Graphical abstract
Keywords: Procedure delay, COVID-19, Electronic health records
Abstract
Objective
During the COVID-19 pandemic, health systems postponed non-essential medical procedures to accommodate surge of critically-ill patients. The long-term consequences of delaying procedures in response to COVID-19 remains unknown. We developed a high-throughput approach to understand the impact of delaying procedures on patient health outcomes using electronic health record (EHR) data.
Materials and Methods
We used EHR data from Vanderbilt University Medical Center’s (VUMC) Research and Synthetic Derivatives. Elective procedures and non-urgent visits were suspended at VUMC between March 18, 2020 and April 24, 2020. Surgical procedure data from this period were compared to a similar timeframe in 2019. Potential adverse impact of delay in cardiovascular and cancer-related procedures was evaluated using EHR data collected from January 1, 1993 to March 17, 2020. For surgical procedure delay, outcomes included length of hospitalization (days), mortality during hospitalization, and readmission within six months. For screening procedure delay, outcomes included 5-year survival and cancer stage at diagnosis.
Results
We identified 416 surgical procedures that were negatively impacted during the COVID-19 pandemic compared to the same timeframe in 2019. Using retrospective data, we found 27 significant associations between procedure delay and adverse patient outcomes. Clinician review indicated that 88.9% of the significant associations were plausible and potentially clinically significant. Analytic pipelines for this study are available online.
Conclusion
Our approach enables health systems to identify medical procedures affected by the COVID-19 pandemic and evaluate the effect of delay, enabling them to communicate effectively with patients and prioritize rescheduling to minimize adverse patient outcomes.
1. Introduction
The coronavirus disease of 2019 (COVID-19) has significantly impacted health systems and patient care. In February 2020, the Center for Disease Control and Prevention (CDC) released guidelines that recommended deferring elective procedures in inpatient settings to conserve beds and personal protective equipment for a likely surge of critically ill patients with COVID-19 [1]. Meanwhile, medical centers experienced fewer admissions for common emergencies such as heart attack and stroke, potentially as a consequence of patient anxiety [2], [3], [4]. Patient anxiety and avoidance of elective or routine care may persist even as health systems lift their restrictions on elective procedures.
Studies prior to COVID-19 have shown that delay of certain procedures is associated with increased risk of adverse outcomes such as complications with the procedure or even death [5], [6]. As health systems begin rescheduling procedures, evidence-based guidelines are needed to prioritize procedures for rescheduling and to help educate patients to minimize the impact of COVID-19 on patient outcomes.
We developed two complementary approaches, one for inpatient surgical procedures and the other for outpatient screening tests, using Vanderbilt University Medical Center electronic health records (VUMC EHRs) to address several clinical needs. Our surgical procedure approach identifies all inpatient surgical procedures performed routinely, helping hospitals evaluate the effect of COVID-19 on delayed procedures. In this study, we focused only on cardiovascular and cancer-related diagnoses and procedures. Adverse outcomes measured include length of hospital stay, mortality during hospitalization, and 6-month readmission rate. A screening approach examines the potential impact of delaying screening tests or diagnostic tests. Using retrospective data, we estimated cancer screening test delay prior to diagnosis and evaluated its impact on patient outcomes, including 5-year survival and cancer stage at diagnosis.
2. Methods
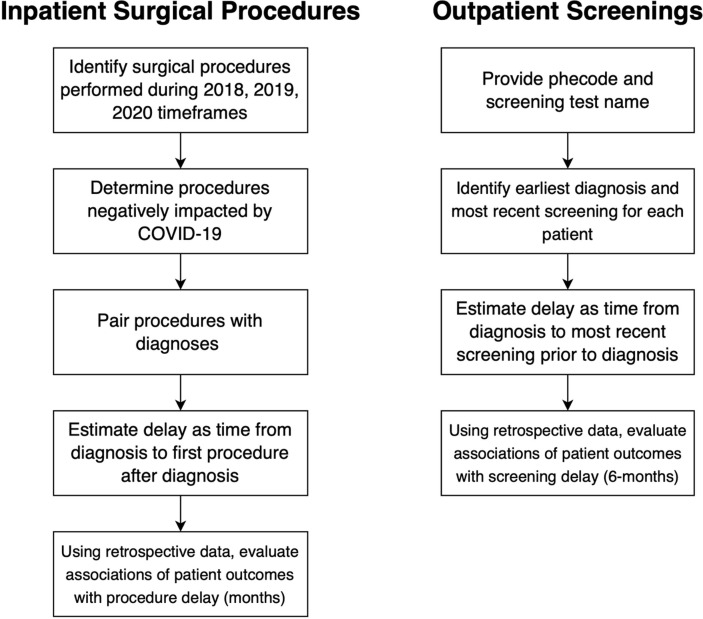
Flowcharts summarizing the approaches to surgical procedure and screening are shown in Fig. 1 . The study was approved by the Institutional Review Board of VUMC (#200731).
Fig. 1.
Flowcharts summarizing the separate approaches to surgical and screening procedures to evaluate the potential impact of surgical or screening delay on patient outcomes. Patients who did not receive the diagnosis or did not receive the relevant procedure or screening were excluded from analysis. Phecodes are manually aggregated diagnosis codes for phenome-wide association studies.
2.1. Data sources
We used VUMC’s Research and Synthetic Derivative, which contains data derived from EHRs between 1993 and 2020 for over three million unique individuals [7]. We identified procedures by Current Procedural Terminology (CPT) – a set of medical codes used to report medical procedures and services – and The International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) – a system of codes used by health insurers to classify medical procedures for billing purposes. All diagnoses that are associated with procedures were converted to ICD-10-CM (Clinical Modification); diagnoses that were coded in ICD-9-CM were mapped to equivalent ICD-10-CM using the Center for Medicare & Medicaid Services' 2018 General Equivalence Mappings (https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs).
2.2. Identifying surgical procedures negatively impacted by COVID-19
VUMC ceased elective procedures and non-urgent in-person visits between March 18, 2020 and April 24, 2020, which will hereafter be referred to as the 2020 timeframe. We selected March 18, 2019 to April 24, 2019 and March 19, 2018 to March 25, 2018 as the 2019 and 2018 timeframes, respectively. Since most procedures are routinely performed on weekdays, we selected timeframes with an equal number of weekdays and weekends and approximately at the same point in the calendar. For each procedure of interest, we counted the number of unique patients who had undergone the procedure while in inpatient care during the 2020, 2019, and 2018 timeframes. We also counted the number of unique patients who received any inpatient care during established timeframes. Inpatient stays were identified by a built-in flag in the EHR database.
The proportion of patients receiving the procedure () approximates the assumption of the binomial distribution, where the patients are independent and the probability each patient receives the procedure () is constant. To check for 2018 and 2019, we calculated the relative ratio and 95% confident intervals for procedures performed in 2018 compared to 2019. We include procedures that have a non-significant change or increased in inpatient proportion between 2018 and 2019, which is indicated when the lower 95% CI for the relative ratio < 1. We then set , the proportion of patients receiving the procedure in 2019.
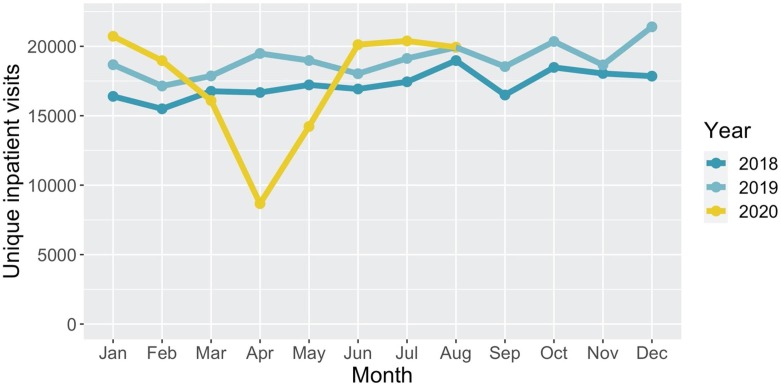
Looking at the number of unique inpatient visits at VUMC for 2018, 2019 and 2020 by month, we observed that the number of inpatient visits during 2020 was greater than during 2019 except for the months of March, April and May (Fig. 2 ). This is consistent with VUMC’s policy of ceasing elective procedures and non-urgent visits through March and April. Therefore, we assumed that the number of inpatient visits in 2020 would have been equivalent or higher than 2019 under non-pandemic circumstance. However, estimating the true number of inpatient visits under a counterfactual scenario for the binomial distribution is difficult.
Fig. 2.
Number of unique inpatient visits by month for 2018, 2019, and 2020. Data was collected on August 25, 2020.
Instead, we set , the total number of unique inpatient patients during the 2019 timeframe. Then the probability that patients will receive the procedure during the 2020 timeframe out of the total of patients is given by the following probability mass function:
We used one-tailed binomial tests (significance level set at α < 0.05) to determine whether a procedure was performed significantly fewer times when comparing the 2020 to 2019 timeframes. Since we assumed , setting means the binomial test provides a conservative estimate for the significance of change in procedure frequency. For this approach, we focused on any surgical procedures (CPT 10004–69990 and any ICD-10-PCS that starts with 0, Supplementary Table 1) and excluded procedures related to imaging and diagnostic tests, which are better addressed by the screening approach.
2.3. Estimating surgical procedure delay
We limited this component of the study to only cardiovascular and cancer-related diagnoses, which were identified by ICD codes (Supplementary Table 1), due to their greater potential risk of adverse outcomes and mortality resulting from delay. We also grouped procedures using the built-in terminology hierarchy for CPT and ICD-10-PCS to ease the computational burden when identifying diagnoses.
To estimate surgical procedure delay, we first identified diagnoses related to each procedure. A procedure can be paired with several diagnoses. For example, coronary artery bypass (ICD-10-PCS 02100Z9) can be paired with ‘Total occlusion of coronary artery’ (ICD-10-CM I25.82) and ‘Atherosclerotic heart disease of native coronary artery with unstable angina pectoris’ (ICD-10-CM I25.110). We assumed that most patients who received a procedure during an inpatient visit also received a corresponding diagnosis code during the same visit. Using historical data from January 1, 1993 to March 18, 2020, we counted the number of co-occurrences between procedures and diagnoses during the same inpatient visit. We kept diagnosis-procedure pairs that co-occurred in 50 or more inpatient visits, which is an arbitrary threshold that is adjustable for future studies to reduce computational burden and unstable statistical estimation due to small sample size.
Then, for each diagnosis-procedure pair, we calculated the proportion of patients with the diagnosis that received the procedure using historical data. This proportion helped filter out common diseases that may co-occur frequently with unrelated procedures only due to their high prevalence. For example, hypertension has high prevalence and co-occurs frequently with many cardiovascular surgical procedures, but the more likely intent of the surgical procedures is to treat a specific cardiovascular disease rather than to treat hypertension. We kept diagnosis-procedure pairs where the proportion of diagnosed patients receiving the procedure ≥ 0.10. This threshold can be reduced to include more uncommon procedures.
After the diagnosis-procedure pairs were identified, we determined the first diagnosis date and first procedure date after diagnosis for each patient across their whole EHR. Patients who did not undergo procedure were excluded from analysis. Procedure delay was defined as the difference between the first diagnosis date and the first procedure date after diagnosis. Patients whose first diagnosis date was the same as their procedure date were assigned a delay of 0 (e.g., emergency surgical procedures). However, VUMC is a tertiary care center and some patients may only visit VUMC for their procedure to treat a chronic condition. Since these patients have no previous EHRs at VUMC, it may appear as if these patients’ first diagnosis dates and procedure dates were equivalent, causing an underestimation of true procedure delay. Therefore, we only included patients with at least one outpatient visit prior to their first diagnosis.
2.4. Estimating screening and diagnostic test delay
Using historical inpatient and outpatient data from January 1, 1993 to March 18, 2020, we investigated the potential impact of delaying screening tests settings prior to breast, colorectal, lung, and prostate cancer diagnosis. Recent studies have shown a significant decrease in cancer screenings and newly diagnosed cancers during the COVID-19 pandemic [8], [9]. Therefore, cancer screening tests were highly likely to have been delayed during COVID-19. We supply phecodes, which are manually aggregated diagnosis codes for phenome-wide association studies, [10], [11] and the name of the relevant screening test. We then identify relevant procedure codes through keyword matching with the name of the screening test. For this demonstration, we used the following diagnosis-screening pairs: breast cancer (phecode 174.11) and ‘mammography’, colorectal cancer (phecode 153) and ‘colonoscopy’, prostate cancer (phecode 185) and ‘prostate specific antigen’, lung cancer (phecode 165.1) and ‘computed tomography, thorax.’
Screening test delay was defined as the time between the earliest diagnosis date and the date of the most recent non-diagnostic screening test prior to diagnosis. For instance, when a patient is diagnosed with colorectal cancer, we estimate screening test delay as the time since their last colonoscopy. Patients may have different recommendations for screening test frequency. Therefore, in our analyses, we adjusted for frequency of screening test, which was defined as years between the age at which clinical guidelines recommend regular testing to age at diagnosis divided by the number of screening tests the patient received prior to diagnosis. Tests that took place within one month of the diagnosis date were likely involved in the diagnosis and were therefore excluded.
2.5. Outcomes
For surgical procedures, the primary outcomes were length of hospital stay, mortality during hospitalization and readmission within 6 months. We defined the hospitalization length to be the days between the procedure date and the discharge date. Mortality during hospitalization was defined as death in hospital following procedure date. Readmission within 6 months was defined as any inpatient or emergency visits within 6 months after discharge from a procedural inpatient stay.
For screening procedures, the primary outcomes were 5-year survival and cancer stage at diagnosis. Since the cause of death was not available for all patients, we determined survival status for the 5-year timeframe after the diagnosis date, assuming that the majority of deaths during this 5-year timeframe can be attributed to the diagnosed cancer. We identified the cancer stage at diagnosis using VUMC’s cancer registry data.
2.6. Statistical analysis of associations between procedure delay and patient outcomes
We performed linear regression analyses between surgical procedure delay (months) and hospitalization length (days), as well as logistic regressions for mortality during hospitalization and readmission within 6 months. Regression models were adjusted for sex, race, age at the first diagnosis, insurance type (Commercial, Medicare, Medicaid, Other Government, Other), and the year that the procedure was performed.
We also evaluated the potential impact per 6-months of delay in screening. We employed Cox proportional hazard models to evaluate 5-year survival, censoring at any-cause death or last follow-up date. We treated the cancer stage as an ordinal outcome variable in logistic regressions. All models were adjusted for sex, race, age at the first diagnosis, insurance type, the year that the procedure was performed, and the frequency of screening test prior to the first diagnosis.
All statistical analyses were performed with R version 3.6.1. Statistical tests were based on 2-tailed probability and a significance level set at α < 0.05.
2.7. Estimating performance with clinician review
Three clinicians independently reviewed all the significant associations between procedure delay and adverse patient outcomes: cardiovascular-related associations were reviewed by one cardiologist and cancer-related associations were reviewed by two oncologists. The reviewers were provided the procedure, diagnosis, outcome and effect size and were asked to indicate whether the association was plausible or implausible. A third clinician reviewed any disagreements between the two oncologists.
3. Results
3.1. Demographics
As of June 2020, there were 3,169,625 individuals with available EHRs in VUMC’s Research and Synthetic derivatives, including data collected from as early as January 1, 1993. Demographic data is presented in Table 1 . Most patients are adults with several years of EHR data available for retrospective analysis.
Table 1.
Demographics for all individuals from VUMC’s Research and Synthetic derivatives included in analyses.
| Demographics | Value |
|---|---|
| Age, median (IQR) | 44 (24 to 66) |
| Female (%) | 53.5 |
| Self-reported Race (%) | |
| White | 61.8% |
| Black | 9.8% |
| Other | 3.2% |
| Unknown | 25.2% |
| EHR length, mean ± SD | 4.4 ± 6.0 |
IQR = interquartile range, SD = standard deviation, EHR = electronic health record.
3.2. Surgical procedures negatively impacted by COVID-19
We identified 2690 inpatient surgical procedures performed during the established timeframes for 2018, 2019, and 2020. The total number of unique patients with inpatient stays during the 2018, 2019, and 2020 timeframes were 20,798, 23,463, and 11,665, respectively. For the top 10 most common inpatient surgical procedures performed during the 2019 timeframe, the percent reduction in the number of procedures performed ranged from 19.7% to 90.1% during the 2020 timeframe compared to the 2019 timeframe. We identified 416 inpatient surgical procedure volumes that were significantly negatively impacted by COVID-19 (Supplementary Table 2). Table 2 shows the 10 inpatient procedures that were most significantly negatively impacted during the 2020 timeframe compared to the 2019 timeframe.
Table 2.
Top 10 inpatient procedures that were most significantly negatively impacted during the 2020 timeframe compared to the 2019 timeframe.
| Procedure Group | 2019 Count | 2020 Count | Reduction (%) | P-value a |
|---|---|---|---|---|
| Transversus abdominis plane (TAP) block (abdominal plane block, rectus sheath block) bilateral | 208 | 71 | 65.9 | 2.11 × 10-28 |
| Chemodenervation of one extremity | 123 | 25 | 79.7 | 4.43 × 10-27 |
| Biopsy, prostate | 55 | 0 | 100.0 | 1.22 × 10-24 |
| Spinal Instrumentation Procedures on the Spine (Vertebral Column) | 82 | 11 | 86.6 | 7.14 × 10-23 |
| Repair and/or Reconstruction Procedures on the Breast | 70 | 14 | 80.0 | 3.60 × 10-16 |
| Introduction Procedures on the Bladder | 102 | 32 | 68.6 | 4.70 × 10-16 |
| Posterior segmental instrumentation (e.g., pedicle fixation, dual rods with multiple hooks and sublaminar wires) | 106 | 46 | 56.6 | 3.95 × 10-11 |
| Arthrodesis, posterior or posterolateral technique, single level | 98 | 46 | 53.1 | 3.45 × 10-9 |
| Repair, Revision, and/or Reconstruction Procedures on the Pelvis and Hip Joint | 59 | 20 | 66.1 | 3.70 × 10-9 |
| Closure of enterostomy, large or small intestine | 23 | 4 | 82.6 | 1.42 × 10-6 |
P-value derived from one-tailed binomial tests.
3.3. Associations between surgical procedure delay and patient outcomes
Of the 416 inpatient surgical procedures, we identified 718 diagnosis-procedure pairs. We found 27 significant associations between surgical procedure delay and patient outcomes (Supplementary Table 3), including 22 associations for adverse patient outcomes such as increased hospitalization length or higher risk of readmission. The top 10 associations between procedure delay (months) and adverse patient outcomes are reported in Table 3 . There are some procedures that are coded both in CPT and ICD-10-PCS and therefore may appear more than once (e.g., coronary artery bypass). Patients may receive both a CPT and ICD-10-PCS code when receiving a procedure.
Table 3.
Top 10 most significant associations between procedure delay (months) and adverse patient outcomes for cardiovascular and cancer-related diseases.
| ICD-10-CM | ICD-10-CM Description a | Procedure | Procedure Description b | Reduction c | N d | Outcome e | Beta/OR (95% CI) f | P-value |
|---|---|---|---|---|---|---|---|---|
| I25.110 | Atherosclerotic heart disease of native coronary artery with unstable angina pectoris | 33,517 | Coronary artery bypass | 24.5% | 273 | Hospital LOS | 0.09 (0.04 to 0.14) | 2.26 × 10-4 |
| C32.8 | Malignant neoplasm of overlapping sites of larynx | 15,734 | Muscle, myocutaneous, or fasciocutaneous flap | 47.2% | 247 | Hospital LOS | 0.16 (0.07 to 0.25) | 6.27 × 10-4 |
| I25.110 | Atherosclerotic heart disease of native coronary artery with unstable angina pectoris | 021009 W | Coronary artery bypass | 25.9% | 289 | Hospital LOS | 0.10 (0.04 to 0.15) | 8.25 × 10-3 |
| I25.82 | Total occlusion of coronary artery | 02100Z9 | Coronary artery bypass | 25.9% | 287 | Hospital LOS | 0.13 (0.05 to 0.22) | 2.42 × 10-3 |
| I48.3 | Typical atrial flutter | 02K83ZZ | Map conduction mechanism | 35.5% | 361 | Hospital LOS | 0.13 (0.04 to 0.21) | 3.25 × 10-3 |
| I49.01 | Ventricular fibrillation | 33,241 | Removal of implantable defibrillator | 32.6% | 224 | 6-month readmission | 0.99 (0.98 to 1.00) | 3.51 × 10-3 |
| I25.82 | Total occlusion of coronary artery | 027034Z | Dilation of Coronary Artery | 47.9% | 243 | 6-month readmission | 1.07 (1.02 to 1.13) | 3.66 × 10-3 |
| I12.0 | Hypertensive chronic kidney disease with stage 5 CKD or ESRD | 50,360 | Renal allotransplantation, implantation of graft | 65.2% | 878 | 6-month readmission | 1.03 (1.01 to 1.05) | 6.01 × 10-3 |
| I71.2 | Thoracic aortic aneurysm, without rupture | 33,863 | Ascending aorta graft | 46.7% | 253 | 6-month readmission | 1.02 (1.00 to 1.03) | 7.02 × 10-3 |
| I25.110 | Atherosclerotic heart disease of native coronary artery with unstable angina pectoris | 027035Z | Dilation of Coronary Artery | 47.9% | 70 | 6-month readmission | 0.17 (0.05 to 0.30) | 7.58 × 10-3 |
CKD = chronic kidney disease; ESRD = end stage renal disease.
Procedure descriptions are abbreviated from description.
Reporting percentage reduction in volume of respective procedure group between 2019 and 2020 timeframes
N represents the number of individuals included in analysis for each diagnosis-procedure pair from historical data.
Hospital LOS = hospitalization length of stay (days); 6-month readmission = any-cause inpatient or emergency room readmission within 6 months after discharge from a procedural inpatient stay.
Reported betas and odds ratios (OR) are per month of procedure delay. Beta and 95% CIs are derived from linear regression models with hospitalization length (days) as the outcome. OR and 95% CIs are derived from logistic regression models with mortality during hospitalization and readmission within 6 months as the outcomes. All models were adjusted for sex, race, age at first diagnosis, insurance type, and year that procedure was performed.
3.4. Potential impact of delaying screening tests
In this study, we selected four common cancer screening tests to demonstrate the screening approach: colonoscopy for colorectal cancer, low-dose computed tomography (CT) for lung cancer, mammography for breast cancer, and prostate specific antigen (PSA) test for prostate cancer. Results from association analyses between delay of cancer screening tests (per 6-months) and patient outcomes are reported in Table 4 . In historical patients who were diagnosed with colorectal cancer, delay of colonoscopy prior to diagnosis was associated with increased 5-year mortality (Hazard ratio [HR] = 1.05; 95% CI = 1.01 to 1.09). Similarly, delay of computed tomography (CT) for lung cancer (HR = 1.04; 95% CI = 1.01 to 1.07) or delay of prostate-specific antigen tests for prostate cancer (HR = 1.05; 95% CI = 1.01 to 1.09) were associated with increased 5-year mortality. Moreover, delayed CT was associated with a more advanced stage of lung cancer at diagnosis (Odds ratio [OR] = 1.07; 95% CI = 1.03 to 1.10). We also found that the delay of mammography was associated with having a more advanced stage of breast cancer at diagnosis (OR = 1.08; 95% CI = 1.05 to 1.11).
Table 4.
Associations between delay of screening tests (per 6-months) and 5-year survival and cancer stage at diagnosis.
| Phecode | Phenotype | Procedure a | N b | HR/OR (95% CI) c | P-value |
|---|---|---|---|---|---|
| 5-year survival | |||||
| 153 | Colorectal cancer | Colonoscopy | 736 | 1.05 (1.01 to 1.09) | 0.019 |
| 165.1 | Lung cancer | Low-dose CT | 1668 | 1.04 (1.01 to 1.07) | 2.26 × 10-3 |
| 174.11 | Breast cancer | Mammography | 1822 | 1.01 (0.94 to 1.08) | 0.803 |
| 185 | Prostate cancer | PSA test | 3196 | 1.08 (1.03 to 1.12) | 3.79 × 10-4 |
| Cancer stage at diagnosis | |||||
| 153 | Colorectal cancer | Colonoscopy | 195 | 1.00 (0.96 to 1.05) | 0.815 |
| 165.1 | Lung cancer | Low-dose CT | 522 | 1.07 (1.03 to 1.10) | 1.07 × 10-4 |
| 174.11 | Breast cancer | Mammography | 936 | 1.08 (1.05 to 1.11) | 1.70 × 10-6 |
| 185 | Prostate cancer | PSA test | 1314 | 1.01 (0.97 to 1.05) | 0.619 |
CT = computed tomography; PSA = prostate-specific antigen.
N represents the number of cancer patients that received screening tests prior to diagnosis that were included in analysis from historical data. Cancer staging data was only available for patients diagnosed at VUMC.
Reported hazard ratios (HR) and odds ratios (OR) are per 6-months of screening test delay. HR and 95% CIs are derived from Cox proportional hazard models for 5-year survival. OR and 95% CIs are derived from ordinal logistic regression models for cancer stage at diagnosis. All models were adjusted for sex, race, age at first diagnosis, insurance type, screening test frequency, and year that diagnostic test was performed.
3.5. Evaluation of approach via clinician review
There were 32 significant associations between procedure delay and adverse patient outcomes, including 27 surgical procedure associations and 5 screening associations. The combined review of the surgical and screening procedure approaches indicated that 24 out of 32 (75.0%) significant associations between procedure or screening delay and patient outcomes were plausible. Clinicians identified 19 of 22 (86.4%) of the associations for surgical procedure delay and adverse patient outcomes (e.g., increased hospital length of stay) as plausible. For the screening approach, both oncologists classified all five of the significant associations between delay of cancer screening and 5-year mortality or cancer stage at diagnosis as plausible and potentially clinically significant.
4. Discussion
Our approaches to evaluate surgical procedures and cancer screenings offer complementary perspectives to help health systems reschedule procedures and educate patients on the potential risks of delaying procedures. Clinician review indicated that the majority of the identified significant associations were plausible and potentially clinically significant. Both approaches are built for EHRs structured with the widely adopted Observational Medical Outcomes Partnership (OMOP) Common Data Model, which will allows medical centers to efficiently implement and adapt the pipeline for their own analyses [12].
The surgical procedure approach provides a high-throughput method to identify procedures negatively impacted by COVID-19 and evaluates potential adverse patient outcomes that may result from delaying procedures. For instance, our top association indicated that delay of coronary artery bypass for patients with atherosclerotic heart disease is associated with increased hospitalization length, which is consistent with previous studies that showed delays of coronary artery bypass are associated with in-hospital mortality [13]. On the other hand, clinician review marked a few associations as implausible, such as the association between one-month delay of removal of implantable defibrillator and reduced 6-month readmission for patients with ventricular fibrillation.
The screening procedure approach allows risk assessment for patients that delayed their screening due to COVID-19. We found that a six-month delay in cancer screening was associated with increased 5-year mortality in patients who were diagnosed with colorectal, lung, and prostate cancer, and with having a more advanced cancer stage at breast and lung cancer diagnosis. Mammography delay was not significantly associated with 5-year mortality, which is likely attributed to the high 5-year survival rate for breast cancers (90% for all stages combined for cases diagnosed between 2008 and 2014) [14]. Studies have shown that early detection and treatment for breast and lung are associated with improved disease-specific and overall survival [15], [16], [17], [18], [19]. A delay in screening due to COVID-19 may delay the date of diagnosis, thereby also influencing the time from diagnosis to treatment.
There is, however, mixed-evidence on the benefits of frequent PSA test for screening asymptomatic, average-risk patients compared to the potential harms [20], [21], [22], [23], [24]. Given that all the individuals included in analysis were eventually diagnosed with cancer, it is possible that some of the administered PSA tests that preceded cancer diagnosis were for surveillance after the initial screening test was indicative of a suspected cancer or high-risk of developing cancer.
Nonetheless, the results suggest that health systems should communicate with the patients about the importance of regular screenings, especially to high-risk patients such as those with family history or other identified risk markers. It is worth pointing out that while our demonstration included only cardiovascular and cancer-related diagnoses, our approach can be easily adapted to analyze any subset of diagnoses. For instance, the screening pipeline can be adapted to evaluate non-cancer outcomes, such as diabetic retinopathy or diabetic foot problems.
There are limitations to our definition of procedure and screening delay. There is no structured variable indicating the date that the healthcare provider recommended a procedure for a patient. Our definition for surgical procedure delay (the difference between first diagnosis date and first procedure date post-diagnosis) may overestimate the true procedure delay. For instance, patients with chronic conditions may not need a procedure immediately and a longer time lag does not necessarily indicate a procedure delay. Additionally, previous studies have demonstrated that the chart fragmentation and lack of longitudinal data may harm phenotyping performance [25], [26]. Since VUMC is a tertiary care center, there may be some patients whose diagnoses or procedures were recorded at another healthcare setting. We only included patients with at least one outpatient visit prior to their first diagnosis to help capture a more comprehensive medical history. Nonetheless, an overestimation of surgical procedure delay likely results in an underestimation of the impact of procedure delay on adverse patient outcomes.
Similarly, there is no structured variable indicating when a patient is due for a recommended screening test and some patients may not have been overdue when diagnosed. We adjusted our analysis by frequency of screening test to control for differences in screening recommendations. Additionally, we used retrospective data to estimate the consequences of delays in procedures on outcomes, where historical delays may not fully reflect delays related to COVID-19. However, delays related to COVID-19 may actually compound upon delays that patient would typically experience under non-pandemic circumstances. Since we estimated delay across hundreds of diagnosis-procedure pairs, validating our delay estimates is difficult in a high-throughput manner. Therefore, our definitions for delay should be treated as the current best estimates using retrospective data. Despite these limitations, a majority (75.0%) of the significant associations by our approach were marked as plausible by clinician reviewers, supporting the potential of our approach as a discovery tool.
While procedure and screening delay may contribute to adverse patient outcomes, there are many other reasons that may also contribute to adverse patient outcomes, such as surgical complications, comorbidities, or disease severity. In our analysis, we adjusted for several important cofounders, such as type of insurance or age at diagnosis, but other factors like disease severity may impact the procedure delay and outcomes. However, identifying additional cofounders across many diseases and procedures, such as disease severity, can be difficult to do in a high-throughput manner. In addition, detailed characterization of patient features with respects to different outcomes would be helpful to better understand the findings but is difficult with outcomes for several hundred diagnosis-procedure pairings. Further study is needed to detail the underlying reasons for associations found in this study. Our analyses are also limited by considering all-cause inpatient readmission and all-cause mortality. Cause of death was only available for those who died during in-hospital stay at VUMC, limiting detailed analyses. Future research investigating disease-specific readmission or mortality may help better explain the observed associations.
There is no standardized list of elective procedures that is readily available to allow a comprehensive evaluation of all procedures that were ceased or delayed across health systems. Some of the associations identified by our surgical procedure pipeline involve procedures that may be considered by the health system to be essential and non-elective. However, several studies have reported fewer hospital admission for life-threatening conditions such as stroke and myocardial infarction during COVID-19 [2], [3], [4]. Therefore, identifying and characterizing procedures that have been affected by COVID-19, elective or non-elective, would help health systems identify and prioritize vulnerable patients and bring them back for required procedure(s) to reduce the risk adverse outcomes related to the delay. While clinicians might have intuitive knowledge of what procedures are being impacted, our approach can enable health systems and leaders to determine what can be safely delayed and to be more proactive about what cannot be delayed at a more granular level. This could help with hospital space and personal protective equipment planning and help take pressure off the clinicians who might have to evaluate “medical necessity” when ordering procedures. In addition, our findings may be valuable during physician-patient communications to help patient make informed decisions when considering the risks of delaying selected procedures and the risk of COVID-19 transmission during inpatient visits.
Our approach also encounters issues that are inherent to how patient data is entered into EHRs. For instance, Papanicolaou tests are a screening for cervical cancer and were likely impacted by COVID-19. However, Papanicolaou tests are typically entered into EHRs using a generic code for preventative medical examination, which makes it difficult to determine if a patient received the screening. Natural language processing on clinical notes may address these issues in the future.
5. Conclusions
In summary, our high-throughput approach can help health systems identify and prioritize procedures and vulnerable patient population. The surgical procedure and screening approaches are portable and flexible, allowing for natural adaptations to each health system’s unique needs or for future pandemic-like events. Moreover, our results demonstrate that informatics approaches can provide critical insights towards minimizing the adverse patient outcomes during unexpected times of crisis.
6. Data availability
The surgical procedure and screening pipelines are made freely available for download at https://github.com/cpmdev/procedure-delay. Both pipelines are written using structured query language (SQL) and R for EHRs structured within the OMOP Common Data Model [12].
7. Funding statement
The study was supported by National Institutes of Health (NIH) under grant numbers P50 GM115305, R01 HL133786, RO1 GM120523, R35 GM131770, and NIH/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA068485. The dataset used for the analyses described were obtained from Vanderbilt University Medical Center's resources, the Research and Synthetic Derivative, which are supported by institutional funding and by the National Center for Advancing Translational Science grant 2UL1 TR000445-06 from NCATS/NIH. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Neil S. Zheng: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft. Jeremy L. Warner: Validation, Writing - review & editing. Travis J. Osterman: Validation, Writing - review & editing. Quinn S. Wells: Validation, Writing - review & editing. Xiao-Ou Shu: Methodology, Writing - review & editing. Stephen A. Deppen: Methodology, Writing - review & editing. Seth J. Karp: Supervision. Shon Dwyer: Supervision. QiPing Feng: Writing - review & editing. Nancy J. Cox: Writing - review & editing. Josh F. Peterson: Writing - review & editing. C. Michael Stein: Writing - review & editing. Dan M. Roden: Writing - review & editing. Kevin B. Johnson: Conceptualization, Methodology, Writing - review & editing, Supervision. Wei-Qi Wei: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbi.2020.103657.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Center for Disease Control and Prevention. Healthcare Facilities: Preparing for Community Transmission. February 19, 2020 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fguidance-hcf.html.
- 2.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheth K. Hospital admissions for strokes appear to have plummeted, a doctor says, a possible sign people are afraid to seek critical help. The Washington Post. April 9, 2020 2020. https://www.washingtonpost.com/national/health-science/hospital-admissions-for-strokes-appear-to-have-plummeted-a-doctors-says-a-possible-sign-people-are-afraid-to-seek-critical-help/2020/04/08/2048b886-79ac-11ea-b6ff-597f170df8f8_story.html.
- 4.Solomon M.D., McNulty E.J., Rana J.S., et al. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 5.Karthik S, Grayson AD, McCarron EE, Pullan DM, Desmond MJ. Reexploration for bleeding after coronary artery bypass surgery: risk factors, outcomes, and the effect of time delay. Ann Thorac Surg 2004;78(2):527-34; discussion 34. doi: 10.1016/j.athoracsur.2004.02.088. [DOI] [PubMed]
- 6.Moran C.G., Wenn R.T., Sikand M., Taylor A.M. Early mortality after hip fracture: is delay before surgery important? J. Bone Joint Surg. Am. 2005;87(3):483–489. doi: 10.2106/JBJS.D.01796. [DOI] [PubMed] [Google Scholar]
- 7.Danciu I., Cowan J.D., Basford M., et al. Secondary use of clinical data: the Vanderbilt approach. J. Biomed. Inform. 2014;52:28–35. doi: 10.1016/j.jbi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin. Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny J.C., Ritchie M.D., Basford M.A., et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu P., Gifford A., Meng X., et al. Mapping ICD-10 and ICD-10-CM Codes to Phecodes: Workflow Development and Initial Evaluation. JMIR Med Inform. 2019;7(4) doi: 10.2196/14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss E.A., Makadia R., Matcho A., et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J. Am. Med. Inform. Assoc. 2015;22(3):553–564. doi: 10.1093/jamia/ocu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobolev B.G., Fradet G., Hayden R., Kuramoto L., Levy A.R., FitzGerald M.J. Delay in admission for elective coronary-artery bypass grafting is associated with increased in-hospital mortality. BMC Health Serv. Res. 2008;8:185. doi: 10.1186/1472-6963-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 15.Bleicher R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018;25(10):2829–2838. doi: 10.1245/s10434-018-6615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soerjomataram I., Louwman M.W., Ribot J.G., Roukema J.A., Coebergh J.W. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res. Treat. 2008;107(3):309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y., Yang Y., Inoue L.Y., Munsell M.F., Miller A.B., Berry D.A. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J. Natl. Cancer Inst. 2005;97(16):1195–1203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 18.Raz D.J., Zell J.A., Ou S.H., Gandara D.R., Anton-Culver H., Jablons D.M. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007;132(1):193–199. doi: 10.1378/chest.06-3096. [DOI] [PubMed] [Google Scholar]
- 19.International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355(17):1763-71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed]
- 20.Hayes J.H., Barry M.J. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311(11):1143–1149. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]
- 21.Carter H.B., Albertsen P.C., Barry M.J., et al. Early detection of prostate cancer: AUA Guideline. J. Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mysliwiec P.A., Brown M.L., Klabunde C.N., Ransohoff D.F. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann. Intern. Med. 2004;141(4):264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lilja H., Ulmert D., Vickers A.J. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat. Rev. Cancer. 2008;8(4):268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman D.A., Rex D.K., Winawer S.J., Giardiello F.M., Johnson D.A., Levin T.R. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Wei W.Q., Leibson C.L., Ransom J.E., et al. Impact of data fragmentation across healthcare centers on the accuracy of a high-throughput clinical phenotyping algorithm for specifying subjects with type 2 diabetes mellitus. J. Am. Med. Inform. Assoc. 2012;19(2):219–224. doi: 10.1136/amiajnl-2011-000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei W.Q., Leibson C.L., Ransom J.E., Kho A.N., Chute C.G. The absence of longitudinal data limits the accuracy of high-throughput clinical phenotyping for identifying type 2 diabetes mellitus subjects. Int. J. Med. Inform. 2013;82(4):239–247. doi: 10.1016/j.ijmedinf.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The surgical procedure and screening pipelines are made freely available for download at https://github.com/cpmdev/procedure-delay. Both pipelines are written using structured query language (SQL) and R for EHRs structured within the OMOP Common Data Model [12].