Abstract
The presence of foreign materials in the tissues, represented in the present study by the insertion of dental implants, creates artificial structures that can sometimes cause adverse consequences, which implies the obligation to perform a complex medical assessment before inserting dental implants. This assessment appreciates the quality of the tissue, depending on which the use of a certain type of biomaterial is indicated and focuses on a certain surgical technique. We assessed the clinical, histopathological (HP) and immunohistochemical (IHC) aspects of peri-implant soft tissue in patients who did not show mobility or imagistic signs of bone resorption, three months after implant insertion, some of them showing no inflammatory clinical signs. Immunohistochemically, on the sections of the peri-implant mucosa, we assessed the presence of mast cells, vascularization and the process of angiogenesis. Mast cells are key cells actively involved in the pathogenesis of peri-implant inflammation, having an immunomodulatory role. Vasodilation and angiogenesis, determined by the release of chemical mediators by degranulation of mast cells under the action of pathogens, induce tissue remodeling, ensuring the healing and formation of a tissue to form a barrier that effectively prevents the development of a bacterial biofilm. Thus, the control of the activity of these cells is important for the management of the local inflammatory process. The correlations between the clinical, HP and IHC behavior of the peri-implant soft tissue bring important information for the clinic, emphasizing the need to identify a therapeutic strategy to modulate mast cell activity, in order to prevent and treat peri-implant disease, to ensure osseointegration and longer survival of the dental implant.
Keywords: dental implant, peri-implant mucosa, mast cells, tryptase, angiogenesis
Introduction
Through oral implantology, dentistry has made significant progress, with endosseous dental implants becoming a treatment option increasingly requested by edentulous patients. This modern prosthetic restoration therapy not only addresses the functional needs of patients (mastication, phonation), but also contributes to obtaining aesthetic results desired and appreciated by the patient. These are the reasons why implant treatment is increasingly used in specialized medical practice.
The presence of foreign materials in the tissues, represented by the insertion of dental implants, creates artificial structures that can sometimes cause adverse consequences, which implies the obligation to perform a complex medical assessment before inserting dental implants. This assessment assesses the quality of the tissue, depending on which the use of a certain type of biomaterial is indicated and orients towards a certain surgical technique. This is important because, despite a high rate of maintenance of dental implants, there are many patients who have complications immediately after implant insertion, after its osseointegration, or in the prosthetic reconstruction phase, complications that can even lead to the loss of the dental implant [1,2].
The etiological factors involved in peri-implant pathology are insufficiently known, but two are considered to be the main factors: bacterial infection and biomechanical overload [3,4]. Experimental studies suggest that peri-implant pathology has, as principal cause, bacterial plaque [5]. These are supported by the findings of clinical studies, histopathological (HP) studies on explanted dental implants and studies that assessed the role of oral hygiene for the long-term success of dental implant treatment [6,7,8]. The possibility that occlusal overload may cause pathological changes in osseointegration [9] has been confirmed by biomechanical overload studies in experimental animal models [10], by finding dental implant mobility without signs of marginal inflammation [11] and through studies that asses the impact of bone quality and of the length of the implant on the long-term success of the implant treatment [12].
Mast cells are key cells actively involved in the pathogenesis of peri-implant inflammation, the control of their activity being important for the management of inflammatory conditions. On the other hand, pathogens induce the release of chemical mediators from mast cells, which causes vasodilation, angiogenesis, cell recruitment, tissue remodeling and fibrosis [13].
Aim
Starting from these aspects, this morphological study aimed at highlighting the presence of mast cells, vascularization and angiogenesis, as well as quantifying these data in relation to the clinical aspects found, in order to achieve a correlation between clinical, HP and immunohistochemical (IHC) behavior of the peri-implant soft tissue.
Patients, Materials and Methods
The study was performed on a number of 10 patients, from whom we harvested, three months after the insertion of dental implants, peri-implant oral mucosa. The number of implants differed from one patient to another, depending on the number of missing teeth. All patients were non-smokers and ranged in age from 30 to 65 years. Imaging examination did not reveal peri-implant bone changes, but three patients showed local inflammatory signs caused by the presence of bacterial plaque.
Paraffin-embedding histological technique was performed for the processing of peri-implant mucosal fragments. The obtained sections were stained with Hematoxylin–Eosin (HE) and Goldner–Szekely (GS) trichrome. To perform the IHC study, 4 μm thick sections were made from the paraffin blocks. IHC processing used the Avidin–Biotin complex/Horseradish peroxidase (ABC/HRP – Avidin complexed with biotinylated peroxidase) technique as a working method. We used the following antibodies: anti-tryptase (monoclonal mouse anti-human mast cell tryptase, clone AA1, 1/500 dilution, Dako) for mast cells; anti-cluster of differentiation (CD) 34 (monoclonal mouse anti-human CD34 Class II, clone QBEnd/10, 1/100 dilution, Dako) for endothelial cells highlighting.
Results
The oral mucosa around natural teeth and around dental implants has common morphological features, but the orientation of collagen fibers and vascularization differs in the connective tissue. The quality of the peri-implant mucosa is important for the long-term success of the dental implant. Thus, an inadequate response can lead to treatment failure, through loss of the implant. Knowledge of the structure of the peri-implant mucosa brings important information to ensure the clinical success of dental implant treatment. On the sections obtained from patients who did not show inflammatory clinical signs, as well as from those who showed obvious clinical signs of inflammation, we highlighted immunohistochemically the presence of mast cells and the appearance of vascularization. This is because it is known that intense vascularization is present in an inflammatory process, being necessary to support the repair processes, through the supply of necessary substances. On the other hand, we aimed to establish correlations between the intensity of vascularization and the degree of representation of mast cells that, as released mediators, have the ability to stimulate this process.
Histological aspects of the peri-implant mucosa
On the examined sections, we identified different histological aspects, both at the level of the epithelium and at the level of the lamina propria of the peri-implant mucosa, compared to the structure of the normal gingival mucosa. Sometimes, the changes were zonal, other times they affected larger areas of the peri-implant mucosa. In the areas where changes in the connective tissue were more intense, they were also associated with changes in the overlaying epithelium. These changes were more intense in patients where inflammation was also clinically present and were more important at the level of the connective tissue, compared to the surface epithelium.
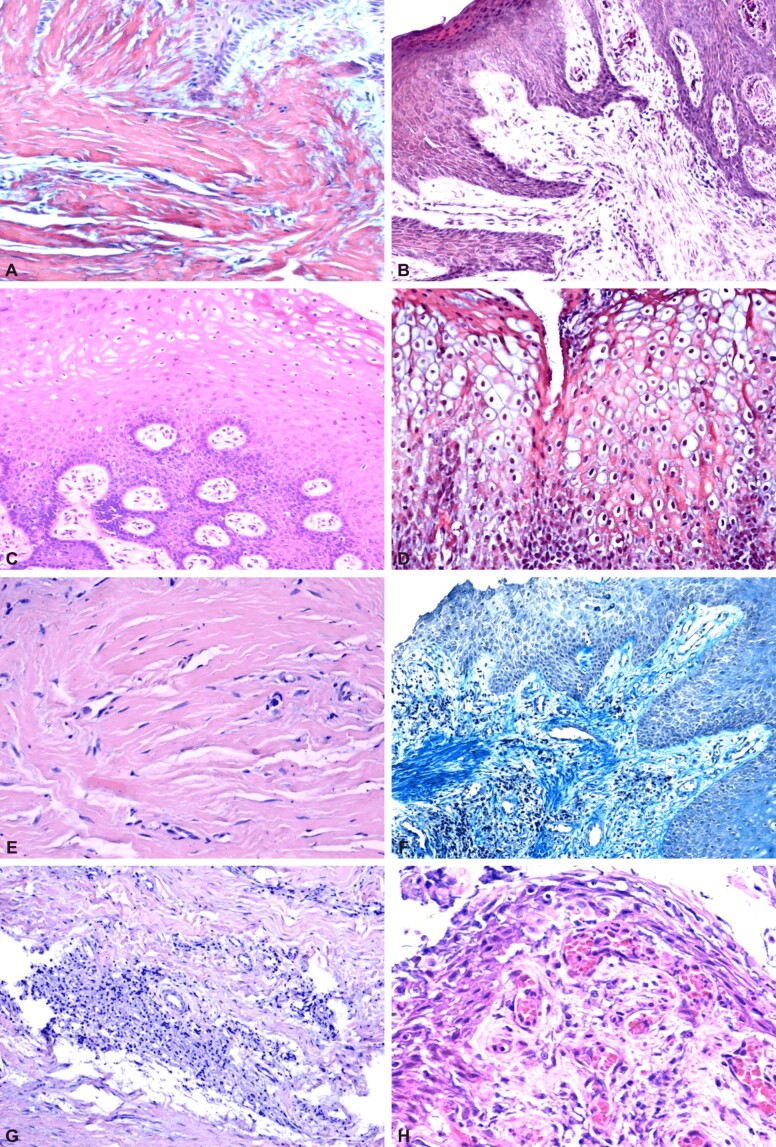
The epithelium was keratinized on all examined sections, sometimes orthokeratinization was present, other times a process of parakeratinization was present, there were no differences compared to the gingival mucosa around the natural teeth (Figure 1A and 1B). We identified the presence of acanthosis, which increased the thickness of the epithelium and the appearance of long, branched and interconnected epithelial extensions (Figure 1C). This aspect, present in the proximity of the lamina propria, ensures an interpenetration of the epithelial projections in the form of ridges with the papillary extensions of the connective tissue. On some sections, the epithelial extensions take on the appearance of long and narrow cell cords. On some of the sections, the epithelial cells from the spinous and superficial layer presented a ballooned appearance due to vacuums of different sizes, sometimes confluent (Figure 1D). Epithelial changes differed from patient to patient, but also varied in the same patient from one implant site to another.
Figure 1.
Peri-implant mucosa: (A) Orthokeratinized epithelium; (B) Parakeratinized epithelium; (C) Acanthosis, deep epithelial ridges, edema of the epithelial cells in the intermediate layer, which, at the level of the superficial layer, have a vacuolar appearance; (D) Edema of cells in all layers of the epithelium; (E) Lamina propria rich in collagen fibers associated with numerous fibroblasts; (F) Diffuse lymphoplasmocytic inflammatory infiltrate; (G) Nodular-looking lymphoplasmocytic inflammatory infiltrate; (H) Numerous capillaries and inflammatory infiltrate in a papilla of the connective tissue. HE staining: (A–C, E and H) ×200; (G) ×100. Masson’s trichrome staining: (D and F) ×100
At the level of the chorion of the peri-implant mucosa, compared to the structure of the normal gingival mucosa, there were changes of all components: cellularity, collagen fibers and blood vessels. In the structure of the chorion, the collagen fibrillar component, specific to a masticatory mucosa, was majoritarily. Overall, the conjunctival papillae took on a high appearance rising between the epithelial ridges. The cell population was represented by fibroblasts, as well as chronic inflammatory cells, of lymphoplasmocytic and macrophage type, which indicates the presence of both a specific and non-specific inflammatory process. The fibroblasts encountered in large numbers justify the presence in large numbers of collagen fibers on the examined sections (Figure 1E). The intensity of the inflammatory process was different from one patient to another and from one implant site to another in the case of the same patient. Inflammatory infiltrate was more pronounced in cases where patients also had a local peri-implant inflammatory reaction clinically visible, with a diffuse appearance (Figure 1F) or sometimes showed a nodular disposition (Figure 1G). The variability of the inflammatory aspect, both in terms of intensity and in terms of disposition, suggests that these changes are caused by bacterial plaque, which is exacerbated by local aggressive factors (Figure 1H). The distribution of blood vessels was different, being more numerous in the conjunctival papillae and in areas with inflammatory infiltrate. The blood vessels were represented by typical capillaries but also by neoangiogenesis vessels. Reduced vascularization was identified on the sections where the collagen fibrillar component predominated and the inflammatory connective cells were numerically reduced, indicating the tendency to extinguish the inflammatory reaction.
The changes we encountered in the peri-implant mucosa, both in the epithelium and in the lamina propria, are non-specific, being present in other periodontal, inflammatory and hyperplastic diseases.
IHC study of the peri-implant mucosa
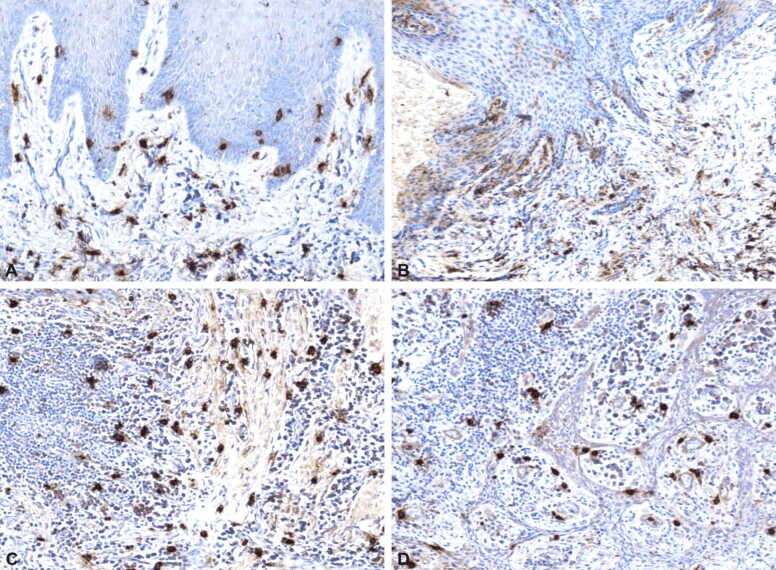
In the IHC study, on the sections of peri-implant mucosa harvested from patients three months after the insertion of dental implants, we monitored the presence of mast cells, the appearance of vascularization and the process of angiogenesis. This is because mast cells are key cells, actively involved in the pathogenesis of peri-implant inflammation, having an immunomodulatory role, along with lymphocytes and plasma cells. The immunomodulatory properties in inflammation are due to the ability to produce cytokines and mediators, increasing the intensity of inflammation. The release of chemical mediators by degranulation of mast cells under the action of pathogens causes vasodilation and angiogenesis, inducing tissue remodeling and ensuring the healing and formation of a tissue that becomes a barrier to effectively prevent the development of a bacterial biofilm. Thus, the control of the activity of these cells is important for the management of the local inflammatory process. To highlight mast cells, we used tryptase, which marks not only immature cells, but also degranulated ones. On the examined sections, mast cells were present in large numbers in inflammatory areas and were also identified in very small numbers in areas with developed collagen fibrillar component, or in areas of the mucosa where no inflammatory process was present. When the inflammatory infiltrate was located in the superficial chorion, mast cells were also identified intraepithelially (Figure 2A and 2B). The degranulation process was more frequently observed in mast cells present in the inflammatory focus, many of them being degranulated (Figure 2C) and located perivascularly (Figure 2D). On the examined sections of peri-implant mucosa, immunolabeling for tryptase was evident in patients with obvious inflammatory clinical signs, indicating the involvement of these cells in the chronic inflammatory process of these patients.
Figure 2.
Peri-implant mucosa: (A) Mast cells located intraepithelially and in the superficial chorion; (B) Mast cells arranged in the superficial chorion; (C) Diffused arranged granulated mast cells; (D) Mast cells with perivascular location. Positive immunolabeling for anti-tryptase antibody: (A–D) ×100
Examination of IHC expression and localization of mast cell tryptase in peri-implant soft tissue from the studied patients, contributes to increasing understanding of the inflammatory phenomenon and its evolution, associated with clinical issues encountered in patients receiving dental implant therapy.
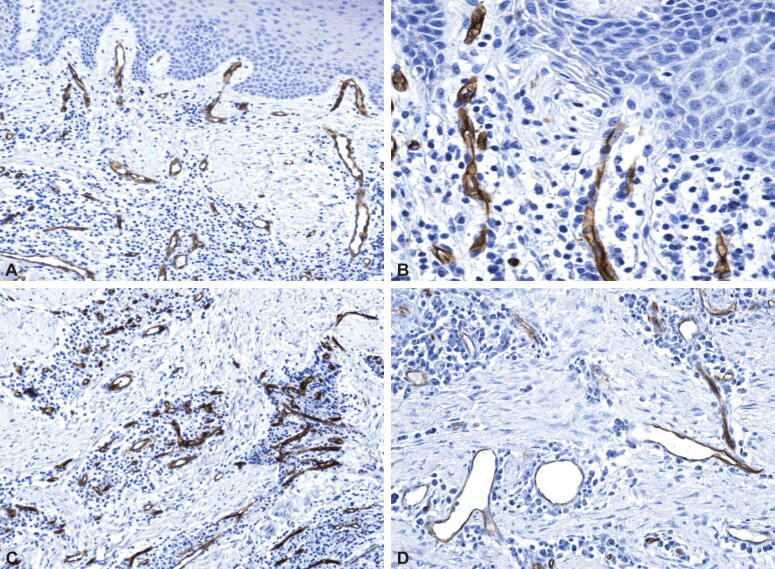
Vascularization and angiogenesis in the peri-implant mucosa were demonstrated immunohistochemically using the anti-CD34 antibody. Blood supply is important in conditions of a healthy mucosa to ensure the functionality of peri-implant tissues and it becomes absolutely necessary in the event of an inflammatory process. In order for the repair and healing processes to take place, the supply of the necessary substances has to be present. Vascularization and angiogenesis were present on all sections examined, but showed different intensities. On microscopic sections from patients who did not show clinical signs of inflammation, inflammatory cells and mast cells were present in very small numbers and were associated with restricted vascularity and an almost non-existent angiogenic process. On the sections from patients who showed clinical signs of inflammation, vascularity and angiogenesis was very intense, correlated to the inflammatory process’s intensity and to the population of mast cells that release chemical mediators and enzymes that cause angiogenesis. The vessels were mainly represented by small-caliber, capillary vessels. The angiogenesis process was of capillary type, starting with the pre-existing vessels, being located in areas with inflammatory infiltrate (Figure 3A, 3B, 3C, 3D).
Figure 3.
Peri-implant mucosa: (A and B) Intense vascularization in the connective tissue’s papillae and superficial chorion; (C) Intense vascularization in areas with inflammatory infiltrates; (D) Capillary angiogenesis in inflammatory infiltrate. Positive immunolabeling for anti-CD34 antibody: (A and C) ×100; (B and D) ×200
These microscopic aspects are specific to the development of a healing process.
Discussion
Clinical studies and experiments on laboratory animals have shown that the accumulation of bacterial plaque around dental implants causes the development of an inflammatory process in the peri-implant mucosa, named mucositis [14]. This inflammatory lesion has many features in common with gingivitis developed in the gingival mucosa around natural teeth. Some authors [15] have analyzed the manner in which the tissue responds to the appearance of bacterial plaque on teeth and dental implants in on partially edentulous persons who volunteered for the study. The increase of clinical signs of inflammation, of inflammatory cell infiltrate’s dimension and the proportion of different markers of inflammatory cells, both in the peri-implant mucosa and in the gingival mucosa, during plaque formation has been shown [16,17].
Pathogens induce the release of chemical mediators from mast cells, causing vasodilation, angiogenesis, cell recruitment, tissue remodeling and fibrosis [13]. The process of tissue remodeling and fibrosis takes place with the participation of mast cells that have an important role regarding the genesis of connective tissue [18,19]. Increased immunolabeling of mast cells with tryptase in various tissues has been described systemically in the aorta [20,21], renal cell carcinoma [22] and calcification areas [23]. Although no studies have been performed on chymase or tryptase-labeled mast cells in peri-implantitis, the involvement of these cells has been shown in human and rodent periodontal [24] disease [25]. Other studies show an increased tryptase activity in cervical fluid in patients diagnosed with periodontal disease. These experimental studies prove that inhibition of tryptase is able to prevent the loss of alveolar bone in periodontitis. Moreover, through their inflammation immunomodulatory properties, mast cells are able to produce cytokines and mediators by increasing the degree of inflammation intensity [26,27]. In our study, too, there was an increase in mast cell tryptase immunolabeling in patients who also showed inflammatory clinical signs, compared with those who did not clinically show these signs, which proves the involvement of these cells in the chronic inflammatory process of the group of patients who showed obvious clinical signs of inflammation. The release of tumor necrosis factor-alpha (TNF-α) by mast cells activates the endothelium and upgrades inflammation. The activation of the endothelium produces greater expression of the CD31 adhesion molecule in the endothelial membrane. Studies in rats have shown an increase in CD31 in lesions located periapically that also confirms the conclusions of our study in which CD34 immunolabeling shows a higher density of blood vessels, especially on sections from patients who also had a clinical inflammatory signs. Thus, where a higher density of blood vessels immunolabeled with anti-CD34 antibody was present, we hypothesized that, in this group, increased neovascularization during chronic inflammation causes a greater influx of leukocytes at the inflammation site, and particularly effector lymphocytes. The effector T-lymphocytes that first arrive at the site of inflammation are T-helper 17 (Th17) cells, which produce interleukin (IL)-17. The pathogenesis of various inflammatory diseases is linked to this cytokine. Among these diseases are rheumatoid arthritis, psoriasis, systemic lupus erythematosus, peri-implantitis [28]. IL-17 is known to play an important part in the immune and the inflammatory response. It regulates the expression of cytokines, chemokines and adhesion molecules, which are mediators of inflammation [29]. However, proper vascularization is also important in the absence of an inflammatory process to ensure the functionality of peri-implant tissues [30,31].
We found that the density of tryptase-positive mast cells and the density of blood vessels immunolabeled with anti-CD34, which is the adhesion molecule present on endothelial cells, are correlated. Chemical mediators and enzymes that cause angiogenesis are released by mast cells, and the presence of mast cells in large numbers determines, in response, osteoclastic resorption. In health conditions of the peri-implant mucosa, IL-13 can act, controlling and regulating the synthesis of IL-17 by T-lymphocytes. In inflammatory processes, there is an increase in IL-17, which contributes to the exacerbation of this process, inhibiting IL-13 activity. In our study, the presence of these cells in large numbers, especially in patients who showed clinical inflammatory appearance, explains the increase in the number of CD34 immunolabeled vessels and suggests the participation of mast cell tryptase in endothelial cell-induced changes. Thus, increased IL-17 could activate osteoclasts and cause bone loss and consequently trigger implant loss. The understanding of the role played by cytokines in the installation of peri-implantitis, could lead to the development of new therapies inhibiting the synthesis of IL-17 and inducing the synthesis of IL-13 in the peri-implant tissue, which contributes to increasing the dental implant’s long-term success rate.
Mast cells are key cells actively involved in the pathogenesis of peri-implant inflammation, the control of their activity being important for the management of inflammatory conditions. Our findings should materialize in the therapeutic implications of building strategies for the possible use of drugs to inhibit and/or influence mast cell activation in order to increase dental implant osseointegration and to prevent and treat peri-implant disease.
The manner in which peri-implant mucosa responds to plaque depends on a local defense mechanism that forms once the mucosa attaches to the implant. The existence in the alveolar mucosa of various leukocytes was analyzed before and after it became peri-implant mucosa [32]. Only inflammatory cells that expressed markers for different types of immunity were identified in the alveolar mucosa, while a higher number of leukocytes was present in the peri-implant mucosa. It has been suggested that an epithelial barrier is formed in the peri-implant mucosa due to antigens that may pass to the mucosa from the oral cavity.
Our study reported the presence of inflammatory lesions in the peri-implant mucosa. These findings are in accordance with other studies that indicate that inflammatory cells are also present in healthy gums and free peri-implant mucosa [33]. Accumulation of bacterial plaque and microbial contamination of peri-implant soft tissue causes inflammation and, consequently, large exudation of inflammatory cells. Our study is consistent with others that have found an increase in vascular permeability and exudation of leukocytes that release enzymes that cause destruction of the vascular wall.
Because angiogenesis is an important part of the healing process, we used the CD34-labeled endothelial cells to identify vascular structures on the examined sections. IHC study of blood vessels and angiogenesis with the help of anti-CD34 antibody showed the presence of a larger number of vessels in patients who showed clinical signs of inflammation. In our study, we found an important positive correlation between the density of tryptase-positive mast cells and the density of blood vessels immunolabeled with anti-CD34 antibody. This is because CD34 is an adhesion molecule present on endothelial cells and mast cells release chemical mediators and enzymes that promote angiogenesis. In response, mast cells present in large numbers may cause osteoclastic resorption.
Conclusions
The changes we encountered in the peri-implant mucosa, both in the epithelium and in the lamina propria are non-specific, being present in other periodontal, inflammatory and hyperplastic diseases. Mast cells are key cells actively involved in the pathogenesis of peri-implant inflammation, the control of their activity being important for the management of inflammatory conditions in patients who have benefited from dental implant therapy. Intense vascularity and the presence of angiogenesis were correlated in patients who showed obvious clinical signs of inflammation, with increased immunoreactivity for tryptase, but also with a higher number of inflammatory cells involved in specific and nonspecific defense. The correlations between the clinical, HP and IHC behavior of the peri-implant soft tissue bring important information for the clinic, emphasizing the need to identify a therapeutic strategy to modulate mast cell activity, in order to prevent and treat peri-implant disease, ensure osseointegration and implicitly the longevity of the dental implant.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
This paper was published under the frame of European Social Found, Human Capital Operational Programme 2014–2020, Project No. POCU/82/3/7/106931.
References
- 1.Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81(2):231–238. doi: 10.1902/jop.2009.090269. [DOI] [PubMed] [Google Scholar]
- 2.Pjetursson BE, Tan K, Lang NP, Brägger U, Egger M, Zwahlen M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. IV. Cantilever or extension FPDs. Clin Oral Implants Res. 2004;15(6):667–676. doi: 10.1111/j.1600-0501.2004.01120.x. [DOI] [PubMed] [Google Scholar]
- 3.de Araújo Nobre M, Azul AM, Rocha E, Maló P. Risk factors of peri-implant pathology. Eur J Oral Sci. 2015;123(3):131–139. doi: 10.1111/eos.12185. [DOI] [PubMed] [Google Scholar]
- 4.Kourtis SG, Sotiriadou S, Voliotis S, Challas A. Private practice results of dental implants. Part I: survival and evaluation of risk factors - Part II: surgical and prosthetic complications. Implant Dent. 2004;13(4):373–385. doi: 10.1097/01.id.0000148564.88384.de. [DOI] [PubMed] [Google Scholar]
- 5.Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182–190. doi: 10.1111/j.1600-0501.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- 6.Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20(2):169–174. doi: 10.1111/j.1600-0501.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 7.Martins MC, Abi-Rached RSG, Shibli JA, Araujo MWB, Marcantonio E. Experimental peri-implant tissue breakdown around different dental implant surfaces: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants. 2004;19(6):839–848. [PubMed] [Google Scholar]
- 8.Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 9.Hsu YT, Fu JH, Al-Hezaimi K, Wang HL. Biomechanical implant treatment complications: a systematic review of clinical studies of implants with at least 1 year of functional loading. Int J Oral Maxillofac Implants. 2012;27(4):894–904. [PubMed] [Google Scholar]
- 10.Heitz-Mayfield LJ, Schmid B, Weigel C, Gerber S, Bosshardt DD, Jönsson J, Lang NP, Jönsson J. Does excessive occlusal load affect osseointegration? An experimental study in the dog. Clin Oral Implants Res. 2004;15(3):259–268. doi: 10.1111/j.1600-0501.2004.01019.x. [DOI] [PubMed] [Google Scholar]
- 11.Esposito M, Thomsen P, Mölne J, Gretzer C, Ericson LE, Lekholm U. Immunohistochemistry of soft tissues surrounding late failures of Brånemark implants. Clin Oral Implants Res. 1997;8(5):352–366. doi: 10.1034/j.1600-0501.1997.080502.x. [DOI] [PubMed] [Google Scholar]
- 12.Strub JR, Jurdzik BA, Tuna T. Prognosis of immediately loaded implants and their restorations: a systematic literature review. J Oral Rehabil. 2012;39(9):704–717. doi: 10.1111/j.1365-2842.2012.02315.x. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- 14.Berglundh T, Jepsen S, Stadlinger B, Terheyden H. Peri-implantitis and its prevention. Clin Oral Implants Res. 2019;30(2):150–155. doi: 10.1111/clr.13401. [DOI] [PubMed] [Google Scholar]
- 15.Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28(6):517–523. doi: 10.1034/j.1600-051x.2001.028006517.x. [DOI] [PubMed] [Google Scholar]
- 16.Obădan F, Crăiţoiu Ş, Manolea HO, Hîncu MC, Iacov-Crăiţoiu MM. The evaluation of the morphological evolution of the tissue integration of dental implants through conventional histology and immunohistochemistry techniques. Rom J Morphol Embryol. 2018;59(3):851–859. [PubMed] [Google Scholar]
- 17.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S313–S318. doi: 10.1002/JPER.17-0739. [DOI] [PubMed] [Google Scholar]
- 18.Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, Levi-Schaffer F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy. 2002;32(2):237–246. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomasi C, Tessarolo F, Caola I, Piccoli F, Wennström JL, Nollo G, Berglundh T. Early healing of peri-implant mucosa in man. J Clin Periodontol. 2016;43(10):816–824. doi: 10.1111/jcpe.12591. [DOI] [PubMed] [Google Scholar]
- 20.Czyzewska-Buczyńska A, Witkiewicz W. Role of mast cells in the pathogenesis of atherosclerosis. Przegl Lek. 2011;68(3):171–174. [PubMed] [Google Scholar]
- 21.Qin Y, Shi GP. Cysteinyl cathepsins and mast cell proteases in the pathogenesis and therapeutics of cardiovascular diseases. Pharmacol Ther. 2011;131(3):338–350. doi: 10.1016/j.pharmthera.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Miyata Y, Matsuo T, Mochizuki Y, Nishikido M, Hayashi T, Sakai H. High density of tryptase-positive mast cells in patients with renal cell carcinoma on hemodialysis: correlation with expression of stem cell factor and protease activated receptor-2. Hum Pathol. 2012;43(6):888–897. doi: 10.1016/j.humpath.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Maślińska D, Laure-Kamionowska M, Deręgowski K, Maśliński S. Association of mast cells with calcification in the human pineal gland. Folia Neuropathol. 2010;48(4):276–282. [PubMed] [Google Scholar]
- 24.Batista AC, Rodini CO, Lara VS. Quantification of mast cells in different stages of human periodontal disease. Oral Dis. 2005;11(4):249–254. doi: 10.1111/j.1601-0825.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 25.Holzhausen M, Balejo RDP, Lara GM, Cortelli SC, Saad WA, Cortelli JR. Nafamostat mesilate, a potent tryptase inhibitor, modulates periodontitis in rats. Clin Oral Investig. 2011;15(6):967–973. doi: 10.1007/s00784-010-0463-1. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira Rodini C, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(1):59–63. doi: 10.1016/s1079-2104(03)00378-0. [DOI] [PubMed] [Google Scholar]
- 27.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61(3):233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- 28.Severino VO, Napimoga MH, de Lima Pereira SA. Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Arch Oral Biol. 2011;56(8):823–828. doi: 10.1016/j.archoralbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Park SJ, Lee YC. Interleukin-17 regulation: an attractive therapeutic approach for asthma. Respir Res. 2010;11(1):78–78. doi: 10.1186/1465-9921-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piattelli A, Degidi M, Di Stefano DA, Rubini C, Fioroni M, Strocchi R. Microvessel density in alveolar ridge regeneration with autologous and alloplastic bone. Implant Dent. 2002;11(4):370–375. doi: 10.1097/00008505-200211040-00017. [DOI] [PubMed] [Google Scholar]
- 31.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 32.Liljenberg B, Gualini F, Berglundh T, Tonetti M, Lindhe J. Some characteristics of the ridge mucosa before and after implant installation. A prospective study in humans. J Clin Periodontol. 1996;23(11):1008–1013. doi: 10.1111/j.1600-051x.1996.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 33.Crăiţoiu MM, Păuna M, Mercuţ V, Crăiţoiu Ş, Niţulescu EA. Clinical, histological and therapeutic study regarding the variations of the edentulous ridge’s mucosa. Rom J Morphol Embryol. 2009;50(3):441–445. [PubMed] [Google Scholar]