Abstract
Iron (Fe) and manganese (Mn) are nutritional components of rice, plays an important role in its physiological processes and can minimize absorption of cadmium (Cd) in rice. Fe, Mn, and Cd transporters such as CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, OsHMA3, and OsNAAT1 regulate uptake of Cd in rice. However, the effect of exogenous application of Fe, and Mn on the accumulation of Cd and relative expression (RE) of these transporters in rice has not been investigated. Therefore, a hydroponic culture experiment was conducted to investigate the impact of Fe and Mn on Cd uptake and RE of these transporters in rice. The results showed that the Fe and Mn application significantly decreased Cd in the roots and shoots of rice. Whereas, Cd concentration in the rice significantly increased with increasing Cd concentration in the solution. The addition of manganese in the culture medium can reduce the cadmium content of rice roots by 11.9–82.3% and shoots by 11.6–85.0%, while the addition of iron in the culture medium can reduce the cadmium content of rice roots and shoots by 26–65% and 9–683% respectively. Meanwhile, application of sufficient doses of Fe and Cd in solution culture increased RE of CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, and OsNAAT1 in roots, whereas expression level of OsHMA3 was decreased. Similarly, expression level of CAL1, OsNRAMP5, and OsNRAMP1 significantly increased in roots in high Cd and Mn deficient treatments. This may be concluded that the Cd increases expression of CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, and OsNAAT1 but decreases OsHMA3 expression in rice roots, which resulted in increased Cd uptake in hydroponically grown rice.
Introduction
Rice (Oryza sativa L.) is the staple food for more than half of the world’s population [1]. Cd pollution in rice has posed a serious threat to human health especially in Asian countries [2]. Cd can severely affect several human organs and systems such as the reproductive system, respiratory system, kidneys and skeletal system and can cause severe health problems such as Itai-Itai disease [2, 3]. Thus, minimizing Cd uptake by rice through fertilizer/nutrient management is an easy and effective method [4, 5].
Fe is an essential micronutrient and has effectively decreased Cd toxicity in different plant species [6–8]. Exogenous application of Fe can reduce Cd content in rice and improve rice growth and yield [8, 9]. Manganese is a key supplement for plant growth, which plays an important role in enzyme activation, biotic redox reactions, splitting of H2O, and decontamination O2 free radicals [10, 11]. Fe and Mn both alleviated Cd toxicity by reducing Cd accumulation and by upholding redox regulation that prevents Cd-inducible damage to root growth and photosynthesis [12]. Fe and Mn can form iron plaque on the surface of rice roots sequester Cd on roots [1, 13]. However, molecular studies showed that Cd translocation into rice occurs via Fe metabolic pathways which may be affected by Fe concentration in substrates [14, 15].
Rice accumulates more Cd than other cereal crops may be due to higher expression and functionality of the OsNRAMP5 gene (responsible for Cd uptake by roots) [3]. Furthermore, several rice genes have been identified which take part in xylem loading and phloem redistribution of Fe and Cd at different locations in the rice plant. For example, OsIRTI and OsNRAMP5 mediate uptake of Cd from the rhizosphere into root cells [15]. OsHMA3 is involved in Cd compartmentalization into vacuoles in root cells [16]. It was found that the expression of OsHMA3 was up-regulated under Cd stress in rice roots than that of control [17]. Several genes have been reported, which affects the uptake, transportation, and accumulation of Mn and Cd in rice. Cd from the root surface is primarily taken into root cells as a ‘hitchhiker’ via the Mn transporter OsNRAMP5 [15]. CAL1 (cadmium accumulation in leaf 1) mainly expressed in root exodermis and xylem parenchyma cells and sequesteres Cd in the cytosol, seems to minimize Cd content in cytosol there by carrying long-distance Cd transport through xylem vessels. CAL1 did not show any effect on the accumulation of Cd in rice grain [18]. Thus, effect of exogenous application of Fe and Mn on the accumulation of Cd as well as relative expression of CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, OsNAAT1, and OsHMA3 in rice has not been investigated. Therefore, the present experiment was conducted to find out the impact of Fe and Mn cations on uptake of Cd in rice and expression level of aforementioned genes under combined application of Fe, Mn and Cd.
Materials and methods
Plant growth and treatments
A hydroponic pot experiment was conducted at greenhouse of the Chinese Academy of Agricultural Sciences Beijing (40° 0' 8.6364'' N, 116° 21' 57.5208'' E). The seeds of the “Huang Hua Zhan” rice variety, collected from Changsha city were surface sterilized for 15 minutes in 30% (v/v) H2O2 solution, thoroughly washed with deionized water and then soaked in distilled water in the dark for 48 hours. Rice seeds were then sandwiched into two filter papers that were placed vertically in petri dishes. The young seedlings were then transferred into beakers containing hydroponic solution for three weeks. After three weeks rice seedlings were transferred to pots for four weeks in a full-strength Hoagland nutrient solution (pH 5.5), consisting of 0.116 mg L−1 NH4NO3, 0.0499 mg L−1 NaH2PO4•2H2O, 0.087 mg L−1 K2SO4, 0.111 mg L−1 CaCl2, 0.418 mg L−1 MgSO4•7H2O, 0.091 mg L−1 (NH4)6MoO24•4H2O, 1.098 mg L−1 H3BO3, 0.0445 mg L−1 ZnSO4•7H2O, 0.0416 mg L−1 CuSO4•5H2O. The pH of the nutrient solution was set to 5.5 using morpholinoethanesulphonic acid (MES) and the solution was changed after every three days. In the full strength, Hoagland solution seedlings were treated with 0, 10, 20, and 30 mg L−1 Cd (supplied as CdSO4). While 0, 0.2, 0.4 and 0.6 mg L−1 Fe as FeSO4.7H2O with full-strength Hoagland solution were supplied for four weeks. The experiment had two factors, the first factor was Fe (Fe0, Fe0.2, Fe0.4 and Fe0.6) and the second was Cd (Cd0, Cd10, Cd20 and Cd30). The interactions of Fe and Cd combination resulted in 16 treatments, each replicated three times. The treatments combinations were Fe0Cd0 (no Fe and Cd applied), Fe0Cd10, Fe0Cd20, Fe0Cd30, Fe0.2Cd0, Fe0.2Cd10, Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd0, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd0, Fe0.6Cd10, Fe0.6Cd20 and Fe0.6Cd30. For Mn and Cd experiment the same procedure was followed. Cd was supplied as 0, 10, 20 and 30 mg L−1 Cd (CdSO4), while Mn treatments were as 0, 0.5, 1.0, and 2.5 in the form of MnSO4. Here the two factors were Mn (Mn0, Mn0.5, Mn1 and Mn1.5) and Cd (Cd0, Cd10, Cd20 and Cd30). Thus, interaction of Mn and Cd treatments also resulted in 16 combination as follows: Mn0Cd0 (no Mn and Cd supply), Mn0Cd10, Mn0Cd20, Mn0Cd30, Mn0.5Cd0, Mn0.5Cd10, Mn0.5Cd20, Mn0.5Cd30, Mn1.0Cd0, Mn1.0Cd10, Mn1.0Cd20, Mn1.0Cd30, Mn2.5Cd0, Mn2.5Cd10, Mn2.5Cd20, and Mn2.5Cd30. The experiment was carried out in a greenhouse with 70% humidity, the temperature varying between 25–35°C, with light exposure of 12–14 h d−1. The photographs of the experiment are shown in S1 Fig.
Chemical analysis of roots and leaves
The rice roots and leaves were harvested at the end of the experiment, cleaned with tap water followed by a wash with distilled water In order to achieve a constant weight, samples were oven-dried at 100°C. A stainless steel grinder was used to crush dried rice plant materials (i.e. roots and shoots) into fine powder and accurately weighed (0.5 g) into clean, dry digestion tubes (100 ml). Then 6 ml of concentrated nitric acid (HNO3) and 3 ml of hydrogen peroxide (H2O2) were added and left overnight in the digestion tubes. The tubes were then placed in a high-pressure sealed digestion microwave where the temperature was set at 200°C and kept for two hours. The tubes were put on a heating block after digestion in the microwave and kept for around two to three hours. The solutions were then cooled at room temperature, diluted to 50 ml with ultra-pure water containing 5% HNO3; the samples were gently shaken and then filtered [19]. The total Fe, Mn Subsequently and Cd in digested solutions were measured by inductively coupled plasma mass spectrometry (ICP-MS).
RNA extraction and QRT-PCR
The total RNA was obtained from four weeks old seedlings. Samples of fresh roots and shoots were collected using Trizol reagent (Life, Japan). For each sample, approximately 2μ of RNA was used for reverse transcription with a PrimerScript 1st strand cDNA synthesis Kit (Takara, Japan). The QRT-PCR assays were carried out using SYBR Premix Ex Taq (Takara, Dalian, China) on the AB17500 PCR system (Life Technologies, USA). The expression levels of target genes were normalized to that of OsActin. All QRT-PCR assays were performed in three independent replications. Relative gene expression levels were detected using the 2–ΔΔCT method [20]. The primers used in this study are listed in Table 1.
Table 1. Primers used for relative genetic expression.
| Primer code | Base sequence (5' to 3') |
|---|---|
| CAL1-Q-F | AGTCGCGTGTTCTCCTTTGT |
| CAL1-Q-R | CATGACAGCAGCTTGCAAAT |
| OsNAAT1-Q-F | GGAGGGAATCCATGATGATG |
| OsNAAT1-Q-R | GGCAGAAGGATTTGATCCTCTC |
| OsIRT1-Q-F | GAACCGCGTCGTCGTTCAG |
| OsIRT1-Q-R | CCATCCCCTCGAACATCTGG |
| OsIRT2-Q-F | TCATGCTCACGTTCCACACG |
| OsIRT2-Q-R | GAGAACCTGCACAATGACGC |
| OsNramp1-Q-F | TCTCTGTCTCCGGCACTGTA |
| OsNramp1-Q-R | CATCAGGTTCCGAAGCCACT |
| OsNramp5-Q-F | GAAGTGGCTTCGGAACCTGA |
| OsNramp5-Q-R | GAAGCTCGTGCTCAGGAAGT |
| OsHMA2-Q-F | GAGGGAGGGAGGTGTCAGAA |
| OsHMA2-Q-R | TGGTGATCTTCTCACTGCCG |
| OsHMA3-Q-F | AGAACAGCAGGTCGAAGACG |
| OsHMA3-Q-R | ATTGCTCAAGGCCATCTGCT |
| OsLCT1-Q-F | GAGTTCTTCGTCAGAGCTAC |
| OsLCT1-Q-R | CAGTGCTGGATGACGAATTG |
Quality control
To ensure the accuracy of the results, a standard reference material (GBW08513) from the National Research Center for Standards of China was used. The metals recovery rate ranged from 95% to 104%.
Statistical analysis
Descriptive statistics were conducted by Microsoft Excel 2016. Analysis of variance (ANOVA) was conducted by XLSTAT software [21]. Mean data was tested by Duncan’s multiple range test at (p < 0.05), conducted with the XLSTAT.
Results
Effect of Fe, Mn and Cd on biomass of rice roots and shoots
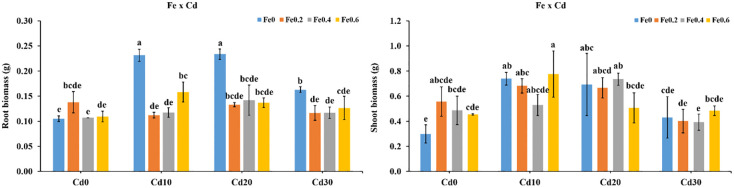
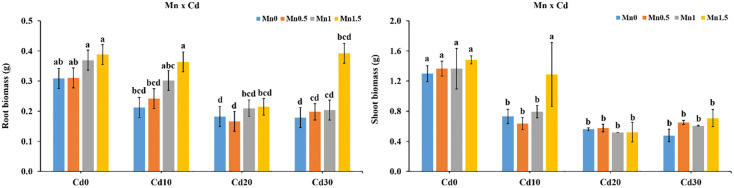
Results showed that the biomass of roots and shoots increased under high Fe and low Cd levels (Fig 1). Relative to Fe0Cd0, the roots biomass was increased by 31.4% in Fe0.2Cd0 while average increased 67.3% was recorded from Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 treatments. However, it can be noted that the high doses of Cd combine with Fe also increased roots and shoots biomass. Application of Mn significantly promoted crop growth. Roots and shoots biomass was increased with increasing Mn concentration in the solution. The roots and shoots biomass increased by 22% and 12.7% on average in Mn0.5Cd0, Mn1Cd0 and Mn1.5Cd0 respectively as compared to Mn0Cd0 treatment (Fig 2).
Fig 1. Effect of Fe and Cd interaction on root and shoot biomass (g).
Here, Fe and Cd indicates 0, 0.2, 0.4 and 0.6 mg L−1 Fe as FeSO4.7H2O and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4). The data presented are mean ± standard deviation (n = 3). Mean values followed by different letter are significantly different using Duncan’s multiple range test at 5% level.
Fig 2. Effect of Mn and Cd interaction on root and shoot biomass (g).
Here, Mn and Cd indicates 0, 0.5, 1 and 1.5 mg L−1 Mn as MnSO4 and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4).
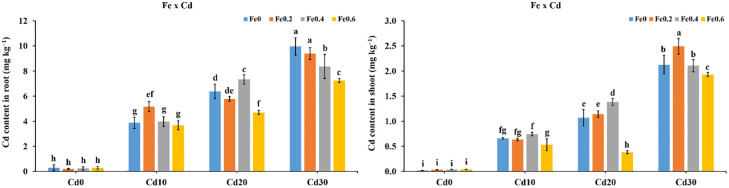
Effect of Fe on Cd uptake by rice root and shoot
Roots and shoots Cd concentration was ranged from 0.20–9.96 mg kg−1 and 0.02–2.49 mg kg−1 respectively. At the low Cd level treatment, the Cd in roots and shoots decreased with increase in Fe concentration. The lower Cd in roots were found in Fe0Cd0, Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 with an average value of 0.29 mg kg−1. The highest Cd 9.96 mgkg−1 in the roots was found in Fe0Cd30 treatment (Fig 3). Moreover, Cd concentrations in shoots were lower in Fe0Cd0, Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 as compared to other treatments. The highest total Cd content in shoot was found in Fe0.2Cd30 treatment 2.49 mg kg−1 followed by Fe0Cd30, Fe0.4Cd30 and Fe0.6Cd30 than Fe0Cd0 (Fig 3).
Fig 3. Effect of Fe and Cd interaction on Cd accumulation in rice root and shoot.
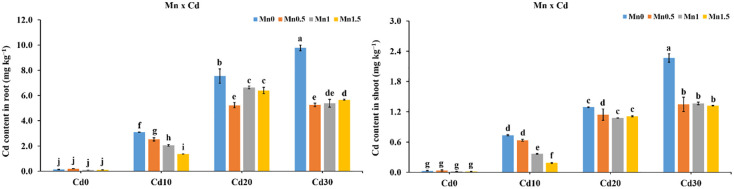
Effect of Mn on Cd uptake by rice roots and shoots
In Mn0Cd0, Mn0Cd10, Mn0Cd20 and Mn0Cd30 treatments, the total Cd content in roots were 0.13, 3.09, 7.54, 9.78 mg kg−1 respectively, while the total Cd concentration in shoots were 0.03, 0.74, 1.29, 2.27 mg kg−1 respectively. Under the low Cd level treatments, the Cd content of roots and shoots decreased with the increase in Mn concentration in solution. The range of total Cd reduce ratio was 11.9–82.3% in roots and 11.6–85.0% in shoots respectively.
The concentration of Cd in roots and shoots under Mn0.5Cd0, Mn1Cd0 and Mn1.5Cd0 treatments was non-significantly different than Mn0Cd0. While, in Mn0Cd10, Mn0Cd20 and Mn0Cd30 treatments, the average Cd content was 49.9 and 44.3 times higher than Mn0Cd0 in roots and shoots respectively. Overall, the lowest Cd contents in the roots were found in plants treated with Mn1.0Cd0, Mn0.5Cd0, Fe0Cd0 and Mn1.5Cd0, while the highest Cd content in roots was found in Mn0Cd30 treatment. In the shoots the concentration of Cd was found lowest in Mn1.5Cd0, Mn1Cd0, Fe0Cd0 and Mn0.5Cd0, and greatest Cd content was found in Mn0Cd30 with the value of 2.27 mg kg−1 (Fig 4).
Fig 4. Effect of Mn and Cd interaction on Cd accumulation in roots and shoots of rice.
Genes expression in rice roots and shoots under Fe and Cd addition
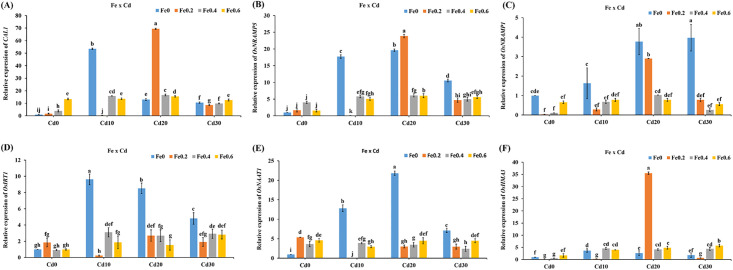
CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, OsNAAT1, and OsHMA3 are considered as important Cd transporters. The results showed that the different levels of Fe and Cd in the solution had a substantial effect on the level of expression of genes in both rice roots and shoots. In the roots CAL1 expression increased significantly by 0.72–12.4 times in the Fe0.2Cd0, Fe0.4Cd0, and Fe0.4Cd0 treatments relative to Fe0Cd0 (Fig 5A). While the expression of CAL1 in the Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments increased by 9.5–52.5 times than Fe0Cd0. Whereas, in Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 the CAL1 expression in roots increased by 7.6–68.4 times. OsNRAMP5 and OsNRAMP1 are crucial transporters of Fe and Cd that carry Fe and Cd from the surface of the root into root cells. OsNRAMP5 expression increased by 0.5–3.0 times in roots treatments under Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 relative to Fe0Cd0. The relative expression of OsNRAMP5 also increased in the roots by 9.5–18.6 times in the Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments. Under Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments, the expression level of OsNRAP5 increased by 3.6–22.8 times in roots compared with Fe0Cd0 (Fig 5B). The expression of OsNRAMP1 in roots decreased by 34.2%-96.5% in Fe0.2Cd0, Fe0.4Cd0, and Fe0.4Cd0 treatments whereas, it increased by 63.2%-296% under Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments. The expression of OsNRAMP1 also decreased by 22.1%-73.2% in Fe0.2Cd10, Fe0.2Cd30, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments than Fe0Cd0 (Fig 6C). While relative expression of OsNRAMP1 enhanced by 190% and 2.85% under Fe0.2Cd20 and Fe0.2Cd40 treatments respectively (Fig 5C). The relative expression of OsIRTI in the roots decreased by 7.65% and 1% in the Fe0.4Cd0, and Fe0.6Cd0 treatments respectively, while it increased by 84.6% under Fe0.2Cd0 treatment as compared to Fe0Cd0 treatment. However, the expression increased by 3.8–8.6 times in Fe absence and Cd sufficient treatments such as Fe0Cd10, Fe0Cd20, and Fe0Cd30. In the Fe and Cd sufficient treatments (Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30) the expression of OsIRT1 in roots enhanced by 54.2%-210% (Fig 5D). Compared to Fe0Cd0, in the Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 treatments, the relative expression of OsNAAT1 increased by 2.6–4.4 times. Whereas, the expression values for OsNAAT1 were 6.1–20.7 times higher in the Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments than Fe0Cd0. In the Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, FE0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments the expression of OsNAAT1 increased by 141%-348% (Fig 5E). The relative expression of OsHMA3 reduced by 81.5% and 41.6% in the Fe0.2Cd0, Fe0.4Cd20 treatments respectively, while the relative expression of OsHMA3 enhanced by 71.2% in Fe0.6Cd0 treatment as compared to Fe0Cd0. The OsHMA3 expression level also decreased by 72.8% and 33.1% under Fe0.2Cd10 and Fe0.2Cd30 treatments respectively (Fig 5F). The greatest expression of OsHMA3 was recorded under the Fe0.2Cd20 treatment and it was enhanced by 34.4 times. Under the treatments Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 the OsHMA3 expression increased by 0.7–4.74 times in roots than that of Fe0Cd0.
Fig 5. Effect of Fe and Cd interaction on relative genetic expression of CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, OsNAAT1, and OsHMA3 in rice roots.
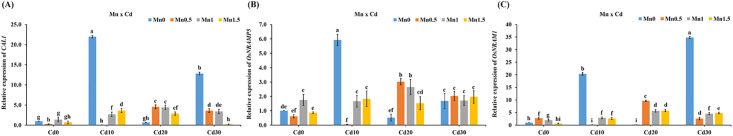
Fig 6. Effect of Mn and Cd interaction on relative genetic expression of CAL1, OsNRAMP5, and OsNRAMP1 in rice roots.
Genes expression in rice roots and shoots under Mn and Cd application
The application of Mn and Cd in the nutrient solution showed a significant impact on the expression levels of CAL1, OsNRAMP5 and OsNRAMP1 in roots. The relative expression of CAL1 in the roots was reduced by 718%, 29.6%, and 32.1% under Mn0.5Cd0, Mn1.5Cd0 and Mn0Cd20 treatments respectively as compared to Fe0Cd0 (Fig 6A). In the Mn0Cd10 and Mn0Cd30 treatments gene expression was increased by 2 and 1.1 fold respectively as relative to Mn0Cd0. Similarly, under the Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, and Mn1.5Cd20 treatments the expression of CAL1 also enhanced by 169%-357% as compared to Mn0Cd0, OsNRAMP5 expression decreased by 38.4%, 13.1% and 47.2% respectively under the treatments Mn0.5Cd0, Mn1.5Cd0 and Mn0Cd20, whereas it was increased by 492% and 68.3% respectively in the treatments Mn0Cd10 and Mn0Cd30. The genes expression of OsNRAMP5 was also increased by 52.7%-202% in Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, Mn1.5Cd20, and Mn1.5Cd30 treatments (Fig 6B). The expression of OsNRAMP1 in Mn0.5Cd0 and Mn1Cd0 treatments enhanced by 177% and 97.6% respectively. Although OsNRAMP1 expression was decreased by 33.4%, 84.5% and 78.5% in Mn1.5Cd0, Mn0.5Cd10, and Mn0Cd20 treatments respectively. Whereas, its expression was enhanced by 159%-868% in Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, Mn1.5Cd20, and Mn1.5Cd30 treatments (Fig 6C).
Discussion
Compared to Fe0Cd0 treatment, the roots biomass was increased by 31.4% in Fe0.2Cd0 treatment, while shoots biomass in Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 was 67.3% higher than Fe0Cd0 on average (Fig 1). Similarly, roots and shoots biomass was increased in Mn0.5Cd0, Mn1Cd0 and Mn1.5Cd0 treatments containing excessive Mn and Cd deficient doses (Fig 2). Adhikari [22], found that supply of both Fe and Cd in hydroponic experiment significantly affected plant growth and yield, as well as accumulation of Cd in plant tissues. Furthermore, it was confirmed that the dry matter production of rice shoot was highest at the highest activity level of Fe [22]. It was found that the addition of Fe in the soil increased root and shoot dry weight of rice [8]. Moreover, it has been reported that the exogenous application of Mn at the rate from 0.05 μM to 800 μM under hydroponic experiment can reduce accumulation of Cd in under and above ground plant parts in rice [23]. However, increasing Cd concentration in solution has reduced roots and shoots biomass under Fe and Mn addition (Figs 1 and 2). It was confirmed that the plant growth was inhibited under Cd stress in four rice cultivars as compared to normal conditions [24]. Root growth was limited and number and length of roots and tillers was also decreased by Cd in the rice cultivars [24].
At the low Cd level treatments, the Cd in roots and shoots decreases with increase in Fe concentration. Such as Cd concentrations in shoots were lower in Fe0Cd0, Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 as compared to other treatments (Fig 3). It is in consistence with that Fe and Mn could decrease Cd uptake and minimize Cd inducible rhizotoxicity [12]. Exogenous Fe application can significantly decrease Cd concentration in rice roots and shoots [25]. It was further confirmed that Fe2+ cations could compete with Cd2+ ions for adsorption sites at the roots surface and as a result uptake of Cd in rice is decreased [26]. Consistence with our results [27, 28], it was observed that under a Fe-sufficient supply, Cd concentration in rice stem and leaves was reduced. Fe fertilization directly and effectively increased Fe content and decreased Cd contamination to some extent. The reason may be Fe2+ competes with Cd for the same binding sites and follow similar transport pathways on the surface of roots cells [29]. Also, Fe oxides have a significant ability for Cd adsorption and can effectively immobilize Cd [30]. However, the highest Cd concentration of 9.96 mg kg−1 in the roots was found in Fe0Cd30 treatment (Fig 3). The highest total Cd content in shoot was found in Fe0.2Cd30 treatment 2.49 mg kg−1 followed by Fe0Cd30, Fe0.4Cd30 and Fe0.6Cd30 than Fe0Cd0 (Fig 3). It was reported that Cd content in DCB extracts enhanced with increasing Cd and Fe addition [25]. Furthermore, roots and shoots Cd concentration enhanced with enhancing Cd supply [25]. It was further reported that FeSO4 fertilizer significantly enhanced cadmium content in roots as well as shoots of rice than CK [31]. The reason was may be due to the Fe transporter OsIRT1 in the cell membrane which mainly expressed in rice roots directly carrying Fe2+ via cell membrane and Cd2+ as well [31–33]. A similar finding was found by [34], which stated that Cd2+ bound to the apoplastic membrane and remains in the root cell wall after desorption while saturated Cd2+ from the solution influx across the root cell plasma membrane was mediated by the transporter. Furthermore, Fe deficiency induces the expression of IRTI in Arabidopsis which may assisted translocation of Cd2+ [34].
The Cd content of the roots and shoots decreased with increase of Mn concentration in solution under the low Cd level treatments. The range of total Cd reduction was 11.9–82.3% in roots and 11.6–85.0% in shoots. In the shoots the concentration of Cd was found the lowest in Mn1.5Cd0, Mn1Cd0, Fe0Cd0 and Mn0.5Cd0 (Fig 4). Qin [35], noted that rice plant height was significantly increased and decreased Cd toxicity to normal level under Mn dose of 0.5 mg L−1. The roots Cd content in two rice genotypes were significantly reduced by the increased MnSO4 and EDTA•Na2Mn application in solution than CK [11]. The findings are in agreement with that the application of 40 μM Fe and 2 μM Mn increased biomass and reduced Cd concentration in rice under Cd stress of 5 and 25 mM. Furthermore, it was concluded that Fe and Mn alleviated Cd toxicity by decreasing Cd content in rice and by regulating redox potential which hinders damage to root growth and photosynthesis caused by Cd [12]. Moreover, application of 0.3 mM MnSO4 in hydroponic solution reduced cadmium absorption in roots and shoots by 40% and 60% respectively in rice seedlings [36]. Because, Mn is a divalent cation that is absorbed by plants through an active transport mechanism which comes in competition with Cd2+ as Mn and Cd contains similar pathways for plant transport and accumulation [36]. While, in Mn0Cd10, Mn0Cd20 and Mn0Cd30 treatments, the average Cd content was 49.9 and 44.3 times higher than Mn0Cd0 in roots and shoots respectively. Moreover, the highest Cd content in the shoot was found in Mn0Cd30 treatment with the value of 2.27 mg kg−1 (Fig 4). It was confirmed that Cd accumulation in roots was directly associated with the Cd content in solution [22]. Therefore, two possible mechanisms may exist for the accumulation of Cd in combination with Fe and Mn. First may be due to the desorption of Cd2+ ions from the root cell wall and then mediated by Cd transporters IRTI [34], such as OsIRT1 and other transporters in our experiment. Secondly, various studies reported that the formation of Fe and Mn plagues on the roots had no significant effect on the uptake of Cd in plants [37–39]. Huang [39] noted that Phytolacca acinosa Roxb (P. acinosa) plants treated with 50 mg L−1 Cd accumulated higher level of Cd as compared to plants exposed to 2 mg L−1 Cd, especially in the plague treatments (p < 0.05). Moreover, it was reported that DCB extractable Cd in the roots and shoots of Kandalar. Obovata (S.L.) significantly enhanced with an increasing Cd supplementation [38]. Thus, the results may depend on several factors such as amount of metal plaque, the concentration of metal and the pH in the culture solution [13, 38, 39].
Relative expression of genes were significantly affected by Fe and Cd in the solution. The expression of CAL1 enhanced by 0.7–12.4 times under the Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments than Fe0Cd0. Whereas, in the Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treated plants the CAL1 expression in roots increased by 7.6–68.4 times (Fig 5A). Luo [18] found CAL1 is a defensin-like protein and localized in root exodermis and xylem parenchyma cells. CAL1 expression in the roots was induced by exposure to Cd, with the near-isogenic line (NIL) containing the TN1 allele showing a greater response to Cd than the NIL(CJ06) control line [18]. In addition, it was reported that CAL1 was preferentially expressed in root and leaf sheath of rice seedlings. It was further confirmed that expression of CAL1 was significantly induced under Cd exposure in near-isogenic line NIL(TNI) in root and expression in various tissues except for leaf blades [18]. The expression of OsNRAMP5 enhanced by 0.5–3.0 times under Fe0.2Cd0, Fe0.4Cd0, and Fe0.6Cd0 treatments in roots than Fe0Cd0 treatment. While, in the Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatment the relative expression of OsNRAMP5 also increased by 9.5–18.6 times in roots. The expression level of OsNRAMP5 enhanced by 3.6–22.8 times in roots under Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments than Fe0Cd0 (Fig 5B). These results suggest that a synergistic effect may have existed between Cd concentration in solution and relative expression of OsNRAMP5. The results are in consistent that gene expression OsNRAMP5 in roots was up-regulated under two doses of Cd treatments (1 μmol L−1 and 5 μmol L−1 Cd) [40]. Meanwhile, OsNRAMP1 expression level reduced by 34.2%-96.5% in Fe0.2Cd0, Fe0.4Cd0, and Fe0.4Cd0 treatments whereas, increased by 63.2%-296% under Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments in roots (Fig 5C). The results are in accordance with that the expression of OsNRAMP which was induced by Cd treatment or by Fe inadequacy in both roots and shoots [41]. Overexpression of OsNRAMP1 in rice roots enhanced the accumulation of Cd in leaves [14]. Cd accumulation in the shoot of rice increased due to higher expression of OsNRAMP1, this showed that OsNRAMP1 could uptake and transport Cd in addition to Fe [42]. It was confirmed that among the seven members of the NRAMP family in rice, expression of OsNRAMP1 was increased in roots and shoots under Cd stress, whereas on contrary our results OsNRAMP5 (Os07g0257200) expression was decreased in both tissues in presence of Cd [43]. It was further confirmed that the expression of OsNRAMP1 in yeast cells significantly increased the accumulation of Cd (5-fold) than that of control [44]. The relative expression of OsIRTI in Fe0Cd10, Fe0Cd20, and Fe0Cd30 treatments increased by 3.8–8.6 times. In the Fe and Cd sufficient treatments (Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30) the expression of OsIRT1 in roots increased by 54.2%-210% (Fig 5D). It has been documented that various transporters regulated Fe transportation in IRT and NRAMP families involved in Cd transport by rice [14, 45]. It was confirmed that Fe(II) mediated transporter such as OsIRT1 and OsIRT2 take part in Cd uptake in Fe-deficient rice grown in hydroponic culture [33]. The expression of OsIRT1 was found higher in roots and was up-regulated under lower Fe conditions [46]. In addition to Fe and Cd also enters into the root cell through OsIRT1 [33]. Compared to Fe0Cd0, the relative expression of OsNAAT1 in the Fe0Cd10, Fe0Cd20, and Fe0Cd30 enhanced by 6.1–20.7 times. Whereas, in the Fe0.2Cd20, Fe0.2Cd30, Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, FE0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments OsNAAT1 expression increased by 141%-348% (Fig 5E). Similar findings have been revealed by [47] which states that OsNAAT1 was highly up-regulated under Fe deficiency and Cd stress both in roots and shoots [47]. Furthermore, it was confirmed that when 1 mM Cd was added to the nutrient solution, Cd concentration in both naat1 roots or shoots was about 50% higher than that those in wild-type seedlings [48]. The relative expression of OsHMA3 reduced by 81.5% and 41.6% in Fe0.2Cd0, Fe0.4Cd20 treatments respectively, while the relative expression of OsHMA3 enhanced by 71.2% in Fe0.6Cd0 treatment as compared to Fe0Cd0. Whereas, its expression was also decreased by 72.8% and 33.1% under Fe0.2Cd10 and Fe0.2Cd30 treatments respectively (Fig 6F). Under treatments Fe0.4Cd10, Fe0.4Cd20, Fe0.4Cd30, Fe0.6Cd10, Fe0.6Cd20, and Fe0.6Cd30 treatments the OsHMA3 expression was increased by 297%-474% than that of Fe0Cd0. It was confirmed that the application of Fe chelates in hydroponic culture did not significantly affect OsHMA3 expression [28]. The results are in accordance with [49], who stated the higher expression of OsHMA3 in the root. Furthermore it was confirmed that expression of OsHMA3 was significantly increased in excessive Fe treatment [27]. Lu [50] reported, that overexpression of OsHMA3 significantly reduced Cd translocation from roots to shoots and enhanced Cd tolerance. When Cd enters into the cytosol, OsHMA3 sequestered Cd into vacuole [51]. However, [49] discovered an allele of OsHMA3 which failed to transport Cd into vacuole in high Cd accumulating cultivars. Therefore in the present experiment, it may be possible that OsHMA3 failed to sequester Cd in a vacuole. That’s why accumulation of Cd was found higher in Fe0.2Cd30, Fe0Cd30, Fe0.4Cd30 and Fe0.6Cd30 treatments. Furthermore, it was confirmed that OsHMA3 expression was directly proportional to Cd concentration in the medium [52].
The application of Mn and Cd in the nutrient solution showed a significant impact on expression levels of CAL1, OsNRAMP5 and OsNRAMP1. Compared to Mn0Cd0 treatment, the relative expression of CAL1 in the Mn0Cd10 and Mn0Cd30 treatments increased by 20.9 and 11.8 times. Meanwhile, the expression of CAL1 under the Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, and Mn1.5Cd20 treatments also enhanced by 169%-357% (Fig 6A). It was confirmed that CAL1 expression in the roots was significantly induced by exposure to Cd [18]. The results are in accordance with that expression of CAL1 was also found high in various tissues except for leaf blades while in node I, and the adjoining flag leaf sheath its expression was significantly higher. In addition, higher Cd concentration was observed in seedling leaf blade and mature plant straws. Thus, it was suggested that CAL1 might specifically carry Cd from roots to shoots, but not from shoot to grain [18]. This shows that the application of Mn significantly affects CAL1 expression, however we don’t know the reason behind this. Therefore, further trials may be conducted to know the mechanism behind it.
Compared to Mn0Cd0, the expression of OsNRAMP5 increased by 492% and 68.3% in Mn0Cd10 and Mn0Cd30 treatments respectively. The expression of OsNRAMP5 also increased by 52.7%-202% in under Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, Mn1.5Cd20, and Mn1.5Cd30 treatments (Fig 6B). It was confirmed that the deficiency of Fe2+ and M2+ induced the up-regulation of OsNRAMP5 [53]. However, [43] reported that OsNRAMP5 was not up-regulated under Mn deficiency. While, a recent study showed that the expression of OsNRAMP5 was remarkably enhanced 2.33–5.67 folds in roots of three rice genotypes at Mn phytotoxicity condition than that of Mn deficient condition [54]. Thus, our experiment showed that both Mn and Cd affects the expression of OsNRAMP5. Therefore, further studies can be carried out to find the mechanism behind. It was suggested that the fluctuation of OsNRAMP5 expression level may be different among rice genotypes and different hydroponic solution systems [54]. As the OsNRAMP1 expression enhanced by 159%-868% in Mn0.5Cd20, Mn0.5Cd30, Mn1Cd10, Mn1Cd20, Mn1Cd30, Mn1.5Cd10, Mn1.5Cd20, and Mn1.5Cd30 treatments than Mn0Cd0 (Fig 6C). Previous studies confirmed that the expression of OsNRAMP1 was up-regulated under Fe deficiency in rice [42] and Mn deficiency in Arabidopsis [55]. Similarly, [43] reported that expression of OsNRAMP1 in roots and shoots of rice increased during Cd exposure. Thus the present experiment indicated that the expression of OsNRAMP1 was affected by both Mn and Cd cations. In a recent study it has been showed that OsNRAMP1 was predominantly expressed in root plus leaf as well as fixed plasma film restricted protein [41]. OsNRAMP1 articulation was instigated by exposure to Cd and Fe inadequacy. Immunostaining demonstrated that OsNRAMP1 confined in all root cell excluding central vasculature, and in leaf mesophyll cell [41]. The knockout of OsNRAMP1 brought about noteworthy reductions in root take-up of Cd and Mn and their amassing in rice shoot and grain, and expanded affectability to Mn insufficiency. The knockout of OsNRAMP1 showed less effect on Cd and Mn take-up compared to knockout of OsNRAMP5, while knockout of the two qualities brought about enormous declines in the take-up of the Cd and Mn. While, OsNRAMP1 plays an essential role in take-up of manganese and cadmium in rice and the role of OsNARAMP5 and OsNRAMP1 are comparative yet not excess [41]. Thus it can be concluded that in the present experiment, variations in expression of all the genes may also be affected by the rice variety and hydroponic solution.
Conclusions
This study demonstrated the expression levels of cadmium-related genes in rice under conditions of cadmium pollution and their relationship with iron and manganese nutrition were ascertained. It may be concluded that the application of Fe and Mn cations in rice under Cd exposure significantly decreased Cd concentration in roots and shoots. Increasing level of Cd in solution significantly increased Cd concentration in rice roots and shoots. Expression level of CAL1, OsNRAMP5, OsNRAMP1, OsIRT1, and OsNAAT1 were increased with sufficient Cd and Fe and/or Mn insufficient treatments while OsHMA3 expression decreased. In order to find the Fe and Mn effects on Cd, further research should be conducted under different conditions and under different doses of Fe and Mn and the deep insight process involved during Cd absorption and transportation.
Supporting information
Fe and Cd indicates 0, 0.2, 0.4 and 0.6 mg L−1 Fe as FeSO4.7H2O and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4).
(PNG)
Mn and Cd indicates 0, 0.5, 1 and 1.5 mg L−1 Mn as MnSO4 and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4).
(PNG)
Acknowledgments
We are thankful to Prof. Keke Yi for providing us with laboratory equipments for gene expressions analysis.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
We are thankful to Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences and National Key Research and Development Program of China for funding and resources. The research was funded by the National Key Research and Development Program of China, under grant no (2016YFD0800406) and project “Research on Migration/Transformation and Safety Threshold of Heavy Metals in Farmland Systems. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhou H, Zhu W, Yang WT, Gu JF, Gao ZX, Chen LW, et al. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages. Ecotoxicology and environmental safety. 2018; 152: 91–97. 10.1016/j.ecoenv.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Yu HY, Liu C, Zhu J, Li F, Deng DM, Wang Q, et al. Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH value. Environmental pollution. 2016; 209: 38–45. 10.1016/j.envpol.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Wang PM, Gu Y, Kopittke PM, Zhao FJ, Wang P. Iron-manganese (oxyhydro) oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environmental Science & Technology. 2019; 53: 2500–2508. 10.1021/acs.est.8b06863 [DOI] [PubMed] [Google Scholar]
- 4.Suda A, Makino T. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: a review. Geoderma. 2016; 270: 68–75. 10.1016/j.geoderma.2015.12.017. [DOI] [Google Scholar]
- 5.Bashir A, Rizwan M, Ali S, Zia Ur Rehman M, Ishaque W, Atif Riaz M, et al. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environmental Science and Pollution Research (International). 2018; 25: 20691–20699. 10.1007/s11356-018-2042-y [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Nakanishi H, Nishizawa NK. Recent insights into iron homeostasis and their application in graminaceous crops. Proceedings of the Japan Academy, Ser. B, Physical and Biological Sciences. 2010; 86: 900–913. 10.2183/pjab.86.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao L, Chang J, Chen R, Li H, Lu H, Tao L, et al. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: prospects for cultivating Fe-rich but Cd-free rice. Rice. 2016; 9: 39 10.1186/s12284-016-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Zhang C, Wang J, Zhou C, Feng H, Mahajan MD, et al. Influence and interaction of iron and cadmium on photosynthesis and antioxidative enzymes in two rice cultivars. Chemosphere. 2017; 171: 240–247. 10.1016/j.chemosphere.2016.12.081. [DOI] [PubMed] [Google Scholar]
- 9.Liu HJ, Zhang JL, Zhang FS. Role of iron plaque in Cd uptake by and translocation within rice (Oryza sativa L.) seedlings grown in solution culture. Environmental and Experimental Botany. 2007; 59: 314–320. 10.1016/j.envexpbot.2006.04.001 [DOI] [Google Scholar]
- 10.Zornoza P, Sanchez-Pardo B, Carpena RO. Interaction and accumulation of manganese and cadmium in the manganese accumulator Lupinus albus. Journal of Plant Physiology. 2010; 167: 1027–1032. 10.1016/j.jplph.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, An H, Yang Y, Liang Y, Shao G. Effects of Mn-Cd antagonistic interaction on Cd accumulation and major agronomic traits in rice genotypes by different Mn forms. Plant Growth Regulation. 2017; 82: 317–331. 10.1007/s10725-017-0261-8 [DOI] [Google Scholar]
- 12.Sebastian A, Prasad M. Iron-and manganese-assisted cadmium tolerance in Oryza sativa L.: lowering of rhizotoxicity next to functional photosynthesis. Planta. 2015; 241: 1519–1528. 10.1007/s00425-015-2276-6 [DOI] [PubMed] [Google Scholar]
- 13.Liu WJ, Zhu YG, Smith FA. Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant and Soil. 2005; 277: 127–138. 10.1007/s11104-005-6453-4 [DOI] [Google Scholar]
- 14.Takahashi R, Ishimaru Y, Nakanishi H, Nishizawa NK. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signaling & Behavior. 2011; 6: 1813–1816. 10.4161/psb.6.11.17587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki A, Yamaji N, Yokosho K, Ma JF. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell. 2012; 24: 2155–2167. 10.1105/tpc.112.096925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki A, Yamaji N, Ma JF. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. Journal of Experimental Botany. 2014; 65: 6013–6021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi G. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant, Cell & Environment. 2011; 34: 994–1008. 10.1111/j.1365-3040.2011.02299.x [DOI] [PubMed] [Google Scholar]
- 18.Luo JS, Huang J, Zeng DL, Peng JS, Zhang GB, Ma HL, et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nature Communication. 2018; 9: 645 10.1038/s41467-018-03088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva YJ, do Nascimento CW, Biondi CM. Comparison of USEPA digestion methods to heavy metals in soil samples. Environmental Monitoring Assessment. 2014; 186: 47–53. 10.1007/s10661-013-3354-5 [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25: 402–408, 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.XLSTAT A. Data analysis and statistics software for Microsoft Excel. New York, NY: Addinsoft. 2013. https://www.xlstat.com/en/.
- 22.Adhikari T, Tel-Or E, Libal Y, Shenker M. Effect of cadmium and iron on rice (Oryza Sativa L.) plant in chelator-buffered nutrient solution. Journal of Plant Nutrition. 2006; 29: 1919–1940. 10.1080/01904160600927435 [DOI] [Google Scholar]
- 23.Yang M, Zhang Y, Zhang L, Hu J, Zhang X, Lu K, et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. Journal of Experimental Botany. 2014; 65: 4849–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang BL, Ouyang YN, Xu JY, Liu K. Cadmium remobilization from shoot to grain is related to pH of vascular bundle in rice. Ecotoxicology and Environmental Safety. 2018; 147: 913–918. 10.1016/j.ecoenv.2017.09.064 [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Zhang J, Christie P, Zhang F. Influence of iron plaque on uptake and accumulation of Cd by rice (Oryza sativa L.) seedlings grown in soil. Science of the Total Environment. 2008; 394: 361–368. 10.1016/j.scitotenv.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Zheng RL, Cai C, Liang JH, Huang Q, Chen Z, Huang YZ, et al. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere. 2012; 89: 856–862. 10.1016/j.chemosphere.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Tang YT, Yao AJ, Cao J, Wu ZH, Peng ZR, et al. Mitigation of Cd accumulation in paddy rice (Oryza sativa L.) by Fe fertilization. Environmental Pollution. 2017; 231: 549–559. 10.1016/j.envpol.2017.08.055. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Tang YT, Zhou C, Xie ST, Xiao S, Baker AJM, et al. Mechanisms of Fe biofortification and mitigation of Cd accumulation in rice (Oryza sativa L.) grown hydroponically with Fe chelate fertilization. Chemosphere. 2017; 175: 275–285. 10.1016/j.chemosphere.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 29.Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, et al. Role of mineral nutrition in minimizing cadmium accumulation by plants. Journal of the Science of Food and Agriculture. 2010; 90: 925–937. 10.1002/jsfa.3916 [DOI] [PubMed] [Google Scholar]
- 30.Liu R. Altschul EB, Hedin RS, Nakles DV, Dzombak DA. Sequestration enhancement of metals in soils by addition of iron oxides recovered from coal mine drainage sites. Soil and Sediment Contamination: An International Journal. 2014; 23: 374–388. 10.1080/15320383.2014.831027 [DOI] [Google Scholar]
- 31.Shao G, Chen M, Wang D, Xu C, Mou R, Cao Z, et al. Using iron fertilizer to control Cd accumulation in rice plants: A new promising technology. Science in China Series C: Life Sciences. 2008; 51: 245–253. 10.1007/s11427-008-0031-y [DOI] [PubMed] [Google Scholar]
- 32.Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. The Plant Journal. 2006; 45: 335–346. 10.1111/j.1365-313X.2005.02624.x [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Science and Plant Nutrition. 2006; 52: 464–469. 10.1111/j.1747-0765.2006.00055.x [DOI] [Google Scholar]
- 34.Cohen CK, Fox TC, Garvin DF, Kochian LV. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiology. 1998; 116: 1063–1072. 10.1104/pp.116.3.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin D, Chen M, Zhou R, Cao Z, Zhu Z, Shao G, et al. Effects of interaction between manganese and cadmium on plant growth and contents of cadmium and manganese in rice. Chinese Journal of Rice Science. 2010; 24: 189–195 (In Chinese). [Google Scholar]
- 36.Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. Comptes Rendus Biologies. 2016; 339: 462–474. 10.1016/j.crvi.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 37.Tripathi RD, Tripathi P, Dwivedi S, Kumar A, Mishra A, Chauhan PS, et al. Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics. 2014; 6: 1789–1800. 10.1039/c4mt00111g [DOI] [PubMed] [Google Scholar]
- 38.Du J, Yan C, Li Z. Formation of iron plaque on mangrove Kandalar. Obovata (S.L.) root surfaces and its role in cadmium uptake and translocation. Marine Pollution Bulletin. 2013; 74: 105–109. 10.1016/j.marpolbul.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, Tang K. Xu X, Cai C. Interaction of Fe-Mn plaque and Arthrobacter echigonensis MN1405 and uptake and translocation of Cd by Phytolacca acinosa Roxb. Chemosphere. 2017; 174: 585–592. 10.1016/j.chemosphere.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Zhang W, Xu S, Shi H, Wen D, Huang Y, et al. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environmental and Experimental Botany. 2018; 155: 734–741. 10.1016/j.envexpbot.2018.08.024. [DOI] [Google Scholar]
- 41.Chang JD, Huang S, Yamaji N, Zhang W, Ma JF, Zhao FJ. OsNRAMP1 contributes to cadmium and manganese uptake in rice. Plant, Cell & Environment. 2020; 43(10): 2476–2491. 10.1111/pce.13843 [DOI] [PubMed] [Google Scholar]
- 42.Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, et al. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. Journal of Experimental Botany. 2011; 62: 4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, et al. Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport. Scientific Reports. 2012; 2: 286 10.1038/srep00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK. Expression in arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant, Cell & Environment. 2014; 37: 140–152. 10.1111/pce.12138 [DOI] [PubMed] [Google Scholar]
- 45.Ishimaru Y, Bashir K, Nakanishi H, Nishizawa NK. OsNRAMP5, a major player for constitutive iron and manganese uptake in rice. Plant Signaling & Behavior. 2012; 7: 763–766. 10.4161/psb.20510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, An G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant, Cell & Environment. 2009; 32: 408–416. 10.1111/j.1365-3040.2009.01935.x [DOI] [PubMed] [Google Scholar]
- 47.Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, et al. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Molecular Biology. 2008; 66: 193–203. 10.1007/s11103-007-9262-8 [DOI] [PubMed] [Google Scholar]
- 48.Cheng L, Wang F, Shou H, Huang F, Zheng L, He F, et al. Mutation in Nicotianamine Aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiology. 2007; 145: 1647–1657. 10.1104/pp.107.107912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytologist. 2011; 189: 190–199. 10.1111/j.1469-8137.2010.03459.x [DOI] [PubMed] [Google Scholar]
- 50.Lu C, Zhang L, Tang Z, Huang XY, Ma JF, Zhao FJ. Producing cadmium-free indica rice by overexpressing OsHMA3. Environment International. 2019; 126: 619–626. 10.1016/j.envint.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 51.Takahashi R, Bashir K, Ishimaru Y, Nishizawa NK, Nakanishi H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signaling & Behavior. 2012; 7: 1605–1607. 10.4161/psb.22454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui J, Liu T, Li Y, Li F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Science of the Total Environment. 2018; 644: 602–610. 10.1016/j.scitotenv.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Chen J, Huang Q, Tang S, Wang J, Hu P, et al. Can liming reduce cadmium (Cd) accumulation in rice (Oryza sativa) in slightly acidic soils? A contradictory dynamic equilibrium between Cd uptake capacity of roots and Cd immobilisation in soils. Chemosphere. 2018; 193, 547–556. 10.1016/j.chemosphere.2017.11.061 [DOI] [PubMed] [Google Scholar]
- 54.Cai Y, Wang M, Chen B, Chen W, Xu W, Xie H, et al. Effects of external Mn2+ activities on OsNRAMP5 expression level and Cd accumulation in indica rice. Environmental Pollution. 2020; 260: 113941 10.1016/j.envpol.2020.113941 [DOI] [PubMed] [Google Scholar]
- 55.Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for arabidopsis growth in low manganese conditions. The Plant Cell. 2010; 22: 904–917. 10.1105/tpc.109.073023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fe and Cd indicates 0, 0.2, 0.4 and 0.6 mg L−1 Fe as FeSO4.7H2O and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4).
(PNG)
Mn and Cd indicates 0, 0.5, 1 and 1.5 mg L−1 Mn as MnSO4 and 0, 10, 20 and 30 mg L−1 Cd (supplied as CdSO4).
(PNG)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.