Abstract
The conservation and management of subterranean biodiversity is hindered by a lack of knowledge on the true distributions for many species, e.g., the Wallacean shortfall. In recent years, several studies have demonstrated the potential of environmental DNA (eDNA) as an effective approach to detect and monitor biodiversity, including rare, threatened, and endangered taxa. However, there are few eDNA studies of groundwater fauna. Here we report the results of the development and implementation of an eDNA assay targeting a short fragment of the mitochondrial CO1 locus of a critically imperiled cave crayfish, the Sweet Home Alabama Cave Crayfish (Cambarus speleocoopi), known from just four cave systems in the Interior Plateau karst region of northern Alabama. We detected C. speleocoopi DNA from water samples collected at 5 of 16 sites sampled (caves and springs), including two historical sites as well as three additional and potentially new sites in Marshall County, Alabama. All three of these sites were within 2 km of historical sites. Our study is the first to detect a groundwater crustacean in the Interior Plateau karst region. Additionally, our study contributes to the growing literature that eDNA is a viable complementary tool for detection and monitoring of a fauna that is difficult to survey and study using traditional approaches.
Introduction
Effective conservation and management of biodiversity is limited by a lack of knowledge on the distributions of species. This biodiversity knowledge gap known as the Wallacean shortfall [1] is particularly prominent for fauna that live in groundwater and other subterranean ecosystems [2, 3]. This is, in part, because subterranean habitats are extremely challenging to access and survey using traditional approaches, such as visual surveys and trapping (i.e., the Racovitzan shortfall; [4]). Most stygofauna—obligate groundwater species—are thought to have small, restricted distributions (i.e., short-range endemics; sensu [5]) and limited dispersal ability [6] and, consequently, are of high conservation concern. Thus, the development of sound management strategies and measurable conservation priorities for most stygofauna is exceedingly difficult.
An increasingly popular complement, or in some cases alternative, to traditional sampling and monitoring approaches for many aquatic species is environmental DNA (eDNA) that leverages DNA shed by organisms into their surrounding habitats. eDNA represents a powerful new tool for ecologists and conservation biologists to detect and monitor biodiversity rapidly, nondestructively, and potentially cost-effectively [7–10]. This approach has been successfully employed in many freshwater habitats, including rivers, streams, lakes, and ponds, and applied to an assortment of vertebrate and invertebrate taxa [9–12; and references therein], including several taxa considered rare, threatened or endangered [e.g., 13–16]. Few studies have employed eDNA for the detection and monitoring of cave and groundwater macrofauna, with research limited to salamanders [17–20], fishes [20–22], amphipods [16], and crayfishes [21, 23]. Korbel et al. [24] applied a metabarcoding approach to characterize the DNA community of prokaryotes and eukaryotes using 16S rDNA and 18S rDNA, respectively. These studies have demonstrated the utility of eDNA for detecting rare and threatened groundwater biodiversity as an effective complement and possible alternative to more invasive and destructive traditional sampling approaches.
The Sweet Home Alabama Cave Crayfish, Cambarus speleocoopi, is a blind, depigmented cave-dwelling crayfish (Fig 1) in the subgenus Aviticambarus recently described by Buhay and Crandall [25] that is closely related to several other groundwater crayfishes in northern Alabama, including C. hamulatus, C. jonesi, and C. laconensis. This obligate cave-dweller is endemic to Marshall County, Alabama, occurring in just four cave systems along both sides of the Tennessee River valley northwest and downstream of Guntersville Dam [25, 26]. Cave bioinventory surveys have not yielded any additional occurrences in recent years. Because of its extremely limited distribution (extent of occurrence 61.5 km2) and presumed rarity, C. speleocoopi is considered a priority species of high conservation concern (Priority 2) in Alabama [26]. Its conservation status has been evaluated as Critically Imperiled (G1) by NatureServe [27] and Endangered under criteria B1ab(v) by IUCN [28]. Potential threats to the species include groundwater pollution associated with urban development and changes to hydrology related to impoundments on the Tennessee River [25, 28].
Fig 1. The Sweet Home Alabama Cave Crayfish (Cambarus speleocoopi) is an obligate groundwater crayfish endemic to just four cave systems in Marshall Co., Alabama, USA.

Photo by Matthew L. Niemiller.
Few cave systems in the Tennessee-Alabama-Georgia (TAG) region have been comprehensively surveyed biologically [29, 30], and there is great potential that species such as C. speleocoopi occur at additional undocumented sites. In fact, cavers regularly report observations of “white and blind crayfishes” from undocumented cave systems in northern Alabama. However, species identification requires specimen collection and genetic analysis, as morphology alone cannot easily distinguish C. speleocoopi, C. laconensis, and C. jonesi [26]. In fact, populations of C. speleocoopi from Beech Spring and Keller’s caves in Marshall County were identified as C. jonesi previously [31]. Consequently, eDNA may be an appealing, nondestructive alternative to rapidly determine species occupancy and identification at a spring, cave, or well for rare, threatened, or endangered groundwater species without the need for specimen collection and expert identification.
In this study, we developed, tested, and validated an eDNA assay for C. speleocoopi and screened water samples collected from springs and cave systems (including historical sites) within and near its distribution to test the applicability of an eDNA approach to detect a karst groundwater crustacean and identify possible new sites of this imperiled crayfish. Our study demonstrates the potential utility of eDNA as an effective surveying and monitoring tool for groundwater biodiversity but also highlights some challenges of this approach when applied to groundwater ecosystems.
Methods
Ethics statement
This research was authorized under Alabama Department of Conservation and Natural Resources scientific collection permit nos. 2018061776268680 and 2019060224868680 and Alabama State Parks scientific permit no. 192213. Cave location data has been intentionally omitted to protect these sensitive ecosystems and their biodiversity. Cave location data are maintained by the Alabama Cave Survey (ACS; http://www.alabamacavesurvey.org) and can be requested from ACS or the corresponding author.
Sampling sites
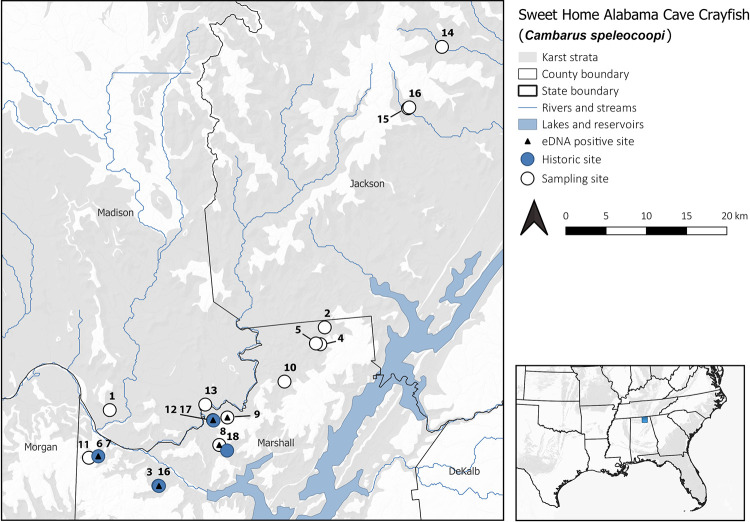
We collected water samples from 13 sites between March 2018 and April 2019 (Table 1), including two historical sites–Cherry Hollow Cave (and the associated spring run) and Beech Spring Cave. Cambarus speleocoopi has been confirmed at these sites within the past three years (Niemiller, unpublished data). We could not obtain permission to access Keller’s Cave (site 17) (but collected water at the nearby spring; site 12) and Porches Spring Cave (site 18). We also sampled 10 additional sites (three caves and seven springs) located within a 20-km radius of historical sites in Jackson and Marshall counties, Alabama (Table 1; Fig 2). Three sites (sites 14–16 in Table 1) outside of the suspected range of C. speleocoopi were included to serve as negative field controls.
Table 1. Sampling sites where water samples were collected in Jackson and Marshall counties, Alabama, USA and results of screening of an eDNA assay for Cambarus speleocoopi.
| Site no. | County | Site name | Collection date | Technical replicates |
|---|---|---|---|---|
| 1 | Marshall | Ashburn Spring | 11 Jan 2019 | 0/12 |
| 2 | Marshall | Babe Wright Spring | 07 Feb 2019 | 0/24 |
| 3 | Marshall | Beech Spring Cave (spring)a | 29 Apr 2019 | 6/12 |
| 4 | Marshall | Cathedral Caverns | 07 Feb 2019 | 0/12 |
| 5 | Marshall | Cathedral Caverns (spring) | 07 Feb 2019 | 0/12 |
| 6 | Marshall | Cherry Hollow Cavea | 18 Jan 2019 | 5/12 |
| 7 | Marshall | Cherry Hollow Cave (spring)a | 18 Jan 2019 | 3/12 |
| 8 | Marshall | Cushion Spring | 11 Jan 2019 | 2/12 |
| 9 | Marshall | Davis Spring | 11 Jan 2019 | 1/18 |
| 10 | Marshall | Guffey Cave | 22 Feb 2019 | 0/12 |
| 11 | Marshall | Kings Spring Cave | 01 Feb 2019 | 0/18 |
| 12 | Marshall | McGehee Spring | 11 Jan 2019 | 1/12 |
| 13 | Marshall | New Hope Spring | 31 Jan 2019 | 0/12 |
| 14 | Jackson | Bluff River Caveb | 18 Aug 2018 | 0/12 |
| 15 | Jackson | Tumbling Rock Caveb | 04 Mar 2018 | 0/12 |
| 16 | Jackson | Tumbling Rock Cave (spring)b | 04 Mar 2018 | 0/12 |
| 17 | Marshall | Keller’s Cave a | ||
| 18 | Marshall | Porches Spring Cave a |
aHistorical sites for C. speleocoopi.
bSites used for negative field controls. Two to four water samples were collected from a site, each with six PCR technical replicates. Sites 17 and 18 are historical sites but we could not gain permission to sample.
Fig 2. Distribution of the Sweet Home Alabama Cave Crayfish (Cambarus speleocoopi) (blue dots) and eDNA sampling sites (white dots and numbered blue dots) in northern Alabama, USA.
Cambarus speleocoopi eDNA was detected (black triangle) at two historical sites and three new sites. Site numbers correspond to those listed in Table 1. Sites 17 and 18 are historical sites but we could not gain permission to sample. Karst and cave-bearing carbonate strata is shown in gray.
Water sampling, filtering, and eDNA filter extraction
We collected 1–2 L of water in total (2–4 500 mL samples) at each site by submerging sterile Nalgene bottles beneath the surface. For spring sites, we collected water samples as close as possible to the point where water emerged from underground. Water samples were collected, placed on ice in a cooler, then transported back to the laboratory at The University of Alabama in Huntsville for filtering.
Water samples were vacuum-filtered within 24 hours of collection in the laboratory using 0.45-μm cellulose-nitrate filters (Thermo ScientificTM NalgeneTM) following Niemiller et al. [16]. After filtration, the filters were folded, transferred to 8-mL sterile tubes, then stored at -20°C until DNA extraction. Multiple filters were needed for a few water samples with substantial amounts of suspended silt and organic matter. For each set of water samples filtered, we also filtered a water sample comprised of distilled water to serve as a negative control.
We extracted DNA from filters in a dedicated laminar flow hood using a modified Qiagen® DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA, USA) protocol. One half of each filter was cut into small pieces using flame-sterilized scissors and transferred into a 2-mL microcentrifuge tube. The remaining half of each filter was stored long-term to serve as a back-up. For environmental water samples that required multiple filters, we extracted DNA from one-half of each filter and then pooled elutions. We added 360 μL ATL Buffer and 40 μL of Proteinase K (Qiagen, Inc.) then incubated filter pieces overnight at 55°C. Samples were then vortexed for 15 s and centrifuged for 1 min (8000 g). The resulting supernatant was transferred into a new 2-mL microcentrifuge and processed following the manufacturer’s protocol, with the exceptions of using 400 μL of AL Buffer and 400 μL of ethanol. The final elution volume for all samples was 125 μL of buffer AE preheated to 70°C. In addition, elutions were treated with the OneStep™ PCR Inhibitor Removal Kit (Zymo Research) to remove potential PCR inhibitors that may be present. Elutions were stored at -20°C until qPCR.
qPCR assay design
Candidate species-specific qPCR assays that included forward and reverse primers and an intervening hydrolysis probe were designed using Integrated DNA Technologies’(IDT) PrimerQuest tool [32] available online at https://www.idtdna.com/PrimerQuest/Home/Index. The default settings for qPCR assay design were used except for modifying the optimal primer temperature (60°C), optimal probe temperature (70°C), and amplicon length (200 bp max). CO1 sequences available on GenBank for C. speleocoopi (accession nos. DQ411780–DQ411781; [33]) as well as other crayfish species that may occur in the study area were aligned using MUSCLE [34] in the program Jalview [35]. A consensus sequence from this alignment for C. speleocoopi was used as input into PrimerQuest.
In silico and in vitro assay validation
We examined specificity of candidate assays both in silico and in vitro. For in silico validation, we used NCBI Primer-BLAST to query forward and reverse primers and probes against the nr/nt database [36]. Candidate assays then were synthesized by IDT; the internal probe was a PrimeTime® double-quenched ZEN™/IOWA Black™ FQ probe labeled with 6-FAM and validated in vitro. We tested specificity in vitro by qPCR on a 678-bp gBlock gene fragment synthesized by IDT based on GenBank accession no. DQ411780 for C. speleocoopi and tissue-derived genomic DNA for C. speleocoopi and other crayfish species in the family Cambaridae, that may potentially occur in caves and springs in the study area, including C. hamulatus, C. jonesi, C. laconensis, C. pecki, C. tenebrosus, and Orconectes australis. We evaluated performance of our candidate assays after optimizing primer-probe concentrations and qPCR reagents and settings for our field samples (see below).
Primer-probe concentration optimization
We optimized concentrations of primers and probe by screening varying concentrations of forward and reverse primers (600 nM, 900 nM, and 1200 nM) and probe (125 nM, 250 nM, and 500 nM) in triplicate with 0.5 ng of target-species synthetic DNA per 20- μL reaction (see next section). Primer-probe concentrations with the greatest peak fluorescence and lower Ct values were employed in screening field-collected water samples.
Field eDNA sample screening
Real-time qPCR amplification of each field-collected water sample was conducted with six replicates in a final volume of 20 μL, using 10.0 μL of TaqMan® Environmental Master Mix 2.0 (Applied Biosystems), 4.7 μL of ddH20, 0.9 μL of forward primer (20 μM), 0.9 μL of reverse primer (20 μM), 0.5 μL of probe (20 μM), and 3.0 μL of template DNA. Final concentrations of primers and probe in each 20-μL reaction were 900 and 250 nM, respectively. Samples were run in 96-well optical plates on a QuantStudio® 3 Real-Time PCR System (Applied Biosystems) under the following conditions: an initial 10-min incubation at 95°C to activate the AmpliTaq Gold® enzyme followed by 50 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. A dilution series of C. speleocoopi synthesized gBlock was used as a positive control standard, ranging from 10−1 to 10−9 ng/μL in concentration. This standard also was used to determine the limit of detection (LOD)–the concentration in which there was at least one positive amplification among technical replicates, and limit of quantification (LOQ)–the concentration in which all technical replicates amplified. Negative controls with all PCR reagents but no template (six replicates) were included on each plate to assess potential contamination. We also screened negative filter controls. Positive amplifications were purified using ExoSAP-IT and specificity confirmed via Sanger sequencing at Eurofins Genomics (Louisville, Kentucky, USA).
Contamination precautions
False positives can result from contamination during every step in the sampling and quantification pipeline from field collection and filtering of water samples to DNA extraction and qPCR amplification. To minimize the potential for contamination, we employed several procedures in addition to those already outlined. Prior to water sample collection in the field, all bottles and collection supplies were sterilized with 10% bleach solution and autoclaved. Filtering occurred in a lab space dedicated for such purposes. All eDNA filter extractions and qPCR preparations were conducted in dedicated laminar flow hoods. Lab space surfaces and equipment (e.g., pipettes, forceps, tubes, and other consumables etc.) were decontaminated before and after use with 10% bleach solution and/or 30-min ultraviolet light (UV) treatment. Filtered tips were used during all protocols that required pipetting. Finally, disposable gloves were worn during field collection, filtering, DNA extraction, and qPCR setup and cleanup. We included negative controls during the filtering, DNA extracting, and qPCR amplification stages to check for potential contamination.
Results
Assay design and validation
The species-specific qPCR assay developed for C. speleocoopi targeted a 163-bp fragment of the mitochondrial CO1 locus (Table 2). In silico assay validation demonstrated that this assay was not likely to amplify non-target crayfish species, particularly other cave-dwelling crayfishes in the genus Cambarus that also occur in the Tennessee River Valley of northern Alabama–C. hamulatus, C. jonesi, C. laconensis, C. pecki, and C. tenebrosus. The forward primer had one mismatch with published sequences of C. jonesi (GenBank accession nos. DQ411777–DQ411779), two mismatches with C. laconensis (accession no. DQ411782) and C. pecki (accession no. JX514434), 1–2 mismatches with C. hamulatus (accession nos. DQ411760–DQ411776) and C. tenebrosus (accession no. EU583576 and JX514444), and 1–3 mismatches with O. australis (accession nos. EF207161–EF207162, EU583506–EU583551, EU583577–EU583580, EU583583–EU583604, EU583607–EU583620, EU583622–EU583626, EU583628). The reverse primer had 2–3 mismatches with published sequences of C. hamulatus, C. laconensis, C. pecki, C. tenebrosus, and O. australis, and one mismatch with published sequences of C. jonesi. Finally, the probe had 4–5 mismatches with published sequences of C. hamulatus, C. jonesi, C. laconensis, C. pecki, and C. tenebrosus, and 3–5 mismatches with published sequences of O. australis. In vitro validation also showed that the designed assay was specific to C. speleocoopi with a LOD of 1–2 copies/μL and LOQ of 13.5 copies/ μL (R2 = 0.995).
Table 2. Primers and probe developed and used in the current study to amplify a 163-bp fragment of CO1 for Cambarus speleocoopi.
| Oligo | Sequence (5’ to 3’) | Direction | Length (bp) | Tm (°C) |
|---|---|---|---|---|
| Forward | TGGGATAGTTGGGACTTCA | Sense | 19 | 60 |
| Reverse | ATTRCCAAACCCTCCAATTA | Antisense | 20 | 60 |
| Probe | TCCGAGTTGAATTGGGTCAGGTAGGAAGG | Sense | 29 | 70 |
Field surveys
We detected C. speleocoopi eDNA at 5 of 16 sites sampled, including two historical sites–Beech Spring Cave at the spring (site 3) and Cherry Hollow Cave (both in the cave and at the spring; sites 6 and 7). We also detected C. speleocoopi at three additional and potentially new sites in Marshall County–Cushion Spring (site 8), McGehee Spring (site 12), and Davis Spring (site 9). All three of these sites were within 2 km of historical sites for the species. Positive detections from Cherry Hollow Cave (in cave and at the spring) were identical to the corresponding region of a CO1 haplotype from the cave sampled previously (accession no. D411780). Positive detections from Beech Spring Cave, Cushion Spring, McGehee Spring, and Davis Spring matched a CO1 haplotype from Keller’s and Porches Spring Cave (accession no. D411781). We did not detect C. speleocoopi eDNA at Bluff River Cave (site 14) and Tumbling Rock Cave (site 15) and associated spring (site 16) in Jackson County. These sites are far removed from the range of C. speleocoopi, but populations of the closely related C. hamulatus occur in these two caves. We found no evidence of contamination in either our field or laboratory controls across qPCR runs. All positive detections were confirmed by Sanger sequencing.
Discussion
The use of eDNA as an effective approach in the detection and monitoring of groundwater fauna is still in its infancy. However, our study contributes to a growing literature that indicates eDNA is a viable monitoring tool for occupancy of a fauna that is otherwise difficult to survey and study using traditional approaches. To date, single-species eDNA assay approaches have been applied successfully in the detection of two groundwater salamanders—Olm (Proteus anguinus) from caves, springs, and wells in Bosnia and Herzegovina, Croatia, Montenegro, and Slovenia [17–19] and Barton Springs Salamander (Eurcyea sosorum) from a spring in the Edwards Aquifer region of Texas, USA [20], four groundwater fishes—Mexican Blindcat (Prietella phreatophila) from a cave in the Edwards Aquifer region of Texas, USA [20], Blind Cave Eel (Ophisternon candidum) from boreholes in northwestern Australia [22], and Ozark Cavefish (Troglichthys rosae) and Eigenmann’s Cavefish (Typhlichthys eigenmanni) from caves, springs, and wells in the Ozarks region of Arkansas, Missouri, and Oklahoma, USA [21], two amphipods—Hay’s Spring Amphipod (Stygobromus hayi) and Potomac Groundwater Amphipod (S. tenuis potomacus) from hypotelminorheic springs in the District of Columbia, USA [16], and two crayfishes—Oklahoma Cave Crayfish (Cambarus tartarus) from caves in the Ozarks region of Oklahoma, USA [21] and Caney Mountain Cave Crayfish (Oroconectes stygocaneyi) from a cave in the Ozarks region of Missouri, USA [21, 23]. This study is the first to successfully detect eDNA of crayfishes from groundwater of the Interior Plateau karst region and demonstrates that eDNA can detect groundwater crustaceans in karst groundwater habitats, such as springs and cave streams.
We designed a species-specific assay that was lab and field validated to discriminate C. speleocoopi from several closely related, morphologically cryptic congeners. Our assay detected C. speleocoopi at two historical sites where occupancy has been confirmed by visual surveys within the last five years. However, we also detected C. speleocoopi eDNA at three potentially new sites in Marshall County, suggesting that an eDNA approach is a rapid and cost-effective method to detect rare subterranean fauna of conservation concern inhabiting karst groundwater. The three positive detections of C. speleocoopi eDNA from Cushion, Davis, and McGehee springs (sites 8, 9, and 12, respectively) appear reasonable given the close proximity to historical sites north of the Tennessee River on the escarpments of Grassy Mountain (Fig 2). Cushion Spring is located 1.2 km northwest of Porches Spring Cave (site 18) on the south side of Grassy Mountain. McGehee Spring is located just 55 m north and is the main resurgence of the stream in Keller’s Cave (site 17), which is the type locality of C. speleocoopi located on the north side of Grassy Mountain. Davis Spring is located 1.9 km east of Keller’s Cave. All of these springs and caves drain into the Paint Rock River. However, eDNA samples from Kings Spring Cave (site 11) located on the south side of Kings Hollow on Brindley Mountain in the Little Cane Creek drainage south of the Tennessee River did not detect C. speleocoopi eDNA, despite being just 1.2 km west of Cherry Hollow Cave (site 6). Cambarus speleocoopi has never been observed at Kings Spring Cave, including several recent surveys since 2018. Hydrological connectivity between these cave and spring sites is not well understood, as dye tracing investigations have yet to be conducted for these particular karst cave systems.
It is possible that C. speleocoopi is present but rare (i.e., in low abundance) at non-detection sites in Marshall County, but our assay was not sensitive enough to detect extremely low abundance. Conducting eDNA studies on groundwater crayfishes (and other groundwater fauna) in an occupancy-modelling framework [37, 38] would benefit future research efforts [e.g., 19]. In addition, we did not assess the effects of water level/flow on detectability. Water samples were collected primarily in late winter and early spring when local water tables were higher, as some spring sites have little to no flow in summer and autumn. However, we only sampled when water conditions were considered normal for the time of the year, and avoided collecting water samples after recent heavy rainfall events that resulted in higher water levels in caves, increased discharge from karst springs, and increased sediment transport, as water volume, flow, and sediment load all can influence eDNA detection [39–42]. Regardless, eDNA concentrations may vary seasonally in caves and springs due to variation in environmental factors, as observed in other aquatic systems [e.g., 43] and suggested for cave systems [21], such as water volume, flow rate, concentrations of potential inhibitors, etc., as well as biological factors, such as population dynamics and seasonality of reproduction. For example, seasonal variation in crayfish life history and behavior may influence detectability using eDNA [44]; however, little is known about the life history of C. speleocoopi. These and other factors can affect the production rate of eDNA. We encourage future researchers to examine seasonal and annual variation in eDNA concentration and detectability.
Several abiotic and biotic factors affect the degradation and persistence time of eDNA in water, such as UV radiation, temperature, pH, and microbial activity [45–52]. A wide range of degradation rates of eDNA have been reported in the literature for aquatic species in surface ecosystems [12, 48]; however, estimates from groundwater habitats are unknown. In general, degradation rates are greater at warmer temperatures [46, 48], higher UV radiation exposure [45, 46, 50, but see 51], and higher levels of microbial activity [45, 46, 49, 52]. Groundwater ecosystems are characterized by lack of light (and UV radiation), cooler and more stable temperatures, lower nutritional resources, and lower microbial activity compared to most surface aquatic ecosystems [53, 54]. Consequently, eDNA is thought to be comparatively stable in groundwater habitats and capable of being detected [18]. However, the persistence time of eDNA in groundwater is unknown but likely is considerably longer than the timescale of hours to days reported for many surface species and habitats. Macro-organismal eDNA has been recovered from dry cave sediments dating back thousands to hundreds of thousands of years before present [e.g., 55–57], demonstrating the potential for subterranean habitats to act as incubators for eDNA.
Determining degradation rates and persistence time of eDNA in various groundwater habitats should be priority of future research, as inaccurate estimates may have implications for conservation and management. Many groundwater and cave-dwelling species have small, restricted distributions (i.e., short-range endemics) and are of conservation concern [58, 59]. An underlying assumption of eDNA approaches for detection and monitoring is that a positive detection represents contemporary occupancy. However, if eDNA persists for weeks to months or even years in groundwater habitats, then we could overestimate the distribution of a species–e.g., a “false positive” in which eDNA is detected at a site where a population has long been extirpated–or fail to detect declines in range size when eDNA is employed as the sole approach in a monitoring program.
The use of eDNA in the detection and monitoring of groundwater fauna is still in its early stages. Here we report the first application of eDNA to detect a stygobitic crayfish in karst groundwater habitats of the Interior Plateau karst region and provide a demonstration for how eDNA can be applied in groundwater biodiversity monitoring of rare and endangered taxa. Our study along with the encouraging results from the few other recent studies to date have shown that eDNA can be a valid and effective complement to traditional sampling approaches for determining occupancy of groundwater species. We envision a quick transition from proof-of-concept studies to experimental approaches examining rates of degradation and persistence in groundwater to development of best practices for long-term monitoring programs of groundwater fauna.
Acknowledgments
We thank the many private landowners, Randall Blackwood (Cathedral Caverns State Park), and the Southeastern Cave Conservancy, Inc. for access to springs and caves on their properties. We thank members of the Niemiller Lab at UAH who assisted with field collection of water samples, filtering, and molecular procedures, including Jashen Bailey, Joseph Benito, Kayla Wilson, and Carson Woodward. We also thank Amata Hinkle, and Joe Lamb for assistance in the field.
Data Availability
Cave location data has been intentionally omitted to protect these sensitive ecosystems and their biodiversity. Cave location data are maintained by the Alabama Cave Survey (http://www.alabamacavesurvey.org/). Please direct data requests to membership@alabamacavesurvey.org.
Funding Statement
This study was supported by funding from the Cave Conservancy Foundation (contract no. 7959) to MLN, The University of Alabama in Huntsville New Faculty Research Award (no. 251353) to MLN, and the Integrated DNA Technologies Sustainability Award to MLN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lomolino MV. Conservation biogeography In Lomolino M.V. & Heaney L.R. (Eds.). Frontiers of biogeography: new directions in the geography of nature. Sunderland, Massachusetts: Sinauer Associates; 2004. pp. 293–296. [Google Scholar]
- 2.Niemiller ML, Bichuette E, Taylor SJ. Conservation of cave fauna in Europe and the Americas In Moldovan O.T., Kovac L., & Halse S.(Eds.), Ecological studies: cave ecology. Dordrecht: Springer; 2018. pp. 451–478. 10.1002/ar.24044 [DOI] [Google Scholar]
- 3.Mammola S, Cardoso P, Culver DC, Deharveng L, Ferreira RL, Fiser C, et al. Scientists’ warning on the conservation of subterranean ecosystems. BioScience. 2019;69: 641–650. [Google Scholar]
- 4.Ficetola GF, Canedoli C, Stoch F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conservation Biology. 2019;33: 214–216. 10.1111/cobi.13179 [DOI] [PubMed] [Google Scholar]
- 5.Harvey MS, Rix MG, Framenau VW, Hamilton ZR, Johnson MS, Teale RJ, et al. Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes. Invertebr Syst. 2011;25: 1–10. [Google Scholar]
- 6.Gibert J, Deharveng L. Subterranean ecosystems: A truncated functional biodiversity. BioScience. 2002;52: 473–481. [Google Scholar]
- 7.Ficetola GF, Miaud C, Pompanon F, Taberlet P. Species detection using environmental DNA from water samples. Biol Lett. 2008;23: 423–425. 10.1098/rsbl.2008.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg CS, Strickler KM, Pilliod DS. Moving environmental DNA methods from concept to practice for monitoring aquatic macroorganisms. Biol Conserv. 2015;183: 1–3. [Google Scholar]
- 9.Thomsen PF, Willerslev E. Environmental DNA—an emerging tool in conservation for monitoring past and present biodiversity. Biol Conserv. 2015;183: 4–18. [Google Scholar]
- 10.Barnes MA, Turner CR. The ecology of environmental DNA and implications for conservation genetics. Conserv Genet. 2016;17: 1–17. [Google Scholar]
- 11.Bohmann K, Evans A, Gilbert MTP, Carvalho GR, Creer S, Knapp M, et al. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol Evol. 2014;29: 358–367. 10.1016/j.tree.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Beng KC, Corlett RT. Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodiversity and Conservation. 2020;29: 2089–2121. [Google Scholar]
- 13.Jerde CL, Mahon AR, Chadderton WL, Lodge DM. “Sight unseen” detection of rare aquatic species using environmental DNA. Conserv Lett. 2011;4: 150–157. [Google Scholar]
- 14.Thomsen PF, Kielgast J, Iversen LL, Wiuf C, Rasmussen M, Gilbert MTP, et al. Monitoring endangered freshwater biodiversity using environmental DNA. Mol Ecol. 2012;21: 2565–2573. 10.1111/j.1365-294X.2011.05418.x [DOI] [PubMed] [Google Scholar]
- 15.Laramie MB, Pilliod DS, Goldberg CS. Characterizing the distribution of an endangered salmonid using environmental DNA analysis. Biol Conserv. 2015;183: 29–37. [Google Scholar]
- 16.Niemiller ML, Porter ML, Keany J, Gilbert H, Fong DW, Culver DC, et al. Evaluation of eDNA for groundwater invertebrate detection and monitoring: a case study with endangered Stygobromus (Amphipoda: Crangonyctidae). Conserv Genet Resour. 2018;10: 247–257. [Google Scholar]
- 17.Gorički Š, Stanković D, Aljančič M, Snoj A, Kuntner M, Gredar T, et al. Searching for black Proteus with the help of eDNA. Nat Sloveniae. 2016;18: 57–58. [Google Scholar]
- 18.Gorički Š, Stanković D, Snoj A, Kuntner M, Jeffery WR, Trontelj P, et al. Environmental DNA in subterranean biology: range extension and taxonomic implications for Proteus. Sci Rep. 2017;7: 45054 10.1038/srep45054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vörös J, Marton O, Schmidt BR, Gal JT, Jelic D. Surveying Europe’s only cave-dwelling chordate species (Proteus anguinus) using environmental DNA. PLoS ONE. 2017;12: e0170945 10.1371/journal.pone.0170945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons KM. Exploring the distribution of groundwater salamanders and catfish with environmental DNA. M.Sc. Thesis. The University of Texas at Austin, Austin, Texas. 2019, pp. 32.
- 21.Mouser J. Examining occurrence, life history, and ecology of cavefishes and cave crayfishes using both traditional and novel approaches. M.Sc. Thesis. Oklahoma State University, Stillwater, Oklahoma. 2019, pp. 122.
- 22.White NE, Guzik MT, Austin AD, Moore GI Humphreys WF, Alexander J, et al. Detection of rare Australian endemic blind cave eel (Ophisternon candidum) with environmental DNA: implications for threatened species management in subterranean environments. Hydrobiologica. 2020. 10.1007/s10750-020-04304-z [DOI] [Google Scholar]
- 23.DiStefano RJ, Ashley D, Brewer SK, Mouser JB, Niemiller ML. Preliminary investigation of the critically imperiled Caney Mountain Cave Crayfish Orconectes stygocaneyi Hobbs III, 2001 (Decapoda: Cambaridae) in Missouri, USA. Freshwater Crayfish. 2020;25: 47–57. [Google Scholar]
- 24.Korbel K, Chariton A, Stephenson S, Greenfield P, Hose GC. Wells provide a distorted view of life in the aquifer: implications for sampling, monitoring and assessment of groundwater ecosystems. Sci Rep. 2017;7: 40702 10.1038/srep40702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buhay JE, Crandall KA. Taxonomic revision of cave crayfish in the genus Cambarus, subgenus Aviticambarus (Decapoda: Cambaridae) with descriptions of two new species, C. speleocoopi and C. laconensis, endemic to Alabama, U.S.A. J. Crustacean Biology. 2009; 29: 121–134. [Google Scholar]
- 26.Huryn A. Sweet Home Alabama Cave Crayfish Cambarus speleocoopi Buhay and Crandall In: Shelton-Nix E, editor. Alabama Wildlife. Tuscaloosa, Alabama: The University of Alabama Press; 2017. p. 113. [Google Scholar]
- 27.NatureServe. 2020 [cited 28 May 2020]. NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available from: https://explorer.natureserve.org/.
- 28.Buhay J, Crandall KA, Cordeiro J. 2010. [cited 28 May 2020]. Cambarus speleocoopi. The IUCN Red List of Threatened Species 2010: e.T164915A5938003 Available from: 10.2305/IUCN.UK.2010-3.RLTS.T164915A5938003.en. [DOI] [Google Scholar]
- 29.Niemiller ML, Ziger KS. Patterns of cave biodiversity and endemism in the Appalachians and Interior Plateau of Tennessee, USA. PLoS One. 2013;8, e64177 10.1371/journal.pone.0064177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigler KS, Niemiller ML, Stephen CDR, Ayala BN, Milne MA, Gladstone NS, et al. Biodiversity from caves and other subterranean habitats of Georgia, USA. J Cave Karst Stud. 2020;82, 125–167. [Google Scholar]
- 31.Hobbs II HH, Hobbs III HH, Daniel MA. A review of the troglobitic crustaceans of the Americas. Smithson Contrib Zool. 1977;244: 1–183. [Google Scholar]
- 32.Owczarzy R, Tataurov AV, Wu Y, Manthey JA, McQuisten KA, Almabrazi HG, et al. IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 2008;36: W163–W169. 10.1093/nar/gkn198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhay ME, Moni G, Mann N, Crandall KA. Molecular taxonomy in the dark: Evolutionary history, phylogeography, and diversity of the cave crayfish in the subgenus Aviticambarus, genus Cambarus. Mol Phylogenet Evol. 2007;42: 435–448. 10.1016/j.ympev.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 34.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13: 134 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt BR, Kery M, Ursenbacher S, Hyman OJ, Collins JP. Site occupancy models in the analysis of environmental DNA presence/absence surveys: a case study of an emerging amphibian pathogen. Meth Ecol Evol. 2013;4: 646–653. [Google Scholar]
- 38.Dorazio RM, Erickson RA. An r package for multiscale occupancy modelling of environmental DNA data. Mol Ecol Res. 2018;18, 368–380. [DOI] [PubMed] [Google Scholar]
- 39.Turner CR, Uy KL, Everhart RC. Fish environmental DNA is more concentrated in aquatic sediments than surface water. Biol Conserv. 2015;183: 93–102. [Google Scholar]
- 40.Jane SF, Wilcox TM, McKelvey KS, Young MK, Schwartz MK, Lowe WH, et al. Distance, flow and PCR inhibition: eDNA dynamics in two headwater streams. Mol Ecol Resour. 2015;15: 216–227. 10.1111/1755-0998.12285 [DOI] [PubMed] [Google Scholar]
- 41.Jerde CL, Olds BP, Shogren AJ, Andruszkiewicz EA, Mahon AR, Bolster D, et al. Influence of stream bottom substrate on retention and transport of vertebrate environmental DNA. Environ Sci Technol. 2016;50: 8770–8779. 10.1021/acs.est.6b01761 [DOI] [PubMed] [Google Scholar]
- 42.Stoeckle BC, Beggel S, Cerwenka AF, Motivans E, Geist J. A systematic approach to evaluate the influence of environmental conditions on eDNA detection success in aquatic ecosystems. PLoS One. 2017;12: e0189119 10.1371/journal.pone.0189119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxton AS, Groombridge JJ, Zakaria NB, Griffiths RA. Seasonal variation in environmental DNA in relation to population size and environmental factors. Sci Rep. 2017;7: 46294 10.1038/srep46294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn N, Priestley V, Herraiz A, Arnold R, Savolainen V. Behavior and season affect crayfish detection and density inference using environmental DNA. Ecol Evol. 2017;7: 7777–7785. 10.1002/ece3.3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilliod DS, Goldberg CS, Arkle RS, Waits LP. Factors influencing detection of eDNA from a stream-dwelling amphibian. Mol Ecol Resour. 2014;14: 109–116. 10.1111/1755-0998.12159 [DOI] [PubMed] [Google Scholar]
- 46.Strickler K M, Fremier AK, Goldberg CS. Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biol Conserv. 2015;183: 85–92. [Google Scholar]
- 47.Goldberg CS, Strickler KM, Fremier AK. Degradation and dispersion limit environmental DNA detection of rare amphibians in wetlands: increasing efficacy of sampling designs. Sci Total Environ. 2018;633: 695–703. 10.1016/j.scitotenv.2018.02.295 [DOI] [PubMed] [Google Scholar]
- 48.Harrison JB, Sunday JM, Rogers SM. Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proceedings of the Royal Society B. 2019;286: 20191409 10.1098/rspb.2019.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eichmiller JJ, Best SE, Sorensen PW. Effects of temperature and trophic state on degradation of environmental DNA in lake water. Environ Sci Technol. 2016;50: 1859–1867. 10.1021/acs.est.5b05672 [DOI] [PubMed] [Google Scholar]
- 50.Andruszkiewicz EA, Sassoubre LM, Boehm AB. Persistence of marine fish environmental DNA and the influence of sunlight. PLoS ONE. 2017;12: e0185043 10.1371/journal.pone.0185043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mächler E, Osathanunkul M, Altermatt F. Shedding light on eDNA: neither natural levels of UV radiation nor the presence of a filter feeder affect eDNA-based detection of aquatic organisms. PLoS ONE. 2018;13: e0195529 10.1371/journal.pone.0195529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zulkefi NS, Kim KH, Hwang SJ. Effects of microbial activity and environmental parameters on the degradation of extracellular environmental DNA from a eutrophic lake. Int J Environ Res Public Health. 2019;16: 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibert J, Danielopol D, Stanford JA. Groundwater Ecology Academic Press; 1994. [Google Scholar]
- 54.Fiser C, Pipan T, Culver DC. The vertical extent of groundwater metazoans: an ecological and evolutionary perspective. BioScience. 2014;64: 971–979. [Google Scholar]
- 55.Willerslev E, Hansen AJ, Binladen J, Brand TB, Gilbert MTP, Shapiro B, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300: 791–795. 10.1126/science.1084114 [DOI] [PubMed] [Google Scholar]
- 56.Hofreiter M, Mead JI, Martin P, Poinar HN. Molecular caving. Current Biology. 2003;13: R693–R695. 10.1016/j.cub.2003.08.039 [DOI] [PubMed] [Google Scholar]
- 57.Haile J, Holdaway R, Oliver K, Bunce M, Gilbert MTP, Nielsen R, et al. Ancient DNA chronology within sediment deposits: are paleobiological reconstructions possible and is DNA leaching a factor? Mol Biol Evol. 2007;24: 982–989. 10.1093/molbev/msm016 [DOI] [PubMed] [Google Scholar]
- 58.Zagmajster M, Eme D, Fišer C, Galassi D, Marmonier P, Stoch F, et al. Geographic variation in range size and beta diversity of groundwater crustaceans: insights from habitats with low thermal seasonality. Glob Ecol Biogeogr. 2014;23: 1135–1145. [Google Scholar]
- 59.Eme D, Malard F, Colson-Proch C, Jean P, Calvignac S, Konecny-Dupré L, et al. Integrating phylogeography, physiology and habitat modelling to explore species range determinants. J Biogeogr. 2014;41: 687–699. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Cave location data has been intentionally omitted to protect these sensitive ecosystems and their biodiversity. Cave location data are maintained by the Alabama Cave Survey (http://www.alabamacavesurvey.org/). Please direct data requests to membership@alabamacavesurvey.org.