Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most common conditions worldwide that targets the liver parenchyma. NAFLD represents an intrahepatic triglyceride accumulation in the absence of excessive alcohol consumption and other diseases that affect the liver parenchyma. The current “gold standard” for evaluating the amount of intrahepatic fat is represented by liver biopsy, but many patients are reluctant and hardly accept undergoing this procedure due to its invasive nature. The current study addresses this aspect by evaluating the reliability of liver magnetic resonance spectroscopy (MRS) in diagnosing NAFLD, compared to the traditional invasive liver biopsy. The present study included a total of 38 patients based on several well-defined inclusion and exclusion criteria. We used the same NAFLD grading system for both liver MRS and liver biopsy: grade 0: <5% hepatocytes are affected; grade I: 5–33% hepatocytes are affected; grade II: 34–66% hepatocytes are affected; grade III: >66% hepatocytes are affected. Regarding the NAFLD grade, over three-quarters of patients were classified as grade I and grade II, with a strong predilection for men. The current results indicated a significant association between the NAFLD grade indicated by liver MRS and the NAFLD grade indicated by liver biopsy. At the end of our study, we recommend using liver MRS for evaluating and grading NAFLD in association with other parameters like serum triglycerides and body mass index grade as this protocol can enhance early detection and provide an accurate grading that will lead to a proper management of this disease.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, liver biopsy, magnetic resonance spectroscopy, body mass index, serum triglycerides
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common diseases worldwide that targets the liver parenchyma. NAFLD is defined by the intrahepatic triglyceride accumulation in the absence of immoderate alcohol consumption (most of today’s guidelines recommend setting the threshold for alcohol intake to 20 g/day for women and 30 g/day for men) and other causes of liver damage (viral hepatitis, intrahepatic cholangiocarcinoma, Cushing’s disease, biliary tract obstruction with superjacent cholestatic changes of the liver parenchyma, hemochromatosis, Wilson’s disease, drug intake – Amiodarone, Methotrexate, corticosteroids) [1,2,3,4,5,6,7]. Non-alcoholic fatty liver (NAFL), or often referred to as simple steatosis, occurs early in the evolution of NAFLD and involves a hepatocellular lipid accumulation that exceeds 5% with no signs of hepatic parenchyma inflammation. As the disease progresses, non-alcoholic steatohepatitis (NASH) may develop in the presence of liver parenchyma inflammation [suggested by the elevated liver blood tests alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] and excessive triglyceride accumulation inside the hepatocytes. Given the chronic liver damage, this condition can progress even further towards liver fibrosis and cirrhosis that are associated with an increased risk of developing hepatocellular carcinoma [8,9,10,11].
Regarding the medical imaging techniques capable to detect the presence of intrahepatic fat, several methods have proven their utility: ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI). However, performing a quantitative assessment of the intrahepatic lipid content using the previously mentioned medical imaging techniques is difficult. The current “gold standard” for evaluating the amount of intrahepatic fat is represented by liver biopsy, but many patients are reluctant and sometimes hardly accept undergoing this procedure due to its extremely invasive nature. NAFLD is currently an underdiagnosed and underestimated condition worldwide mainly due to the lack of a simple, non-invasive diagnostic tool [12,13,14,15].
Aim
The current study addresses this aspect by evaluating the reliability of liver magnetic resonance spectroscopy (MRS) in diagnosing NAFLD compared to the traditional invasive liver biopsy.
Patients, Materials and Methods
The present study received the local Ethics Committee approval. All patients freely expressed their consent to take part in this study after being presented with a standardized form and understood all key elements of the research.
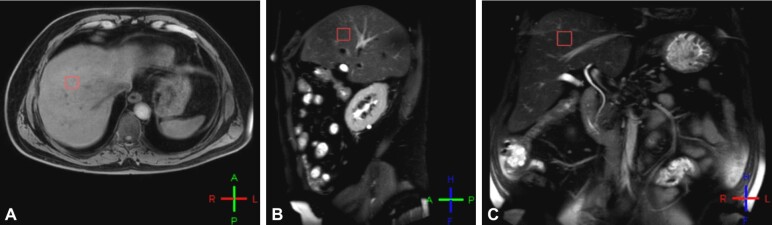
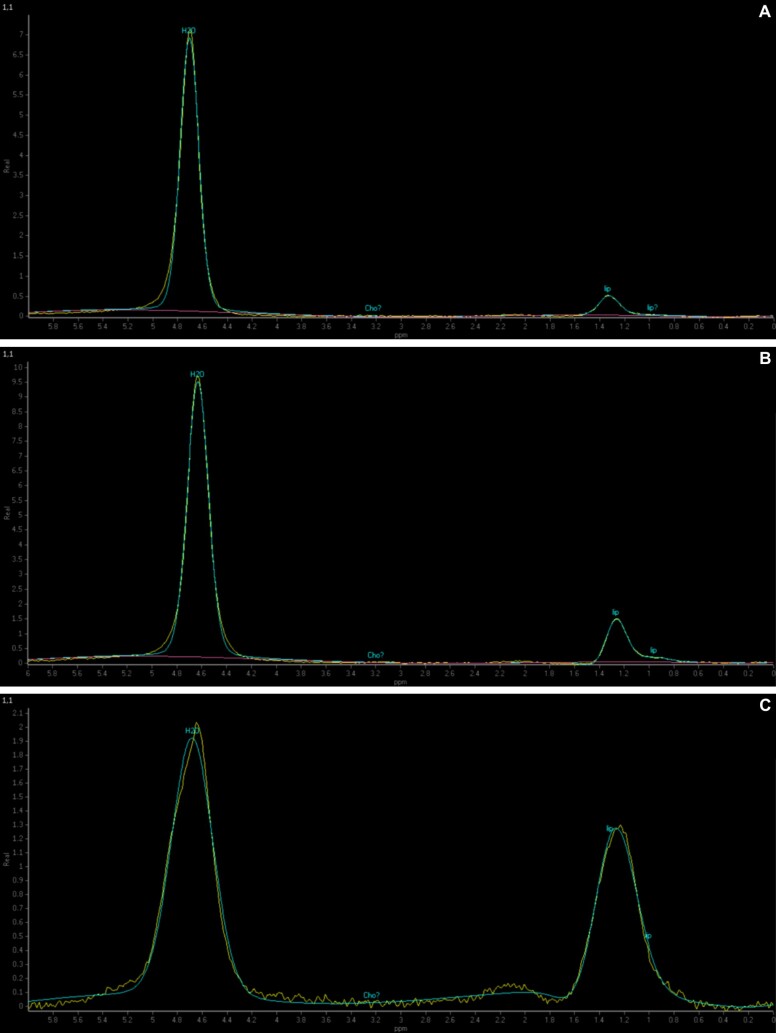
Between 2017 and 2019, we included 38 patients in the study. Initially, all patients underwent a liver MRS in the Department of Medical Imaging, University of Medicine and Pharmacy of Craiova, Romania, in order to quantitatively assess the fat fraction (FF) in the right hepatic lobe using multiple regions of interest (ROIs). The selection of ROIs in the right hepatic lobe was performed carefully and involved targeting areas that did not include the edge of the liver, bile ducts or vessels inside the liver parenchyma (Figure 1).
Figure 1 – (A–C).
The figure illustrates how the ROI inside the liver parenchyma was selected on MRS. ROI: Region of interest; MRS: Magnetic resonance spectroscopy
The MRS examinations were performed on a Philips Ingenia 3T machine. Subsequently, the next step of the protocol that we used involved obtaining multiple tissue samples from the parenchyma of the right hepatic lobe in order to perform a quantitative evaluation of the liver fat content using the current “gold standard”. The tissue samples were obtained in one to 14 days after the liver MRS examination in the Department of General Surgery, Emergency County Hospital of Craiova. The Hematoxylin–Eosin (HE) slides that were obtained from the tissue samples were scanned in the Research Center for Microscopic Morphology and Immunology, University of Medicine and Pharmacy of Craiova, with a 10× objective utilizing a Nikon 90i microscope equipped with a Prior ES111 OptiScan motorized stage, a Nikon DS-Ri2 complementary metal–oxide–semiconductor (CMOS) 16 Mp color camera and the Nikon NIS-Elements Advanced Research imaging and control software.
The patient inclusion criteria comprised the following: patient’s consent to take part in the study, age between 20–70 years, diffuse distribution of intrahepatic fat previously detected through abdominal US or CT, no alcohol consumption (or lower than the currently recommended threshold – 20 g/day for women and 30 g/day for men), no other identifiable causes of liver damage (viral hepatitis, intrahepatic cholangiocarcinoma, Cushing’s disease, biliary tract obstruction with superjacent cholestatic changes of the liver parenchyma, hemochromatosis, Wilson’s disease or certain drug intake – Amiodarone, Methotrexate, corticosteroids), no identifiable contraindications for liver biopsy. The patient exclusion criteria included the following: patient’s refusal to take part in the study, age <20 years or >70 years, focal distribution or absence of intrahepatic fat previously confirmed through abdominal US or CT, alcohol consumption that exceeds the currently recommended threshold – 20 g/day for women and 30 g/day for men, presence of other causes of liver damage (viral hepatitis, intrahepatic cholangiocarcinoma, Cushing’s disease, biliary tract obstruction with superjacent cholestatic changes of the liver parenchyma, hemochromatosis, Wilson’s disease or certain drug intake – Amiodarone, Methotrexate, corticosteroids), presence of contraindications for liver biopsy.
We used the same NAFLD grading system for both liver MRS and liver biopsy: grade 0: <5% hepatocytes are affected; grade I: 5–33% hepatocytes are affected; grade II: 34–66% hepatocytes are affected; grade III: >66% hepatocytes are affected.
The body mass index (BMI) grading system that we used in the present study included the following: normal: 18.5–24.9 kg/m2; overweight: 25–29.9 kg/m2; obesity grade I: 30–34.9 kg/m2; grade II: 35–39.9 kg/m2; grade III: ≥40 kg/m2.
Several parameters were of interest when evaluating the entire group of patients included in the study: age, gender, FF indicated by liver biopsy, NAFLD grade indicated by liver biopsy, FF indicated by MRS, NAFLD grade indicated by MRS, serum triglycerides, total serum cholesterol, serum ALT, serum AST, serum glucose, presence/absence of diabetes mellitus, BMI and BMI grade.
The statistical processing of the entire data acquired from the patients included in the study was performed with the help of Excel 2016 (developed by Microsoft) and Statistical Package for the Social Sciences (SPSS) Statistics version 20 (developed by IBM). In the current study, p-values lower than 0.05 indicated a statistically significant association between the investigated parameters. Continuous data was expressed as mean ± standard deviation (SD).
Results
Patient demographics
The present study included 38 patients (25 men, 13 women).
The age ranged between 32 years and 69 years in the entire population of the study group, with a mean ± SD of 51.81±10.35 years. In men, the age varied between 32 years and 65 years (50.4±9.85 years), while in women ages were between 37 and 69 years (54.53±11.14 years).
If all patients included in the study group are taken into account, the FF indicated by liver biopsy ranged between 2% and 64% (31.23±18.43%). Among men, the FF indicated by liver biopsy ranged recorded a minimum value of 2%, a maximum of 64% (29.24±16.79%), while among women the FF indicated by liver biopsy ranged between 2% and 64%, with a mean ± SD value of 35.07±21.44%.
The FF indicated by liver MRS varied between 3% and 63% in the entire study group (31.78±18.53%). In men, the FF indicated by liver MRS recorded a minimum value of 3% and a maximum value of 62%, with a mean ± SD value of 29.88±17.12%, while in women the FF indicated by liver MRS ranged between 4% and 63% (35.46±21.21%).
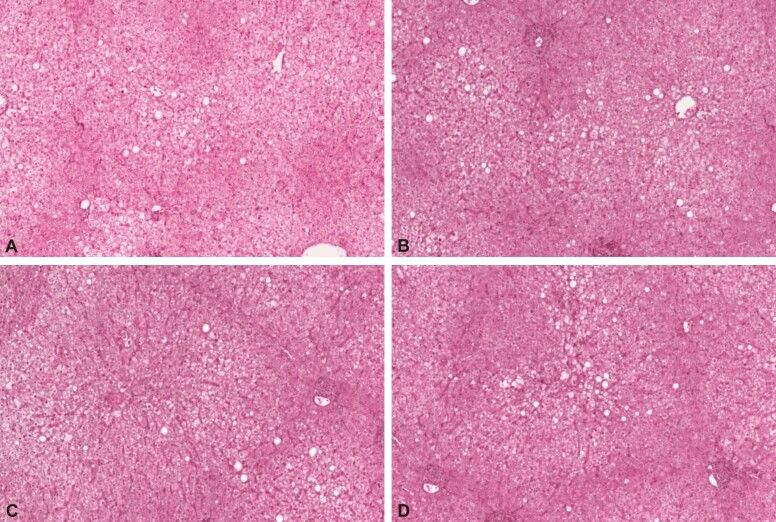
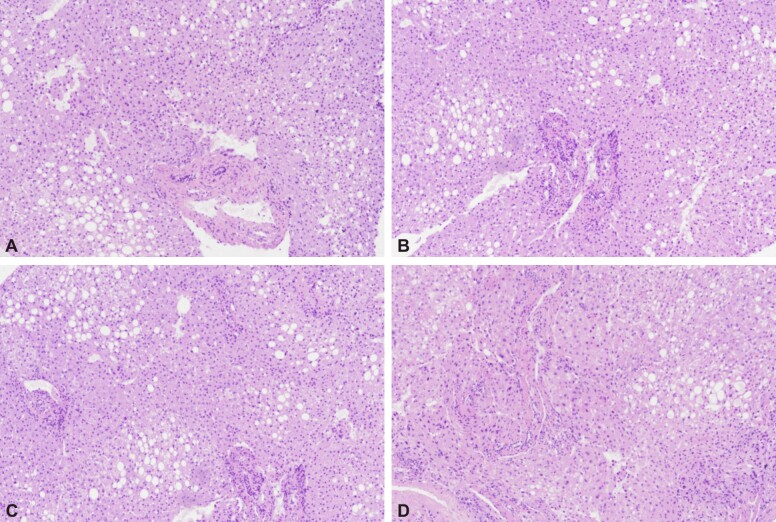
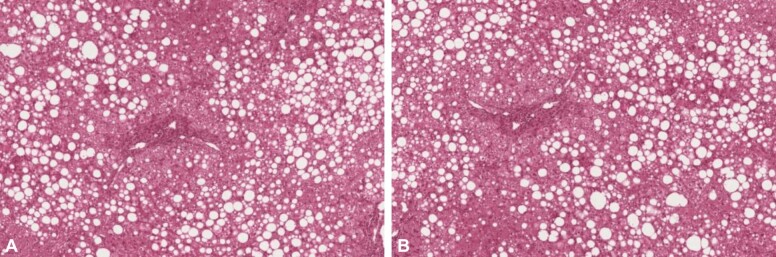
According to both liver MRS and liver biopsy, all the patients included in the study were categorized into the following grades: grade 0: five patients (three men, two women) (Figure 2); grade I: 17 patients (12 men, five women) (Figure 3); grade II: 16 patients (10 men, six women) (Figure 4); grade III: 0 patients (Figure 5).
Figure 2.
The HE stained slides (A–D, ×100) indicated generalized periportal and centrolobular stasis and the presence of panlobular steatosis in less than 5% of hepatocytes corresponding to grade 0 NAFLD. HE: Hematoxylin–Eosin; NAFLD: Non-alcoholic fatty liver disease
Figure 3.
The HE stained slides (A–D, ×100) indicated the presence of stasis, moderate fibrosis in the periportal space and steatosis in 5–33% of hepatocytes corresponding to grade I NAFLD. HE: Hematoxylin–Eosin; NAFLD: Non-alcoholic fatty liver disease
Figure 4.
The HE stained slides (A and B, ×100) indicated the presence of stasis, moderate fibrosis in the periportal space and steatosis in 34–66% of hepatocytes corresponding to grade II NAFLD. HE: Hematoxylin–Eosin; NAFLD: Non-alcoholic fatty liver disease
Figure 5.
Liver MRS aspect of various degrees of NAFLD: (A) Grade 0 – fat content in less than 5% of hepatocytes; (B) Grade I – fat content in 5–33% of hepatocytes; (C) Grade II – fat content in 34–66% of hepatocytes. MRS: Magnetic resonance spectroscopy; NAFLD: Non-alcoholic fatty liver disease
The serum triglycerides varied between 150 mg/dL and 347 mg/dL in the study group (239.36±62.52 mg/dL). The serum triglycerides ranged between 150 mg/dL and 343 mg/dL among men (233.92±60.08 mg/dL), while among women the serum triglycerides ranged between 157 mg/dL and 347 mg/dL (249.84±68.2 mg/dL).
The total serum cholesterol values ranged between 170 mg/dL and 412 mg/dL in the study group (255.47±63.66 mg/dL). We found total serum cholesterol between 170 mg/dL and 367 mg/dL in men, with a mean ± SD value of 254.08±60.41 mg/dL, while in women the total serum cholesterol varied between 178 mg/dL and 412 mg/dL (258.15±72.01 mg/dL).
The serum ALT values recorded in the study group presented a minimum value of 26 U/L, a maximum value of 82 U/L and a mean ± SD value of 47.21±16.73 U/L. Among men, the serum ALT varied between 26 U/L and 82 U/L (46.8±16.92 U/L), while among women between 28 U/L and 75 U/L (48±17.01 U/L).
In the study group, the serum AST values ranged between 24 U/L and 69 U/L (40.39±13.50 U/L). In men, the minimum serum AST value was 25 U/L, while the maximum value of this parameter was 69 U/L, recording a mean ± SD value of 40.04±13.27 U/L. In women, the serum AST values ranged between 24 U/L and 61 U/L (41.07±14.45 U/L).
From our 38 patients, 16 patients (11 men and five women) also had type 2 diabetes mellitus. The serum glucose values recorded in the study group varied between 81 mg/dL and 205 mg/dL and presented a mean ± SD value of 118.36±34.86 mg/dL. The minimum serum glucose value obtained in male subjects was 87 mg/dL, while the maximum value was 205 mg/dL, recording a mean ± SD value of 120.16±35.98 mg/dL. The serum glucose values recorded in women ranged between 81 mg/dL and 195 mg/dL (114.92±33.74 mg/dL).
The BMI of all subjects included in the current study varied between 23 kg/m2 and 44 kg/m2 and presented a mean ± SD value of 32.57±5.65 kg/m2. Among men, the BMI presented a minimum value of 24 kg/m2 and a maximum value of 44 kg/m2 (32.32±5.52 kg/m2). Among women, the BMI ranged between 23 kg/m2 and 43 kg/m2 (33.07±6.08 kg/m2).
According to the BMI grading system, all patients included in the study group were classified as presented in Table 1. The variability of all the investigated parameters between NAFLD grades is represented in Table 2.
Table 1.
Patient classification based on the BMI grade
|
BMI grade |
Men |
Women |
Total |
|
Normal |
1 |
2 |
3 |
|
Overweight |
8 |
1 |
9 |
|
I |
5 |
3 |
8 |
|
II |
9 |
6 |
15 |
|
III |
2 |
1 |
3 |
BMI: Body mass index
Table 2.
Mean values ± SD of all the investigated parameters in the study based on the NAFLD group
|
Parameter (mean value ± SD) |
Grade 0 |
Grade I |
Grade II |
|
|
Age [years] |
T |
59±6.2 |
45.7±8.57 |
56.06±9.85 |
|
M |
57.33±3.21 |
46.58±9.6 |
52.9±10.08 |
|
|
W |
61.5±10.6 |
43.6±5.68 |
61.33±7.42 |
|
|
FF indicated by liver biopsy [%] |
T |
3±1 |
22.88±7.12 |
48.93±10.25 |
|
M |
3±1 |
22.25±6.86 |
45.5±10.27 |
|
|
W |
3±1.41 |
24.4±8.32 |
54.66±7.89 |
|
|
FF indicated by liver MRS [%] |
T |
3.6±0.54 |
23±6.95 |
49.93±9.58 |
|
M |
3.33±0.57 |
22.41±6.84 |
46.8±9.87 |
|
|
W |
4±0 |
24.4±7.82 |
55.16±6.88 |
|
|
Serum triglycerides [mg/dL] |
T |
155.2±3.96 |
210.47±43.09 |
296.37±29.72 |
|
M |
152.66±2.51 |
210.41±48.23 |
286.5±27.8 |
|
|
W |
159±1.41 |
210.6±32.08 |
312.83±27.17 |
|
|
Total serum cholesterol [mg/dL] |
T |
265.8±29.54 |
255.35±75.57 |
252.37±60.23 |
|
M |
257.33±38.42 |
242.91±65.76 |
266.5±61.47 |
|
|
W |
278.5±0.7 |
285.2±96.81 |
228.83±54.95 |
|
|
Serum ALT [U/L] |
T |
31.6±2.6 |
49.05±15.38 |
50.12±18.51 |
|
M |
32.66±2.3 |
46.91±15.35 |
50.9±19.73 |
|
|
W |
30±2.82 |
54.2±15.84 |
48.83±17.98 |
|
|
Serum AST [U/L] |
T |
29±2.34 |
41.47±11.97 |
42.81±15.62 |
|
M |
30.33±2.08 |
39.25±11.74 |
43.9±15.84 |
|
|
W |
27±0 |
46.8±12 |
41±16.56 |
|
|
Serum glucose [mg/dL] |
T |
99.6±3.04 |
111.41±36.23 |
131.62±35.15 |
|
M |
98.66±3.78 |
116.25±39.43 |
131.3±35.16 |
|
|
W |
101±1.41 |
99.8±27.1 |
132.16±38.49 |
|
|
BMI [kg/m2] |
T |
25.2±2.38 |
32±4.96 |
35.5±4.83 |
|
M |
26.33±2.51 |
31.58±4.83 |
35±5.53 |
|
|
W |
23.5±0.7 |
33±5.7 |
36.33±3.66 |
|
SD: Standard deviation; NAFLD: Non-alcoholic fatty liver disease; FF: Fat fraction; MRS: Magnetic resonance spectroscopy; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; T: Total (men and women); M: Men; W: Women
Using One-Way Analysis of Variance (ANOVA) test in IBM SPSS Statistics version 20 to process the results obtained in this study revealed significant associations from a statistical standpoint between: (i) the NAFLD grade indicated by liver biopsy and the NAFLD grade indicated by MRS (p<0.001); (ii) the NAFLD grade (liver biopsy/MRS) and serum triglycerides (p<0.001); (iii) the NAFLD grade (liver biopsy/MRS) and the BMI value (p<0.001). On the other hand, the results of our study indicated no statistically significant associations between: (i) the NAFLD grade (liver biopsy/MRS) and total serum cholesterol (p=0.461); (ii) the NAFLD grade (liver biopsy/MRS) and serum ALT (p=0.43); (iii) the NAFLD grade (liver biopsy/MRS) and serum AST (p=0.346).
Discussion
In our study group, NAFLD was more common in men and affected them nearly twice more often than women. Another aspect worth mentioning is represented by the slightly increased mean age of women compared to men. Regarding the NAFLD grade, over three-quarters of patients were classified as grade I and grade II, with a strong predilection for men in all groups. Both liver biopsy and liver MRS revealed a higher mean value of FF in women compared to men. Regarding the serum triglycerides, there was a slightly increased mean value in women compared to men and contributed to the evaluation of the NAFLD grade alongside other diagnostic tools. On the other hand, total serum cholesterol did not seem to offer the same benefits as the results of the study indicated no statistically significant association between the NAFLD grade and the total serum cholesterol. Another important aspect is represented by the statistically significant association between the NAFLD grade and the BMI value, which can result in an increased utility of the BMI value in grading NAFLD alongside the value of serum triglycerides and liver MRS. Last but certainly not least, the current results indicated a significant association between the NAFLD grade indicated by liver MRS and the NAFLD grade indicated by liver biopsy. Regarding the ALT and AST liver enzymes, the data provided by the present study indicated a non-specific high variability of these two parameters between patients with different NAFLD grades.
All patients diagnosed with grade 0 of NAFLD presented the highest values regarding the mean age. Also, if referring only to women, the mean age was similar between patients diagnosed with grade 0 of NAFLD and grade II of NAFLD, but significantly higher when compared to the mean age of women diagnosed with grade I of NAFLD. The mean FF indicated by both liver biopsy and liver MRS revolved around 3% in patients with NAFLD grade 0, around 22% in patients with NAFLD grade I and around 46% in patients with NAFLD grade II. The results of the study indicated an increased mean value of serum triglycerides as the grade of NAFLD also increased. However, this rule did not apply to the mean values of total serum cholesterol. The mean values of serum ALT and AST recorded a significant increase in patients with grade I of NAFLD when compared to patients with grade 0 of NAFLD. However, there were no significant differences regarding the mean serum ALT and AST values between patients diagnosed with NAFLD grade I and grade II. It seemed that this rule only applied to the male subjects included in the study. Our study indicated an increased mean value of serum glucose as the grade of NAFLD also increased. However, it seemed that this rule was valid only in male subjects. The results provided by the current study highlighted an increased mean value of BMI as the NAFLD grade also increased. This seemed to apply to both male and female subjects.
The currently available literature data considers NAFLD to be the manifestation of the metabolic syndrome at hepatocellular level and is estimated to affect up to one-third of the general population worldwide with several regional differences. Regarding the gender distribution of this disease, some of the most recently published studies provide contradictory results [16,17,18,19]. Summart et al. presented the NAFLD prevalence values for each gender in one of the most recent studies conducted in Thailand. The study group included over 34 000 subjects, out of which 27 073 were women and 7636 were men. The prevalence of NAFLD recorded in women reached values of 22.9%, while in men it revolved around 18.3%. The highest difference in prevalence between genders was recorded in the 56–60 years age group [19]. The prevalence of NAFLD was also studied by Amarapurkar et al. and published the final results of their research in 2007. Their study included over 1150 Asian patients. Some of the most relevant exclusion criteria encountered in this study included: excessive alcohol consumption, presence of hepatitis B surface antigen, presence of viral hepatitis C antibodies, presence of other liver diseases, hepatotoxic drug intake. According to the results of this study, the prevalence of NAFLD was higher in men (24.6%) compared to women (13.6%) [20]. In 2017, Ballestri et al. published a review [21] which confirmed the increased prevalence of NAFLD in men compared to women. Moreover, the authors indicated that the prevalence values of NAFLD start to increase from young adulthood up until approximately 50–60 years old, when it starts to decline. In women, the prevalence of NAFLD is reduced below 50 years old and reaches the maximum value in the 60–69 years interval. After 70 years old, the prevalence of NAFLD is low in both genders [22,23,24].
The reliability of liver MRS in grading NAFLD compared to liver biopsy is also suggested by Tang et al. in an original paper that was published in 2013. The aforementioned study included 77 patients with NAFLD who underwent both liver biopsy and liver MRS. The results indicated a significant correlation between the NAFLD grade indicated by liver MRS and the NAFLD grade indicated by liver biopsy [24]. The importance of liver MRS in staging NAFLD is also indicated by Georgoff et al. in a study that was published in 2012 and included a total of 52 subjects that underwent liver MRS and liver biopsy. At the end of the study, the authors concluded that liver MRS is a powerful and effective non-invasive tool that can quantitatively assess NAFLD [14].
One study that was published in 2018 by Li et al. indicated that serum levels of ALT and AST liver enzymes may not necessarily be elevated in all patients with NAFLD [5]. A systematic review published in 2015 by Rinella [25] indicated that patients with an advanced stage of NAFLD usually have normal ALT levels. Moreover, elevated liver enzymes (ALT>AST) might indicate inflammation inside the liver parenchyma in patients with NASH [26,27,28]. In 2003, Mofrad et al. presented the results of their study, which included two different groups of patients. The first group consisted of 51 patients with NAFLD who presented normal serum values of ALT. The second group included 50 subjects with NAFLD who presented elevated serum values of ALT. The two study groups were similar regarding gender, ethnicity and age distribution. The authors concluded that the entire arsenal of histopathological changes can be highlighted in both groups regardless of the serum ALT values [27].
In 2014, Abangah et al. presented the results of an Iranian study that included a total of 213 patients (140 men and 73 women). The authors of the study highlighted a statistically significant association between the severity of hepatic steatosis described by US and the BMI values. Also, the results of the study indicated the presence of another statistically significant association between the severity of hepatic steatosis described by US and the serum values of triglycerides. Some other parameters like ALT, AST, total serum cholesterol, serum glucose were investigated, but the results provided by the authors did not indicate any significant association between the severity of hepatic steatosis described by US and these parameters [28].
The main limitation of our study is represented by the extremely invasive nature of the liver biopsy that determined several potential subjects to avoid undergoing this procedure, thus resulting in a somewhat reduced number of patients but still enough in our opinion to reveal the true potential of liver MRS in grading NAFLD.
Based on the results of the current study, we highly recommend using liver MRS as a quantitative assessment tool for NAFLD as it can result in a higher degree of acceptance among patients due to its non-invasive character. Moreover, this aspect will lead to an earlier diagnosis of NAFLD that can benefit from multiple therapeutic options due to a reduced chronic liver damage.
Conclusions
We recommend using liver MRS for evaluating and grading NAFLD in association with other parameters like serum triglycerides and BMI, as this protocol can enhance early detection and provide an accurate grading that will lead to a proper management of this disease.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
Grant support
This work was partially supported from the Research Grant No. 1334/17.12.2015, ClinicalTrials.gov ID: NCT02669641.
References
- 1.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Byrne CD. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease. Proc Nutr Soc. 2013;72(4):412–419. doi: 10.1017/S0029665113001249. [DOI] [PubMed] [Google Scholar]
- 4.Florescu LM, Gheonea IA, Ene D, Florescu DN, Braia N, Pirici D, Şandru V, Forţofoiu MC, Ciurea T. An extremely rare case of distal common bile duct adenocarcinoma in a 65-year-old male patient. Rom J Morphol Embryol. 2018;59(1):297–302. [PubMed] [Google Scholar]
- 5.Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10(8):530–542. doi: 10.4254/wjh.v10.i8.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musetescu AE, Cioroianu A, Ciurea PL, Florescu DN, Georgescu CV, Florescu LM, Bumbea AM. Iron synovitis in hemochromatosis associated arthropathy - a great mimicker. Rev Chim (Bucharest) 2018;69(4):971–974. [Google Scholar]
- 7.Traussnigg S, Kienbacher C, Gajdošík M, Valkovič L, Halilbasic E, Stift J, Rechling C, Hofer H, Steindl-Munda P, Ferenci P, Wrba F, Trattnig S, Krššák M, Trauner M. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017;37(10):1544–1553. doi: 10.1111/liv.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheonea IA, Streba CT, Cristea CG, Stepan AE, Ciurea ME, Sas T, Bondari S. MRI and pathology aspects of hypervascular nodules in cirrhotic liver: from dysplasia to hepatocarcinoma. Rom J Morphol Embryol. 2015;56(3):925–935. [PubMed] [Google Scholar]
- 9.Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(Suppl 4):64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 10.Ungureanu BS, Pirici D, Margaritescu C, Gheonea IA, Trincu FN, Fifere A, Saftoiu A. Endoscopic ultrasound guided injection of iron oxide magnetic nanoparticles for liver and pancreas: a feasibility study in pigs. Med Ultrason. 2016;18(2):157–162. doi: 10.11152/mu.2013.2066.182.eus. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23(4):290–301. doi: 10.3350/cmh.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgoff P, Thomasson D, Louie A, Fleischman E, Dutcher L, Mani H, Kottilil S, Morse C, Dodd L, Kleiner D, Hadigan C. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012;199(1):2–7. doi: 10.2214/AJR.11.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 16.Streba LAM, Cârstea D, Mitruţ P, Vere CC, Dragomir N, Streba CT. Nonalcoholic fatty liver disease and metabolic syndrome: a concise review. Rom J Morphol Embryol. 2008;49(1):13–20. [PubMed] [Google Scholar]
- 17.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 18.Băloşeanu CL, Streba CT, Vere CC, Comănescu V, Rogoveanu I. Association between liver histology, carotid ultrasonography and retinal vascular changes in patients with nonalcoholic fatty liver disease (NAFLD) Rom J Morphol Embryol. 2012;53(3):609–614. [PubMed] [Google Scholar]
- 19.Summart U, Thinkhamrop B, Chamadol N, Khuntikeo N, Songthamwat M, Kim CS. Gender differences in the prevalence of nonalcoholic fatty liver disease in the Northeast of Thailand: a population-based cross-sectional study. Version 2. F1000Res. 2017;6:1630–1630. doi: 10.12688/f1000research.12417.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, Baijal R, Lala S, Chaudhary D, Deshpande A. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6(3):161–163. [PubMed] [Google Scholar]
- 21.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43(3):508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T; Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multi-center large retrospective study. J Gastroenterol. 2012;47(5):586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 24.Ayuob NN, Abdel-Hamid AAHM, Helal GMM, Mubarak WA. Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom J Morphol Embryol. 2019;60(2):479–486. [PubMed] [Google Scholar]
- 25.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 26.Vere CC, Neagoe D, Streba CT, Prejbeanu I, Ianoşi G, Comănescu V, Pirici D. Steatosis and serum lipid patterns in patients with chronic viral hepatitis: differences related to viral etiology. Rom J Morphol Embryol. 2010;51(3):509–514. [PubMed] [Google Scholar]
- 27.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 28.Abangah G, Yousefi A, Asadollahi R, Veisani Y, Rahimifar P, Alizadeh S. Correlation of body mass index and serum parameters with ultrasonographic grade of fatty change in non-alcoholic fatty liver disease. Iran Red Crescent Med J. 2014;16(1):e12669–e12669. doi: 10.5812/ircmj.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]