Abstract
Autologous Chimeric Antigen Receptor (CAR) T cell manufacturing involves the modification and expansion of T cells obtained by apheresis collection from a patient. The mechanism of apheresis collection and the specific clinical features seen in these patients combine to generate apheresis products with high variability of content. Manufacturers often attempt to minimize this variability such that processes can be standardize in accordance with Good Manufacturing Practices (GMP). Such standardization improves efficiency and helps to ensure robustness of the overall process. Apheresis product variability can negatively impact T cell manufacturing success. Patient and collection driven variability often leads to non-T cells entering the apheresis product. Many of these cells can directly or indirectly impair T cell activation and expansion, decreasing the manufacturing success rate. Therefore, patient driven variability observed in apheresis products, must be mitigated through downstream processing. T cell enrichment is one step in the manufacturing cycle that can reduce process variability by generating more uniform downstream material. However, current T cell enrichment methods have limitations. Much of this type of variability can be avoided by collecting patients earlier in their disease or treatment course, this is not current, widespread or standard practice. While variability poses challenges to successful CAR T cell manufacturing and mitigation strategies can be successful, more work is needed in this area.
Lay summary
Apheresis collection is a method of obtaining large numbers of cells to start the CAR T cell manufacturing process. Because patients are highly variable in their presentation, some with more or less cells in their blood, the apheresis product will reflect this with more or less cells in the bag. This poses problems with the manufacturer who wants to perform uniform processing. There are ways to combat this variability, however, current techniques are limited and additional work is needed to develop better ways to do this. Collecting patients earlier may improve this, but it is not standard practice to date.
1. Introduction
There is an inherent tension in academic cell manufacturing. A core tenet of industrial sciences is the standardization of manufacturing processes. This standardization enables greater efficiency and reproducibility. When successful, this approach ultimately generates a robust process for scaled-up and scaled-out manufacturing. This type of standardization is so crucial, that many Good Manufacturing Practices (GMP) focus on standardized process to meet quality metrics. Cell biology, on the other hand, is often highly variable with different populations of cells behaving in unexpected and unpredictable ways. This is particularly the case when working with primary cells from human donors. In many cases, these cells act according to acquired or inherent differences. Often the root of these differences is genetic, but just as frequently the etiology is unknown. The tension between industrial level standardization and the observed variability in cell biology is particularly challenging to academic cell manufacturing facilities. Often, such facilities simultaneously support different protocols, using source materials, and obtained from different patient populations. Further, academic facilities are more likely to manufacture for early phase clinical trials in which the manufacturing feasibility of a particular therapeutic cell type from a specific subject population has yet to be established. To maximize the chance of manufacturing and clinical success, academic facilities must carefully observe and document the variability that they encounter in different patient populations. Once described in detail, this variability can serve as a roadmap for process improvement, allowing targeted process modifications to better standardize the overall manufacturing cycle. As manufacturing of CAR T and other engineered cell therapies rapidly expands as a field, academic cell manufacturing facilities will benefit from collaborative efforts to share and learn from this variability. Here I describe the sources, consequences and mitigation strategies of variability in the autologous cell manufacturing cycle.
2. Upstream sources of variability in the autologous cell manufacturing cycle
The autologous cell manufacturing cycle most often begins with apheresis collection of the patient. These patients are highly variable in their clinical presentations at the time of collection. Further, the performance of the apheresis collection itself can differ from patient to patient. Both patient-to-patient and collection-to-collection variability can contribute to the diversity seen in the resulting apheresis product. As the primary source of starting material for cell manufacturing, apheresis product variability is highly impactful to downstream processes.
It is primarily due to the mechanism of apheresis collection that patient-to-patient viability is directly reflected in the product itself. In brief, apheresis collection begins with obtaining peripheral or central vascular access. Whole blood flows from the patient, through a sterile tubing set into the apheresis machine where it is mixed with an anti-coagulant, typically citrate. The anticoagulated blood continues to flow through the tubing, which is seated in a rotated ring. During rotation, blood components separate based on density. The separation of the plasma layer from the white blood cell and red blood cell layers can be detected by digital optical sensors, included in many current generation apheresis machines. In a continuous or semi-continuous fashion, the target cell layer is identified and collected in a separate bag. For the purposes of cellular immunotherapy manufacturing, apheresis collection targets the mononuclear cell (MNC) layer. This layer includes cells with specific gravities around 1.060–1.070[1]. Notable cell types that may be obtained in MNC products include T lymphocytes, B lymphocytes, Monocytes, Basophils, Promyelocytes, Myelocytes, Metamyelocytes and Reticulocytes. Non-target cells and plasma are returned to the patient along with the anti-coagulant. However, if flow rates fluctuate significantly during the collection, the layer being collected may drift to include non-target cells such as granulocytes, red cells or platelets. Because extra-corporeal blood volumes are low [2], apheresis collection as opposed to whole blood donation, uniquely allows for multiple blood volumes to be processed in a single procedure. In sum, apheresis collection is the most reliable method for obtaining large numbers of cells without putting patients at serious risk from depletion of non-target cells.
a. Pre-collection source of variability
Autologous apheresis isolates and collects cells in the peripheral blood with certain specific gravities. Therefore, the collection will only ever be able to obtain cells that are in the peripheral blood at the moment of the procedure. Many factors have been described that influence the relative or absolute frequency of circulating cell types in the peripheral blood. Therefore, any of these factors can also impact the MNC product content and downstream manufacturing. Few studies have been published on peripheral blood and MNC content diversity in cellular immunotherapy patients to date [3]. Nonetheless, the available data and expert consensus have identified several potential pre-collection sources of MNC product variability. These drivers of variation include patient demographics, underlying clinical indication, and prior treatment. At the time of collection, these features are immutable, and therefore the most effective strategies to combat these sources of variability ought to be employed early in the patient’s disease and treatment course.
It is believed that increased frequency of memory, memory-like or naïve T cells in the MNC product may be associated with improved manufacturing and clinical outcomes for autologous engineered T cell patients. High frequencies of certain T cell subsets with memory-like features in MNC products have already been shown to be associated with sustained remissions [4]. Further, it has been shown in vitro that primary CD8+ T cell responses decline with age [5]. Many other changes also occur in the immune system and T cell compartment with age [6]. On the other hand, in the very young, there are practical difficulties in obtaining adequate T cells as well as possible inherent T cell growth defects. Taken together, it is likely age is a demographic factor that may be a source of variability seen in apheresis products.
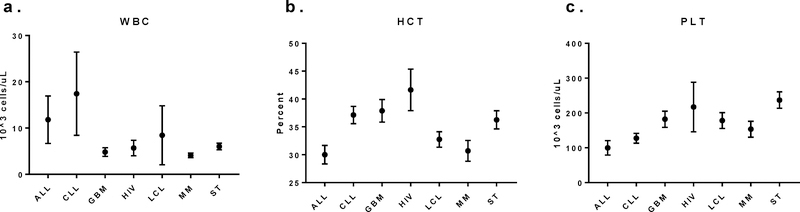
Clinical indication also impacts apheresis product variability. Differences among absolute and relative peripheral blood cell counts in cellular immunotherapy patients with different indications have been observed. At our institution complete blood counts (CBC) with differential are routinely performed on patients on the day of collection on the standard clinical hematology analyzer. While other testing may be intermittently performed on these patients, we do not consistently gather these data to date. CBC data, however, were available for 298 unique collections from cellular immunotherapy patients with a variety of underlying indications (Fig 1). CBCs were obtained from 62 patients with acute lymphoblastic leukemia (ALL); 62 patients with chronic lymphocytic leukemia (CLL), 15 patients with glioblastoma (GBM), 7 patients with human immunodeficiency virus (HIV), 66 patients with large B cell lymphoma (LCL) 51 patients with multiple myeloma (MM) and 34 patients with various solid tumors including mesothelioma and prostate, ovarian, and pancreatic cancer.
Figure 1:
Peripheral blood counts from cellular immunotherapy patients at the time of collection show difference amongst indications (bars = 95% confidence interval). a. White blood cell count (WBC) demonstrates leukocytosis in patients with ALL. b. Hematocrit (HCT) demonstrates anemia more pronounced in patients with ALL, LCL and MM. c. Platelet (PLT) counts show thrombocytopenia more commonly in patients with ALL and CLL.
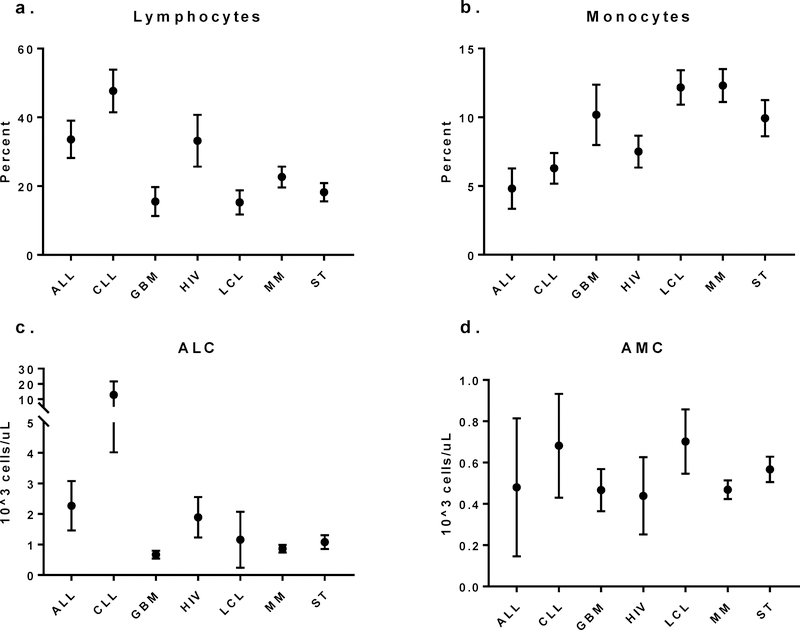
Differences amongst cell counts were observed and consistent with known clinical features of underlying disease. Patients with acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) demonstrate leukocytosis in their peripheral blood (Fig 1a), whereas patients with ALL, large B cell lymphoma (LCL) and multiple myeloma (MM) are more likely to be anemic (Fig 1b) and patients with ALL and CLL were more likely to be thrombocytopenic at the time of collection (Fig 1c). Comparison of WBC differentials show relative and absolute lymphocytosis in patients with ALL and CLL as well as monocytopenia in those same patients whereas patients with GBM, LCL, MM and ST were more likely to be lymphopenic (Fig 2). These differences in peripheral blood counts are reflect in the resulting MNC product with higher peripheral blood absolute lymphocyte counts (ALC) being associated with higher lymphocyte content in the MNC product (data not shown).
Figure 2:
Peripheral blood count white blood cell differentials from cellular immunotherapy patients at the time of collection show differing absolute and proportional lymphocyte and monocyte counts. a. Proportions of circulating lymphocytes are highest in patients with ALL and CLL whereas b. relative frequency of monocytes is lower in ALL and CLL. Absolute lymphocytosis is common in patients with ALL and CLL whereas GBM, MM and ST patients frequently display absolute lymphopenia. d. Absolute monocyte counts are relatively similar amongst indications, however there is a slight trend toward higher counts in ALL, CLL and LCL patients.
Engineered T cell therapy is a relatively nascent treatment modality and it is therefore primarily restricted to refractory/relapsed patients at this time. Such patients have usually endured years of a combination of cytotoxic treatment including chemotherapy, radiation and/or bone marrow transplant. It is unsurprising, therefore, that at the time of collection, refractory/relapsed malignancy patients are frequently lymphopenic. Lympopenia at the time of collection is a common occurrence and associated with decrease T cell yield and decreased T cell purity in the MNC product. In a recent clinical trial of CD19-redirect CART cells against non-Hodgkin lymphoma (NHL) 17 of 45 MNC collections occurred in patients with ALC <500/uL [7]. Low ALC or peripheral blood CD3 at the time of collection decreases the likelihood of collection of adequate numbers of cells to initiate T cell culture [3].
Specific cytotoxic chemotherapy may impair future ex vivo T cell growth. Singh et al. demonstrated that early lineage T cells were selectively depleted by cyclophosphamide and cytarabine in pediatric patients with ALL and NHL [8]. This depletion was associated with worsening ex vivo T cell expansion throughout the course of treatment. By maintenance phase, less than half of samples from standard risk ALL patients and less than one-quarter of samples from high-very high risk patients were able to expand in test expansions. These findings are consistent with our overall experience in clinical manufacturing with more heavily pre-treated patients demonstrating growth defects.
Both malignancy and anti-cancer therapy can increase the circulating frequency of myeloid-derived suppressor cells (MDSCs). In vivo MDSCs are a crucial mediator of tumor-mediate immunosuppression [9]. In ex vivo T cell cultures, MDSCs can significantly impair T cell activation and expansion. We have shown that a higher frequency of MDSCs in MNC products is associated with failure of T cells to expand in culture and that a selective depletion of MDSCs is associated with reversal of this defect [7].
b. Collection sources of variability
Local apheresis center practices for MNC collection may differ from site to site. MNC collections may occur via central or peripheral venous access. Procedure duration and total target volumes to be processed may differ based on local clinical practice, scheduling or patient tolerance. For apheresis collection to yield an acceptable MNC product to enter the manufacturing cycle, not only must the patient present for collection with adequate peripheral blood counts, but also the collection must proceed without significantly difficulty. Difference amongst collection practices or difficulty during the collection procedure itself can introduce variability into the MNC product and therefore into the manufacturing process in general.
Successful apheresis collection is heavily reliant upon obtaining adequate inlet and return vascular access. Collections can occur via peripheral venous access if the patient’s vasculature can support the multi-hour procedure. In these cases, a 16–18 gauge steel needle is inserted and remains in place for the duration of the collection. Inlet flow rates must be maintained at 50–100 mL/min, however and if the patient and his/her vasculature cannot tolerate the full duration of collection, central venous access may be required. At many institutions, these central lines are placed in the internal jugular vein under image guidance by interventional radiologists.
The decision as to whether to place a central line must take into account a number of factors. Line placement should be specified as an option in the clinical protocol under which the patient is being treated. A determination must be made that the patient’s peripheral vasculature will not be adequate to complete the necessary collection. When more than one collection is required across several days, central line placement may be preferred. However, many cell therapy protocols require a single collection and therefore peripheral access should be an option in the majority of cases.
While peripheral access often provides adequate flow, central access provides more consistency. Consistent flow rate are critical to maintain product purity. As blood components enter the apheresis centrifuge, cells layers are separated and interfaces between these layers are established. It is the detection and maintenance of these interfaces that allow the machine or operator to identify and collect specific cell layers. Depending on the machine, either the operator or an automated system will then attempt to collect only the target cell layer. If the inlet flow is inconsistent, the interface may fluctuate and therefore layer being collected may shift allowing non-target cells into the collection bag and increasing product variability. Peripheral collections are subject to significant fluctuations in flow rates due to vasoconstriction, fluid shifts or even movement of the patient, disrupting access. For these reasons, central access provides more consistent flow and therefore collection by central access is less likely to lose the interface and possibly reduce variability. Central venous access is, of course, not without risks. Mechanical and infectious complications limit the universal use of this type of access [10]. Nonetheless, in experienced centers with image guided techniques and short in-dwelling time, central venous access can be a safe method to obtain access for MNC collection.
A major advantage of apheresis collection is the ability to process multiple blood volumes in a single visit, however, not all patients can tolerance lengthy collections. During a single collection, a patient’s entire blood volume may circulate through the machine several times. We typically perform a 2–3 blood volume collection for cell therapy manufacturing. Collection efficiencies vary significantly depending on site, instrument used, procedure parameters, target cell type or method of calculation. Lymphocyte collection efficiencies of around 60% are common in non-mobilized donors [11]. It is also important to note that while total yield with each passing blood volume decreases, recruitment into the peripheral blood is also observed improving overall yield in larger collections. Extending the duration of the procedure beyond 3 blood volumes exposes the patient to additional citrate which has been associated with an increased incidence of citrate toxicity [12]. Apheresis instruments must be anti-coagulated to prevent clotting of the circuit. Citrate is the most common method of anti-coagulation used for MNC collections. Citrate reversibly chelated divalent cations such as Ca2+, preventing it from participating as a co-factor in coagulation. Citrate in the form of acid-citrate-dextrose (ACD-A or B) is mixed with whole blood decreasing calcium levels and functionally anticoagulating it in the machine. Citrate along with non-target cell and plasma components are returned to the patient. Particularly in large-volume apheresis patients may be exposed to high levels of citrate. Due to rapid metabolism by mitochondria in cells of the kidney, liver and muscle, citrate is rapidly degrade with a half-life of 36+/− 18 minutes [13]. With such a short half-life, most patients are not functionally anti-coagulated when exposed to high levels of citrate, though they may experience a transient hypocalcemia. This is typically manifested by perioral or acral paresthesia, flushing and lightheadedness, progressing to GI distress, tremors and if untreated hypotension, arrhythmia and seizure [14]. Such severe manifestations are extraordinarily rare as most mild citrate reactions can be easily and quickly managed with oral or intravenous calcium and/or discontinuation of the procedure.
In large volume MNC collections, citrate exposure may exceed patient tolerance. In some of these cases at other institutions, unfractionated heparin may be used in conjunction with citrate to allow lowering of the rate of citrate exposure. Heparin binds to antithrombin, catalyzing rapid inactivation of thrombin and Factor Xa. While the half-life of heparin is only 60–90 minutes, the powerful anti-coagulant is associated with significant bleeding complications [15]. Given the relative safety profiles, citrate is the preferred anti-coagulant, recognizing that citrate alone may not always be adequate. It should also be noted that while the local use of heparin in the setting of hypocalcemia, at our institution we have rarely if ever encounter refractory hypocalcemia requiring administration of heparin.
Whether citrate or heparin is used to anticoagulated the apheresis circuit, adverse reactions to the anti-coagulation may limit procedure duration. If patients receiving citrate experience severe hypocalcemia that is refractory to intravenous calcium or patients receiving heparin develop bleeding, the procedure may need to be discontinued before reaching the target volume processed. Less common adverse events may also negatively impact procedure tolerance. Patients may experience hypotension, vasovagal response, pain at the site of access, or allergic reactions [16]. In nearly all cases, advanced malignancy patients are predisposed to apheresis toxicities that are rare in other patient populations. Several studies have shown that overall yield correlates with total volume processed [3, 11]. Therefore, regardless of the underlying reason, discontinue apheresis prior to reaching target volume process will decrease the expected total MNC yield and increase product-to-product variability.
c. Downstream challenges posed by variable input
Upstream variability is primarily driven by patient and collection sources and has significant downstream impact on T cell culture. Efficient T cell enrichment can be achieved through a variety of techniques, though proper implementation of these methods can be challenging. Different enrichment methods are specifically effective at depletion of particular cell types. Therefore, to optimize efficiency, selection of enrichment techniques may vary to match the make-up of the apheresis product. Variable processing directly contradicts standardization of manufacturing, potential even impacting final product characteristics. In light of this, validation of each permutation of the processing pathway is required and can be complex, costly and time consuming. Nonetheless, for the overall manufacturing cycle to be robust, variability introduced by the apheresis product must be reduced at some point in the vein-to-vein process.
Enrichment by density gradient can effectively deplete an MNC product of granulocytes and red blood cells. Such non-MNC contaminants often find their way into the apheresis product when the interface is lost during collection and non-MNC layers are diverted into the collect line. Because apheresis is a density-based separation method itself, non-MNCs should only rarely enter an MNC product if the interface is maintained consistently throughout the collection. Non-T cell MNCs such a monocytes, however, will be definition be collected in MNC products. Both Non-MNCs as well as non-T cell MNCs can inhibit T cell activation and expansion. Non-MNCs such as granulocytes [17] and red blood cells [18, 19] have been shown to suppress T cell proliferation and so elimination of these cell types is important to enhance T cell culture.
Elutriation, on the other hand, can separate cells based on size and shape as well as density. During elutriation, cells are packed in a rotation cone, while elutriation buffer is pumped in a direction opposite the centrifugal force. The flow rate and different viscosities of the buffer and cell material allow for separation based on cell size, shape and density rather than a single parameter. Cells are pumped out of the cone over time and collected in different fraction with some fractions. Elutriation is particularly effective at separating lymphocytes form monocytes. As noted above, the density of monocytes and lymphocytes are similar, making density-based separations typically ineffective. If left in culture, monocytes are notoriously problematic for engineered T cell. Monocytes and monocyte-derived cells can impair T cell activation [20], mediate T cell anergy [21] and induce apoptosis of activated T cells [22]. In addition, they may bind and engulf anti-CD3/anti-CD28 beads, depriving co-cultured T cells of activation signal. Elimination of monocytes from T cell culture greatly improves chances of T cell manufacturing success. Several closed system elutriators are available for GMP manufacturing including the TerumoBCT Elutra Cell Separation System and Beckman Coulter Centrifugal Elutriation System.
Antibody-bead conjugates can be used for positive and negative selections. While this has potential to be the most specific method, it requires availability of high-quality reagents, which are often expensive if readily available at all. This method can be particularly useful in selection of T cells in a background of leukemic blasts. Much like monocytes, leukemic blasts can directly inhibit T cell activation and proliferation in culture [23, 24]. Further, as blasts die or are killed in culture, toxic cell debris can accumulate, leading to clumping of cell material. Removal of blasts early in cell processing is ideal.
While it is true that T cell yield is critical for a successful culture, purity appears to be just as if not more important. Removal of non-T cells from culture early in cell processing improved the likelihood of efficient activation, transduction, and expansion. Incoming apheresis material varies in the absolute number and relative proportions of non-T cells. In light of this variability, there is no universally optimal T cell enrichment process currently available. In addition, on-going clinical manufacturing continues to reveal new sources of T cell inhibition. We recently demonstrated that MDSCs were associated with decreased T cell proliferation in CAR T cell culture and that these cells are selectively depleted via cryopreservation [7]. As more is learned about non-T cell types and their impact on T cell culture, enrichment techniques will need to be further optimized.
Due to this variable of incoming apheresis material, selection of an enrichment process that matches the non-T compartment must balance increased purity with decreasing yield. No enrichment technique recovers 100% of T cells and therefore must work within the context of a target culture seeding cell number. This further complicates matters as both T cell yield and purity will vary significantly from apheresis product to product, making standardized cell processing extremely difficult.
d. Process driven minimization of variability
Much of the art and science of cell manufacturing focuses on mitigating upstream variability. While there are a variety of approaches, we largely rely on a sequential algorithm to incrementally reduce variability throughout the manufacturing process. This means that at each step of our process, we aim to generate a more standardized downstream product. The end goal is to generate a therapeutic product that is a consistent as possible in meeting our pre-defined release specifications.
In practice, this process to reduce variability as applied to lentiviral CAR T cell manufacturing begins with an enrichment pathway that reflects the apheresis content. For example, in cases with excessive monocytes, elutriation is performed, whereas if the monocyte proportion is low, elutriation is bypassed. Based on historical and engineering run growth curves a maximum number of cells will be seeded into culture. T cells will be activated by anti-CD3, anti-CD28 beads and transduction with the lentiviral vector at the specific multiplicity of infection will be performed. T cells will preferentially grow in the setting of this activation. The final product will be harvested 9–11 days later and relevant quality control testing will be performed. At enrichment and seeding, incoming apheresis product variability may be decreased. For example, most products being seeded into culture will have few monocytes either due to few being present in the apheresis product or after depletion by elutriation. In addition, having a maximum number of cells to seed in culture ensures that for cases with ample post-enrichment cells, a standard number of cells enter culture and a uniform amount of vector can be used.
This approach has several advantages. Because no single process is universally applied, the particular pathway followed is more likely to be appropriate for the specific material being processed. Pre-defined flexibility allows for batch-to-batch tailored manufacturing, while remaining under tight control overall. The sequential reduction in variability means that the tolerances of downstream processing can be more restrictive. In practice, for example, if a hypothetical apheresis product might yield 1E9 to 10E9 total nucleated cells, and enrichment yields range from 10% to 90%, setting a maximum culture seed number of 1E8 means that the majority of cultures will start with the same number of cells despite variable performance of apheresis and enrichment. In doing so, as the process reaches final formulation, this increases the uniformity of the therapeutic product.
On the other hand, this approach poses significant operational and scientific challenges. Allowing incoming product variability to dictate the processing pathway means that daily tasks are uncertain until receipt of the product. This can make for challenging staffing, training, assignment of duties and preparation of reagents and materials. The precious nature of the apheresis product and the often tenuous clinical condition of the patient, means that preparation of manufacturing processes must err on the side of excess. Ultimately, this can lead to costly operational inefficiencies. Scientifically, different upfront processing makes it difficult to compare run-to-run data. Each additional decision point increases the number of possible overall processing pathways (2^n where n is the number of binary decisions made). Given the number of possible pathways, it is often difficult to compare manufacturing outcomes as two products may have been processed by different techniques. While each technique is individually validated prior to use in clinical manufacturing, most trials are not powered to determine subtle differences in manufacturing by more than a dozen possible pathways.
A single, universally applicable, highly efficient, upfront processing step is the holy grail of T cell manufacturing. There are a number of promising technologies on the horizon that may bring us closer to this goal.
Selection by GMP-grade anti-CD4 and anti-CD8 beads are able to achieve median purities of around 90% [25] and improve T cell expansion by efficient depletion of leukemic blasts[26]. These reagents and compatible closed-system equipment are currently available to manufacturing (Miltenyi Biotec). Non-bead based cell separation is also an area of active investigation. Because different cell types differ in polarizability and density, dielectropohoresis and surface acoustic waves can be used to isolate specific cell populations [27]. When employed in a microfluidics environment, multi-pass separation technology can generate highly pure cell populations. While promising, these techniques are still under development and will require additional time and cost to fully deploy in clinical manufacturing.
Perhaps the most attractive process modification to reduce variability is to reconsider timing of apheresis collection. As noted above, many factors drive variability in apheresis content. Most of these factors are disease or treatment related. It follows that collection earlier in the disease or treatment course could yield an apheresis product with higher yield, higher purity and functionally robust T cells. While current clinical trials and FDA approved indications for CAR T cells are focused on refractory/relapsed malignancies, this does not preclude prophylactic collection early after diagnosis, in first-remission or even prior to development of disease in the first place. Umbilical cord blood banking as an industry essentially provides an insurance policy against future need for cord blood transplant. In the setting of cord blood, it is clear that the ability to obtain and bank the material is time limited and therefore, must occur prior to need. Apheresis for CAR T cell therapy is different, in that manufacturing to date has only been performed at time of need. However, as these therapies evolve and expand to include greater numbers of patients, we will continue to learn how critical the quality of incoming apheresis material is to manufacturing and overall clinical success. For autologous T cell therapy, the only way to comprehensively avoid the challenges posed by disease and treatment is to collect prior to these cytotoxic insults. Logistical and scientific hurdles still exist to this approach. It is unclear the manufacturing consequences of long-term cryopreservation on T cells and or whether this approach would be cost effective if applied more broadly. Nonetheless, at least some data suggest that manufacturing and clinical success could be improved if apheresis were performed earlier in the patient’s disease and treatment course[8]. However, no head to head studies have been performed to date showing that large scale, clinical manufacturing would be more successful or generate superior clinical products with early apheresis collection.
e. Conclusions
Variability is a major challenge in T cell manufacturing. To date, engineered T cell therapies have largely been autologous and generated from apheresis starting material. Apheresis products reflect the donor circulating cells at the time of collection. For patients, the absolute and relative numbers of these cells vary significantly based on a variety of clinical factors and collection variables can alter apheresis product yield or purity (Table 1). We continue to learn about which factors drive this variability and are associated with manufacturing and clinical success, but more work is needed. We recognize that adaptive manufacturing processes challenge core tenets of GMP manufacturing. There are many ways to standardize current manufacturing processes, however, applicability to non-cell manufacturing protocols does not guarantee success when applied to cell manufacturing. Nonetheless, the ultimate goal is to develop a universally applicable standard cell manufacturing approach. Real progress is being made in this domain with the recent development of several closed-system, large scale, cell processing and culture platforms. In the near future, apheresis variability may be overcome by the ability to apply standard techniques to variable inputs and still achieve a high quality final product. To date, no such universally applicable platform exists, but there is promise on the horizon given the high level of interest and active development.
Table 1:
Pre-collection and collection variables
| Pre-collection variables (peripheral blood cell counts) | Collection variables (apheresis product yield or purity) |
|---|---|
| -Clinical Indication and disease status | -Access type and volume status |
| -Prior treatment type and timing | -Procedural tolerance |
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Manual Technical, 19th Edition. 19th ed 2017, Bethesda: AABB. [Google Scholar]
- 2.Brauninger S, et al. , Mobilized allogeneic peripheral stem/progenitor cell apheresis with Spectra Optia v.5.0, a novel, automatic interface-controlled apheresis system: results from the first feasibility trial. Vox Sang, 2011. 101(3): p. 237–46. [DOI] [PubMed] [Google Scholar]
- 3.Allen ES, et al. , Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion, 2017. 57(5): p. 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraietta JA, et al. , Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med, 2018. 24(5): p. 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn KM, et al. , Age-Related Decline in Primary CD8(+) T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8(+) T Cells. Cell Rep, 2018. 23(12): p. 3512–3524. [DOI] [PubMed] [Google Scholar]
- 6.Fry TJ and Mackall CL, Current concepts of thymic aging. Springer Semin Immunopathol, 2002. 24(1): p. 7–22. [DOI] [PubMed] [Google Scholar]
- 7.Davis MM, et al. , Predictors of Manufacturing (Mfg) Success for Chimeric Antigen Receptor (Car) T Cells in Non-Hodgkin Lymphoma. Cytotherapy, 2017. 19(5): p. S79–S80. [Google Scholar]
- 8.Singh N, et al. , Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med, 2016. 8(320): p. 320ra3. [DOI] [PubMed] [Google Scholar]
- 9.Veglia F, Perego M, and Gabrilovich D, Myeloid-derived suppressor cells coming of age. Nat Immunol, 2018. 19(2): p. 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGee DC and Gould MK, Preventing complications of central venous catheterization. N Engl J Med, 2003. 348(12): p. 1123–33. [DOI] [PubMed] [Google Scholar]
- 11.Anyanwu A, et al. , Low-Volume Leukapheresis in Non-Cytokine-Stimulated Donors for the Collection of Mononuclear Cells. Transfus Med Hemother, 2018. 45(5): p. 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip J, Sarkar RS, and Pathak A, Adverse events associated with apheresis procedures: Incidence and relative frequency. Asian J Transfus Sci, 2013. 7(1): p. 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer L, et al. , Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med, 2003. 31(10): p. 2450–5. [DOI] [PubMed] [Google Scholar]
- 14.Lee G and Arepally GM, Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J Clin Apher, 2012. 27(3): p. 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsh J, et al. , Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest, 2008. 133(6 Suppl): p. 141S–159S. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan A, Complications of apheresis. Semin Dial, 2012. 25(2): p. 152–8. [DOI] [PubMed] [Google Scholar]
- 17.Munder M, et al. , Suppression of T-cell functions by human granulocyte arginase. Blood, 2006. 108(5): p. 1627–34. [DOI] [PubMed] [Google Scholar]
- 18.Bernard A, et al. , Packed red blood cell-associated arginine depletion is mediated by arginase. J Trauma, 2007. 63(5): p. 1108–12; discussion 1112. [DOI] [PubMed] [Google Scholar]
- 19.Long K, et al. , T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion, 2014. 54(5): p. 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charron L, et al. , Monocyte:T-cell interaction regulates human T-cell activation through a CD28/CD46 crosstalk. Immunology and cell biology, 2015. 93(9): p. 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoves S, et al. , Monocyte-Derived Human Macrophages Mediate Anergy in Allogeneic T Cells and Induce Regulatory T Cells. The Journal of Immunology, 2006. 177(4): p. 2691. [DOI] [PubMed] [Google Scholar]
- 22.Munn DH, et al. , Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. A potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol, 1996. 156(2): p. 523–32. [PubMed] [Google Scholar]
- 23.Chiao JW, et al. , Suppression of lymphocyte activation and functions by a leukemia cell-derived inhibitor. Proc Natl Acad Sci U S A, 1986. 83(10): p. 3432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orleans-Lindsay JK, et al. , Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function--implications for the adoptive immunotherapy of leukaemia. Clin Exp Immunol, 2001. 126(3): p. 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aleksandrova K, et al. , Functionality and Cell Senescence of CD4/ CD8-Selected CD20 CAR T Cells Manufactured Using the Automated CliniMACS Prodigy® Platform . Transfusion Medicine and Hemotherapy, 2019. 46(1): p. 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Highfill S, et al. , CD4 and CD8 T-cell positive selection increases the robustness of the CD22 CAR T-cell manufacturing process. Cytotherapy, 2017. 19(5): p. S14. [Google Scholar]
- 27.Smith AJ, et al. , Rapid cell separation with minimal manipulation for autologous cell therapies. Scientific Reports, 2017. 7: p. 41872. [DOI] [PMC free article] [PubMed] [Google Scholar]