K., Kurihara T. Lactoferrin Has a Therapeutic Effect via HIF Inhibition in a Murine Model of Choroidal Neovascularization. Front. Pharmacol. 2020;11:174. doi: 10.3389/fphar.2020.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibuki M., Shoda C., Miwa Y., Ishida A., Tsubota K., Kurihara T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019;20:5049. doi: 10.3390/ijms20205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoda C., Miwa Y., Nimura K., Okamoto K., Yamagami S., Tsubota K., Kurihara T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients. 2020;12:1055. doi: 10.3390/nu12041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miwa Y., Hoshino Y., Shoda C., Jiang X., Tsubota K., Kurihara T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019;128:21–31. doi: 10.1016/j.neuint.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee D., Miwa Y., Wu J., Shoda C., Jeong H., Kawagishi H., Tsubota K., Kurihara T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules. 2020;10:1405. doi: 10.3390/biom10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunimi H., Miwa Y., Inoue H., Tsubota K., Kurihara T. A Novel HIF Inhibitor Halofuginone Prevents Neurodegeneration in a Murine Model of Retinal Ischemia-Reperfusion. Int. J. Mol. Sci. 2019;20:3171. doi: 10.3390/ijms20133171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew E.Y. Nutrition effects on ocular diseases in the aging eye. Investig. Ophthalmol. Vis. Sci. 2013;54:ORSF42–ORSF47. doi: 10.1167/iovs13-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Pinilla F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrenson J.G., Downie L.E. Nutrition and Eye Health. Nutrients. 2019;11:2123. doi: 10.3390/nu11092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCann J.C., Ames B.N. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005;82:281–295. doi: 10.1093/ajcn/82.2.281. [DOI] [PubMed] [Google Scholar]
- 25.Hanyuda A., Rosner B.A., Wiggs J.L., Willett W.C., Tsubota K., Pasquale L.R., Kang J.H. Low-carbohydrate-diet scores and the risk of primary open-angle glaucoma: Data from three US cohorts. Eye. 2020;34:1465–1475. doi: 10.1038/s41433-020-0820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurihara T., Omoto M., Noda K., Ebinuma M., Kubota S., Koizumi H., Yoshida S., Ozawa Y., Shimmura S., Ishida S., et al. Retinal phototoxicity in a novel murine model of intraocular lens implantation. Mol. Vis. 2009;15:2751–2761. [PMC free article] [PubMed] [Google Scholar]
- 27.Malek G., Busik J., Grant M.B., Choudhary M. Models of retinal diseases and their applicability in drug discovery. Expert Opin. Drug Discov. 2018;13:359–377. doi: 10.1080/17460441.2018.1430136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayyad Z., Sirohi K., Radha V., Swarup G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017;7:16855. doi: 10.1038/s41598-017-17241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellinen L., Hagström M., Knuutila H., Ruponen M., Urtti A., Reinisalo M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 2019;9:13761. doi: 10.1038/s41598-019-50324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spilsbury K., Garrett K.L., Shen W.Y., Constable I.J., Rakoczy P.E. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaauwgeers H.G., Holtkamp G.M., Rutten H., Witmer A.N., Koolwijk P., Partanen T.A., Alitalo K., Kroon M.E., Kijlstra A., van Hinsbergh V.W., et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am. J. Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ablonczy Z., Dahrouj M., Marneros A.G. Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels. FASEB J. 2014;28:2369–2379. doi: 10.1096/fj.13-248021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forooghian F., Razavi R., Timms L. Hypoxia-inducible factor expression in human RPE cells. Br. J. Ophthalmol. 2007;91:1406–1410. doi: 10.1136/bjo.2007.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takei A., Ekström M., Mammadzada P., Aronsson M., Yu M., Kvanta A., André H. Gene Transfer of Prolyl Hydroxylase Domain 2 Inhibits Hypoxia-inducible Angiogenesis in a Model of Choroidal Neovascularization. Sci. Rep. 2017;7:42546. doi: 10.1038/srep42546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahrami B., Shen W., Zhu L., Zhang T., Chang A., Gillies M.C. Effects of VEGF inhibitors on human retinal pigment epithelium under high glucose and hypoxia. Clin. Exp. Ophthalmol. 2019;47:1074–1081. doi: 10.1111/ceo.13579. [DOI] [PubMed] [Google Scholar]
- 36.Chu C.-Y., Jin Y.-T., Zhang W., Yu J., Yang H.-P., Wang H.-Y., Zhang Z.-J., Liu X.-P., Zou Q. CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer. Int. J. Oncol. 2016;48:271–280. doi: 10.3892/ijo.2015.3253. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto T., Kawashima H., Osada H., Toda E., Homma K., Nagai N., Imai Y., Tsubota K., Ozawa Y. Dietary Spirulina Supplementation Protects Visual Function From Photostress by Suppressing Retinal Neurodegeneration in Mice. Transl. Vis. Sci. Technol. 2019;8:20. doi: 10.1167/tvst.8.6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki M., Yuki K., Kurihara T., Miyake S., Noda K., Kobayashi S., Ishida S., Tsubota K., Ozawa Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012;23:423–429. doi: 10.1016/j.jnutbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Gul K., Yousuf B., Singh A.K., Singh P., Wani A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre. 2015;6:24–30. doi: 10.1016/j.bcdf.2015.06.002. [DOI] [Google Scholar]
- 40.Park H.Y., Lee K.W., Choi H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017;8:935–943. doi: 10.1039/C6FO01763K. [DOI] [PubMed] [Google Scholar]
- 41.Kurihara T., Westenskow P.D., Bravo S., Aguilar E., Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Investig. 2012;122:4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhutto I., Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann G.L., Benedicto I., Philp N.J., Rodriguez-Boulan E. Plasma membrane protein polarity and trafficking in RPE cells: Past, present and future. Exp. Eye Res. 2014;126:5–15. doi: 10.1016/j.exer.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford K.M., Saint-Geniez M., Walshe T., Zahr A., D’Amore P.A. Expression and role of VEGF in the adult retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011;52:9478–9487. doi: 10.1167/iovs.11-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparrow J.R., Ueda K., Zhou J. Complement dysregulation in AMD: RPE-Bruch’s membrane-choroid. Mol. Asp. Med. 2012;33:436–445. doi: 10.1016/j.mam.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noël A., Jost M., Lambert V., Lecomte J., Rakic J.-M. Anti-angiogenic therapy of exudative age-related macular degeneration: Current progress and emerging concepts. Trends Mol. Med. 2007;13:345–352. doi: 10.1016/j.molmed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Espinosa-Heidmann D.G., Reinoso M.A., Pina Y., Csaky K.G., Caicedo A., Cousins S.W. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp. Eye Res. 2005;80:369–378. doi: 10.1016/j.exer.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Spaide R.F. Rationale for combination therapies for choroidal neovascularization. Am. J. Ophthalmol. 2006;141:149–156. doi: 10.1016/j.ajo.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Mansour S.E., Browning D.J., Wong K., Flynn H.W., Jr., Bhavsar A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020;14:653–678. doi: 10.2147/OPTH.S236637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grunwald J.E., Daniel E., Huang J., Ying G.-S., Maguire M.G., Toth C.A., Jaffe G.J., Fine S.L., Blodi B., Klein M.L., et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saint-Geniez M., Maharaj A.S.R., Walshe T.E., Tucker B.A., Sekiyama E., Kurihara T., Darland D.C., Young M.J., D’Amore P.A. Endogenous VEGF Is Required for Visual Function: Evidence for a Survival Role on Müller Cells and Photoreceptors. PLoS ONE. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamed Q., Gillies M.C., Wong T.Y. Management of Diabetic RetinopathyA Systematic Review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 53.Simó R., Hernández C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32:1556–1562. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giovannitti J.A., Trapp L.D. Adult sedation: Oral, rectal, IM, IV. Anesth. Prog. 1991;38:154–171. [PMC free article] [PubMed] [Google Scholar]