Abstract
Objectives/Hypothesis:
The use of a short 10-mm/10-electrode cochlear implant to preserve low-frequency residual hearing was investigated. This report describes the 12-month outcomes of this multicenter clinical trial.
Study Design:
Single-subject design.
Methods:
Twenty-eight subjects with low-frequency hearing at or better than 60 dB HL at 500 Hz and severe high-frequency hearing loss were implanted with a Nucleus Hybrid S12 implant in their poorer ear. Speech perception in quiet using Consonant-Nucleus-Consonant (CNC) words and sentences in noise using AzBio sentences was collected pre- and postoperatively at 3, 6, and 12 months. Subjective reporting using the Speech, Spatial, and Qualities of Hearing Scale (SSQ) questionnaire was also collected pre- and postoperatively.
Results:
Functional hearing preservation was accomplished in 96% of subjects. At 3 and 6 months, 86% of the 28 subjects had maintained functional hearing. By 12 months, 23 out of 27 subjects (85%) had maintained functional hearing (one subject with functional hearing at 6 months withdrew from the study prior to the 12-month visit). Speech perception results demonstrated that 81% of the participants on CNC words and 77% with AzBio sentences in noise had significant improvements using their everyday listening condition at 12 months compared to preoperative performance with bilateral hearing aids. Furthermore, preoperative to 12 months postoperative subjective ratings showed significant improvements for the SSQ.
Conclusions:
This study demonstrates that a high degree of hearing preservation enabling acoustic-electric hearing and improvement in speech understanding in quiet and in noise can be accomplished using a short-electrode 10-mm cochlear implant.
Keywords: Hybrid, residual hearing, cochlear implant, short electrode
INTRODUCTION
For nearly the past 20 years, we and others have been investigating the use of combined acoustic-electric processing (A+E) to address the needs of individuals who have functional residual low-frequency (LF) hearing and severe to profound high-frequency sensorineural hearing losses.1–14 In 2014, the Nucleus Hybrid L24 cochlear implant (CI)15 and in 2016, the MED-EL FLEX 20 and 24 CIs16 were approved by the Food and Drug Administration (FDA) for implantation in those with these indications. In both clinical trials (CTs)15,16 and in other subsequent research studies,1,7,10,13–15,17 outcomes demonstrate that by preserving the LF hearing, listeners can utilize fine-timing information11,18 to aid speech perception in noise and music,9,11 and maintain localization abilities.19–22 Subsequently, the electrically encoded high-frequency information provides important spectral information.
Recently, the American Academy of Otolaryngology has adopted a minimum reporting standard for CIs in adults23 and includes specifications to describe residual hearing. Consistent with their approach, in this article we specify a functionally relevant LF pure-tone average (PTA) at 125 Hz, 250 Hz, 500 Hz as ≤85 dB HL.24–27
In this study, we report outcomes for the Nucleus Hybrid S12 CI (S12) CT. The Hybrid S12 multicenter CT was initiated in 2008, completed in 2015, and was conducted across five centers in the United States.
MATERIALS AND METHODS
Participants
Twenty-eight subjects in the United States received the S12 under FDA Investigational Device Exemption #G070016. The study was conducted under the institutional review board at each implant center. The subjects ranged in age from 33.1 to 86.8 years (mean (M) = 62.3 years; SD = 11.6 years). Thirteen (46%) of the subjects were female. Average duration of severe/profound high-frequency hearing loss was 14.2 years (SD = 9.2 years).
Much of our understanding regarding the importance of patient demographics in the use of a short electrode came from the first version of the short electrode (S8).1,28,29 In the S8 study, severity of hearing loss at 1 year postactivation was significantly correlated with age, gender, and noise-induced hearing loss. Therefore, in this study, these predictor factors were considered prior to inclusion in the study. Detailed demographic data and etiology for each subject are shown in Table I.
Table I.
Demographic Data and Etiology for Each Study Subject.
| Subject ID | Gender | Implanted Ear | Age at Implant (yr) | Duration of Hearing Loss (yr), CI Ear | Duration of Hearing Loss (yr), Non-CI Ear | Duration of Severe/Profound High-Frequency Hearing Loss (yr), CI Ear | Duration of Severe/Profound High-Frequency Hearing Loss (yr), Non-CI Ear | Etiology (Both Ears) |
|---|---|---|---|---|---|---|---|---|
| US18-S12–1001 | F | L | 33 | 10 | 10 | 3 | 3 | Familial |
| US14-S12–1001 | F | L | 41 | 18 | 18 | 16 | 16 | Familial |
| US09-S12–1001 | M | R | 54 | 14 | 14 | 8 | 0 | Familial |
| US15-S12–1001 | F | L | 54 | 27 | 27 | 10 | 10 | Familial |
| US17-S12–1001 | M | L | 59 | 43 | 43 | 19 | 19 | Familial |
| US07-S12–1001 | F | L | 60 | 22 | 22 | 4 | 4 | Familial |
| US06-S12–1001 | M | L | 65 | 44 | 44 | 25 | 25 | Familial |
| US14-S12–1460 | M | L | 65 | 27 | 27 | 12 | 12 | Familial/noise |
| US02-S12–1001 | M | R | 60 | 24 | 20 | 8 | 8 | Noise |
| US15-S12–1460 | M | L | 65 | 20 | 20 | 9 | 11 | Noise |
| US05-S12–1460 | M | L | 65 | 37 | 37 | 24 | 24 | Noise |
| US01-S12–1202 | F | R | 67 | 27 | 27 | 22 | 22 | Ototoxic |
| US13-S12–1001 | F | R | 45 | 13 | 13 | 8 | 8 | Trauma |
| US04-S12–1460 | F | R | 51 | 24 | 24 | 24 | 24 | Unknown |
| US05-S12–1139 | F | L | 57 | 49 | 49 | 4 | 4 | Unknown |
| US16-S12–1001 | M | R | 57 | 42 | 42 | 27 | 27 | Unknown |
| US11-S12–1001 | F | L | 58 | 8 | 8 | 2 | 3 | Unknown |
| US01-S12–1001 | F | R | 61 | 11 | 10 | 4 | 4 | Unknown |
| US10-S12–1001 | F | L | 62 | 57 | 57 | 22 | 31 | Unknown |
| US12-S12–1001 | M | R | 63 | 13 | 13 | 10 | 10 | Unknown |
| US10-S12–1460 | F | R | 67 | 35 | 35 | 23 | 23 | Unknown |
| US01-S12–1247 | M | L | 67 | 27 | 27 | 7 | 7 | Unknown |
| US07-S12–1139 | M | R | 70 | 32 | 19 | 19 | 5 | Unknown |
| US03-S12–1139 | M | L | 74 | 34 | 34 | 16 | 16 | Unknown |
| US06-S12–1139 | M | R | 76 | 51 | 51 | 8 | 8 | Unknown |
| US09-S12–1139 | M | R | 86 | 21 | 21 | 10 | 10 | Unknown |
| US04-S12–1139 | M | R | 86 | 30 | 30 | 4 | 4 | Unknown |
| US19-S12–1001 | F | R | 64 | 38 | 38 | 38 | 38 | Viral |
CI = cochlear implant; F = female; ID = identification; L = left; M = male; R = right.
Inclusion criteria included LF PTA thresholds between 125 Hz and 500 Hz ≤60 dB HL; threshold average at 2,000 Hz, 3,000 Hz, and 4,000 Hz ≥ 75 dB HL; and aided Consonant-Nucleus-Consonant (CNC)30 word scores between 10% and 60% in the ear to be implanted and ≤80% in the contralateral ear. Audiometric thresholds are shown in Table II. A no-response threshold is written as 121. The ear with the poorer hearing, as determined by word recognition score and/or audiometric thresholds, received the CI. All subjects were required to have used optimally fitted bilateral hearing aids (HAs) for a minimum of 2 weeks prior to baseline testing. Subject exclusionary criteria included a congenital hearing loss and severe high-frequency hearing loss duration >30 years.
Table II.
Preoperative CI Ear and Non-CI Ear Air- and Bone-Conduction Thresholds (dB HL).
| Subject | Ear | Mode | 125 | 250 | 500 | 750 | 1,000 | 1,500 | 2,000 | 3,000 | 4,000 | 6,000 | 8,000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US01-S12–1001 | CI | Air | 30 | 30 | 45 | 50 | 60 | 80 | 80 | 100 | 121 | 121 | 121 |
| Bone | 25 | 35 | 60 | 55 | 75 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 30 | 35 | 45 | 65 | 65 | 80 | 105 | 100 | 121 | 121 | |
| Bone | 25 | 35 | 60 | 55 | 75 | 121 | 121 | ||||||
| US02-S12–1001 | CI | Air | 35 | 40 | 55 | 65 | 70 | 95 | 121 | 121 | 115 | 121 | 121 |
| Bone | 30 | 40 | 60 | 65 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 30 | 35 | 45 | 55 | 65 | 85 | 110 | 110 | 110 | 121 | 121 | |
| Bone | 15 | 35 | 50 | 55 | 121 | 121 | 121 | ||||||
| US06-S12–1001 | CI | Air | 30 | 25 | 50 | 50 | 60 | 65 | 75 | 100 | 105 | 95 | 121 |
| Bone | 15 | 40 | 50 | 65 | 70 | 70 | 121 | ||||||
| Non-CI | Air | 25 | 25 | 30 | 40 | 45 | 60 | 70 | 90 | 95 | 100 | 121 | |
| Bone | 15 | 20 | 30 | 30 | 70 | 70 | 121 | ||||||
| US07-S12–1001 | CI | Air | 20 | 30 | 45 | 55 | 70 | 100 | 110 | 115 | 115 | 121 | 121 |
| Bone | 25 | 45 | 60 | 65 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 20 | 30 | 35 | 45 | 50 | 60 | 90 | 100 | 105 | 100 | 121 | |
| Bone | 30 | 45 | 40 | 50 | 60 | 70 | 70 | ||||||
| US09-S12–1001 | CI | Air | 40 | 35 | 30 | 50 | 75 | 121 | 121 | 121 | 121 | 121 | 121 |
| Bone | 25 | 25 | 121 | 121 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 15 | 20 | 20 | 20 | 40 | 75 | 80 | 75 | 70 | 65 | 60 | |
| Bone | 0 | 10 | 10 | 25 | 121 | 121 | 121 | ||||||
| US10-S12–1001 | CI | Air | 20 | 25 | 45 | 45 | 70 | 90 | 100 | 95 | 105 | 100 | 121 |
| Bone | 20 | 121 | 50 | 121 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 10 | 20 | 20 | 40 | 55 | 80 | 85 | 75 | 75 | 70 | 55 | |
| Bone | 0 | 5 | 25 | 40 | 121 | 121 | 121 | ||||||
| US11-S12–1001 | CI | Air | 40 | 45 | 35 | 35 | 45 | 60 | 90 | 121 | 110 | 121 | 121 |
| Bone | 121 | 25 | 30 | 40 | 65 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 30 | 25 | 30 | 50 | 65 | 95 | 115 | 105 | 100 | 121 | |
| Bone | 25 | 25 | 30 | 40 | 65 | 121 | 121 | ||||||
| US12-S12–1001 | CI | Air | 30 | 35 | 40 | 60 | 60 | 80 | 105 | 121 | 105 | 121 | 121 |
| Bone | 40 | 35 | 60 | 60 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 30 | 35 | 45 | 55 | 65 | 80 | 100 | 121 | 110 | 121 | 121 | |
| Bone | 40 | 35 | 60 | 60 | 121 | 121 | 121 | ||||||
| US13-S12–1001 | CI | Air | 25 | 25 | 55 | 65 | 75 | 90 | 100 | 110 | 115 | 105 | 121 |
| Bone | 25 | 45 | 70 | 121 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 35 | 55 | 70 | 75 | 70 | 85 | 100 | 115 | 121 | 121 | |
| Bone | 25 | 45 | 55 | 60 | 75 | 121 | 121 | ||||||
| US14-S12–1001 | CI | Air | 20 | 25 | 35 | 45 | 60 | 105 | 115 | 115 | 115 | 105 | 121 |
| Bone | 20 | 30 | 40 | 55 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 20 | 25 | 30 | 35 | 45 | 80 | 115 | 115 | 115 | 105 | 121 | |
| Bone | 20 | 30 | 25 | 40 | 121 | 121 | 121 | ||||||
| US15-S12–1001 | CI | Air | 25 | 30 | 50 | 60 | 75 | 80 | 95 | 95 | 95 | 85 | 85 |
| Bone | 25 | 50 | 60 | 70 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 10 | 25 | 55 | 65 | 75 | 85 | 90 | 85 | 85 | 95 | 95 | |
| Bone | 30 | 50 | 60 | 70 | 70 | 121 | 121 | ||||||
| US16-S12–1001 | CI | Air | 10 | 15 | 35 | 45 | 55 | 80 | 100 | 110 | 115 | 100 | 121 |
| Bone | 15 | 30 | 40 | 50 | 70 | 121 | 121 | ||||||
| Non-CI | Air | 10 | 10 | 30 | 50 | 50 | 70 | 105 | 110 | 105 | 100 | 121 | |
| Bone | 15 | 30 | 40 | 55 | 65 | 121 | 121 | ||||||
| US17-S12–1001 | CI | Air | 20 | 20 | 20 | 45 | 65 | 75 | 85 | 95 | 95 | 90 | 75 |
| Bone | 15 | 25 | 35 | 50 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 45 | 40 | 40 | 45 | 55 | 85 | 100 | 105 | 105 | 121 | 121 | |
| Bone | 10 | 30 | 35 | 45 | 121 | 121 | 121 | ||||||
| US18-S12–1001 | CI | Air | 40 | 30 | 20 | 15 | 20 | 85 | 80 | 85 | 90 | 90 | 85 |
| Bone | 30 | 15 | 5 | 10 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 35 | 25 | 20 | 20 | 15 | 20 | 45 | 95 | 95 | 90 | 85 | |
| Bone | 15 | 15 | 10 | 5 | 25 | 45 | 121 | ||||||
| US19-S12–1001 | CI | Air | 30 | 25 | 35 | 40 | 40 | 70 | 80 | 90 | 100 | 121 | 121 |
| Bone | 15 | 25 | 30 | 35 | 65 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 20 | 30 | 35 | 40 | 65 | 75 | 85 | 110 | 121 | 121 | |
| Bone | 5 | 25 | 25 | 35 | 65 | 121 | 121 | ||||||
| US03-S12–1139 | CI | Air | 45 | 45 | 40 | 50 | 60 | 75 | 95 | 105 | 115 | 121 | 121 |
| Bone | 35 | 35 | 50 | 60 | 75 | 121 | 121 | ||||||
| Non-CI | Air | 35 | 40 | 40 | 45 | 50 | 70 | 90 | 121 | 110 | 121 | 121 | |
| Bone | 35 | 40 | 45 | 45 | 70 | 121 | 121 | ||||||
| US04-S12–1139 | CI | Air | 45 | 55 | 55 | 65 | 75 | 80 | 90 | 110 | 115 | 121 | 121 |
| Bone | 40 | 50 | 60 | 70 | 80 | 121 | 121 | ||||||
| Non-CI | Air | 45 | 55 | 55 | 65 | 70 | 85 | 85 | 110 | 121 | 121 | 121 | |
| Bone | 40 | 55 | 60 | 60 | 80 | 121 | 121 | ||||||
| US05-S12–1139 | CI | Air | 25 | 25 | 15 | 40 | 50 | 85 | 110 | 115 | 105 | 95 | 121 |
| Bone | 20 | 20 | 35 | 50 | 80 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 20 | 15 | 35 | 55 | 80 | 105 | 115 | 110 | 95 | 121 | |
| Bone | 15 | 15 | 25 | 45 | 80 | 121 | 121 | ||||||
| US06-S12–1139 | CI | Air | 20 | 35 | 50 | 60 | 65 | 70 | 80 | 90 | 95 | 95 | 121 |
| Bone | 30 | 45 | 65 | 60 | 60 | 75 | 121 | ||||||
| Non-CI | Air | 30 | 45 | 55 | 65 | 70 | 70 | 70 | 90 | 100 | 121 | 121 | |
| Bone | 40 | 50 | 65 | 60 | 60 | 75 | 121 | ||||||
| US07-S12–1139 | CI | Air | 30 | 35 | 45 | 55 | 80 | 121 | 121 | 121 | 121 | 121 | 121 |
| Bone | 30 | 40 | 50 | 75 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 30 | 35 | 40 | 50 | 70 | 121 | 121 | 121 | 121 | 121 | 121 | |
| Bone | 35 | 40 | 50 | 70 | 121 | 121 | 121 | ||||||
| US09-S12–1139 | CI | Air | 50 | 50 | 50 | 45 | 50 | 70 | 75 | 85 | 95 | 100 | 90 |
| Bone | 121 | 40 | 35 | 40 | 60 | 65 | 75 | ||||||
| Non-CI | Air | 45 | 45 | 45 | 45 | 55 | 70 | 75 | 80 | 90 | 105 | 121 | |
| Bone | 121 | 40 | 35 | 45 | 60 | 70 | 85 | ||||||
| US01-S12–1202 | CI | Air | 45 | 35 | 20 | 35 | 45 | 85 | 90 | 100 | 110 | 105 | 121 |
| Bone | 25 | 15 | 25 | 30 | 75 | 75 | 121 | ||||||
| Non-CI | Air | 35 | 30 | 25 | 30 | 50 | 80 | 90 | 110 | 121 | 121 | 121 | |
| Bone | 20 | 20 | 25 | 30 | 70 | 80 | 121 | ||||||
| US01-S12–1247 | CI | Air | 55 | 55 | 45 | 45 | 50 | 75 | 70 | 90 | 95 | 90 | 90 |
| Bone | 40 | 40 | 35 | 25 | 55 | 70 | 121 | ||||||
| Non-CI | Air | 60 | 55 | 40 | 35 | 40 | 65 | 65 | 90 | 100 | 95 | 121 | |
| Bone | 121 | 35 | 20 | 20 | 50 | 70 | 121 | ||||||
| US04-S12–1460 | CI | Air | 15 | 35 | 40 | 65 | 80 | 115 | 110 | 115 | 121 | 121 | 121 |
| Bone | 35 | 45 | 65 | 70 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 20 | 30 | 45 | 60 | 75 | 110 | 121 | 115 | 121 | 121 | 121 | |
| Bone | 35 | 45 | 65 | 70 | 121 | 121 | 121 | ||||||
| US05-S12–1460 | CI | Air | 15 | 30 | 35 | 70 | 85 | 90 | 95 | 90 | 121 | 121 | 121 |
| Bone | 25 | 30 | 121 | 121 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 30 | 25 | 35 | 50 | 55 | 90 | 105 | 121 | 121 | |||
| Bone | 20 | 30 | 45 | 45 | 121 | 121 | 121 | ||||||
| US10-S12–1460 | CI | Air | 20 | 20 | 40 | 60 | 60 | 80 | 90 | 105 | 110 | 121 | 121 |
| Bone | 15 | 45 | 60 | 55 | 70 | 80 | 121 | ||||||
| Non-CI | Air | 20 | 15 | 40 | 60 | 65 | 85 | 95 | 95 | 110 | 105 | 121 | |
| Bone | 15 | 45 | 60 | 55 | 70 | 80 | 121 | ||||||
| US14-S12–1460 | CI | Air | 50 | 60 | 55 | 75 | 75 | 90 | 115 | 121 | 105 | 121 | 121 |
| Bone | 121 | 60 | 121 | 70 | 85 | 121 | 121 | ||||||
| Non-CI | Air | 25 | 30 | 40 | 55 | 60 | 70 | 75 | 121 | 105 | 121 | 121 | |
| Bone | 40 | 45 | 55 | 50 | 70 | 121 | 121 | ||||||
| US15-S12–1460 | CI | Air | 25 | 30 | 35 | 65 | 90 | 110 | 115 | 121 | 115 | 121 | 121 |
| Bone | 20 | 30 | 65 | 121 | 121 | 121 | 121 | ||||||
| Non-CI | Air | 15 | 25 | 30 | 50 | 70 | 105 | 115 | 115 | 115 | 105 | 121 | |
| Bone | 10 | 30 | 45 | 90 | 80 | 121 | 121 |
CI = cochlear implant.
Test Materials
Speech perception, audiometry, and a subjective questionnaire were administered pre- and postoperatively at 3, 6, and 12 months. To test in the most ecological condition, speech perception was tested in the subject’s everyday listening condition, defined as the condition that they used on a daily basis. For subjects with functional acoustic hearing in the implanted ear, the everyday listening condition included the acoustic component (AC) and CI and a HA on the contralateral ear. If a subject did not maintain functional hearing on the implanted ear, testing was completed using the CI programmed with a full-frequency range and an HA on the opposite ear.
The recorded CNC word test in quiet and the AzBio sentence test31 played in a multitalker babble at a +5 dB signal-to-noise ratio (SNR) at 0° azimuth were the primary speech perception measures. Two lists of CNC words and AzBio sentences were administered at 60 dBA. Scores were calculated as a percentage of the total number of words correct per list and averaged across the two lists. Unaided pure-tone air conduction thresholds were collected at 125 Hz to 8,000 Hz, and bone-conduction thresholds were collected between 250 Hz and 4,000 Hz for each ear. The Speech, Spatial, and Qualities of Hearing Scale (SSQ)32 was also administered in a one-on-one format.
Statistical Analysis
Longitudinal changes in the LF PTA were analyzed using linear mixed models. The fixed effects were LF PTA and time since activation. A random intercept model was used, and changes over time were the primary tests of interest.
This study was conducted as a within-subject design. Speech perception results were analyzed using a binomial confidence interval to compare pre- and postoperative outcomes. Linear mixed models were used to relate 12-month postoperative LF PTA hearing categories and time since activation, to CNC, AzBio, and SSQ. Duration of deafness was not significant in any models and was not included in the final analyses. An unstructured covariance structure on the residuals was included to account for within subject correlation. Pairwise comparisons between time points and LF PTA categories were made, and a Tukey-Kramer adjustment was used to adjust for multiple comparisons. A Satterthwaite adjustment was used to compute degrees of freedom. Residuals from all models were evaluated visually, and there was no evidence of model assumptions being violated. All statistical analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC).
Surgical Procedure
The 10-mm, 10-electrode S12 has a diameter of 0.2 × 0.4 mm and was designed to enter the descending basal turn of the scala tympani between 190° and 200°. This electrode is identical to the Nucleus Hybrid S8,1 except that the electrode array has four additional active electrode contacts. The purpose of the additional electrodes is to provide an increased spectral density of electrodes across the basal region of the cochlea. The S12 electrode array also incorporates a platinum ring immediately proximal to the collar. The platinum ring is intended to encourage tissue growth to promote sealing of the cochleostomy after insertion, and to fixate the array, at the cochleostomy, so that it does not migrate into or out of the cochlea. This electrode was placed through a cochleostomy located anterior and caudal to the round-window annulus using a soft surgical technique.1 Diamond burs of 1 mm and 0.5 mm were used to create an opening in the otic capsule without disturbing the endosteum. The opening into the cochlea was created with micro footplate hooks. Full insertions were accomplished on each subject.
Postoperative Programming of Electric and Acoustic Devices
All patients’ processors were programmed with a continuous interleaved sampling (CIS)-type (ACE with the number of maxima = number of channels) processing strategy. For the electrical domain, MAPs were chosen to begin at the upper frequency cutoff of functional residual hearing (90 dB HL) in the implanted ear. All electrodes were active at initial activation. The amplification characteristics for the implanted and contralateral ear were based on targets derived from the National Acoustic Laboratories HA fitting procedure. Adjustments to amplification were made postoperatively as necessary.
RESULTS
Hearing Preservation
Figure 1A–E shows the individual and averaged thresholds from 125 Hz to 8,000 Hz preoperatively, at initial activation (IA), and 3, 6, and 12 months postoperatively. Figure 1F shows the averaged thresholds over time for the range 125 Hz to 500 Hz. The averaged change in LF PTA preoperatively to IA was 14 dB HL. Between IA and 3 months postactivation, there was an additional change in LFPTA of 10 dB HL. From the linear mixed model for longitudinal changes in threshold, we were interested in pairwise comparisons between each of the time points. Using a Tukey adjustment for multiple comparisons, there was a statistically significant difference from pre- to all postoperative times (differences >14 dB, all P < .0001). There was also a statistically significant difference from IA to 3 months (difference = −9.80 dB, t = −4.12, P = .0005), 6 months (difference = −8.71 dB, t = −3.62, P = .0031) and 12 months (difference = −9.52 dB, t = −3.91, P = .001). However, there were no statistically significant changes from 3 months to 6 months (difference = 1.09 dB, t = 0.45, P = .9912), 3 months to 12 months (difference = 0.28 dB, t = 0.12, P = .99), or 6 months to 12 months (difference = −0.81 dB, t = −0.33, P = .99). This demonstrates stabilization of hearing after 3 months of CI use. Overall at 12 months, there was a 24 dB HL LF PTA change in hearing. Thresholds at 125 Hz and 250 Hz were not significantly different from each other (difference = −3.33 dB, t = −1.79, P = .1745), unlike 125 Hz and 500 Hz (difference = −11.53 dB, t = −6.19, P < .0001), and 250 Hz and 500 Hz (difference = −8.20 dB, t = −4.41, P < .0001).
Fig. 1.
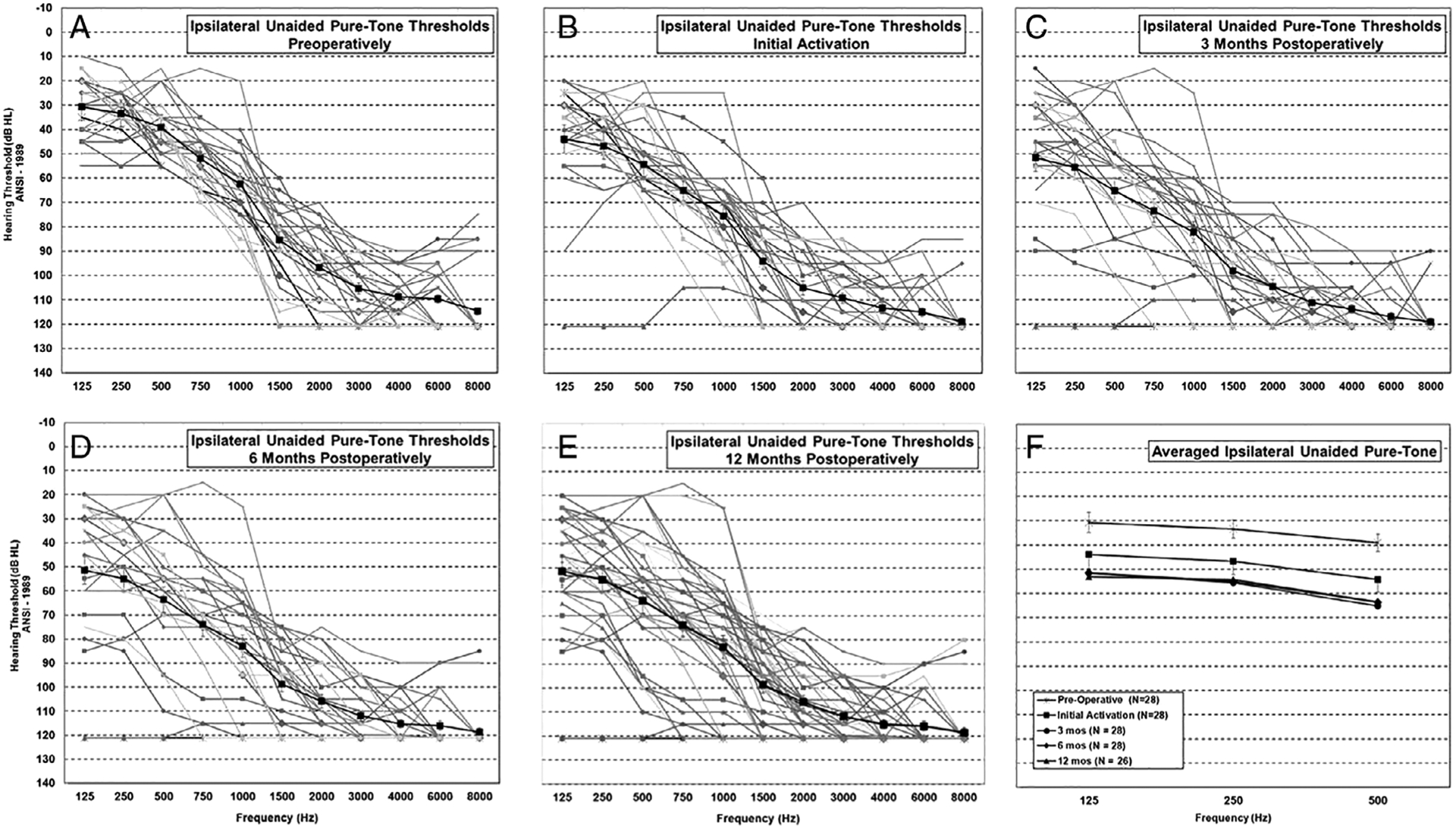
Individual unaided thresholds over the range 125 Hz to 8,000 Hz in the implanted ear shown preoperatively (A) and postoperatively at initial activation (B), 3 months (C), 6 months (D), and 12 months (E). Averaged thresholds with ±1 standard error bars (A–E) are shown with a dark black line. (F) Averaged unaided thresholds over time at 125 Hz, 250 Hz, and 500 Hz are shown. ANSI = American National Standards Institute.
Figure 2 shows the change in LF PTA at IA through 12 months postactivation for each subject. In each panel, the preoperative LF PTA is on the horizontal axis, and the postoperative LF PTA is on the vertical axis. The black diagonal line indicates no change in LF PTA. The horizontal dashed line shows the cutoff for functional hearing at 85 dB HL.1,33 In Figure 2A, we show the LF PTA for 28 subjects at IA. Evaluation of individual thresholds at IA showed that 96% (n = 27/28) of the subjects maintained functional hearing from 125 Hz to 500 Hz. In Figure 2B, C, and D, we show the LF PTA at 3, 6, and 12 months postactivation. At 3 and 6 months, 24/28 subjects (86%) maintained a functional LF PTA with no further subjects with nonfunctional hearing loss at 12 months. At 12 months, we only show the LF PTA for 27 subjects. Two subjects withdrew from the study after 6 months; one subject lost all residual hearing by 3 months, and this subject’s audiometric results were carried forward to the 12-month visit. The other subject had good hearing preservation, but was explanted due to a device failure and reimplanted with a Nucleus Hybrid L24. A case study has been published on this subject.34
Fig. 2.
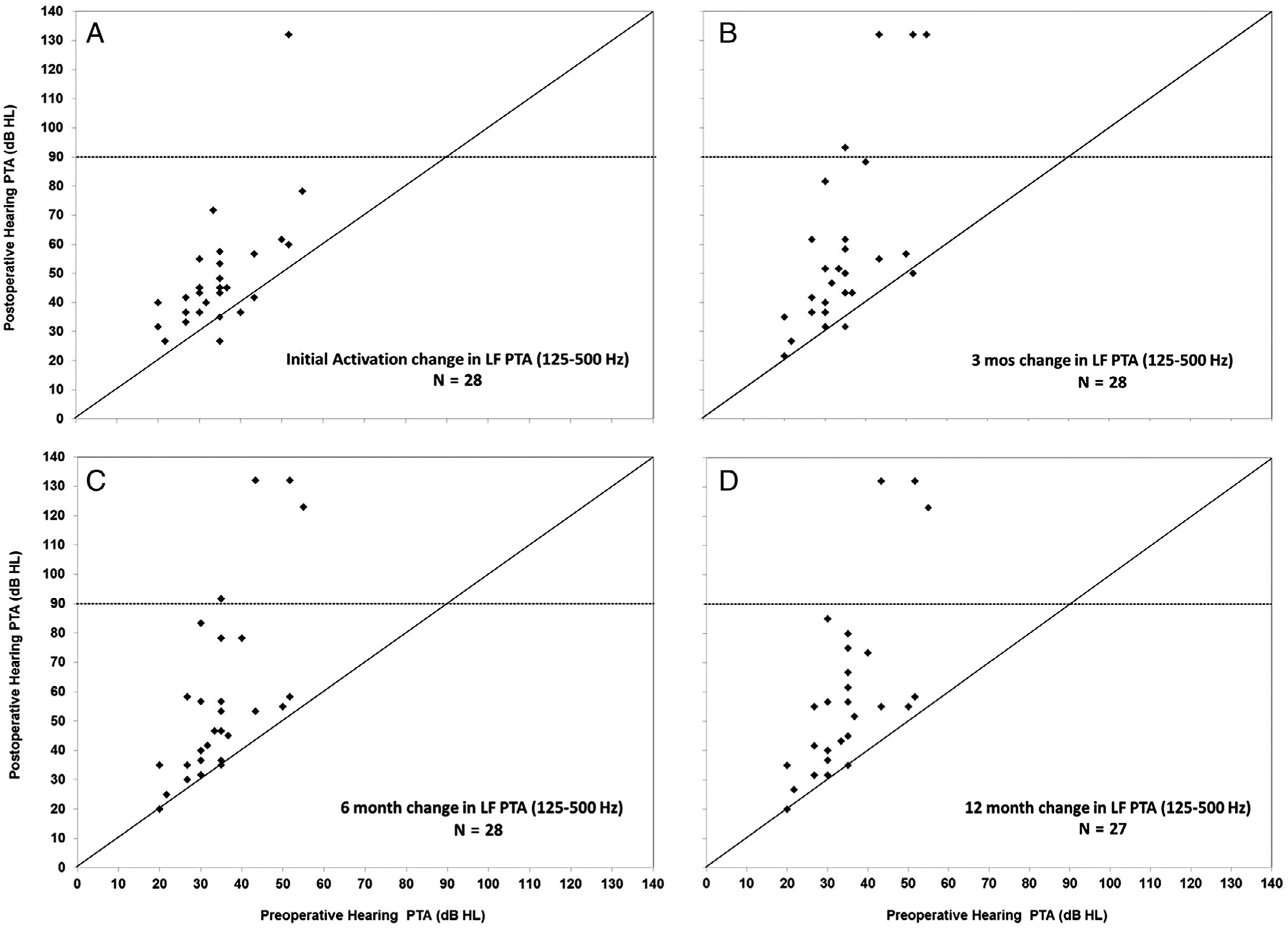
Change in individual subject low-frequency (LF) pure-tone average (PTA) at initial activation and longitudinally through 12 months postimplantation. Horizontal axis in each panel shows the preoperative LF PTA versus postoperative LF PTA at initial activation (A), 3 months (B), 6 months (C), and 12 months (D) on the vertical axis.
Speech Perception
Figure 3A shows the average scores over time for the CNC words and AzBio sentences. In Figure 3B and C, we show change in CNC words (Fig. 3B) and AzBio sentences in noise (Fig. 3C) by comparing scores with bilateral HAs preoperatively (horizontal axis) and postoperative scores at 12 months (vertical axis). Two subjects did not have 12-month scores for the reasons discussed above. Three subjects (denoted by triangles) lost acoustic hearing on the implanted ear and discontinued the AC. In Figure 3B, 21/26 (81%) of the subjects of the subjects had significant improvements (black filled circles); four subjects had no significant change in performance (open circles). Of the four subjects who did not show an improvement, one had a profound LF PTA and two had a severe LF PTA. Two subjects who lost all hearing on the implanted ear displayed substantial percentage-point improvement (39% and 45%) in CNC word understanding compared to preoperative scores. None of the subjects experienced a significant postoperative decline in word recognition.
Fig. 3.
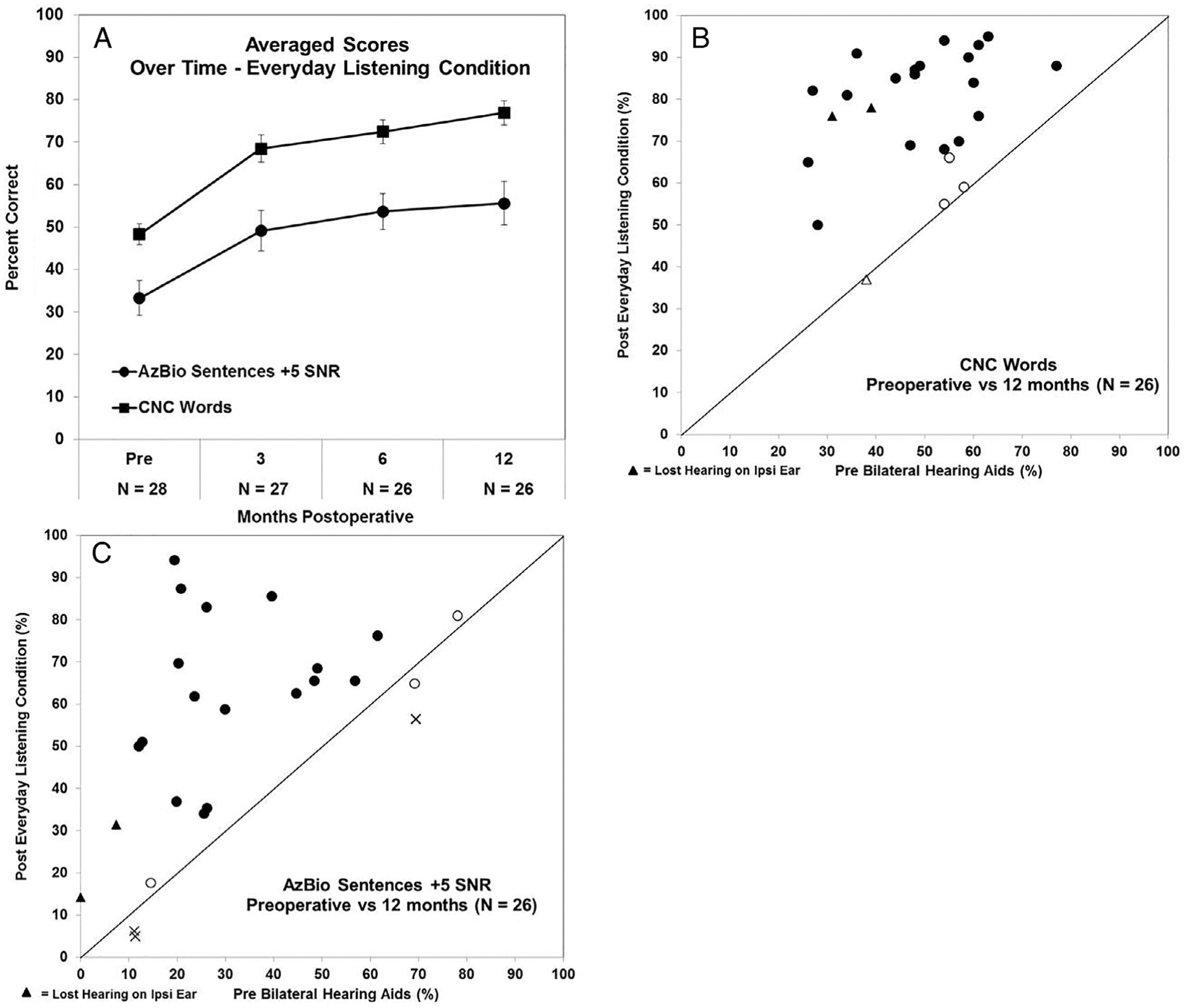
(A) Averaged Consonant-Nucleus-Consonant (CNC) word and AzBio sentence in noise scores preoperatively and 3, 6, and 12 months postoperatively in the subjects everyday listening condition with ±1 standard error bars are shown. (B, C) Change in speech perception from preoperative scores with bilateral hearing aids to 12 months in the subjects everyday listening condition. The horizontal axis in (B) (CNC words) and (C) (AzBio sentence in noise) shows the individual subject’s preoperative score, with the subsequent postoperative score at 12 months shown on the vertical axis. Individual scores that are depicted as black filled circles indicate those with a significant improvement in performance; scores that are open circles indicate no change; and scores that have an X indicate those with a significant decrement in performance. Scores with a triangle indicate those subjects who lost residual hearing on the ipsilaterally (Ipsi) implanted ear.
In Figure 3C, we evaluated the percentage of participants who improved their AzBio scores postoperatively versus those with AzBio scores that decreased or were unchanged. Twenty of 26 (77%) of the subjects had significant improvements (black filled circles); three (12%) had a significant decrement (shown as an X) and three (12%) had no significant change in performance (open circles). Of the six subjects who did not show an improvement, one had a profound LF PTA. The other five subjects had a mild (two), moderate (one), or moderately severe (two) LF PTA. Two of the subjects who did not have a significant improvement had preoperative scores of 78% and 69% and changed postoperatively at 12 months to 81% and 65%, respectively. One subject with a mild LF PTA at 12 months had a significant decrement between the preoperative score (69%) and 12 months score (56%).
To evaluate the impact of residual hearing on CNC words (Fig. 4A) and AzBio sentences (Fig. 4B), LF PTA in the implanted ear was included in the linear mixed model. Scores in Figure 4 are shown by amount of functional hearing postoperatively at 12 months where the subjects’ LF PTA was in the mild, moderate, moderately severe, severe, or profound hearing range. Each box represents the interquartile range, and the horizontal line within each box represents the median. Separate linear mixed models were constructed for the CNC words and AzBio sentences. Because the profound hearing loss group included only two individuals at 12 months, it was not included in the mixed model. Visual representation of this group shows lower speech recognition performance in both quiet and noise compared to the other groups with any amount of residual hearing.
Fig. 4.
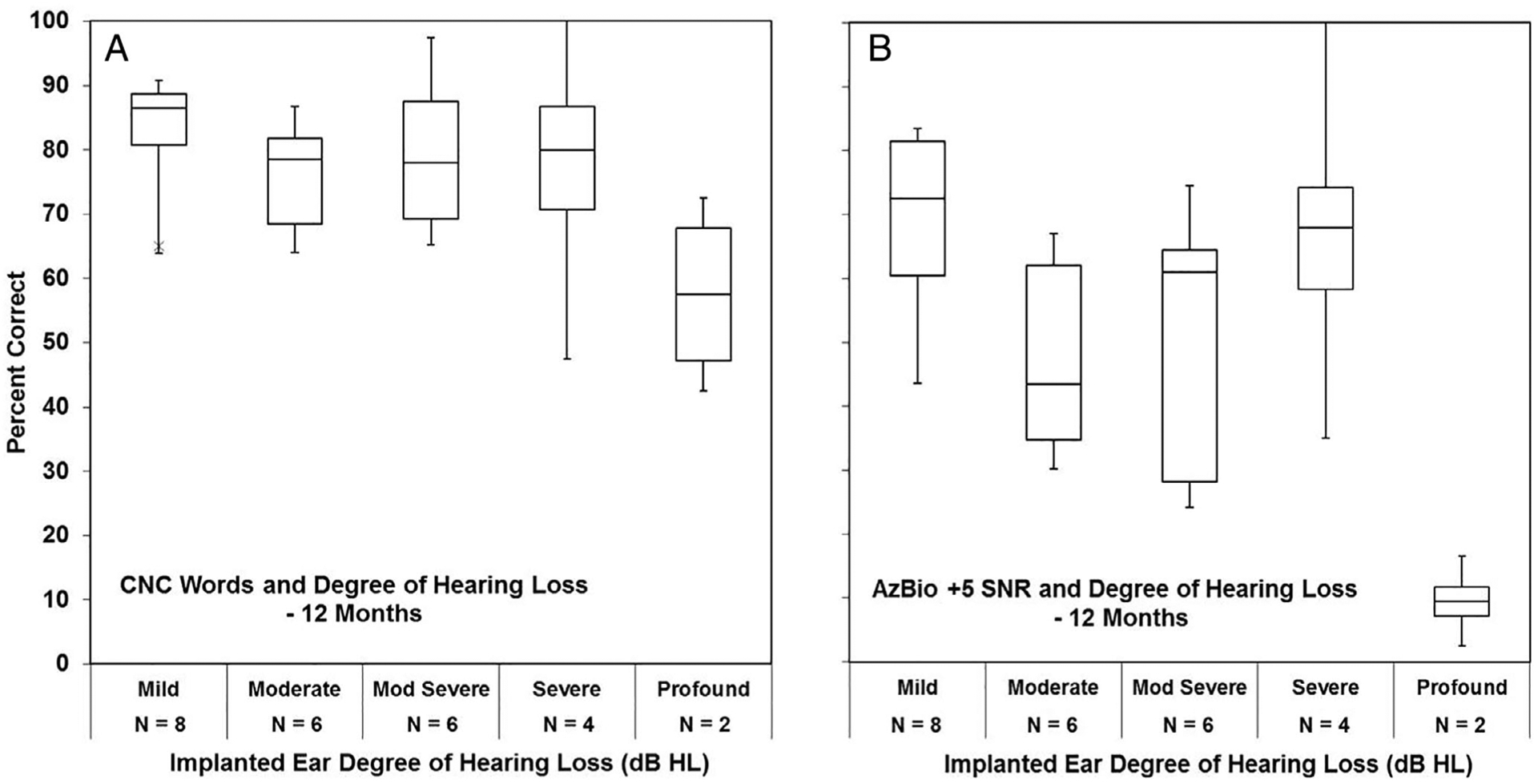
The influence of low-frequency (LF) pure-tone average (PTA) on Consonant-Nucleus-Consonant (CNC) word scores (A) and AzBio sentences in noise (B) at the 12 months postoperative time point is shown. The scores are shown by amount of functional hearing where the subjects’ implanted ear LF PTA was in the mild, moderate, moderately (Mod) severe, severe, or profound hearing range. SNR = signal-to-noise ratio.
For CNC words (Fig. 4A), only time since activation was statistically significant (F = 30.66, P < .0001), whereas LF PTA (F = 0.52, P = .6736) was not. After a Tukey-Kramer adjustment for multiple comparisons, there was a statistically significant change from preimplant to 3 months postimplant in CNC words (increase = 20.69, t = 5.15, P < .0002), but not from 3 to 6 months (increase = 4.59, t = 1.86, P = .2717) or from 6 months to 12 months (increase = 3.71, t = 1.98, P = .2241).
Results for AzBio sentences found that time (F = 8.98, P < .001) was significant, but LF PTA (F = 2.03, P = .1423) was not significant. After a Tukey-Kramer adjustment for multiple comparisons, there was a statistically significant change from preimplant to 3 months postimplant (increase = 4.92, t = 3.61, P < .001), but not for 3 to 6 months (increase = 1.19, t = 1.19, P = .6403) or from 6 months to 12 months (increase = 1.33, t = 0.44, P = .9704).
Subjective Ratings
In Figure 5, results for the subjective ratings using the SSQ are shown. Preoperative scores are shown on the horizontal axis, and postoperative scores at 12 months are shown on the vertical axis. To understand the influence of the residual hearing on each subscale, we added LF PTA into the linear mixed model. For the speech subscale (Fig. 5A), there is a significant change over time (F = 33.90, P < .0001) and effect of LF PTA (F = 5.47, P < .01). After a Tukey-Kramer adjustment for multiple comparisons, there were significant changes in SSQ-Speech scores between preimplant to 12 months (increase = 1.43, t = 4.75, P < .001). There were significant differences between mild and severe (difference = 2.17, t = 3.69, P < .01) and moderately severe to severe (difference = 1.87, t = 2.82, P < .05).
Fig. 5.
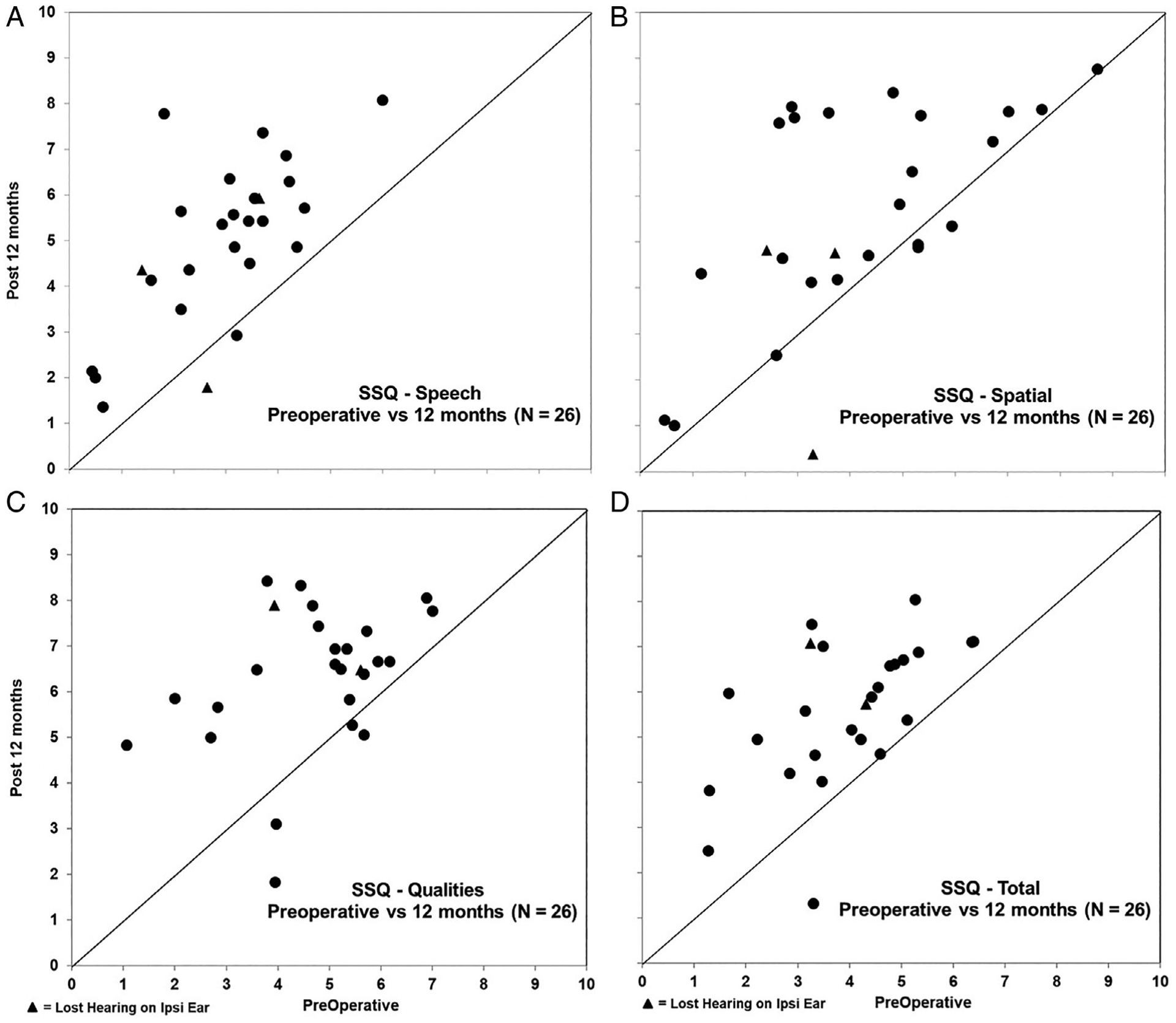
We show change in subjective Speech, Spatial, and Qualities of Hearing Scale (SSQ) ratings preoperatively with bilateral hearing aids to 12 months in the subjects everyday listening condition for the Speech (A), Spatial (B), and Qualities (C) subscales, as well as total score (D). The horizontal axis in the panels represents the individual subject’s preoperative rating, with the subsequent postoperative score at 12 months shown on the vertical axis. Individual scores with a triangle denote those subjects who lost residual hearing on the ipsilaterally (Ipsi) implanted ear.
For spatial, there is a significant change over time (F = 6.431, P < .01) and effect of LF PTA (F = 4.96, P < .01). After a Tukey-Kramer adjustment for multiple comparisons, there was a significant change in SSQ-Spatial scores between preimplant to 12 months (increase = 1.11, t = 2.82, P < .05). The only significant difference is between moderate and severe (increase = 3.49, t = 3.84, P < .01).
For qualities, there is a significant change over time (F = 13.72, P < .0001), but not for LF PTA (F = 1.97, P = .1483). After a Tukey-Kramer adjustment for multiple comparisons, there were significant changes in SSQ-Quality scores between preimplant to 12 months (increase = 1.24, t = 3.86, P < .01).
DISCUSSION
In this study, hearing preservation was considered successful. Of the 28 subjects implanted in this study, only one subject encountered profound hearing loss at initial activation. By 3 months, the LF PTA was considered statistically stable, with no statistical differences in hearing loss between three and 12 months. Overall, at 12 months, 85% (n = 23) of the 27 subjects had maintained aidable LF hearing in all or some of the LFs at 125 Hz, 250 Hz, and 500 Hz enabling the use of the AC. Two subjects in this study lost hearing very early after activation, which could possibly be attributed to cochlear toxicity caused by a high charge associated with afferent cochlear innervation injury.28 Relative to this, we now manage the programming of the CI differently by widening the pulse width rather than continuing to increase the C-level to obtain loudness growth.
Currently, two other CTs have investigated hearing preservation with Nucleus CIs and have published results. Results using data from the Nucleus Hybrid L24 CT15 calculated for frequencies 125 Hz, 250 Hz, and 500 Hz demonstrated that 74% of the 46 subjects (available to the first author) had preserved residual hearing and could use an AC at 12 months. Within the Nucleus Hybrid S8 CT,1 83% subjects (n = 81) had preserved residual hearing at those same LFs and could use an AC at 12 months. Results from this study mimic outcomes from the S8 CT, demonstrating that with the Nucleus electrodes, shorter electrodes might have superior hearing preservation outcomes. In a longitudinal study documenting outcomes associated with the L24 CT, using 125 Hz, 250 Hz, and 500 Hz, 32 subjects had reportable data available 5 years postimplantation.33 Of those, 87.5% had functional residual hearing and continue to make use of combined A+E speech processing. Furthermore, longitudinal data have been published on a cohort of subjects from the Iowa CI Clinical Research Center implanted with Nucleus Hybrid S8, S12, or L24.35 Subjects in this report show that 83% of the S8 subjects, 92% of the S12 subjects, and 86% of the L24 subjects maintained a functional hearing PTA (125 Hz–500 Hz) for up to 15 years. Some of the subjects documented in the longitudinal study by Gantz et al.35 are also being reported upon in this S12 CT update and were also subjects in the L2415 and S81 CTs.
Similar to the previously published Nucleus Hybrid CT articles,1,15 scores in both quiet and in noise for those with residual hearing were better than those without any aidable residual hearing. Our data also demonstrate that regardless of the extent of loss extending from mild to severe, even a small amount of residual hearing results in a significant improvement in speech perception in quiet and in noise compared to ears with profound or nonfunctional acoustics.
These data are further reflected in the SSQ that was assessed in this study. Data from this study showed significant subjective benefit postoperatively. Although there was a significant improvement overall in speech and spatial perceived benefit, those with mild to moderately severe degrees of LF PTA perceived more benefit on speech perception and spatial acuity than did those with a severe or profound LF PTA. Furthermore, patients felt that their perceived quality of sound was significantly better postoperatively than it was preoperatively.
CONCLUSION
The data from this study support the use of a shorter electrode for patients with severe-to-profound high-frequency hearing loss. With other studies demonstrating positive longevity results for shorter electrodes, it might be helpful to consider the impact that these devices might have on younger patients who are experiencing hearing loss who without acoustic-electric intervention might be more susceptible to other comorbidities such as dementia.
ACKNOWLEDGMENTS
The authors acknowledge the centers that participated in and contributed data to this multicenter trial: University of Iowa, University of Washington, University of San Francisco Cochlear Implant Center, Dallas Otolaryngology Associates, and the University of Miami.
This research was supported in part by research grant 2P50DC000242 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health; the Lions Clubs International Foundation; and the Iowa Lions Foundation.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope 2016;126:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mowry SE, Woodson E, Gantz BJ. New frontiers in cochlear implantation: acoustic plus electric hearing, hearing preservation, and more. Otolaryngol Clin North Am 2012;45:187–203. [DOI] [PubMed] [Google Scholar]
- 3.Kiefer J, Pok M, Adunka O, et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol 2005;10:134–144. [DOI] [PubMed] [Google Scholar]
- 4.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The Hybrid cochlear implant: a review. Adv Otorhinolaryngol 2010;67:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luetje CM, Thedinger BS, Buckler LR, Dawson KL, Lisbona KL. Hybrid cochlear implantation: clinical results and critical review in 13 cases. Otol Neurotol 2007;28:473–478. [DOI] [PubMed] [Google Scholar]
- 6.James CJ, Fraysse B, Deguine O, et al. Combined electroacoustic stimulation in conventional candidates for cochlear implantation. Audiol Neurootol 2006;11:57–62. [DOI] [PubMed] [Google Scholar]
- 7.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol 2005;125: 481–491. [DOI] [PubMed] [Google Scholar]
- 8.Gifford RH, Dorman MF, McKarns SA, Spahr AJ. Combined electric and contralateral acoustic hearing: word and sentence recognition with bimodal hearing. J Speech Lang Hear Res 2007;50:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol 2006;11: 12–15. [DOI] [PubMed] [Google Scholar]
- 10.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope 2003;113:1726–1730. [DOI] [PubMed] [Google Scholar]
- 11.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 2005;115:796–802. [DOI] [PubMed] [Google Scholar]
- 12.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurootol 2006;11:63–68. [DOI] [PubMed] [Google Scholar]
- 13.Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol 2004;24:344–347. [DOI] [PubMed] [Google Scholar]
- 14.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol 2009;14: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roland JT Jr, Gantz BJ, Waltzman SB, Parkinson AJ, Multicenter Clinical Trial Group. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope 2016;126:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillsbury HC III, Dillon MT, Buchman CA, et al. Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol 2018;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gifford RH, Dorman MF, Sheffield SW, Teece K, Olund AP. Availability of binaural cues for bilateral implant recipients and bimodal listeners with and without preserved hearing in the implanted ear. Audiol Neurootol 2014;19:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak MA, Black JM, Koch DB. Standard cochlear implantation of adults with residual low-frequency hearing: implications for combined electroacoustic stimulation. Otol Neurotol 2007;28:609–614. [DOI] [PubMed] [Google Scholar]
- 19.Dorman MF, Loiselle LH, Cook SJ, Yost WA, Gifford RH. Sound source localization by normal-hearing listeners, hearing-impaired listeners and cochlear implant listeners. Audiol Neurootol 2016;21:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loiselle LH, Dorman MF, Yost WA, Gifford RH. Sound source localization by hearing preservation patients with and without symmetrical low-frequency acoustic hearing. Audiol Neurootol 2015;20:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear Res 2014;312:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol 2010;21:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. Minimum reporting standards for adult cochlear implantation. Otolaryngol Head Neck Surg 2018;159:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan CA, Turner CW. High-frequency audibility: benefits for hearing-impaired listeners. J Acoust Soc Am 1998;104:432–441. [DOI] [PubMed] [Google Scholar]
- 25.Hornsby BW, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding.II. Sloping hearing loss. J Acoust Soc Am 2006;119:1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summers V Do tests for cochlear dead regions provide important information for fitting hearing aids? J Acoust Soc Am 2004;115:1420–1423. [DOI] [PubMed] [Google Scholar]
- 27.Vickers DA, Moore BC, Baer T. Effects of low-pass filtering on the intelligibility of speech in quiet for people with and without dead regions at high frequencies. J Acoust Soc Am 2001;110:1164–1175. [DOI] [PubMed] [Google Scholar]
- 28.Kopelovich JC, Reiss LA, Etler CP, et al. Hearing loss after activation of hearing preservation cochlear implants might be related to afferent cochlear innervation injury. Otol Neurotol 2015;36:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopelovich JC, Reiss LA, Oleson JJ, Lundt ES, Gantz BJ, Hansen MR. Risk factors for loss of ipsilateral residual hearing after hybrid cochlear implantation. Otol Neurotol 2014;35:1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillman TW, Carhart R. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66–55. Tech Rep SAM-TR 1966:1–12. [DOI] [PubMed] [Google Scholar]
- 31.Spahr AJ, Dorman MF. Performance of subjects fit with the Advanced Bionics CII and Nucleus 3G cochlear implant devices. Arch Otolaryngol Head Neck Surg 2004;130:624–628. [DOI] [PubMed] [Google Scholar]
- 32.Gatehouse S, Noble W. The speech, spatial and qualities of hearing scale (SSQ). Int J Audiol 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roland JT Jr, Gantz BJ, Waltzman SB, Parkinson AJ. Long-term outcomes of cochlear implantation in patients with high-frequency hearing loss. Laryngoscope 2018;128:1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn CC, Etler C, Hansen M, Gantz BJ. Successful hearing preservation after reimplantation of a failed hybrid cochlear implant. Otol Neurotol 2015;36:1628–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gantz BJ, Dunn CC, Oleson J, Hansen MR. Acoustic plus electric speech processing: long-term results. Laryngoscope 2018;128:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]


