Abstract
Bungarus multicinctus is the most venomous snake distributed in China and neighboring countries of Myanmar, Laos, north Vietnam and Thailand. The high mortality rate of B. multicinctus envenomation is attributed to the lethal components of α-, β-, γ- and κ- bungarotoxins contained in the venom. Although anti-B. multicinctus sera were produced in Shanghai, Taiwan and Vietnam, the most widely clinic used product was term as B. multicinctus antivenin and manufactured by Shanghai Serum Bio-technology Co. Ltd. In the present investigation, high purity α-, β- and γ-bungarotoxins were separately isolated from B. multicinctus crude venom. Rabbit anti- α-, β- and γ-bungarotoxin antisera were prepared by common methods, respectively. LD50 values of α-, β- and γ-bungarotoxins were systematically determined via three administration pathways (intraperitoneal, intramuscular and intravenous injections) in Kunming mice. LD50 values of β-bungarotoxin were closely related with injection routines but those of both α- and γ-bungarotoxins were not dependent on the injection routines. Commercial B. multicinctus antivenin showed strong immunoreaction with high molecular weight fractions of the B. multicinctus but weakly recognized low molecular weight fractions like α- and γ-bungarotoxins. Although B. multicinctus antivenin showed immunoreaction with high molecular weight fractions of Bungarus fasciatus, Naja atra, Ophiophagus hannah venoms but the antivenin only demonstrated animal protection efficacy against O. hannah venom. These results indicated that the high molecular weight fractions of the O. hannah played an important role in venom lethality but those of B. fasciatus and N. atra did not have such a role.
Author summary
Snakebite envenoming is an important public health problem around the world. Bungarus multicinctus envenomation is regarded as most dangerous animal bitten disease in China and neighboring countries. At present, the most widely clinic used B. multicinctus antisera was term as B. multicinctus antivenin and manufactured by Shanghai Serum Bio-technology Co. Ltd. By using high purity α-, β- and γ-bungarotoxins isolated from B. multicinctus crude venom and prepared corresponding rabbit anti- α-, β- and γ-bungarotoxin antisera, we evaluated the efficacy of B. multicinctus antivenin against the crude venom, isolated bungarotoxins, as well as other 8 medical important Chinese venomous snake venoms. B. multicinctus antivenin showed strong immunoreaction with high molecular weight fractions of the B. multicinctus venom but weakly recognized low molecular weight fractions like α- and γ-bungarotoxins. Moreover, B. multicinctus antivenin can neutralize Ophiophagus hannah venom but not B. fasciatus venom was demonstrated.
Introduction
The study of toxins and venomous animals has a long history, it affects human life and health [1]. Snakebite envenoming is an important public health problem around the world and was classified as category A neglected tropical disease in 2017 [2]. Recently, a 5 × 5 km resolution contemporary range maps covering global medical important 278 snake species were provided and the vulnerability to snakebite envenoming in subnational scale was given precisely. Combined with antivenom availability, accessibility to urban centers as well as healthcare access and quality index, authors identify the most vulnerable to snakebite morbidity and mortality populations. Unfortunately, China ranks the first in country level count of vulnerable people living within the range of one or more medically important venomous snake species and as many as 33,499,658 Chinese people affected nowadays [2]. It was reported that up to 2.7 million snakebites occur worldwide per year which were responsible for 81,000–138,000 deaths annually as well as 400,000 amputations and other permanent disabilities [2–4]. At present, national level snakebite and venomous snake envenomation epidemiology of mainland China is still largely unknown. A comparative comprehensive snakebite epidemiological survey of Guangxi Zhuang Autonomous Region, one of the provincial level snakebite hotspots in southern China, was carried out co-operatively by Guangxi Medical University and Japan Snake Institute. The investigation results indicated that 37,600 snakebites occurred and 1019 deaths each year in Guangxi around 1990s. Further analysis of the epidemiological data revealed that there were 993 snakebite cases happened in Guangxi in 1990. Farmers 715 cases (72%), students 100 cases (10.1%) and workers 84 cases (8.5%) were the most vulnerable victims. Only 62 cases received antivenom serum treatment at that time and totally 27 victims died from snake-bite out of the 993 cases were reported. Notably, 4 cases out of 62 antivenom serum treatment cases were eventually died because of delayed treatment, in which 3 cases were bitten by Bungarus multicinctus and 1 case by Bungarus fasciatus [5]. However, the number of snakebites might be underestimated because snakebites often occurred in remote, poor rural or mountainous areas that many cases of snakebite unreported.
There are more than 60 species of venomous snakes in China, of which 10 most harmful species are regarded as follows: B. multicinctus, B. fasciatus, Naja atra, Ophiophagus hannah, Deinagkistrodon acutus, Gloydius brevicaudus, Trimeresurus stejnegeri, Daboia russelii siamensis, Protobothrops mucrosquamatus and Laticauda colubrina [6]. Among the Chinese top ten venomous snakes, B. multicinctus represents the most venomous snake in mainland China. It was reported that B. multicinctus bites accounted for 8.12% of all snakebites in China but the lethality of B. multicinctus bite ranked the first [7]. B. multicinctus widely distributed in south China and neighboring countries of Myanmar, Laos, north Vietnam and Thailand globally. The high mortality rate of B. multicinctus envenomation is attributed to presynaptic neurotoxins and postsynaptic neurotoxins contained in the venoms, which can block neurotransmitter at motor nerve terminals and eventually leads to respiratory failure and death [8].
The high lethal effects of B. multicinctus are attributed to neurotoxins existed in the venom. B. multicinctus venom contains four different lethal neurotoxic components classified as α-bungarotoxin (α-BGT), β-bungarotoxin (β-BGT), γ-bungarotoxin (γ-BGT) and κ-bungarotoxin (κ-BGT). β-BGT is composed by a phospholipase A2 subunit and a kunitz-type protease inhibitor subunit linked by an S-S band. β-BGT acts as presynaptic toxin with phospholipase A2 subunit that is similar to most presynaptic neurotoxin while the kunitz-type protease inhibitor subunit plays a key role to guide the toxin to its target [9–11]. α-BGT, γ-BGT and κ-BGT act on postsynaptic membrane. α-BGT mainly interacts with α7 acetylcholine receptor and GABA receptor [12–13]. γ-BGT and κ-BGT act on M2 and α3β2 acetylcholine receptors, respectively. All of the three postsynaptic toxins can directly block the nerve transmission between neuromuscular junction [14–17].
In present investigation, we focus on the neutralization capacity evaluation of the commercial B. multicinctus antivenin produced in mainland China. Systematical LD50 values of the purified bungarotoxins via three administration routines in Kunming mice were also determined.
Materials and methods
Ethics statement
All experiments on animals meet the requirements of National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023) and has been reviewed and approved by Animal Care and Use Committee of Kunming Institute of Zoology, Chinese Academy of Sciences (Approval ID: SMKX-2017023).
Kunming mice (male, 20 ± 2 g) and rabbit (2.5 kg) were provided by Hunan SJA Laboratory Animal Co., Ltd. The animals were fed in animal house of Kunming Institute of Zoology, Chinese Academy of Sciences with a dark/photo period of 12 hours.
Venoms and antivenin
Pooled B. multicinctus venom was purchased from a snake farm in Zhejiang Province, China. Snake venoms of B. fasciatus, N. atra, O. hannah, D. acutus, T. stejnegeri, D. russelii siamensis, G. brevicaudus and P. mucrosquamatus were stocks of our lab at Kunming Institute of Zoology, the Chinese Academy of Sciences. All the used venoms are collected from corresponding adult snakes which are born and grow in snake farms. B. multicinctus antivenin was purchased from Shanghai Serum Bio-technology Company (Batch No. S10820179, Expire date: 16/03, 2020). Presently, only four kinds of monovalent antivenins are available in mainland China as follows: B. multicinctus (10000 U/vial), Naja atra (1000 IU/vial), G. brevicaudus (6000 U/vial) and D. acutus (2000 U/vial) antivenins. All of them are equine immune globulin F(abʹ)2 fragments digested by gastric enzymes and provided in liquid solutions (10 ml/vial). All of the 4 kinds of monovalent antivenins are produced by Shanghai Serum Bio-technology Co. Ltd, the sole anti-snake venom sera producer in mainland China (http://www.serum-china.com.cn/enprodetail/187.html).
Purification of α-, β- and γ-BGTs
Lyophilized B. multicinctus venom (250 mg) was dissolved in 2 ml 0.05 M PBS (pH 7.0) and centrifuged at 10,000 g for 20 min at 4°C. Then the supernatant was loaded onto a Superdex G75 gel filtration column (100×2.6 cm, XK 26, GE Health, USA) which was equilibrated with the same buffer and eluted at a flow rate of 1 ml/min on an Akta Purifier system (GE Healthcare, Uppsala, Sweden). For β-BGT purification, the peak IV of gel filtration was collected and dialyzed against large volume of 0.05 M sodium acetate-acetic acid buffer, pH 5.0. Then, the dialyzed sample was loaded onto a source 15S cationic ion-exchange chromatography column (16×2 cm, GE Health, USA) pre-equilibrated with the same buffer. Elution was carried out at a flow rate of 2 ml/min by a NaCl gradient. The target fraction was collected and further purified by the source 15S cationic ion-exchange chromatography column with 0.02 M phosphate buffer, pH 7.3. Finally, the target fraction collected from second ion-exchange step was freeze-dried and dissolved in Milli-Q water before being loaded onto a reverse C18 HPLC column (300×4.6 mm, Hypersil BDS, Elites, Daliang, China). The C18 column was pre-equilibrated with 0.1% (v/v) trifluoroacetic acid (TFA) and eluted with an acetonitrile (containing 0.1% TFA) gradient. For α-BGT and γ-BGT purification, the peak V of gel filtration was collected and treated the same as the first ion exchange step of β-BGT purification. High purity α-BGT and γ-BGT were isolated from peak II and peak IV of the ion exchange steps by a single HPLC step on the reverse C18 HPLC column, respectively.
Construction and expression of maltose binding protein fusion γ-BGT
The amount of γ-BGT contained in the B. multicinctus venom is too low to get enough toxin to immunize rabbits. Thus, a maltose binding protein (MBP) fusion γ-BGT was constructed and expressed in prokaryotic expression system to prepare γ-BGT antiserum. Briefly, the mature peptide sequence of γ-BGT was retrieved from UniProt (UniProt: Q9YGJ0). The synthetic gene coding for γ-BGT was optimized and inserted into the pMAL-p2X (using BamH I and Sac I) vectors [New England Biolabs (Beijing) Ltd., Beijing, China] by Shanghai Generay Biotech Co., Ltd (Shanghai, China). Finally, the MBP-γ-BGT expression plasmid was subcloned into Escherichia coli (TB1). The TB1 culture was shaken at 180 rpm, 37°C, IPTG was added at a final concentration of 0.5 mM when the OD600 of the culture medium reach 0.6. The cultures were grown for 24 h in shaking flasks at 180 rpm, 16°C. The recombinant MBP-γ-BGT protein was successfully expressed in the periplasm of TB1. The periplasmic fractions were extracted according to the protocols provided by the manufacturer and the fusion protein was purified by amylose affinity resin [New England Biolabs (Beijing) Ltd., Beijing, China].
Protein quantification
Protein quantification was determined by Pierce BCA assay kit (Thermo Scientific, Rockford, USA) using bovine serum albumin as standard, according to manufacturer’s instructions.
Mass spectrometry
The mass spectrometry analysis was performed according to the reported methods [18–19]. Briefly, the molecular weights of purified bungarotoxins were determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (AUTOFLEX III MALDI-TOF, Bruker Corporation, Germany). For de novo mass sequencing of internal peptides, purified neurotoxins were separately isolated by SDS-PAGE under non-reducing conditions. Then, the target bands were cut and digested by trypsin at 37°C. The detailed sample handling was the same as our recently reported methods [18]. Operation was carried out on positive ion mode using α-Cyano-4-hydroxycinnamic acid as matrix. The MS/MS spectrums were analyzed by Biotools software provided by the manufacturer and combined with manual annotation for MS/MS spectra interpretation.
SDS-PAGE and western blotting
SDS-PAGE was performed according to the method of Laemmli [20]. The gel was stained with Coomassie blue R-250 and de-stained with 10% acetic acid in 40:50 (v/v) water/ethanol solution. Different snake venoms were first run on a 15% SDS-PAGE gel under reducing or non-reducing conditions and then transferred onto a PVDF membrane (0.45 μm, Millipore). Then, each PVDF membrane was subsequently blocked with 5% nonfat milk in TBST (20 mM Tris-HCl, pH 7.4, contained 0.5% Tween-20) for 2 h at room temperature. The PVDF membrane was completely washed in TBST and incubated with antivenin or our prepared antiserum (1:5000 dilution) overnight at 4°C. The PVDF membrane was washed three times in TBST to remove the primary antibody and then incubated with 1:5000 dilution secondary antibody at room temperature for 2 h. Finally, the membrane was visualized by using SuperSignal West Pico Chemiluminescent Substrate Kit (34080, Thermo Scientific, IL, USA). Normal horse and rabbit IgG used for commercial B. multicinctus antivenin and prepared antiserum controls were products from Proteintech.
Median lethal dose (LD50) determination
The LD50 value was determined according to the method of Meier & Theakston [21]. Briefly, groups of 6 mice were used per dose for each sample and injected in a final volume of 200 μl through intravenous (i.v), intraperitoneal (i.p) and intramuscular (i.m) routines. The LD50 values were further accurately determined using different sample concentrations around the approximate LD50 for different routines within 24 hours after administration, respectively.
Rabbit specific antiserum preparation
The purified β-BGT, α-BGT and recombinant MBP-γ-BGT protein were separately used to immune rabbits according to common methods. Briefly, β-BGT and α-BGT were diluted to a final concentration of 2 mg/ml with PBS containing 0.4% (w/v) formaldehyde for detoxification and kept in dark for 7 days at 37°C until completely detoxified. Then, each detoxified toxin (1.5 ml) and recombinant MBP-γ-BGT protein (2 mg/ml, 1.5 ml) was separately mixed with an equal volume of Freund’s complete adjuvant and used for the first subcutaneous immunization on rabbits. After that, each sample was mixed with an equal volume of Freund’s incomplete adjuvant for subsequent subcutaneous immunization every 7 days, four immunizations were performed afterward. Finally, polyclonal antisera were purified from rabbit blood by a Protein A Sepharose column [New England Biolabs (Beijing) Ltd., Beijing, China], respectively [22]. Freund’s complete adjuvant and incomplete adjuvant used were products from Sigma-Aldrich (MO, USA).
Immunoreactivity study of commercial antivenin and prepared antisera
The immunoreactivity of antisera was defined as half of the maximum dilution factor at which the immunological binding can be observed [23]. Briefly, 100 μl of B. multicinctus venom, purified β-BGT, α-BGT and γ-BGT at a constant final concentration of 10 μg/ml were separately coated to a 96-well ELISA plate (Corning, USA) overnight at 4°C. After blocking by 3%BSA (B2064, Simga-Aldrich), different dilutions of specific antiserum (1:1,000~500,000) were added to the plate (100 μl/well) and incubated 2 h at 37°C. Then, 1:5000 diluted goat against horse the secondary antibody (for commercial antivenin) or goat against rabbit the secondary antiserum (for our prepared α-BGT, β-BGT and γ-BGT) were separately added to each well and incubated at 37°C for 1 h. All secondary antibodies were labeled with horseradish peroxidase (HRP) and bought from Proteintech (SA00001, USA). Finally, the absorbance at 450 nm was obtained on an Infinite Tecan M200 Pro micro-plate Reader (Austria) after adding HRP detecting substrates provided by Beyotime Biotechnology (P0209, China) according to manufacturer’s instructions.
Determination of 50% effective doses (ED50)
According to WHO recommendation, the neutralizing capability of the antivenom in our experiments was expressed as 50% effective doses (ED50) that the dose of antivenom required to protect 50% of mice when injected with 3LD50 of venom [24]. Briefly, mice were intraperitoneal injected with a mixture (200 μl) of different doses of rabbit antisera containing 3LD50 of B. multicinctus venom (n = 6/group), the mixture was incubated at 37°C for 30 min prior to inject. The mice were observed up to 48 h after injection. The ED50 values were calculated according to the Spearman-Karber equation as follows [25–27]:
Definition of term: log X100 = log dose giving 100% survival and having 100% survival for all higher doses. log FD = the log dilution factor. n = mice used at each dose level. t = mice alive at each dose level. Σ = the sum of mice surviving at every dose level. The weight of mice used in antivenin protection test is calculated as 20 g. ED50 values of antivenoms were expressed as mg of antivenoms per kg body weight of mouse to neutralize the challenge dose of venom.
Results
Purification and characterization of α-, β- and γ-BGTs
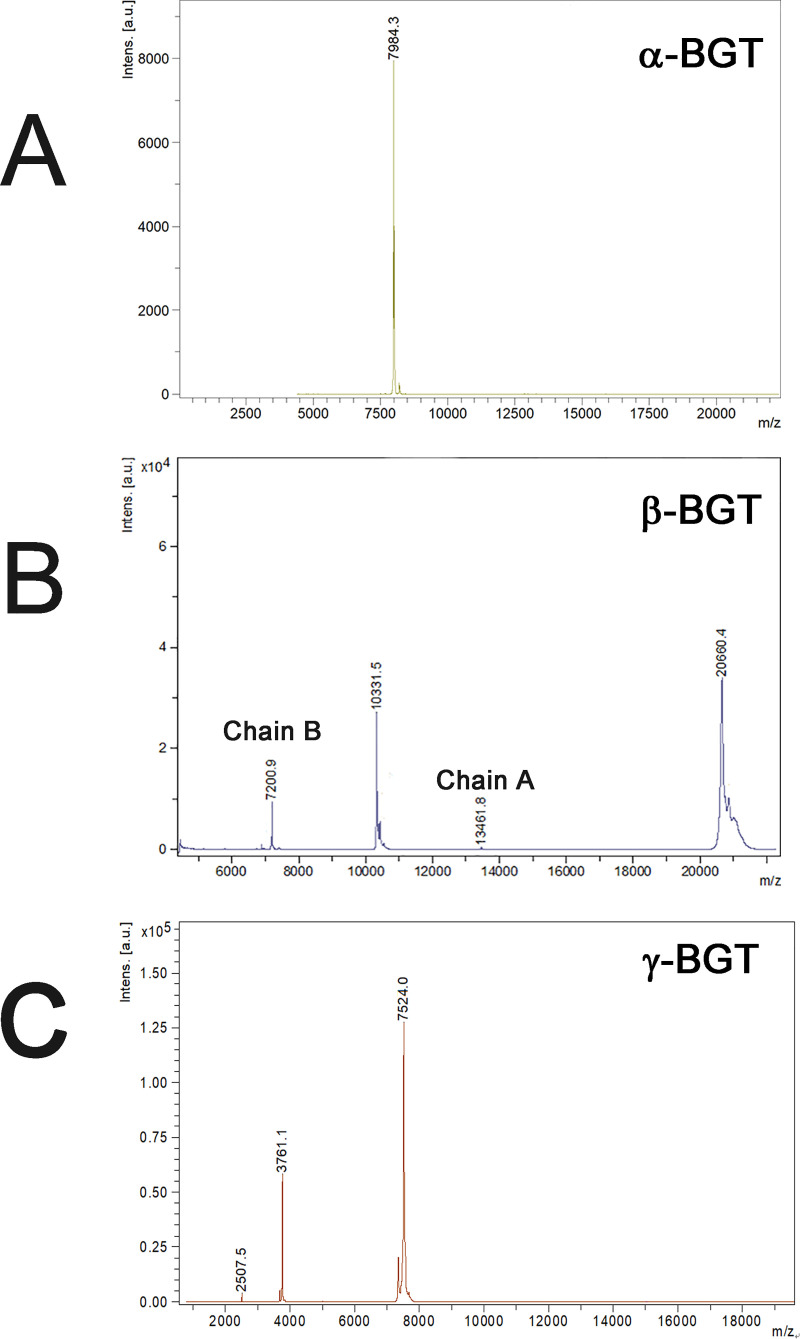
By a combination of gel filtration, ion exchange and HPLC procedures, high purity α-, β- and γ-BGTs were isolated from B. multicinctus venom. The amounts of the isolated α-, β- and γ- BGTs from 1 g of B. multicinctus venom were estimated to be about 30 mg, 10 mg and 0.3 mg, respectively (S1 Fig). β-BGTs were mainly existed in peak IV of gel filtration step as the elution peaks (I-VIII) of the first ion exchange of gel filtration peak IV (S1B Fig) all gave β-BGT signal in SDS-PAGE (S3 Fig). Finally, we isolated a high purity β-BGT by additional 2 purification steps (S1C and S1D Fig). Both α-BGT and γ-BGT existed in peak V of gel filtration step and could be easily purified by an ion exchange step followed by a HPLC step, respectively (S1E–S1G Fig). The determined amino acid sequences of purified bungarotoxins by de novo MS/MS sequencing match well with corresponding toxins found in UniProt database (S2A–S2C Fig). The determined masses of purified β-BGT, α-BGT and γ-BGT were 20660.4 Da, 7984.3 Da and 7524.0 Da, respectively (Fig 1). Meanwhile, 13461.8 Da and 7200.9 Da corresponding to A-chain and B-chain of purified β-BGT were also determined (Fig 1B). Combing molecular weight and de nove internal peptide sequence determinations, the purified α-BGT and γ-BGT are identical to UniProt database of P60615 and Q9YGJ0, respectively. However, only the kunitz-type protease inhibitor subunit identical to UniProt database of P00989 was found for purified β-BGT, the phospholipase A2 subunit could not match that of known identified β-BGTs but the de nove sequences of FGNSEYIEGHKNIDTAR and TIICYGAAGTCGR conserved in the known phospholipase A2 subunit of β-BGTs. Blast search results suggested that the beta-bungarotoxin A chain of our purified β-BGT best match that of UniProt P00617.
Fig 1. Molecular weight determination of purified bungarotoxins by MALDI/TOF mass spectrometer.
α-BGT (A). β-BGT (B). γ-BGT (C).
The recombinant MBP-γ-BGT protein was expressed in TB1 and successively purified from periplasmic fractions by an affinity column (S4 Fig). Approximately 50 mg MBP-γ-BGT protein can be obtained from 1 liter of the culture medium. The purified recombinant MBP-γ-BGT fusion protein had no lethal activity when injected i.v at high dose of 1 mg/mouse.
Median lethal dose (LD50) determination
The LD50 values of α-BGT, β-BGT and γ-BGT were determined to be 0.2 μg/g, 0.004 μg/g and 0.091 μg/g via i.p injections, 0.24 μg/g, 0.015 μg/g and 0.088 μg/g via i.m injections, 0.17 μg/g, 0.007 μg/g and 0.074 μg/g via i.v injections, respectively (Table 1). Table 1 also provided currently available LD50 data on bungarotoxins, a slight difference of the data might be caused by the test animal used [28–38].
Table 1. Summary of the available LD50 values of bungarotoxins via different administration routines.
| LD50 (μg/g) | Types of mice | Reference | ||||
|---|---|---|---|---|---|---|
| i.p | i.m | i.v | subcutaneous | |||
| B. multicinctus venom | 0.09 | - | - | - | Kunming mice | Present work |
| - | - | - | 0.16 | Mice (15–20 g) | Chang CC (1963) | |
| 0.08 | - | 0.071 | - | White mice (20–25 g) | Kocholaty WF (1971) | |
| - | - | 0.014 | - | ICR mice (20–25 g) | Ratanabanangkoon K (2016) | |
| α-BGT(A31) | 0.2 | 0.24 | 0.17 | - | Kunming mice | Present work |
| α-BGT | 0.23 | - | - | - | Outbreeding mice(18–20 g) | Kuch U (2003) |
| α-BGT | 0.14 | - | - | - | NIH strain(20–22 g) | Wu SH (1983) |
| α-BGT | - | - | - | 0.3 | Mice (15–20 g) | Chang CC (1963) |
| α-BGT | - | - | - | 0.14 | Swiss mice | Eterovic VA (1975) |
| α-BGT | 0.11 | - | - | - | Swiss mice | |
| α-BGT | 0.25 | - | - | - | Female ICR mice (20–30 g) | Crosland RD (1989) |
| β-BGT | 0.004 | 0.015 | 0.007 | - | Kunming mice | Present work |
| β-BGT | - | - | - | 0.089 | Mice (15–20 g) | Chang CC (1963) |
| β1-BGT | 0.019 | - | - | - | mice | Kondo K(1982) |
| β2-BGT | 0.028 | - | - | - | ||
| β3-BGT | 0.066 | - | - | - | ||
| β4-BGT | 0.072 | - | - | - | ||
| β5-BGT | 0.013 | - | - | - | ||
| SPI | 0.123 | - | - | - | Albino mice (20–25 g) | Chu CC (1994) |
| SPII | 0.043 | - | - | - | ||
| SPIII | 0.012 | - | - | - | ||
| β-BGT | - | - | 0.05 | - | Swiss mice (20–25 g) | Rosenberg P (1989) |
| β-BGT | 0.0097 | - | - | - | Female ICR mice (20–30 g) | Crosland RD (1989) |
| γ-BGT | 0.091 | 0.088 | 0.074 | - | Kunming mice | Present work |
| γ-BGT | - | - | 0.15 | - | Swiss mice | Aird SD (1999) |
| γ-BGT | - | - | - | 0.12 | Mice (15–20 g) | Chang CC (1963) |
| κ-BGT | - | - | - | - | ||
Abbreviations: α-BGT, α-bungarotoxin; β-BGT, β-bungarotoxin; γ-BGT, γ-bungarotoxin; κ-BGT, κ-bungarotoxin; SP I, SP II and SP III are different subtypes of β-bungarotoxin; “-”, no data available.
Immunoreactivity of bungarotoxin-specific antisera
Both prepared anti-β-BGT and anti-α-BGT antisera could well recognize crude venom and corresponding purified β-BGT or α-BGT, respectively. However, anti-γ-BGT antiserum could only recognize purified γ-BGT but not crude venom because the γ-BGT contained in the crude venom was too low to be detected (Fig 2B).
Fig 2. Specificity of three prepared antisera.
SDS-PAGE of B. multicinctus venom under non-reducing conditions, 25 μg/sample (A). Western-blot profile of prepared anti-α-BGT, anti-β-BGT and anti-γ-BGT antisera against crude venom and corresponding bungarotoxins (B). For Western-blot, 2 μg of crude venom or purified neurotoxins were added in each lane. Bm: Crude venom of B. multicinctus.
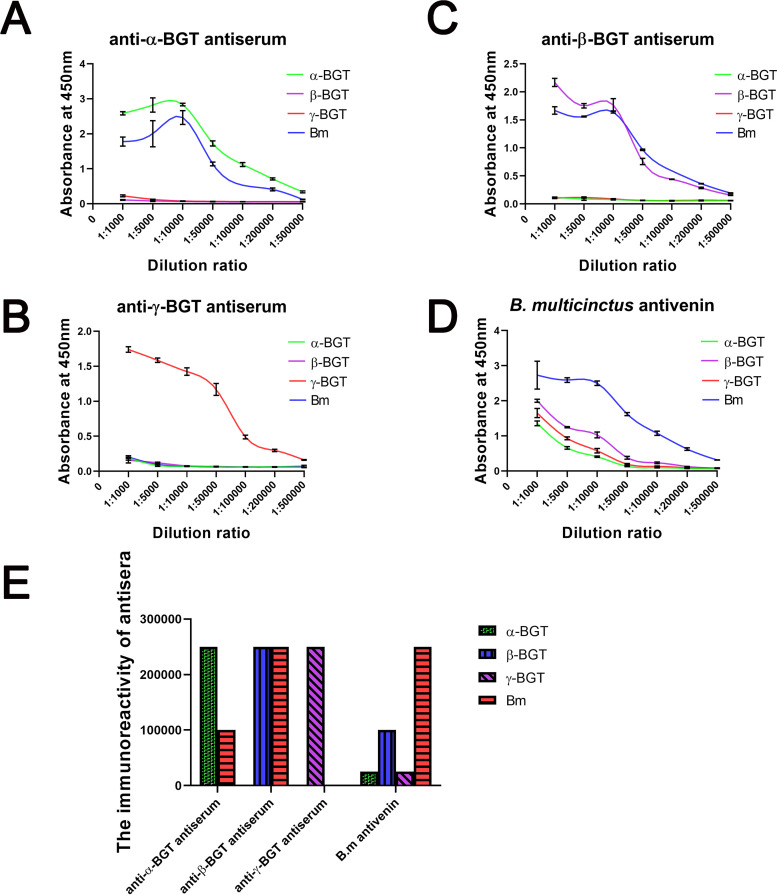
To further characterize the specificity of our prepared antisera, the absorbance at 450 nm of different antiserum against different antigens were determined by indirect ELISA experiments. A fixed concentration of 2 mg/ml was used for both antisera and B. multicinctus antivenin. The results revealed that the specificities of all the prepared rabbit antisera were quite well since no cross-reaction with other purified bungarotoxins was detected for each prepared antiserum (Fig 3A–3C). Again, present used commercial B. multicinctus antivenin showed better neutralizing capacity against crude venom, but obvious weak immunoreactivity against γ-BGT and α-BGT were found (Fig 3D). The immunoreactivity determination demonstrated that the prepared anti-α-BGT antiserum well recognized purified α-BGT but showed weak immunoreactivity against crude venom. Meanwhile, the prepared anti-β-BGT antiserum had the same immunoreactivity when immunobinding with B. multicinctus venom and purified β-BGT. Notably, the immunoreactivity of the commercial B. multicinctus antivenin against B. multicinctus venom was 2.5 times higher than that of immunobinding with purified β-BGT. B. multicinctus antivenin showed very weak immunoreactivity against purified α-BGT and γ-BGT (Fig 3E).
Fig 3. The immunoreactivity of antisera comparison by indirect ELISA.
Prepared anti-α-BGT antiserum against different antigens (A). Prepared anti-γ-BGT antiserum against different antigens (B). Prepared anti-β-BGT antiserum against different antigens (C). Commercial B. multicinctus antivenin against different antigens (D). The immunoreactivity determination of prepared antisera and the B. multicinctus antivenin (E). Antigens of 1μg/well were coated on a 96-well plate, results were expressed as mean ± SD.
Immunoreactivity of the commercial B. multicinctus antivenin and prepared antisera
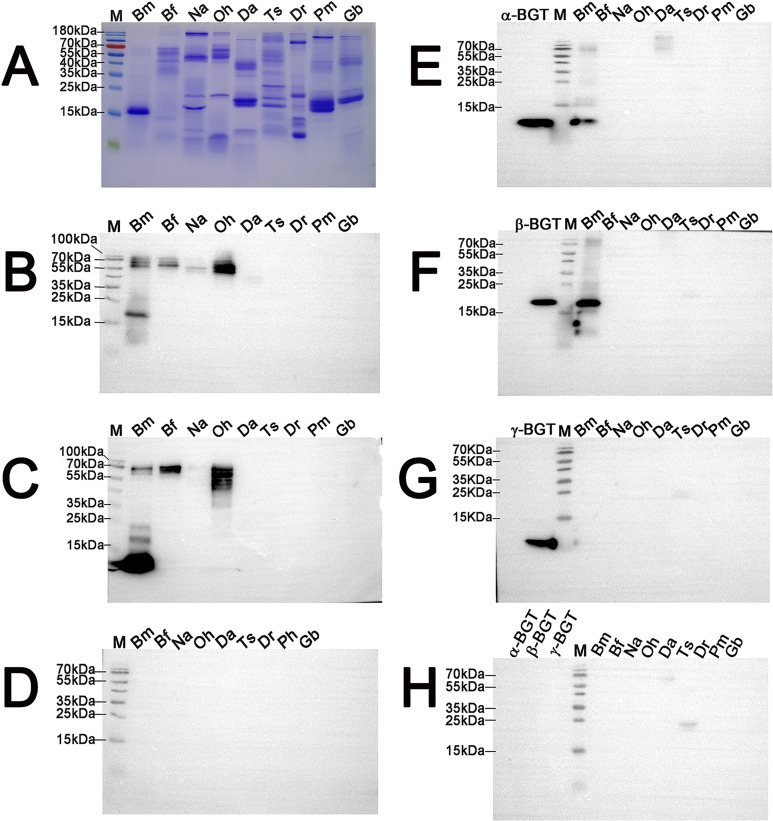
To investigate whether commercial B. multicinctus antivenin has immunological binding activities with other venomous terrestrial snake venoms, eight other dangerous Chinese snake venoms were used. SDS-PAGE stained with Coomassie blue under non-reducing conditions showed the natural profiles of proteins/peptides contained in the nine used snake venoms (Fig 4A). The immunoreactivity between venoms and commercial B. multicinctus antivenin had obvious difference under reducing or non-reducing conditions. High immunoreactivity existed in B. multicinctus high MW (molecule weights) bands (~55–100 kDa) and ~20 kDa β-BGT fractions; similar high MW bands (~ 55–100 kDa) also found in B. fasciatus but not 20 kDa fractions; a weak band of ~ 55 kDa exited in N. atra; strong immunoreactivity in high MW bands (~ 55–70 kDa) was found in O. hannah under non-reducing conditions, respectively (Fig 4B). However, high immunoreactivity existed in B. multicinctus low MW (13 kDa) corresponding to A-chain of β-BGT and high MW bands (~ 55–70 kDa); high MW bands (~55–70 kDa) still found in B. fasciatus; no signal in N. atra; strong immunoreactivity in high MW bands (~ 45–70 kDa) in O. hannah were observed under reducing conditions, respectively (Fig 4C). No immunoreactivity was detected for the B. multicinctus antivenin against other used snake venoms under reducing or non-reducing conditions (Fig 4B and 4C). Since anti-serum recognized antigens better under non-reducing conditions, the cross-neutralization evaluation of prepared antiserum was carried out under non-reducing conditions. The results indicated that the specificities of the prepared antiserum were quite well and no cross-reactions with other snake venoms were found (Fig 4E–4G).
Fig 4. Immunoreactivity of the commercial B. multicinctus antivenin and the prepared antiserum against top Chinese dangerous terrestrial snake venoms.
SDS-PAGE of nine used snake venoms under non-reducing conditions, 25 μg/sample (A). Western blot revealed by B. multicinctus antivenin under non-reducing conditions (B). Western blot revealed by B. multicinctus antivenin under reducing conditions (C). Western blot revealed by normal horse IgG under non-reducing conditions (D). Western blot revealed by prepared anti-α-BGT antiserum under non-reducing conditions (E). Western blot revealed by prepared anti-β-BGT antiserum under non-reducing conditions (F). Western blot revealed by prepared anti-γ-BGT antiserum under non-reducing conditions (G). Western blot revealed by normal rabbit IgG under non-reducing conditions (H). Bm: B. multicinctus; Bf: B. fasciatus; Na: N. atra; Oh: O. hannah; Da: D. acutus; Ts: T. stejnegeri; Dr: Daboia russelii siamensis; Pm: P. mucrosquamatus; Gb: G. brevicaudus. 10 μg/sample was loaded for Western blot experiments.
ED50 values of the commercial used B. multicinctus antivenin and prepared antisera
According to the determined LD50 values of the B. multicinctus venom and purified bungarotoxins, the protection capability (ED50) of each antiserum was separately calculated by injection with 3LD50 of different toxins via i.p injections (Table 2).
Table 2. ED50 determination of B. multicinctus antivenin and the prepared bungarotoxin antiserum against B. multicinctus venom and purified bungarotoxins.
| Antiserum | Toxins | LD50 (mg/kg) i.pa | Antiserum Dose (μg/mouse) | Number of mice (n = 6) | ED50a (mg/kg) | |
|---|---|---|---|---|---|---|
| Died | Lived | |||||
| B. multicinctus antivenin | B. multicinctus venom | 0.09 | 62.5 | 6 | 0 | 17.68 |
| 125 | 6 | 0 | ||||
| 250 | 6 | 0 | ||||
| 500 | 0 | 6 | ||||
| α-BGT | 0.2 | 1000 | 6 | 0 | 178.18 | |
| 2000 | 6 | 0 | ||||
| 4000 | 2 | 4 | ||||
| 8000 | 0 | 6 | ||||
| β-BGT | 0.004 | 2.5 | 6 | 0 | 0.5 | |
| 5 | 5 | 1 | ||||
| 10 | 4 | 2 | ||||
| 20 | 0 | 6 | ||||
| β-BGT antiserum | B. multicinctus venom | 0.09 | 62.5 | 6 | 0 | 12 |
| 125 | 6 | 0 | ||||
| 250 | 3 | 3 | ||||
| 500 | 0 | 6 | ||||
| β-BGT | 0.004 | 2.5 | 6 | 0 | 0.314 | |
| 5 | 4 | 2 | ||||
| 10 | 1 | 5 | ||||
| 20 | 0 | 6 | ||||
| α-BGT antiserum | α-BGT | 0.2 | 62.5 | 6 | 0 | 11.14 |
| 125 | 4 | 2 | ||||
| 250 | 4 | 2 | ||||
| 500 | 0 | 6 | ||||
| B. multicinctus venom | 0.09 | 4000 | 6 | 0 | >200 | |
| α-BGT antiserum plus β-BGT antiserum (1:1) | B. multicinctus venom | 0.09 | 62.5 | 6 | 0 | 17.68 |
| 125 | 6 | 0 | ||||
| 250 | 6 | 0 | ||||
| 500 | 0 | 6 | ||||
a ED50 values of antivenoms were expressed as mg of antivenoms per kg body weight of mouse to neutralize the challenge dose of venom.
Moreover, since commercial B. multicinctus antivenin also showed immunoreactivity against B. fasciatus, N. atra and O. hannah venoms, the ED50 values of B. multicinctus antivenin against these three Elapidae venoms were also determined (Table 3).
Table 3. The protective effects of antiserum against different snake venoms.
| antiserum | venoms | LD50 (mg/kg) i.pa | Antivenin Dose (μg/mouse) | Number of mice | ED50b (mg/kg) | |
|---|---|---|---|---|---|---|
| Died | Lived | |||||
| B. multicinctus antivenin | Bungarus fasciatus | 1.5 | 1000 | 6 | 0 | >800 |
| 2000 | 6 | 0 | ||||
| 8000 | 6 | 0 | ||||
| 16000 | 5 | 1 | ||||
| Naja atra | 0.5 | 1000 | 6 | 0 | >800 | |
| 2000 | 6 | 0 | ||||
| 8000 | 6 | 0 | ||||
| 16000 | 5 | 1 | ||||
| Ophiophagus hannah | 0.44 | 1000 | 6 | 0 | 400 | |
| 2000 | 6 | 0 | ||||
| 8000 | 3 | 3 | ||||
| 16000 | 0 | 6 | ||||
a LD50 values of the crude venoms were determined by present work in Kunming mice.
b ED50 values of antivenoms were expressed as mg of antivenoms per kg body weight of mouse to neutralize the challenge dose of venom.
Discussion
The first edition of “China Expert Consensus on the management of snake-bites” launched in 2018. The morbidity and mortality of snake-bite are still high in mainland China nowadays. Shortages of antivenom and overdependence on folk medicine to treat snake-bites are common in China, especially in rural areas and remote mountainous areas [39]. Snake species of B. multicinctus, B. fasciatus, N. atra, O. hannah, D. acutus, G. brevicaudus, T. stejnegeri, D. russelii siamensis, P. mucrosquamatus as well as sea snake of L. colubrina are listed as the top 10 venomous snakes in mainland China. Among them, B. multicinctus is regarded as the most virulent and deadly species [12]. According to recent snakebite epidemiological investigation in China, farmers still have been the largest proportion of snakebite victims (65%-68%) while the percentage of snake-dealers is about 2.4%-6.94% [40–43]. An act, issued by Chinese government on thoroughly banning the illegal trading of wildlife and eliminating the consumption of wild animals, has initiated since Feb. 24, 2020 in China. Consequently, the morbidity of bitten by venomous snakes might enhance in the near future since China is still an agricultural country. Thus, it is helpful to evaluate current available B. multicinctus antivenin against this most dangerous snake species in mainland China.
It is well known that lethal components responsible for the high morbidity and mortality of B. multicinctus envenomation are attributed to β-, α-, γ- and κ- BGTs contained in the venom, the latter three bungarotoxins all belong to three finger toxin (TFT) family [29,44]. Except γ-BGT, the rest three kinds of bungarotoxins all have various isoforms. High purity α-, β- and γ-BGTs isolated by currently reported methods greatly facilitate systemic LD50 assays and specific antiserum preparations. Unfortunately, κ-BGT was not purified in present work because the used crude venom might not contain this component, as mass determination of Superdex 75 gel filtration peak IV and peak V gave no κ-BGT signal (data not known). Interestingly, quantitative proteomic analysis as well as their components of B. multicinctus crude venoms were independently investigated by different research groups. β-BGT and TFT quantities contained in the crude venoms were reported to be 58.3%-32.6% (Guangxi, China) and 45%-28% (Vietnam), respectively [45–46]. However, γ-BGT was not detected in their used crude venoms. Taken together, β- and α- BGTs were constant contained in the B. multicinctus crude venoms, but γ- and κ-BGTs had the greatest variations in the crude venoms.
Previously, LD50 values of B. multicinctus crude venoms and isolated bungarotoxins were determined by different labs and most of them were carried out via single injection routines. For crude venom LD50 determination, a significant difference was observed by different groups via i.v injections (0.071 vs 0.014 μg/g). This difference might be caused by crude venom or experimental animals used. For isolated α-BGTs, the determined LD50 values varied weakly among different labs via different routines. However, the determined LD50 values of β-BGTs varied greatly. Notably, different β-BGTs showed obvious differences were found by different labs even using same tested animals (Table 1). Thus, using the same animals to evaluate toxins via different routine might be helpful to preciously explain the experimental results. Present work reported the systemic investigated the LD50 values of purified bungarotoxins via i.p, i.m and i.v injection routines in Kunming mice. Notably, administration routines did have effects on the lethal activity of bungarotoxins were first demonstrated. β-BGT had the highest lethal activity via i.p injection followed by i.v and i.m injections, respectively. Four times lethal activity difference existed in β-BGT via different injection routines. On the contrary, both α-BGT and γ-BGT showed the highest lethal activity via i.v injection. The lethal activity of both α-BGT and γ-BGT did not related to injection routines since the determined results were almost at the same levels for each of them. However, approximate 2–3 times high lethal activities of γ-BGT over α-BGT were found for different injection routines (Table 1). The calculated ED50 values of commercial B. multicinctus antivenin against B. multicinctus venom and α-BGT were 17.68 mg/kg and 178.18 mg/kg, respectively. ED50 value of prepared β-BGT antiserum against B. multicinctus venom was 12 mg/kg. ED50 value of prepared α-BGT antiserum against purified α-BGT was 11.14 mg/kg. Interestingly, ED50 value of equal quantity of prepared α-BGT and β-BGT antisera against B. multicinctus venom was identical to that of the clinic used B. multicinctus antivenin. Definitely, β-BGT is the highest lethal component of the crude venom, its ED50 values show 50 and 22.75 times via i.p, 150 and 5.86 times via i.m, 24 and 10 times stronger over α-BGT and γ-BGT, respectively (Table 2). Meanwhile, the ED50 value of B. multicinctus antivenin against O. hannah venom was determined to be 400 mg/kg, but maximal B. multicinctus antivenin used (original solution without dilution) could not protect B. fasciatus and N. atra envenomations and ED50 >800 mg/kg were given for these 2 venoms (Table 3).
At present, commercial anti-B. multicinctus serum was produced in mainland China (Shanghai), Taiwan and Vietnam [47]. However, WHO recommended B. multicinctus antivenom were produced by Shanghai and Taiwan (https://apps.who.int/bloodproducts/snakeantivenoms/database/). The B. multicinctus antivenom was termed as B. multicinctus antivenin by Shanghai Serum Bio-technology Company and is the only commercially available medicine in China (except Taiwan). B. multicinctus antivenin was monovalent F(abʹ)2 fragments prepared by traditional methods on horses and used in present works. To better evaluate the efficiency of B. multicinctus antivenin, anti-α-BGT antiserum and anti-β-BGT antiserum were prepared by using purified components as antigens in rabbits, anti-γ-BGT antiserum was prepared by using recombinant MBP-γ-BGT.
The best way to treat snakebite is to use specific antivenom in time. However, lacking of antivenoms or no specific antivenom available was common in clinic. This problem is very obvious in China since only 4 kinds of commercial antivenin available but approximately 60 venomous snake species existed. Thus, commercial antivenin is recommended to be used in clinic to treat snakebite patients of closely related snake species [48–49]. For example, “China Expert Consensus on the management of snake-bites” suggested using B. multicinctus antivenin to treat B. fasciatus victims, B. multicinctus plus N. atra antivenin to treat king cobra patients [39]. Thus, it’s necessary and helpful to evaluate the cross immunoreactivity of the available antivenom to prevent misuse of antivenoms in clinic. Present investigation evaluated immunoreactivity of commercial B. multicinctus antivenin and prepared antisera with eight other dangerous Chinese terrestrial snake venoms. The immunoreactivity between venoms and clinic B. multicinctus antivenin had obvious difference under reducing or non-reducing conditions. Surprisingly, current used B. multicinctus antivenin showed very strong immunoreactivity with high MW of B. multicinctus venom under non-reducing condition but very weak immunoreactivity with these fractions under reducing conditions. These results consistent with the conclusion that B. multicinctus antivenin well recognize crude venom but poorly recognize α-BGT, β-BGT and γ-BGT as depicted in Fig 1D. Previously, Gao et al evaluated the immunoreactivity between four venoms and commercial antivenoms produced by Shanghai Serum Bio-technology Company [50]. Comparing their western-blotting results of B. multicinctus antivenin against B. multicinctus crude venom with present results, a big difference existed in the immunoreactivity of B. multicinctus antivenin recognize B. multicinctus high MW fractions. The B. multicinctus antivenin used by Gao et al reacted strongly with β-BGTs but not high MW fractions under non-reducing conditions, which was contrary with our present results. The most possible reason might be caused by the B. multicinctus antivenin used by Gao et al was further purified by a HiTrap Protein G column or caused by the used crude venoms in producing of different batches of the antivenins. Although B. multicinctus antivenin could recognize high MW of B. fasciatus venom both under non-reducing and reducing conditions, these fractions were not the lethal components of the venom and ED50 >800 mg/kg demonstrating this conclusion. B. multicinctus antivenin showed very weak reaction with high MW of N. atra venom under non-reducing conditions but weak signals were seen under reducing conditions. However, B. multicinctus antivenin showed strong immunoreactivity with high MW of O. hannah venom both under non-reducing and reducing conditions. Since no anti-king cobra venom antiserum available in China, using single N. atra antivenin / B. multicinctus antivenin or combining both antivenins to treat O. hannah envenomation was recommended by China Expert Consensus on the management of snake-bites and has practiced in clinic for many decades in China [39]. Thus, present results strongly indicated that high MW of O. hannah venom might contribute in the lethal activity of the venom. Furthermore, our previously reported L-amino acid oxidase [51] and blood coagulation factor X activator [52] represented the high MW of O. hannah venom components that could be reacted with commercial B. multicinctus antivenin were determined. The L-amino acid oxidase and blood coagulation factor X activator are identical to UniProt database of P81383 and A3R0T9 respectively by de novo MS/MS sequencing (S5 Fig).
Conclusions
In accordance with previous results that β-BGTs are the most lethal and abundant components contained in the B. multicinctus venom. The determined ED50 values of prepared β-BGT antiserum and commercial B. multicinctus antivenin against B. multicinctus venom demonstrated that prepared β-BGT antiserum showed a better animal protection efficiency over commercial B. multicinctus antivenin (12 mg/kg vs 17.68 mg/kg). Our present results suggested that it might be feasible for commercial B. multicinctus antivenin producer to add a single gel filtration step and us both β-BGT and α-BGT fractions to produce the B. multicinctus antivenin to largely avoid unnecessary high MW reaction antibodies (Figs 4B and S1). The LD50 values of purified α-, β- and γ-BGTs via i.p, i.m and i.v injection routines were systematically determined in Kunming mice. Administration routines did have effects on the lethal activity of different bungarotoxins were first revealed. Importantly, the recommended using B. multicinctus antivenin to treat king cobra victims was supported but using B. multicinctus antivenin to treat B. fasciatus envenomation in clinic practice in China was not supported by present evidences.
Supporting information
The purification process of β-bungarotoxin (A-D). The purification process of α-bungarotoxin (A, E, F). The purification process of γ-bungarotoxin (A, E, G). The buffer used in the experiments were PBS (A), sodium acetate-acetate buffer solution (0.05M, pH 5.0) (B, E), phosphate buffer solution (0.02M, pH 7.3) (C).
(TIF)
The bungarotoxins were digested by trypsin. The MS/MS data of α-BGT (A). The MS/MS data of β-BGT (B). The MS/MS data of γ-BGT (C). Mass tolerance for MS/MS ions spectrum was±0.5 Da.
(TIF)
SDS-PAGE under non-reducing conditions, 1.5 μg/sample (A). Western blot revealed by prepared anti-β-BGT antiserum under non-reducing conditions, 1.5 μg/sample (B).
(TIF)
M: Molecular marker. 1: Whole lysate of uninduced cells. 2: Whole lysate of IPTG induced cells. 3: Periplasmic extract. 4: Purified proteins from periplasmic extracts by amylose affinity column eluted with maltose.
(TIF)
SDS-PAGE of O. hannah venom under non-reducing conditions, 25 μg/sample (A). Western-blot profile of commercial B. multicinctus antivenin against O. hannah venom (B). The MS/MS data of L-amino acid oxidase and blood coagulation factor X activator (C). Mass tolerance for MS/MS ions spectrum was±0.5 Da.
(TIF)
Acknowledgments
We would like to thank Dr. Lin Zeng from the Public Technology Service Center, Kunming Institute of Zoology, Chinese Academy of Sciences for her help in mass spectrometry determination and analysis.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by Science and Technology Department of Yunnan Province (2019ZF003, 202003AD150009) to WHL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhang Y. Why do we study animal toxins? Zoological Research. 2015;36(4):183–222. 10.13918/j.issn.2095-8137.2015.4.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longbottom J, Shearer FM, Devine M, Alcoba G, Chappuis F, Weiss DJ, et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392(10148):673–84. 10.1016/S0140-6736(18)31224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:17063 10.1038/nrdp.2017.63 [DOI] [PubMed] [Google Scholar]
- 4.Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl Trop Dis. 2019;13(2):e0007059 10.1371/journal.pntd.0007059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naiping Wang, Qibin Li, Bingyi Li, Zhaoyan Li, Huaipeng Li, Shengxi Tang, et al. An epidemiological study on the snakebites in GuangXi province, China in 1990. Journal of Snake. 1993;5(1):10–7. [Google Scholar]
- 6.Qin GP. China poisonous snake research. Guangxi Science and Technology Press, Nanning, China: 1988. [Google Scholar]
- 7.Li hong-jian, Li gui-min, Li pei-rong, Zhao wen. Treatment and research progress of Bungarus multicinctus bites (in Chinese). Journal of Snake. 2007;19(3):205–8. [Google Scholar]
- 8.Mao YC, Liu PY, Chiang LC, Liao SC, Su HY, Hsieh SY, et al. Bungarus multicinctus multicinctus Snakebite in Taiwan. Am J Trop Med Hyg. 2017;96(6):1497–504. 10.4269/ajtmh.17-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigoni M, Paoli M, Milanesi E, Caccin P, Rasola A, Bernardi P, et al. Snake phospholipase A2 neurotoxins enter neurons, bind specifically to mitochondria, and open their transition pores. J Biol Chem. 2008;283(49):34013–20. 10.1074/jbc.M803243200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetto O, Montecucco C. Presynaptic neurotoxins with enzymatic activities. Handb Exp Pharmacol. 2008(184):129–70. 10.1007/978-3-540-74805-2_6 [DOI] [PubMed] [Google Scholar]
- 11.Rowan EG. What does beta-bungarotoxin do at the neuromuscular junction? Toxicon. 2001;39(1):107–18. 10.1016/s0041-0101(00)00159-8 [DOI] [PubMed] [Google Scholar]
- 12.Nirthanan S. Snake three-finger alpha-neurotoxins and nicotinic acetylcholine receptors: molecules, mechanisms and medicine. Biochem Pharmacol. 2020:114168 10.1016/j.bcp.2020.114168 [DOI] [PubMed] [Google Scholar]
- 13.McCann CM, Bracamontes J, Steinbach JH, Sanes JR. The cholinergic antagonist alpha-bungarotoxin also binds and blocks a subset of GABA receptors. Proc Natl Acad Sci U S A. 2006;103(13):5149–54. 10.1073/pnas.0600847103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang LS, Chung C, Wu BN, Yang CC. Characterization and gene organization of Taiwan banded krait (Bungarus multicinctus) gamma-bungarotoxin. J Protein Chem. 2002;21(4):223–9. 10.1023/a:1019760401692 [DOI] [PubMed] [Google Scholar]
- 15.Duerrschmidt N, Hagen A, Gaertner C, Wermke A, Nowicki M, Spanel-Borowski K, et al. Nicotine effects on human endothelial intercellular communication via alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(6):621–32. 10.1007/s00210-012-0738-y [DOI] [PubMed] [Google Scholar]
- 16.Barber CM, Isbister GK, Hodgson WC. Alpha neurotoxins. Toxicon. 2013;66:47–58. 10.1016/j.toxicon.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 17.daCosta CJ, Free CR, Sine SM. Stoichiometry for alpha-bungarotoxin block of alpha7 acetylcholine receptors. Nat Commun. 2015;6:8057 10.1038/ncomms9057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Bian X, Zeng L, Pan F, Liu L, Liang J, et al. A cellular endolysosome-modulating pore-forming protein from a toad is negatively regulated by its paralog under oxidizing conditions. J Biol Chem. 2020;295(30):10293–306. 10.1074/jbc.RA120.013556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KY, Tan CH, Tan KY, Quraishi NH, Tan NH. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J Proteomics. 2018;175:156–73. 10.1016/j.jprot.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 21.Meier J, Theakston RD. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 1986;24(4):395–401. 10.1016/0041-0101(86)90199-6 [DOI] [PubMed] [Google Scholar]
- 22.Grodzki AC, Berenstein E. Antibody purification: affinity chromatography—protein A and protein G Sepharose. Methods Mol Biol. 2010;588:33–41. 10.1007/978-1-59745-324-0_5 [DOI] [PubMed] [Google Scholar]
- 23.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200. 10.1016/j.vaccine.2007.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. 2016. [DOI] [PubMed] [Google Scholar]
- 25.Progress in the characterization of venoms and standardization of antivenoms. WHO Offset Publ. 1981(58):1–44. [PubMed] [Google Scholar]
- 26.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber Method for Estimating Median Lethal Concentrations in Toxicity Bioassays. Environ Sci Technol. 1977;11(7):714–9. [Google Scholar]
- 27.Wang Y, Zhang J, Zhang D, Xiao H, Xiong S, Huang C. Exploration of the Inhibitory Potential of Varespladib for Snakebite Envenomation. Molecules. 2018;23(2). 10.3390/molecules23020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratanabanangkoon K, Tan KY, Eursakun S, Tan CH, Simsiriwong P, Pamornsakda T, et al. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl Trop Dis. 2016;10(4):e0004565 10.1371/journal.pntd.0004565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CC, Lee CY. Isolation of neurotoxins from the venom of Bungarus Multicinctus and their modes of neuromuscular blocking action. Arch Int Pharmacodyn Ther. 1963;144:241–57. [PubMed] [Google Scholar]
- 30.Kocholaty WF, Ledford EB, Daly JG, Billings TA. Toxicity and some enzymatic properties and activities in the venoms of Crotalidae, Elapidae and Viperidae. Toxicon. 1971;9(2):131–8. 10.1016/0041-0101(71)90006-7 [DOI] [PubMed] [Google Scholar]
- 31.Kuch U, Molles BE, Omori-Satoh T, Chanhome L, Samejima Y, Mebs D. Identification of alpha-bungarotoxin (A31) as the major postsynaptic neurotoxin, and complete nucleotide identity of a genomic DNA of Bungarus candidus from Java with exons of the Bungarus multicinctus alpha-bungarotoxin (A31) gene. Toxicon. 2003;42(4):381–90. 10.1016/s0041-0101(03)00168-5 [DOI] [PubMed] [Google Scholar]
- 32.Wu SH, Chen CJ, Tseng MJ, Wang KT. The modification of alpha-bungarotoxin by digestion with trypsin. Arch Biochem Biophys. 1983;227(1):111–7. 10.1016/0003-9861(83)90353-3 [DOI] [PubMed] [Google Scholar]
- 33.Eterovic VA, Hebert MS, Hanley MR, Bennett EL. The lethality and spectroscopic properties of toxins from Bungarus multicinctus (Blyth) venom. Toxicon. 1975;13(1):37–48. 10.1016/0041-0101(75)90156-7 [DOI] [PubMed] [Google Scholar]
- 34.Crosland RD. Effect of chlorpromazine and quinacrine on the lethality in mice of the venoms and neurotoxins from several snakes. Toxicon. 1989;27(6):655–63. 10.1016/0041-0101(89)90016-0 [DOI] [PubMed] [Google Scholar]
- 35.Kondo K, Toda H, Narita K, Lee CY. Amino acid sequences of three beta-bungarotoxins (beta 3-, beta 4-, and beta 5- bungarotoxins) from Bungarus multicinctus venom. Amino acid substitutions in the A chains. J Biochem. 1982;91(5):1531–48. 10.1093/oxfordjournals.jbchem.a133844 [DOI] [PubMed] [Google Scholar]
- 36.Chu CC, Chu ST, Chen SW, Chen YH. The non-phospholipase A2 subunit of beta-bungarotoxin plays an important role in the phospholipase A2-independent neurotoxic effect: characterization of three isotoxins with a common phospholipase A2 subunit. Biochem J. 1994;303 (Pt 1):171–6. 10.1042/bj3030171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg P, Ghassemi A, Condrea E, Dhillon D, Yang CC. Do chemical modifications dissociate between the enzymatic and pharmacological activities of beta bungarotoxin and notexin? Toxicon. 1989;27(2):137–59. 10.1016/0041-0101(89)90128-1 [DOI] [PubMed] [Google Scholar]
- 38.Aird SD, Womble GC, Yates JR 3rd, Griffin PR. Primary structure of gamma-bungarotoxin, a new postsynaptic neurotoxin from venom of Bungarus multicinctus. Toxicon. 1999;37(4):609–25. 10.1016/s0041-0101(98)00199-8 [DOI] [PubMed] [Google Scholar]
- 39.Consensus group of Chinese experts on snake-bites rescue and treatment. 2018 China expert consensus on the management of snake-bites. Chinese Journal of Emergency Medicine. 2018;27(12):1315–22. [Google Scholar]
- 40.Juanjuan Mao. Epidemiological characteristics of 692 cases of venomous snakebites in Ningbo area (Chinese). mordern practical medicine. 2020;32(02):270–1+80. [Google Scholar]
- 41.Yanai Liang, Rongde Tang, Yue Zhang, Jiacheng Y. Clinical materials analysis on 1139 cases 0f venomous snake bite patients (in Chinese). Chinese Medical Record. 2017;18(01):104–7. [Google Scholar]
- 42.Zhong-zhi Tang, Su-hua Shao, Dan-ting Fei, Qing Cheng, Jie Liu, Qing Fang, et al. Epidemiological analysis of 1080 snakebites cases (in Chinese). Military Medical Journal of South China 2017;031(006):363–7. [Google Scholar]
- 43.Yu Chen, Dong-lin Z. Epidemiological analysis of venomous snake bite in Pingxiang Area. Journal of Snake. 2018. [Google Scholar]
- 44.Nirthanan S, Gwee MC. Three-finger alpha-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J Pharmacol Sci. 2004;94(1):1–17. 10.1254/jphs.94.1 [DOI] [PubMed] [Google Scholar]
- 45.Shan LL, Gao JF, Zhang YX, Shen SS, He Y, Wang J, et al. Proteomic characterization and comparison of venoms from two elapid snakes (Bungarus multicinctus and Naja atra) from China. J Proteomics. 2016;138:83–94. 10.1016/j.jprot.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 46.Ziganshin RH, Kovalchuk SI, Arapidi GP, Starkov VG, Hoang AN, Thi Nguyen TT, et al. Quantitative proteomic analysis of Vietnamese krait venoms: Neurotoxins are the major components in Bungarus multicinctus and phospholipases A2 in Bungarus fasciatus. Toxicon. 2015;107(Pt B):197–209. 10.1016/j.toxicon.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 47.Ha TH, Hojer J, Trinh XK, Nguyen TD. A controlled clinical trial of a novel antivenom in patients envenomed by Bungarus multicinctus. J Med Toxicol. 2010;6(4):393–7. 10.1007/s13181-010-0051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Qing sheng, Shao H, Liu Z. Combination of naja naja antivenin and bugarus multicinctus antivenin in the treatment of ophiophagus hannah bite(in Chinese). Chinese Critical Care Medicine. 2000;12:76–9. [Google Scholar]
- 49.Jie Zhao, Qi-hong S. Observation on the effect of Agkistrodon acutus antivenin combined with hormone in the treatment of blood circulation toxins (in Chinese). Journal of clinical medical literature 2018;5(38):173. [Google Scholar]
- 50.Gao JF, Wang J, Qu YF, Ma XM, Ji X. Immunoreactivity between venoms and commercial antiserums in four Chinese snakes and venom identification by species-specific antibody. J Immunol Methods. 2013;387(1–2):211–8. 10.1016/j.jim.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 51.Jin Y, Lee WH, Zeng L, Zhang Y. Molecular characterization of L-amino acid oxidase from king cobra venom. Toxicon. 2007;50(4):479–89. 10.1016/j.toxicon.2007.04.013 [DOI] [PubMed] [Google Scholar]
- 52.Lee WH, Zhang Y, Wang WY, Xiong YL, Gao R. Isolation and properties of a blood coagulation factor X activator from the venom of king cobra (Ophiophagus hannah). Toxicon. 1995;33(10):1263–76. 10.1016/0041-0101(95)00077-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The purification process of β-bungarotoxin (A-D). The purification process of α-bungarotoxin (A, E, F). The purification process of γ-bungarotoxin (A, E, G). The buffer used in the experiments were PBS (A), sodium acetate-acetate buffer solution (0.05M, pH 5.0) (B, E), phosphate buffer solution (0.02M, pH 7.3) (C).
(TIF)
The bungarotoxins were digested by trypsin. The MS/MS data of α-BGT (A). The MS/MS data of β-BGT (B). The MS/MS data of γ-BGT (C). Mass tolerance for MS/MS ions spectrum was±0.5 Da.
(TIF)
SDS-PAGE under non-reducing conditions, 1.5 μg/sample (A). Western blot revealed by prepared anti-β-BGT antiserum under non-reducing conditions, 1.5 μg/sample (B).
(TIF)
M: Molecular marker. 1: Whole lysate of uninduced cells. 2: Whole lysate of IPTG induced cells. 3: Periplasmic extract. 4: Purified proteins from periplasmic extracts by amylose affinity column eluted with maltose.
(TIF)
SDS-PAGE of O. hannah venom under non-reducing conditions, 25 μg/sample (A). Western-blot profile of commercial B. multicinctus antivenin against O. hannah venom (B). The MS/MS data of L-amino acid oxidase and blood coagulation factor X activator (C). Mass tolerance for MS/MS ions spectrum was±0.5 Da.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.