Abstract
Background
Understanding racial influences on HPV distribution in women with Atypical Squamous Cells of Undetermined Significance (ASC-US) cytology using partial genotyping in a statewide population can inform HPV-based prevention efforts.
Methods
Women aged 21–65 with any cytology result and partial HPV genotyping for ASC-US triage between January 1, 2014 and December 31, 2017 were included. All women attended a Mississippi Department of Health (MSDH) clinic. Age, race, cytopathologic and HPV data were extracted from the electronic health record and analyzed. Cytologic specimens were processed with ThinPrep and HPV testing with cobas4800®. HPV genotypes were evaluated in hierarchical categories. Chi-square tests and multinomial logistic regression models evaluated associations between race and type prevalence.
Results
43,106 women underwent cervical cancer screening with cytology and ASC-US triage. Of these, 34,363 (80.2%) had normal cytology, 4,672 (10.9%) ASC-US, 2,683 (6.3%) Low-Grade Squamous Intraepithelial Lesion (LSIL), and 633 (1.5%) High-Grade Squamous Intraepithelial Lesion (HSIL). Blacks represented 69.3% of the sample and had a higher proportion of HPV-positive ASC-US (6.5%), compared to Whites (5.6%). Blacks had significantly decreased odds of HPV 16 (OR 0.66; 95% CI, 0.6–0.9; p=0.002) and significantly increased odds for 12 other types (OR 1.37 95% CI, 1.2–1.5; p<0.0001) compared to Whites.
Conclusions
In a diverse population, we show significant differences in HPV genotypes by race. Importantly, Blacks with ASCUS are less likely to be HPV 16 positive compared to Whites. Ongoing work is evaluating individual genotype prevalence and genotype-specific risk of precancer by race.
Keywords: Human Papillomavirus, HPV genotypes, Cervical Cancer, Cervical Cancer Screening, Atypical Squamous Cells of Undetermined Significance
Precis:
Among women with ASC-US cytology, black women have fewer HPV 16 infections, but more other HR type infections than White women.
Introduction
Persistent infections with human papillomaviruses (HPV) and progression to precancer are the necessary steps of cervical carcinogenesis.1,2 Among over 200 known HPV genotypes, 12 are considered carcinogenic.3 Within this group, the carcinogenicity differs vastly between types, with HPV 16 and HPV 18 causing over 70% of invasive cancers, while several other types are rarely found in cancers.4,5 International HPV prevalence surveys have shown different HPV genotype distributions in different regions of the world.6 Recent data suggest that HPV genotype distributions also differ in cervical precancers in the United States (U.S) between Black and White women,7–9 but existing studies are small and do not cover the whole continuum of natural history. To evaluate racial influences on HPV genotype distributions, large observational studies in diverse populations undergoing screening are needed.
In Mississippi (MS), higher rates of cervical disease are observed among racial and ethnic minorities with mortality rates consistently two to three times greater in Black women than White women, (6.9 vs. 2.3 per 100,000 population).10 It is important to understand the underlying biological and behavioral factors responsible for these disparities. Examining the HPV genotype prevalence in this population with high risk of precancer and cancer is central to understanding possible biological differences between racial/ethnic groups.
Only recently, primary screening provided by the state of MS changed from cytology to HPV and cytology co-testing. However, for many years, HPV testing with partial genotyping for HPV 16 and HPV 18 has been performed in women with atypical squamous cells of undetermined significance (ASC-US) cytology results, the most common cytologic abnormality. 11 Approximately 50% of ASC-US cases are HPV positive and 10–15% of this subset is associated with high-grade lesions.12,13
Here we evaluate HPV genotype distributions by race in a large, population-based sample of women undergoing cervical cancer screening with partial HPV genotyping. This statewide study addresses an important research gap and provides insight into HPV prevalence in a previously understudied population in the Southern U.S.
Materials and Methods
Study Population and Clinical Procedures
All women attended a Mississippi State Department of Health (MSDH) clinic for cervical cancer screening. Data from women ages 21–65 with all cytologic interpretations between January 1, 2014 and December 31, 2017, were included. During this time frame, the MSDH screening and management guidelines included cytology alone with ASC-US HPV triage; therefore, only women with ASC-US and partial HPV genotyping results were available for analyses by HPV genotype.
Data were abstracted from databases at the University of Mississippi Medical Center (UMMC). The UMMC Department of Pathology conducted all the cytologic interpretations and HPV testing. Demographic, cytopathologic, and molecular data were extracted from the electronic clinical record and stored in REDCap. In women with a cytologic result of ASC-US, any HPV test result in the medical record within 28 days of the screening cytology order was extracted.
The population represents both rural and urban areas of the state. The majority of women were uninsured and received publicly funded preventative screening. Race was self-reported and stemmed from data recorded on the laboratory order, only three choices were available: White, Black, and Other. The definition of “Other” race was not defined in the pathology reporting system. Ethnicity data was not available. The study was approved by the Institutional Review Boards of both UMMC and MSDH.
Clinical Routine HPV Testing and Cytologic Screening
Cytologic specimens were processed using the ThinPrep (Hologic) liquid-based cytology systems. Cytology results were classified using The Bethesda System for Cervical Cytology as revised in 2014.14 All results were based on the diagnosis reported in the electronic record. HPV testing was done using the sample collected during the Pap test and processed using the cobas4800® HPV genotyping assay (Roche Molecular Systems, Pleasanton, CA) that includes nucleic acid isolation with a real-time polymerase chain reaction.15 The assay targets 14 HPV genotypes and provides type-specific identification of types 16 and 18 and pools 12 ‘Other’ high risk HPV genotypes (HR 12): 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 (HR 12).15
Statistical Analysis
We used descriptive statistics to evaluate demographics of the study population. Between 2014–2017, only three categories of race were reported in the electronic record: White, Black, and a combined category of “Other” races. Because of a small sample size (n=119), we excluded the Other race category in analyses evaluating racial differences in genotype prevalence. Age was divided into three groups: <25, 25–29, and 30–65 years; the age groups are consistent with U.S. based guidelines.
Hierarchical categories were created to evaluate genotypes: HPV16 (including single and multiple infections), else HPV18 (including single and multiple infections), else other HR 12 infections, else HPV negative. Descriptive statistics and Pearson’s chi-square statistics were used to evaluate the relationship between hierarchical HPV categories and age and race.
Crude and continuous age-adjusted binary and multinomial logistic regressions were used to estimate odds ratios (OR) and 95% confidence intervals (CI) to assess associations between race and type prevalence (HPV positive vs. HPV negative and HPV 16, HPV 18, and HR 12 vs. HPV negative, respectively). All reported p-values were two-sided, a p-value <0.05 was considered significant. Statistical analyses were conducted using STATA/SE, version 16.0.
Results
Study Population
A total of 43,106 women undergoing cervical cancer screening received a Pap test at the MSDH between 2014–2017 (Figure 1, Table 1). The participants’ ages ranged from 21–65 years, with a mean age of 29 years and a standard deviation of ±8 years. The population included 11,681 (27.1%) Whites, 26,941 (62.5%) Blacks, and 4,484 (10.4%) with Other race. In the overall screening population, 34,363 (80.2%) tested negative for intraepithelial lesion or malignancy (NILM), 4,672 (10.9%) had ASC-US, 2,683 (6.3%) had a low-grade intraepithelial lesion (LSIL), and 633 (1.5%) had a high-grade intraepithelial lesion (HSIL). Black women had a higher proportion of ASC-US (11.5%) compared to Whites (10.8%), and women with Other race (7.9%). This was driven by a higher proportion of HPV-positive ASC-US (6.5%), compared to Whites (5.6%) and others (1.4%).
Figure 1.
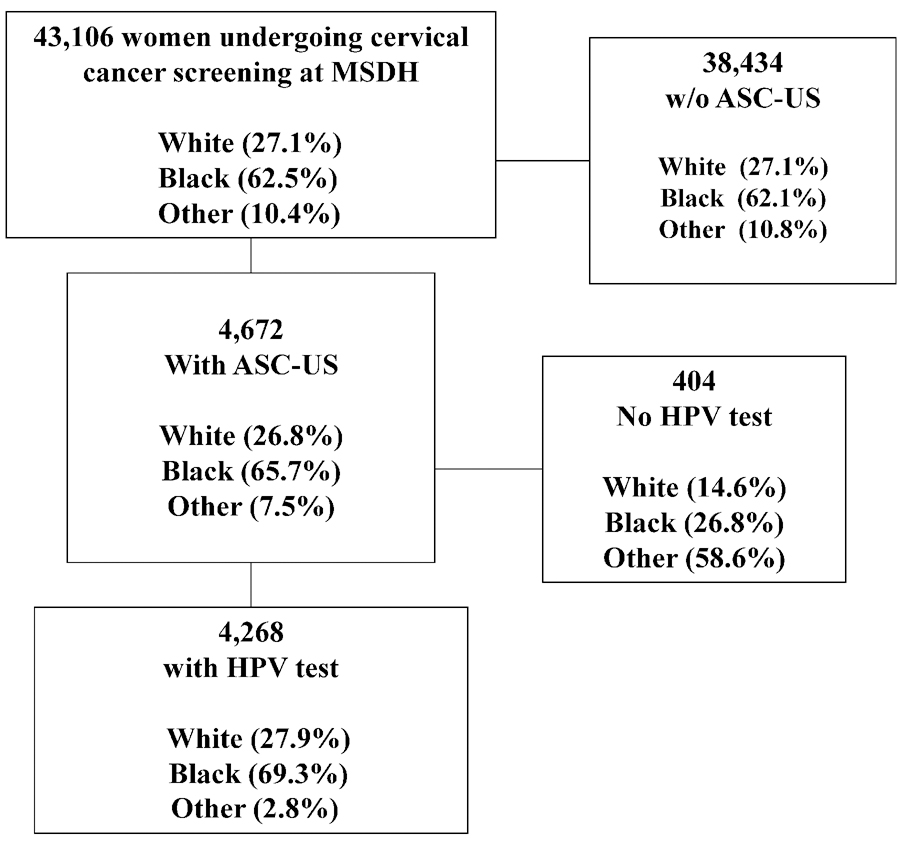
Consort Diagram
Table 1.
Cytology and HPV ASC-US Triage Testing in the Study Population
| Total N (%) |
White n (%) |
Black n (%) |
Other n (%) |
|
|---|---|---|---|---|
|
Total N (%) |
43,106 (100) |
11,681 (27.1) |
26,941 (62.5) |
4,484 (10.4) |
|
Unsatisfactory |
293 (0.7) |
113 (1.0) |
142 (0.5) |
38 (0.9) |
|
NILM |
34,363 (80.2) |
9,159 (79.0) |
21,343 (79.7) |
3,861 (86.4) |
|
ASC-US |
4,672 (10.9) |
1,250 (10.8) |
3,070 (11.5) |
352 (7.9) |
|
HPV negative |
1,786 (4.1) |
537 (4.6) |
1,191 (4.4) |
58 (1.2) |
|
HPV positive |
2,482 (5.7) |
655 (5.6) |
1,766 (6.5) |
61 (1.4) |
|
HPV Untested |
404 (0.9) |
59 (0.5) |
108 (0.4) |
233 (5.2) |
|
LSIL |
2,683 (6.3) |
779 (6.7) |
1,724 (6.4) |
180 (4.0) |
|
ASC-H |
187 (0.4) |
55 (0.5) |
118 (0.4) |
14 (0.3) |
|
HSIL |
633 (1.5) |
230 (2.0) |
382 (1.4) |
21 (0.5) |
|
AGC |
22 (0.1) |
7 (0.1) |
13 (0.1) |
2 (0.04) |
|
Cytology Missing |
253 (0.6) |
88 (34.8) |
149 (58.9) |
16 (6.3) |
Abbreviations:
NILM – Negative for Intraepithelial Lesion or Malignancy
ASC-US – Atypical Squamous Cells of Undetermined Significance
LSIL – Low Grade Squamous Intraepithelial Lesion
ASC-H – Atypical Squamous Cells of Undetermined Significance High Grade
HSIL – High Grade Squamous Intraepithelial Lesion
AGC- Atypical Glandular Cells
HPV Untested – women without an HPV test result
Most women with ASC-US who had an HPV result were under age 30 (64.7%) (Table 2). More Black women were under age 25 compared to the other racial groups with ASC-US. A higher proportion of White women had HPV 16 (12.7%) compared to Black women (7.8%). Conversely, Black women had a higher proportion of HR 12 (47%) compared to White women (38.1%).
Table 2.
Race and Age Distribution of HPV Test Results Among All Women with ASC-US
| Total N (%) |
White n (%) |
Black n (%) |
Other n (%) |
|
|---|---|---|---|---|
| Total N (%) | 4,268 (100.0) | 1,192 (27.9) | 2,957 (69.3) | 119 (2.8) |
| Age | ||||
| <25 | 1,557 (36.5) | 393 (33.0) | 1,126 (38.1) | 38 (32.0) |
| 25–29 | 1,203 (28.2) | 349 (29.2) | 832 (28.1) | 22 (18.5) |
| 30–65 | 1,508 (35.3) | 450 (37.8) | 999 (33.8) | 59 (49.5) |
| HPV Status | ||||
| HPV 16 positive | 392 (9.2) | 151 (12.7) | 232 (7.8) | 9 (7.6) |
| HPV 18 positive | 197 (4.6) | 49 (4.1) | 143 (4.8) | 5 (4.2) |
| HPV HR12 positive | 1,893 (44.4) | 455 (38.1) | 1,391 (47.0) | 47 (39.5) |
HPV 16 hierarchical category = 16 single, 16/other, 16+18, 16+18+other
HPV 18 hierarchical category = 18 single, 18/other
HPV HR 12 hierarchical category = HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68
HR = high risk
Racial differences in HPV prevalence
For subsequent analyses, we excluded 119 women with Other race/ethnicity leaving a total population of 4,149 (Figure 1). In Table 2, we summarize racial differences in HPV prevalence overall and within age groups. Overall, the HPV positivity decreased from 70.2% among women <25 years old to 42.0% among women age 30–65. A similar decrease was observed for both Black and White women in the race-stratified analysis. Overall, Black women were more likely to be positive for HPV (59.7%) compared to Whites (54.9%) (p=0.005). We observed significant racial differences in the distribution of HPV genotypes, with Black women being significantly less likely positive for HPV 16 (13.1% vs. 23% in White women) and more likely to be positive for HR 12 (78.8% vs 69.5% in White women) (p <0.0001). In the age-stratified analysis, a significant difference in overall HPV positivity was only observed in the 25–29 age group (Blacks 65.1% versus Whites 58.2%; p=0.023). In contrast, we saw a significant difference in HPV genotype distribution with lower HPV 16 and higher HR 12 among Black women compared to White women across all age groups (p <0.0001, 0.03 and 0.015 for age groups <25, 25–30, and 30–65, respectively; Table 3).
Table 3.
Race and Age Stratified HPV Positive Associations Among Women with ASC-US.
| Total N (%) | White (n %) | Black (n %) | P-Value | |
|---|---|---|---|---|
| Total | 4,149 (100) | 1,192 (100) | 2,957 (100) | |
| HPV negative n (%) | 1,728 (41.7) | 537 (45.1) | 1,191 (40.3) | |
| HPV positive n (%) | 2,421 (58.3) | 655 (54.9) | 1,766 (59.7) | 0.005 |
| HPV DNA Genotype | <0.0001 | |||
| HPV 16 positive | 383 (15.8) | 151(23.0) | 232 (13.1) | |
| HPV 18 positive | 192 (7.9) | 49 (7.5) | 143 (8.1) | |
| HPV HR12 positive | 1,846 (76.3) | 455 (69.5) | 1,391 (78.8) | |
| Age <25 | 1,519 (36.6) | 393 (33.0%) | 1,126 (38.1%) | P-Value |
| HPV negative n (%) | 452 (29.8) | 128 (32.6) | 324 (28.8) | |
| HPV positive n (%) | 1,067 (70.2) | 265 (67.4) | 802 (71.2) | 0.156 |
| HPV DNA Genotype | <0.0001 | |||
| HPV 16 positive | 165 (15.5) | 65 (24.5) | 100 (12.5) | |
| HPV 18 positive | 73 (6.8) | 15 (5.7) | 58 (7.2) | |
| HPV HR12 positive ONLY | 829 (77.7) | 185 (69.8) | 644 (80.3) | |
| Age 25–29 | 1,181 (28.5) | 349 (29.3%) | 832 (28.1%) | P-Value |
| HPV negative n (%) | 436 (36.9) | 146 (41.8) | 290 (34.9) | |
| HPV positive n (%) | 745 (63.1) | 203 (58.2) | 542 (65.1) | 0.023 |
| HPV DNA Genotype | 0.030 | |||
| HPV 16 positive | 128 (17.2) | 47 (23.1) | 81 (15.0) | |
| HPV 18 positive | 60 (8.1) | 15 (7.4) | 45 (8.3) | |
| HPV HR12 positive ONLY | 557 (74.8) | 141 (69.5) | 416 (76.8) | |
| Age 30–65 | 1,449 (34.9) | 450 (37.8%) | 999 (33.8 %) | P-Value |
| HPV negative n (%) | 840 (58.0) | 263 (58.4) | 577 (57.8) | |
| HPV positive n (%) | 609 (42.0) | 187 (41.6) | 422 (42.3) | 0.806 |
| HPV DNA Genotype | 0.015 | |||
| HPV 16 positive | 90 (14.8) | 39 (20.8) | 51 (12.1) | |
| HPV 18 positive | 59 (9.7) | 19 (10.2) | 40 (9.5) | |
| HPV HR12 positive ONLY | 460 (75.5) | 129 (69.0) | 331 (78.4) |
Abbreviations:
HPV 16 positive = 16 single, 16/other, 16+18, 16+18+other
HPV 18 positive = 18 single, 18/other
HPV HR 12 positive = HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68
HR = high risk
Only 119 women who self-reported as “Other” race had an HPV test result reported. Of these, 58 (49%) were HPV negative and 61 (51%) HPV positive (Table 1). Among those who were HPV positive, the proportions of HPV 16, 18, and the HR 12 looked similar to that of Black women: HPV 16 – 14.8%, HPV 18 – 8.1%, HPV HR 12 – 77%.
We ran multinomial regression models to evaluate the associations of race with HPV genotype results (Table 4). In crude models, Black race was significantly associated with lower odds of HPV 16 (OR 0.66; 95% CI, 0.6–0.9; p=0.002). Conversely, Black women were 1.37 times more likely to be positive for HR 12; (95% CI, 1.2–1.5; p-value <0.0001) compared to Whites. No significant differences were noted by race for HPV18 infections. The age-adjusted odds ratios were very similar to the crude odds ratios, with only slight attenuation.
Table 4.
Associations of Race with HPV Test results among Women with ASC-US
| HPV DNA Genotype | Crude OR (95% CI) |
P-Value | Age Adjusted OR (95% CI) |
P-Value |
|---|---|---|---|---|
| HPV negative | Ref | Ref | ||
| HPV positive | 1.22 (1.1–1.4) |
0.005 | 1.16 (1.0–1.3) |
0.039 |
| HPV 16 positive | 0.69 (0.6–0.9) |
0.002 | 0.66 (0.5–0.8) |
<0.0001 |
| HPV 18 positive | 1.31 (0.9–1.8) |
0.114 | 1.26 (0.9–1.8) |
0.176 |
| HPV HR 12 Positive | 1.37 (1.2–1.5) |
<0.0001 | 1.31 (1.1–1.5) |
<0.0001 |
HPV 16 positive category = 16 single, 16/other, 16+18, 16+18+other
HPV 18 positive category = 18 single, 18/other
HPV HR 12 positive category = HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68
HR = high risk, OR = Odds Ratio, CI = Confidence Interval
Discussion
In this large, population-based study with limited HPV genotyping among women with ASC-US cytology results, we show significant differences in HPV genotypes by race. Importantly, HPV 16 shows a lower proportion and HR 12 shows a higher proportion among all HPV infections in Black women compared to Whites. To our knowledge, these results represent the largest sample of U.S. Black women undergoing cervical cancer screening with partial HPV genotyping results.
Two previous population-based studies, the Kaiser Permanente Northern California and New Mexico cohort, have reported extensive genotyping data in various disease stages but have limited representation of Black women in the cohorts. In our study of women with ASC-US cytology, we found higher a proportion of HPV positivity (2,421 58.3%) compared to these two studies and particularly Blacks in our study being significantly more likely to be HPV positive compared with Whites. In KNPC, HPV ASC-US positivity was 49.2% of 51,527 women and the New Mexico Pap Registry was 41.0% of 15,724; 16 analysis of HPV status and type by race was not presented. These differences are likely multifactorial, with differences in the age distribution having major effects.
HPV natural history studies are lacking in diverse populations (Schiffman & Wentzensen, 2013). Worldwide variations in HPV type prevalence in women with infections, precancers, and cancers have been described. For example, in parts of Africa, Bruni et al. (2019) note that HPV 35 contributes to a higher positivity of precancer cases than what has been previously described.17, 18 In data from Louisiana, Saraiya et al. (2015) described racial differences in 90 histologic specimens from women with carcinoma in situ (CIS), the immediate precursor to invasive cancer.8 Black women had significantly lower rates of HPV 16 (the HPV genotype most commonly associated with invasive cervical carcinoma) and all other vaccine types than Whites. A study from North Carolina also noted similar significant racial differences in HPV prevalence by type in histologically confirmed CIN 1 and CIN2/3 specimens.9 Vidal et al. showed that African Americans were two times less likely to harbor HPV 16/18 (OR 0.48, 95 % CI 0.21–0.94, p = 0.03) compared to Whites. Importantly, these authors noted a similar association when examining CIN 2/3 lesions (OR 0.22, 95 % CI 0.05–0.95, p = 0.04).9
In our study, Black women were more likely to be positive for HR 12. Future research needs to include extended genotyping to determine the identity of the individual high-risk HPV types and assess their risk of progression to precancer.19 Some studies have suggested that extended genotyping may contribute to improved risk stratification for cervical precancer. 13,20,21 In current screening guidelines, management differs only for women with HPV 16/18 types infections.22 However, these risk estimates are lacking in diverse populations that have different HPV genotype distributions. To better inform clinicians of the best practices for this population, additional data is required to calculate the risk of HPV types by race.
Strengths of the study include the large population-based sample and the diversity of the population. Due to a recent change of electronic pathology data systems, histologic outcomes are currently not accessible for this population. Reporting of ethnicity was limited or missing in the electronic record, and could not be evaluated. Our current study was limited to evaluating HPV positivity and partial genotyping using in women with ASC-US, not precancer or cancer. Although ASC-US is the most common epithelial abnormality in screening cytology, there is no direct histologic correlate. 11 Yet, analysis of ASC-US provides a window into this population as it is a representative sample of the full population. Approximately 11% of women with HPV-positive ASC-US are found to have CIN 2/3 on subsequent biopsy.12 Another limitation is that HPV vaccination status was not known. However, uptake of the HPV vaccine in MS is among the lowest in the nation and full impacts on genotype distributions in a screening population are not expected for many years.21 Furthermore, a similar association of lower HPV 16 in Blacks was found prior to widespread vaccination in the U.S. and in Africa.7, 17, 24
In conclusion, Black women had a significantly lower proportion of HR 16 and higher proportion of HR 12 among all HPV infections compared to White women. Identifying possible biological racial differences that contribute to lower HPV 16 and higher HR 12 prevalence in Blacks with ASC-US in this high-risk population requires further study, particularly additional genotyping of the HR 12 group. Studying associations with disease outcomes are crucial to evaluate whether HR 12 types are merely more common in this group, or whether these types have a higher risk of progression to precancer among Blacks. Observational cohort studies are needed to evaluate whether clinical guidelines are generalizable to Black women. Recent guidelines released by the United States Preventative Task Force (USPTF) advised that additional studies are needed in minority populations.25 To that end, subsequent studies in Mississippi are currently being conducted in collaboration with the National Cancer Institute.
Acknowledgments
Funding statement: This work was supported by a grant from the Mississippi Nurses Foundation (MNF), the University of Mississippi Medical Center School of Nursing and the Department of Pathology, the Mississippi Center for Translational Research Center - National Institute of General Medical Sciences of the National Institutes of Health under Award Number 1U54GM115428, the National Institute of Health Graduate Partnership Program.
Abbreviations:
- HPV
Human Papillomavirus
- ASC-US
Atypical Squamous Cells of Undetermined Significance
- HrHPV
High Risk HPV
- HR
High Risk
- HPV16
HPV genotype 16
- HPV18
HPV genotype 18
- HR12
high risk HPV 12 other genotypes
- MS
Mississippi
- UMMC
University of Mississippi Medical Center
- MSDH
Mississippi State Department of Health
Footnotes
Conflict of interest statement:Drs. Nicolas Wentzensen and Mark Schiffman are employed by the National Cancer Institute (NCI). NCI has received cervical cancer screening assays in-kind or at reduced cost from BD and Roche for studies that Drs. Wentzensen and Schiffman are working on. Drs. Carolann Risley, Megan Clarke, Kim Geisinger, Mary Stewart, Lei Zhang, Kim Hoover, Laree Hiser, Maria DeMarco and Kenyata Owens do not have any conflicts of interest to report.
References
- 1.Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nature reviews Disease primers. 2016;2:16086. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976;36 (2 pt 2):794. [PubMed] [Google Scholar]
- 3.IARC. Human Papillomaviruses. 2018; https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100B-11.pdf. Published 2007. Accessed December 28, 2019.
- 4.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018. February;47:2–13. doi: 10.1016 doi: 10.1016 [DOI] [PubMed] [Google Scholar]
- 6.Bruni LAG, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019.
- 7.Hariri S, Steinau M, Rinas A, et al. HPV genotypes in high grade cervical lesions and invasive cervical carcinoma as detected by two commercial DNA assays, North Carolina, 2001–2006. PLoS One. 2012;7(3):e34044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal AC, Smith JS, Valea F, et al. HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA. Cancer Causes Control. 2014;25(8):1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MSDH. Mississippi Cancer Registry. Cervical Mortality. 2014; http://www.cancer-rates.info/ms/index.php, Published 2017. Accessed December 28, 2019.
- 11.Geisinger KR. Modern Cytopathology. Churchill Livingstone; 2003. [Google Scholar]
- 12.Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Archives of pathology & laboratory medicine. 2003;127(8):946–949. [DOI] [PubMed] [Google Scholar]
- 13.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76 Suppl 1:S49–s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123(5):271–281. [DOI] [PubMed] [Google Scholar]
- 15.Roche. Cobas. 2018; https://diagnostics.roche.com/us/en/products/systems/cobas-4800-system.html. Updated December 28, 2019 Accessed December 28, 2019.
- 16.Gage JC, Hunt WC, Schiffman M, et al. Similar Risk Patterns After Cervical Screening in Two Large U.S. Populations: Implications for Clinical Guidelines. Obstet Gynecol. 2016;128(6):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–2359. [DOI] [PubMed] [Google Scholar]
- 18.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Africa. Summary Report 17 June 2019.
- 19.Demarco M, Carter-Pokras O, Hyun N, et al. Validation of a Human Papillomavirus (HPV) DNA Cervical Screening Test That Provides Expanded HPV Typing. Journal of clinical microbiology. 2018;56(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright TC Jr., Stoler MH, Parvu V, et al. Detection of Cervical Neoplasia by Human Papillomavirus Testing in an Atypical Squamous Cells-Undetermined Significance Population: Results of the Becton Dickinson Onclarity Trial. Am J Clin Pathol. 2018. [DOI] [PMC free article] [PubMed]
- 21.Schiffman M, Boyle S, Raine-Bennett T, et al. The Role of Human Papillomavirus Genotyping in Cervical Cancer Screening: A Large-Scale Evaluation of the cobas HPV Test. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffman M, Hyun N, Raine-Bennett TR, et al. A cohort study of cervical screening using partial HPV typing and cytology triage. International journal of cancer. 2016;139(11):2606–2615. [DOI] [PubMed] [Google Scholar]
- 23.Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2018. MMWR Morbidity and mortality weekly report. 2019;68(33):718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi LF, Kiviat NB, Hildesheim A, et al. Human Papillomavirus Type 16 and 18 Variants: Race-Related Distribution and Persistence. JNCI: Journal of the National Cancer Institute. 2006;98(15):1045–1052. [DOI] [PubMed] [Google Scholar]
- 25.Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Soulsby MA. . Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing: A Systematic Evidence Review for the U.S. Preventive Services Task Force Evidence Synthesis No. 158. AHRQ Publication No. 17–05231-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]


